Abstract
The SNP A6986G of the CYP3A5 gene (*3) results in a non-functional protein due to a splicing defect whereas the C3435T was associated with variable expression of the ABCB1 gene, due to protein instability. Part of the large interindividual variability in tacrolimus efficacy and toxicity can be accounted for by these genetic factors. Seventy-two individuals were examined for A6986G and C3435T polymorphism using a PCR-RFLP-based technique to estimate genotype and allele frequencies in the Jordanian population. The association of age, hematocrit, platelet count, CYP3A5, and ABCB1 polymorphisms with tacrolimus dose- and body-weight-normalized levels in the subset of 38 pediatric renal transplant patients was evaluated. A Markov model was used to evaluate the time-dependent probability of an adverse event occurrence by CYP3A5 phenotypes and ABCB1 genotypes. The time-dependent probability of adverse event was about double in CYP3A5 non-expressors compared to the expressors for the first 12 months of therapy. The CYP3A5 non-expressors had higher corresponding normalized tacrolimus levels compared to the expressors in the first 3 months. The correlation trend between probability of adverse events and normalized tacrolimus concentrations for the two CYP3A5 phenotypes persisted for the first 9 months of therapy. The differences among ABCB1 genotypes in terms of adverse events and normalized tacrolimus levels were only observed in the first 3 months of therapy. The information on CYP3A5 genotypes and tacrolimus dose requirement is important in designing effective programs toward management of tacrolimus side effects particularly for the initial dose when tacrolimus blood levels are not available for therapeutic drug monitoring.
KEY WORDS: ABCB1, adverse events, CYP3A5, Markov, tacrolimus
INTRODUCTION
Pharmacogenomics play an important role in the disposition of tacrolimus. Tacrolimus is a substrate of the gastrointestinal tract p-glycoprotein and is metabolized by CYP3A4 and 3A5 in the gut wall and the liver (1,2). Mutations in genes coding for these proteins can lead to altered pharmacokinetics, as has been established for the phenotypic impact of CYP3A5*3 polymorphism (3). CYP3A5 expressor individuals with at least one wild-type allele would require a higher dose of tacrolimus in order to achieve their therapeutic goal (4–6). The variant CYP3A5*3 polymorphisms in transplant recipients predispose them to tacrolimus-related adverse events or even failure due to insufficient immunosuppression, depending on their genotype.
The ABCB1 gene codes for the gastrointestinal tract p-glycoprotein and polymorphisms in this gene could also possibly affect tacrolimus pharmacokinetics. The ABCB1 3435TT variant genotype may lead to a decreased protein expression in the intestinal tract, whereas individuals with the CC genotype had an approximately two-fold the level of p-glycoprotein expression of the TT genotype in the small intestine (7,8). Because the function of p-glycoprotein is to pump drug out of the cell, the variant genotype is thought to lead to an increase in the absorption of calcineurin inhibitors (9). ABCB1 polymorphisms and haplotypes, specifically C3435T and G2677T, were shown to be associated with tacrolimus-related nephrotoxicity in the early post-transplant period, as well as short-term remission rates in patients with ulcerative colitis on tacrolimus (10,11). Several studies, however, have found no effect of the ABCB1 haplotype on tacrolimus pharmacokinetics (12,13). When controlling for CYP3A5 genotype, specifically in the non-CYP3A5 expressors, the effects of ABCB1 haplotypes on tacrolimus pharmacokinetics were shown to be statistically significant (14). It was argued that the reason why these studies did not find an effect was due to the fact that CYP3A5 could possibly mask the effects of ABCB1 genotypes (14).
The effects that these phenotypes and genotypes can have on the concentration-profile of tacrolimus might be related to the adverse events caused by high levels of tacrolimus. The estimation of the initial dose to give to a patient is critical, given that no prior estimation of blood levels is available to determine patient’s clearance for the drug. Several investigators proposed using pharmacogenetic testing for CYP3A5 genotype as an additional factor in determining the initial dose of tacrolimus (e.g., 0.30 mg/kg/day for CYP3A5*1-allele carriers and 0.15 mg/kg/day for CYP3A5 *3/*3 homozygous patients) (15,16). Despite using a genotype-guided dosing strategy, many patients did not achieve the targeted tacrolimus levels during the initial treatment period and this could possibly be a reason for not achieving a significant improvement in the genotype-guided dosing (16). It was later argued that the fact that CYP3A5 is not the only determinant of tacrolimus disposition could be an underlying reason why a substantial number of patients did not achieve their targeted levels (17). The combination of CYP3A4 activity and CYP3A5 genotype was shown to contribute to 56–59% of variability in tacrolimus dose requirement and hematocrit accounted for 4% to 14% (17).
Many of these studies focused on the effect of polymorphisms on tacrolimus pharmacokinetics. Recently, we looked at adverse events as a relevant measure of pharmacogenomics effects on tacrolimus safety and efficacy (18). That study established the effect of both CYP3A5 and ABCB1 polymorphisms on tacrolimus safety. CYP3A5 expressors were associated with only 36% relative risk of the non-expressors and ABCB1 3435TT genotype had less overall risks than the CC and CT genotypes for adverse events, excluding graft loss, acute, and chronic rejection which are associated with insufficient immunosuppression. One of the limitations of the Cox-proportional hazard model, which was used in that analysis, is that it does not account for the change in probability of risk over time. This study evaluates the probability of developing adverse events due to CYP3A5 and ABCB1 pharmacogenomics using a Markov chain model. Because the study was carried out over a 2-year period in pediatric renal transplant patients and tacrolimus dosing is subject to rigorous therapeutic drug monitoring in these patients, the monthly risk should decrease over time due to dose adjustments. For this reason, the probability was determined in a time-dependent manner. The pediatric renal transplant population represents a unique population that also allowed us to evaluate the age-related changes in tacrolimus dosing requirement.
METHODS
The renal transplant study is a retrospective study wherein the tacrolimus blood concentration data from 38 pediatric renal transplant patients were collected for a period of approximately 22 ± 15 months. The transplant recipients were recruited from those undergoing routine therapeutic drug monitoring study of tacrolimus. Informed consent for the pediatric patients was signed by the patient’s parents or guardians and was conducted prior to their study initiation. The clinical design and outcome of the study was previously described (19). An additional 34 male adult healthy volunteers were included in this study to evaluate genotype frequencies in the Jordanian population. The adult volunteers were recruited for a bioequivalence study evaluating quetiapine tablets. Written informed consents were obtained from all adult subjects prior to inclusion in the study. Ethical approval for the study was obtained from the Institutional Review Board/independent ethics committee and the regulatory authorities in Jordan. The protocol conformed to the Declaration of Helsinki. The genetic information from subjects from two separate studies was pooled in order to provide a larger sample size.
Detection of Genetic Polymorphism and Bioanalysis
The genotyping procedure for CYP3A5 A6986G and ABCB1 C3435T and the determination of tacrolimus blood concentrations were previously described (18).
Markov Chain Model
A Markov chain model is a probability model in which the distribution of future outcomes depends only on the current state and not on the whole history (20). In other words, the probability of a certain state to occur in the following time interval is only dependent on the state in the current time interval. Markov models have been broadly applied in clinical studies (21–23). The adverse event data for the development of Markov chain model was from the pediatric renal transplant study. In this study, the transition probabilities defined were based on two states: without (state 0) and with (state 1) adverse event. This was coded per week in the observed time interval of each patient; if the subject reported at least one adverse event in that week, state 1 was assigned. Contrary to a linear regression model, a Markov chain model does not assume independence between observations, an approach that was chosen because in this case the occurrence of an adverse event is related to the concentration of the drug; an adverse event caused by a high drug concentration might not disappear if the dose is not altered. The state of the subject at a given visit is therefore conditioned on the state of his previous visit. For subjects who did not report an adverse event at a particular visit, the two transitions to the next observation are “00” if they remained without adverse event or “01” if they acquired an adverse event (Fig. 1). The properties of transition probabilities were adhered such that the sum of the transition probabilities from state 0 is 1. The same holds true for the sum of transition probabilities from state 1, so that Eq. 1 holds:
| 1 |
Fig. 1.
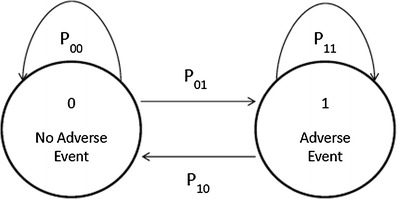
Transitions between the two states (no adverse event, adverse event, defined by the scores 0 and 1, respectively). The P xx are the transition probabilities from the different states
The transition probabilities P01 and P10 per CYP3A5 phenotypes and per ABCB1 genotypes by months were directly determined by odd ratios in the corresponding logit function:
| 2 |
| 3 |
where logiti reflects the baseline logit score for the ith transition after the first observation and θi the corresponding typical population probability operator. Pi is the probability for the ith transition. All models were fitted using two likelihood approximation algorithms, the first-order (FOCE) and second-order (LAPLACE) conditional estimation methods. No random effects were estimated as the data did not support more complex models than the structural model. After this model was developed, the probabilities for the different transitions could be computed. The probability of an adverse event happening given the patient is experiencing no adverse event was estimated in two different analyses. The first divided the patients into two groups based on their CYP3A5 phenotype, non-expressors (CYP3A5*3/3) and expressors (CYP3A5*1/3 or CYP3A5*1/1). The fixed effect parameter (theta) “P01” was estimated separately for these two groups. The second analysis divided patients into three groups, based on their ABCB1 polymorphisms, having high, medium or low expression of the p-glycoprotein (3435CC, 3435CT, and 3435TT, respectively). Here too, P01 was estimated separately for the three groups. For both analyses the probabilities were estimated per block of 3 months in order to see the time dependence of the probability of adverse events occurring. Weighting factors were introduced to the probability model based on the severity of the adverse events (Table I): 0.25 if adverse event is mild, 0.5 if moderate, 0.75 for severe and 1.0 for very severe. The attribute in Table I defines whether the adverse event was related to tacrolimus (TAC-related) or due to the combination of immunosuppressants (combination). Weighting factors for the attributes were 1 for TAC-related and 0.5 for combination. For no adverse events in both previous and current time, the weighting factors were set to 1 for both attribute and severity in order to avoid division by 0. The weighted Markov chains were established as described previously (24). Given that the probabilities P01 and P10 were determined from each adverse event, the weighting functions were incorporated to the estimation of the probabilities by multiplying the estimated probabilities by the product of the two weighting factor system based on severity (rsevr) and attribute (rattr):
| 4 |
Table I.
List of Adverse Events and Their Classification by Severity and Attribute in the Pediatric Renal Transplant Patients
| Adverse events | Severity | Attributea | Total |
|---|---|---|---|
| Gastrointestinal disorder | |||
| Gastritis (viral) | Moderate | Combination | 4 |
| Gastritis (bacterial) | Moderate | Combination | 5 |
| Vomiting, diarrhea, dyspepsia | Moderate | Combination | 10 |
| Colic abdominal pain | Mild | Combination | 1 |
| Gum hypertrophy | Mild | TAC-related | 1 |
| Gastrointestinal bleeding and constipation per rectum | Moderate | Combination | 1 |
| Infections and infestations | |||
| Urinary tract infection | Mild | Combination | 15 |
| Acute otitis media | Mild | Combination | 2 |
| Respiratory infection | Moderate | Combination | 6 |
| Gram negative/positive multiresistant acinobacter septicemia | Moderate | Combination | 3 |
| Pyelonephritis | Moderate | Combination | 2 |
| Chicken pox (varicella zoster virus) | Moderate | Combination | 2 |
| Metabolic disorders | |||
| Diabetes mellitus | Severe | TAC-related | 5 |
| Diabetic ketoacidosis | Severe | TAC-related | 4 |
| Hypokalemia | Mild | TAC-related | 2 |
| Blood and lymphatic system disorders | |||
| Anemia | Moderate | Combination | 4 |
| Leucopoenia | Moderate | Combination | 3 |
| Thrombocytopenia | Mild | Combination | 1 |
| Renal and urinary disorders | |||
| Relapse (recurrence of old disease) | Severe | Combination | 1 |
| Hydronephrosis | Severe | TAC-related | 4 |
| Nephrotoxicity | Severe | TAC-related | 4 |
| Acute rejection | Severe | TAC-related | 6 |
| Chronic rejection | V. severe | TAC-related | 6 |
| Graft loss | V. severe | Combination | 2 |
| Requiring hemodialysis after transplant | V. severe | Combination | 5 |
| General disorders | |||
| Lower limb edema | Mild | Combination | 1 |
| Perth’s disease; deformity in lower limbs | Moderate | Combination | 1 |
| Death | V. severe | Combination | 1 |
| Nervous system disorders | |||
| Organic psychosis | Severe | Combination | 2 |
| Dermatologic | |||
| Skin fungal infection | Mild | Combination | 5 |
| Skin and oral viral lesions | Mild | Combination | 8 |
| Skin bacterial infection (abscess) S. aureus and S. epidermis | Mild | Combination | 2 |
| Erythematous allergic rash; itching of upper part of the chest | Mild | Combination | 2 |
| Cardiovascular | |||
| Chest pain | Severe | TAC-related | 1 |
| Hypertensive encephalopathy | Severe | TAC-related | 1 |
| Other disorders | |||
| Visual disturbances | Mild | TAC-related | 1 |
| Dental abscess | Mild | TAC-related | 1 |
| Dehydration | Mild | TAC-related | 2 |
| Gingivitis with puffy eyes | Mild | TAC-related | 1 |
aAttribute list whether the adverse event was related to tacrolimus (TAC-related) or due to the combination of immunosuppressants (combination)
The weighted average of the probabilities is then computed as:
| 5 |
Statistical Analysis
The dose requirements were computed from trough concentrations divided by the total dose and body weight. The resulting values were then tested for normal distribution using Kolmogorov–Smirnov test. The distribution of the non-transformed values was heavily skewed. The log-normal transformed data, though still not normally distributed by the same test, was not skewed; the histogram showed a bimodal distribution (plot not shown). The transformed data were compared using a generalized linear mixed model (SAS 9.3; SAS Institute Inc., Cary, NC). The fixed effects of CYP3A5 phenotype, ABCB1 genotype, time post-transplant in months, age, hematocrit, and platelet counts were investigated with stepwise selection. Subjects were set as random effects. Appropriate covariance structures were selected to account for the repeated measures from each subject by 3-month period. The covariates were introduced with stepwise forward algorithm at the p value of 0.05, corrected for multiple testing. Tukey–Kramer’s test was used for post-hoc multiple comparisons.
Genotype and allele frequencies were computed directly using the gap and genetics packages within the statistical software R (version 2.14.0). The Hardy–Weinberg equilibrium was assessed by computing the expected values and the gene frequency deviation from this expectation, using a contingency table χ2 statistic. The R × C contingency table analysis was used to evaluate association between ABCB1 and CYP3A5 genotypes and the χ2 test was used for the evaluation. A p value of ≤0.05 was considered statistically significant.
RESULTS
Demographics
Thirty-eight pediatric kidney transplant patients, comprising 17 males and 21 females, were enrolled in the study. The demographics and clinical information of the patients are presented in Table II. All 38 patients received immunosuppressive therapy consisting of tacrolimus and either prednisone, mycophenolate mofetil (n = 27), azathioprine (n = 8) or mycophenolate sodium (n = 3). Mocyphenolate mofetil was substituted with azathioprine in five cases, while mycophenolate mofetil replaced azathioprine in another four cases due to intolerable adverse events encountered during the first year; 18.4% of the pediatric population were CYP3A5 expressors (*1/*1 and *1/*3). The adverse events experienced by the pediatric renal transplant patients are listed in Table I and described previously (19). Each adverse event is categorized by their severity and attribute. Attribute characterizes whether the specific adverse event is related to the combination of immunosuppressive drugs or is specific for tacrolimus, since all the patients were administered at least three immunosuppression drugs.
Table II.
Characteristics of Pediatric Kidney Transplant Patients and Adult Healthy Volunteers
| Patient characteristics (units) | Pediatric kidney transplant patients | Adult healthy volunteers | ||||
|---|---|---|---|---|---|---|
| Number | Mean | Range | Number | Mean | Range | |
| Gender (male/female) | 17/21 | 34/0 | ||||
| Age (year)ª | 11.3 | 4.5–19 | 36 | 19–46 | ||
| Weight (kg)ª | 29.6 | 13.5–53 | 72 | 57–94 | ||
| Height (cm)a | 128.5 | 93–153 | 173 | 160–190 | ||
| Serum creatinine (mg/dL)ª | 6.4 | 1.2–13.9 | ||||
| BUN (mg/dL)a | 75.2 | 17–201 | ||||
| Hematocrit (%)a | 26.1 | 17.1–38.2 | ||||
| Hemoglobin conc (g/dL)a | 8.6 | 5.5–12.3 | ||||
| Albumin (gm/L)a | 37.4 | 17–48 | ||||
| Total bilirubin (mg/dL) | 0.64 | 0.08–1.2 | ||||
| Modality of dialysis (n) | ||||||
| HD | 22 | |||||
| PD | 3 | |||||
| HD and PD | 2 | |||||
| Duration of dialysis (months) | 16.2 | 1.5–60 | ||||
| Pre-emptive transplant (n) | 11 | |||||
| Tacrolimus initial dose (mg, twice daily) | 7.22 | 3–14 | ||||
| Tacrolimus initial dose (mg/kg, twice daily) | 0.25 | 0.11–0.32 | ||||
| Pharmacokinetic data | ||||||
| Samples (n) | 715 | |||||
| Concentration (ng/mL) | 7.93 | 2.1–30 | ||||
| Samples per patient (n) | 18.82 | 6–49 | ||||
| Follow up period (months) | 22 | 6–64 | ||||
| Co-medication | ||||||
| Prednisone (mg/day) | 38 | 40.8 | 5–60 | |||
| Tacrolimus-azathioprineb | 8 (21.1%) | |||||
| Azathioprine (mg/day) | 75 | 50–100 | ||||
| Tacrolimus-MMFb | 27 (71.1%) | |||||
| MMF (mg/day) | 887.1 | 500–1500 | ||||
| Tacrolimus-MPSb | 3 (7.9%) | |||||
| MPS (mg/day) | 725 | 360–1440 | ||||
| Antihypertensive medications | 33 | |||||
| CYP3A5 genotype | ||||||
| *1/*1 (wild-type) | 3 (7.9%) | 3 (8.8%) | ||||
| *1/*3 | 4 (10.5%) | 6 (17.6%) | ||||
| *3/*3 | 31 (81.6%) | 25 (73.5%) | ||||
| ABCB1 genotype | ||||||
| 3435CC (wild-type) | 15 (39.5%) | 11 (32.4%) | ||||
| 3435CT | 15 (39.5%) | 17 (50%) | ||||
| 3435TT | 8 (21%) | 6 (17.6%) | ||||
BUN blood urea nitrogen; HD hemodialysis; PD peritoneal dialysis; MMF mycophenolate mofetil; MPS mycophenolate sodium
ªAt time of transplant
bInitial trial combination therapy
The 34 adult volunteers recruited for a bioequivalence study evaluating quetiapine tablets were all male with a mean age of 36 years; 26.4% of the adults were CYP3A5 expressors; 50% of the subjects had the heterozygous ABCB1 C3435T genotype; 32.4% and 17.6% were homozygous wild-type and variant genotypes, respectively. The limited demographic information of these individuals is shown in Table II.
Markov Probabilities of Adverse Events
The probability of getting an adverse event when experiencing no adverse event for CYP3A5 expressors and non-expressors over time is shown in Fig. 2. The adverse events that were severe and tacrolimus-specific had higher weights in the probability computation. The probability, in fact, is a measure of both the severity and specificity, as well as the occurrence of adverse event. In the first 12 months, non-expressors have almost double the probability of getting an adverse event compared to expressors. For both groups, the probability then declines after the first 3 months. There does not seem to be a significant overall difference between 3 and 12 months for the non-expressors. In the expressor individuals, the probability decreased over time between 3 and 12 months. The results suggest that the non-expressor individuals tend to experience more adverse event with greater severity and specificity for tacrolimus.
Fig. 2.
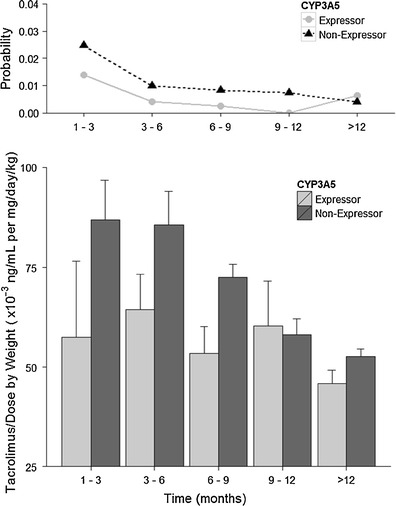
The probability of an adverse event occurring per block of 3 months given the subject has no adverse event at the current moment (P 01; top) and dose- and body-weight-normalized tacrolimus trough concentrations (bottom), stratified by CYP3A5 phenotype
Separated on the basis of ABCB1 genotype, the probability of getting an adverse event when experiencing no adverse event is plotted in Fig. 3. Weighting factors were also incorporated in the probability computation. In the first 3 months, the 3435TT genotype seems to give a lower probability of adverse events compared to the other two genotypes. The probability then declines for all three genotypes.
Fig. 3.
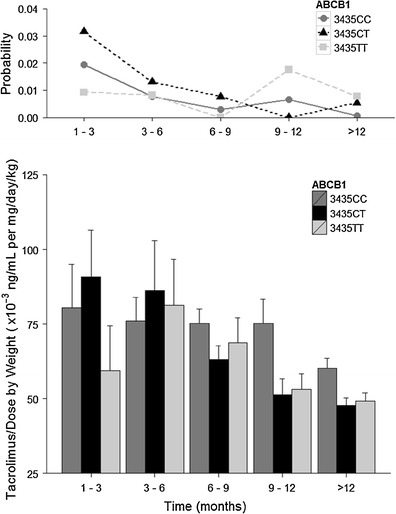
The probability of an adverse event occurring per block of 3 months given the subject has no adverse event at the current moment (P 01; top) and dose- and body-weight-normalized tacrolimus trough concentrations (bottom), stratified by ABCB1 genotype
Plotted on the lower panels of Figs. 2 and 3 were the mean ± SD of dose- and weight-normalized tacrolimus concentrations, by CYP3A5 phenotypes and ABCB1 genotypes, respectively. The CYP3A5 non-expressors had relatively higher normalized tacrolimus levels compared to the expressors in the first 3 months. This may consequently have resulted in higher observed probability of adverse events in the non-expressors. There is a good agreement in the trend between probability of adverse events and normalized tacrolimus concentrations for the two CYP3A5 phenotypes that persisted for the first 9 months of therapy. The normalized tacrolimus concentrations in the CYP3A5 non-expressors decreased over time to the level comparable to that in the expressors.
In Fig. 3, the ABCB1 3435TT genotype individuals had lower normalized tacrolimus concentrations than CC or CT. The difference diminished quickly after the third month of therapy. Similar to that observed in Fig. 2 for CYP3A5 phenotypes, the normalized tacrolimus levels decreased over time for all ABCB1 genotypes. We note that the analysis included both CYP3A5 expressors and non-expressors; this inclusion could potentially mask the contribution of ABCB1 genotypes.
Tacrolimus Dose Requirement and Influential Covariates
The first tacrolimus dose was 0.3 mg/kg in order to achieve trough concentrations between 10 and 20 ng/mL during the first month after transplant and 0.1 mg/kg thereafter to maintain a trough level of 5 to 10 ng/mL. The dose was further adjusted on the basis of patient’s response, adverse events and targeted trough drug concentration. The trough concentrations during the first month were 8.90 ± 4.55 ng/mL, ranging from 3 to 30 ng/mL. After the first month, the trough levels were 7.58 ± 3.04 ng/mL with a range between 2.1 and 25.2 ng/mL. Given that the tacrolimus dose was administered on body weight basis, the dose- and body-weight-normalized drug concentrations were deemed to be better predictors of genotype effect.
Of the tested covariates, CYP3A5 phenotype, ABCB1 genotype, hematocrit, time post-transplant, age and platelet counts were influential predictors of dose- and body-weight-normalized tacrolimus trough concentrations. Figure 4 shows the normalized tacrolimus levels by comparators which were influential covariates. The least square means (95% confidence interval, CI) of the dose-normalized by body weight concentration in the expressors and non-expressors were 37 (26–52) and 51 (41–63.4) × 10−3 ng/mL per mg/kg. The comparisons were tested by Tukey–Kramer method and adjusted for multiple comparisons (p = 0.008; Table III). For ABCB1 genotypes, the 3435TT (33, CI: 24–47) is significantly different from 3435CC (51, CI: 42–63) and CT (48, CI: 36–64), whereas CC and CT were not significantly different from each other with respect to tacrolimus dose requirement (TT vs. CT, p = 0.0001; TT vs. CC, p = 0.0008; CT vs. CC, p = 0.56; Table I). Hematocrit, separated into high (≥33%) and low (<33%) levels, was an influential factor of normalized tacrolimus levels (p = 0.0035; 48, CI: 37–62 vs. 39, CI: 30–52 × 10−3 ng/mL per mg/kg, respectively). Time after transplant in the first 9 months were significantly different from 9 to 12 and >12 months period (1–3 vs. 9–12, p = 0.0218; 1–3 vs. >12, p = 0.0005; 6–9 vs. 9–12, p < 0.0001). In this study population, the patients undergoing renal transplantation ranged from 4 to 19 years of age. The youngest patient at 4 years of age, who is also a CYP3A5 non-expressor, had the highest normalized tacrolimus. The single patient’s normalized tacrolimus levels were significantly higher than other patients who are older. However, due to small sample size (n = 1), the significance could be due to random chance.
Fig. 4.
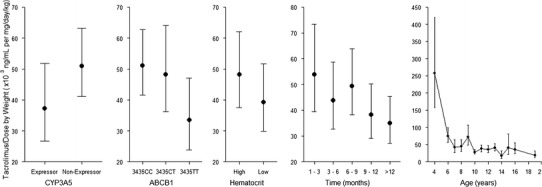
Influential covariates of normalized tacrolimus trough concentrations
Table III.
Differences of CYP3A5-Phenotype- and ABCB1-Genotype-Least-Squares Means Adjustment for Dose- and Weight-Normalized Tacrolimus Trough Concentration and Multiple Comparisons by Tukey–Kramer Post-Hoc Test
| Parameter | Estimate | SE | t value | p value | Adjusted p value |
|---|---|---|---|---|---|
| CYP3A5 phenotypes | |||||
| Expressor vs. non-expressor | −0.3160 | 0.1185 | −2.67 | 0.008 | 0.008 |
| ABCB1 genotypes | |||||
| 3435CC vs. 3435CT | 0.06045 | 0.1042 | 0.58 | 0.5621 | 0.8308 |
| 3435CC vs. 3435TT | 0.4230 | 0.1250 | 3.38 | 0.0008 | 0.0023 |
| 3435CT vs. 3435TT | 0.3626 | 0.09285 | 3.91 | 0.0001 | 0.0003 |
Frequencies of ABCB1 C3435T and CYP3A5 A6986G Polymorphism in Jordanian Population
The allele and genotype frequencies for CYP3A5 and ABCB1 in the Jordanian population are summarized in Table IV. ABCB1 C3435T variant allele frequency was 39%. In this population, the most common ABCB1 genotype, the heterozygous CT, was found in 45% of the subjects, followed by the homozygous wild-type CC with 36%. The observed genotype distributions were consistent with Hardy–Weinberg equilibrium (p > 0.1, Table IV).
Table IV.
Allele and Genotype Frequencies of CYP3A5 and ABCB1 Variants in the Jordanian Population
| SNP | Allele frequency | Genotype frequency | Expected | ||||||
|---|---|---|---|---|---|---|---|---|---|
| wild-type | Variant | Homozygous wild-type | Heterozygous | Homozygous variant | Homozygous wild-type | Heterozygous | Homozygous variant | HWE p valuea | |
| ABCB1 C3435T | 0.61 | 0.39 | 0.36 | 0.45 | 0.19 | 0.34 | 0.49 | 0.17 | 0.47 |
| CYP3A5 A6986G | 0.13 | 0.87 | 0.08 | 0.14 | 0.78 | 0.02 | 0.26 | 0.72 | 0.0007 |
SNP single nucleotide polymorphism; HWE Hardy–Weinberg equilibrium
a P value based on the exact test for Hardy–Weinberg equilibrium
The CYP3A5 A6986G (*3) variant allele frequency was estimated to be approximately 87%. Approximately 78% of the individuals were the homozygous *3/*3 variant genotype, followed by the heterozygous *1/*3 with 14% in frequency and 8% for the wild-type expressor genotype *1/*1. The *1/*1 genotype frequencies were not in Hardy–Weinberg equilibrium, wherein a greater than expected number of the wild-type homozygotes was observed (p = 0.0007). There were contradicting reports on whether CYP3A5*3 polymorphism follows Hardy–Weinberg equilibrium in various ethnic groups (25–28). The likely explanation for the deviation from Hardy–Weinberg equilibrium is that differential selection process in some geographical region could be operating against the *3 allele, given that the CYP3A5 activity is associated with xenobiotic detoxification and the lack of activity could pose a significant risk (27).
The contingency table to evaluate whether there was any association between ABCB1 and CYP3A5 genotypes is shown in Table V. In this population, all of the ABCB1 3435TT individuals also were CYP3A5 *3/*3 genotype. This could be attributed to random chance, given that the majority of this population was CYP3A5 non-expressors. The test of association using the χ2-statistic had a critical value of 7.5 at 4 degrees of freedom, which was not significant.
Table V.
Contingency Table to Evaluate Association Between ABCB1 C3435T and CYP3A5 A6986G Genotype Patterns
| CYP3A5 genotype | ABCB1 genotype | ||
|---|---|---|---|
| 3435CC | 3435CT | 3435TT | |
| 6986AA (*1/*1) | 0.0556 | 0.0278 | 0 |
| 6986AG (*1/*3) | 0.0417 | 0.0972 | 0 |
| 6986GG (*3/*3) | 0.264 | 0.319 | 0.194 |
χ 2 = 7.508; df = 4; overall P = 0.1114
DISCUSSION
This analysis shows the application and use of a Markov chain model for estimating the probability of getting an adverse event on tacrolimus when a patient has no adverse event. The CYP3A5 phenotype seems to have an influence on this probability, as in the first-year post-transplant, it was estimated to be almost doubled for the non-expressors compared to the expressors. So patients with this phenotype have a higher risk to get an adverse event related to tacrolimus toxicity and greater severity, probably due to lower clearance of the drug and therefore higher plasma concentrations. After the first 3 months, the probability of getting an adverse event in both groups declined and the difference between the two groups was consistently distinct during the first year. An explanation for this observation is that all patients were under strict therapeutic drug monitoring during the study. This means the dose was adjusted according to measured plasma levels and was therefore optimized over time to reach target plasma concentrations. However, this appears to be insufficient in the first 3 months of treatment, so that genotyping patients for their CYP3A5 polymorphism before treatment could improve safety, especially in the early phase of treatment.
Although in the first 3 months the 3435TT genotype has a lower probability for the occurrence of an adverse event, overall there is no clear trend in the effect of the ABCB1 genotype on the probability of an adverse event happening. Because the 3435CC genotype leads to higher p-glycoprotein levels in the intestine and therefore supposedly to higher export of the drug, it would lead to lower concentrations, but a lower probability of adverse events is not observed. However, many factors determine the PK of a drug, possibly obscuring this effect by other pathways, such as the CYP3A5 phenotype, which might have resulted in the inconclusiveness of this analysis. It cannot be concluded that the ABCB1 genotype has a clear effect on risk for adverse events in this study. As there are several reported inconsistencies regarding the effect of ABCB1 polymorphisms and tacrolimus-related adverse events (4,5,10–14), the mechanism underlying the association between ABCB1 variants and tacrolimus disposition is still uncertain. Further studies are necessary to elucidate the function of specific variations in the p-glycoprotein expression on the absorption process of tacrolimus in the gut and also other tissues.
There are some drawbacks in this study, one of which is that the phenotypes/genotypes are not evenly distributed. For the CYP3A5 phenotype, 18% were expressors and 82% non-expressors. The small number of patients in one group might introduce bias in the results as the variability can be high. The same holds true for the ABCB1 genotype, where approximately 20% of patients is 3435TT, compared to 40% for 3435CT and 3435CC. Additionally, the sample size is small for detecting effects of genotype; the smaller groups consist of seven or eight patients, compromising the power of this study.
For both stratifications, a probability of zero can occur as seen in Figs. 2 and 3. No patient in this specific group had an adverse event in this period and therefore the group was omitted from analysis in this time interval in order for the model to converge. This does not mean, however, that the probability was zero in this time interval, the data does not support an estimation of the probability.
In Shilbayeh et al. (19), the outcome of the pediatric renal transplantation was described without a stratified analysis based on specific genetic polymorphism. We then used a Cox-proportional hazard model to evaluate the relative risk of adverse event occurrence by CYP3A5 phenotype (18). The expressor phenotype had a significantly lower risk than the non-expressors. The results of the present Markov chain model were consistent with that observed using the Cox-proportional hazard model. The Cox-proportional hazard model, however, lacks the facility to show improvement in the management of adverse events over time, which is the primary endpoint and the reason for therapeutic drug monitoring. The application of Markov chain allowed one to visualize the time-dependent changes in the probability of adverse event occurrence. The significant decrease in the number of adverse events and severity was observed in this patient population over the 1-year period. Thus, the methodology fulfils our need for a metric to monitor whether our therapeutic monitoring program is achieving its goal and is broadly applicable for evaluating the effectiveness of therapeutic drug monitoring.
The dose requirement for tacrolimus measured by dose- and body-weight-normalized blood levels is significantly higher in CYP3A5 non-expressor than the expressor phenotype. There is a good correlation between adverse event probability and tacrolimus dose- and body-weight-normalized levels. CYP3A5 expressor individuals with at least one wild-type allele would require a higher dose of tacrolimus in order to achieve their therapeutic goal (4–6). The dose-normalized tacrolimus blood levels were significantly different between the expressors (*1/*1 or *1/*3) versus the non-expressors (*3/*3). The trend was evident in the first 9 months after transplant. In pediatric kidney transplant patients, CYP3A5 genotype in the organ recipient is more important to account for the variability in tacrolimus dosing requirement probably due to the fact that the variation in hepatic metabolism plays a greater role in tacrolimus disposition. The result of this study supports various investigations that called for genotyping to provide useful information in guiding the initial dose where prior tacrolimus blood levels may not be available for dose guidance and adjustments (29).
The effect of age in this study population was significant and consistent with that reported in the literature (30–32). The patient population in our study, however, was mainly older than 5 years of age. The pediatric patients in the study were above the neonatal age that the effect of ontogeny on drug metabolism is unlikely. One individual with 4 years of age had a significantly larger normalized tacrolimus level than the other individuals in the study. With the exception of the youngest individual, the dose- and weight-adjusted trough concentrations for pediatric patients between 5 and 19 years in this study were consistently below 0.1 ng/mL per mg/day/kg. Numerous age-related differences in drug disposition including CYP3A4/5 metabolism and p-glycoprotein transport have been reported, contributing to large interindividual variability in CYP3A activities (33–35). In heart, kidney, and liver transplant patients, children younger than 5 years of age were shown to require a larger dose of tacrolimus than older children (30–32,36–38). CYP3A4 activity is very low before birth and rapidly increases to approximately 50% of adult levels between 6 and 12 months of age whereas no developmental patterns were observed for CYP3A5 expression (39–41). The likely explanation for the larger dose requirement in pediatric patients below 5 years of age could be due to ontogeny effect of CYP3A4 rather than 3A5.
The proportion of CYP3A5 genotypes in the Jordanian population was analyzed in this study wherein the non-expressor *3/*3 genotype and those who are heterozygous *1/*3 represent 78% and 14% of study population, respectively. The variant allele in this population was detected at a frequency of approximately 87%, which is consistent with data from the Caucasian populations (29,42–45). The ABCB1 C3435T genotype frequency distribution in the Jordanian population is closer to the Caucasian and Saudi Arabian populations (7,46). The variant allele frequency in the Jordanian population was 39% which is comparable to most of the Caucasian population ranging from 32% to 60% (7,46–48). On the basis of the regional genotype frequencies for CYP3A5 A6986G and ABCB1 C3435T and in the absence of routine genotyping facilities in Jordan, we do not recommend a different dosing consensus from that in the Western countries; therapeutic drug monitoring is highly recommended as our study shows the decrease in adverse events over time due to dose adjustments.
CONCLUSION
This study shows that the probability of an adverse event occurrence in the first 3 months in the CYP3A5 non-expressors is twice in magnitude of that in the CYP3A5 expressors. This observation is consistent with the difference in tacrolimus dose requirement between the two phenotypes, measured by the tacrolimus trough concentrations. This information on CYP3A5 phenotypes and tacrolimus dose requirement is important in designing effective programs toward management of tacrolimus side effects particularly for the initial dose when tacrolimus blood levels are not available for therapeutic drug monitoring purposes.
ACKNOWLEDGMENTS
Jules Heuberger was supported by a fellowship of the Saal van Zwanenberg Stichting in the Netherlands.
REFERENCES
- 1.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43(10):623–653. doi: 10.2165/00003088-200443100-00001. [DOI] [PubMed] [Google Scholar]
- 2.Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: part II. Clin Pharmacokinet. 2010;49(4):207–221. doi: 10.2165/11317550-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Dai Y, Hebert MF, Isoherranen N, Davis CL, Marsh C, Shen DD, et al. Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug Metab Dispos. 2006;34(5):836–847. doi: 10.1124/dmd.105.008680. [DOI] [PubMed] [Google Scholar]
- 4.Zheng H, Webber S, Zeevi A, Schuetz E, Zhang J, Bowman P, et al. Tacrolimus dosing in pediatric heart transplant patients is related to CYP3A5 and MDR1 gene polymorphisms. Am J Transplant. 2003;3(4):477–483. doi: 10.1034/j.1600-6143.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 5.Zheng H, Zeevi A, Schuetz E, Lamba J, McCurry K, Griffith BP, et al. Tacrolimus dosing in adult lung transplant patients is related to cytochrome P4503A5 gene polymorphism. J Clin Pharmacol. 2004;44(2):135–140. doi: 10.1177/0091270003262108. [DOI] [PubMed] [Google Scholar]
- 6.Thervet E, Anglicheau D, King B, Schlageter MH, Cassinat B, Beaune P, et al. Impact of cytochrome p450 3A5 genetic polymorphism on tacrolimus doses and concentration-to-dose ratio in renal transplant recipients. Transplantation. 2003;76(8):1233–1235. doi: 10.1097/01.TP.0000090753.99170.89. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000;97(7):3473–3478. doi: 10.1073/pnas.97.7.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fellay J, Marzolini C, Meaden ER, Back DJ, Buclin T, Chave JP, et al. Response to antiretroviral treatment in HIV-1-infected individuals with allelic variants of the multidrug resistance transporter 1: a pharmacogenetics study. Lancet. 2002;359(9300):30–36. doi: 10.1016/S0140-6736(02)07276-8. [DOI] [PubMed] [Google Scholar]
- 9.Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: part I. Clin Pharmacokinet. 2010;49(3):141–175. doi: 10.2165/11317350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Hawwa AF, McKiernan PJ, Shields M, Millership JS, Collier PS, McElnay JC. Influence of ABCB1 polymorphisms and haplotypes on tacrolimus nephrotoxicity and dosage requirements in children with liver transplant. Br J Clin Pharmacol. 2009;68(3):413–421. doi: 10.1111/j.1365-2125.2009.03461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrlinger KR, Koc H, Winter S, Teml A, Stange EF, Fellermann K, et al. ABCB1 single-nucleotide polymorphisms determine tacrolimus response in patients with ulcerative colitis. Clin Pharmacol Ther. 2011;89(3):422–428. doi: 10.1038/clpt.2010.348. [DOI] [PubMed] [Google Scholar]
- 12.Choi JH, Lee YJ, Jang SB, Lee JE, Kim KH, Park K. Influence of the CYP3A5 and MDR1 genetic polymorphisms on the pharmacokinetics of tacrolimus in healthy Korean subjects. Br J Clin Pharmacol. 2007;64(2):185–191. doi: 10.1111/j.1365-2125.2007.02874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mai I, Perloff ES, Bauer S, Goldammer M, Johne A, Filler G, et al. MDR1 haplotypes derived from exons 21 and 26 do not affect the steady-state pharmacokinetics of tacrolimus in renal transplant patients. Br J Clin Pharmacol. 2004;58(5):548–553. doi: 10.1111/j.1365-2125.2004.02182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Zeevi A, McCurry K, Schuetz E, Zheng H, Iacono A, et al. Impact of ABCB1 (MDR1) haplotypes on tacrolimus dosing in adult lung transplant patients who are CYP3A5 *3/*3 non-expressors. Transpl Immunol. 2006;15(3):235–240. doi: 10.1016/j.trim.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 15.MacPhee IA, Fredericks S, Tai T, Syrris P, Carter ND, Johnston A, et al. The influence of pharmacogenetics on the time to achieve target tacrolimus concentrations after kidney transplantation. Am J Transplant. 2004;4(6):914–919. doi: 10.1111/j.1600-6143.2004.00435.x. [DOI] [PubMed] [Google Scholar]
- 16.Thervet E, Loriot MA, Barbier S, Buchler M, Ficheux M, Choukroun G, et al. Optimization of initial tacrolimus dose using pharmacogenetic testing. Clin Pharmacol Ther. 2010;87(6):721–726. doi: 10.1038/clpt.2010.17. [DOI] [PubMed] [Google Scholar]
- 17.de Jonge H, de Loor H, Verbeke K, Vanrenterghem Y, Kuypers DR. In vivo CYP3A4 activity, CYP3A5 genotype, and hematocrit predict tacrolimus dose requirements and clearance in renal transplant patients. Clin Pharmacol Ther. 2012;92(3):366–375. doi: 10.1038/clpt.2012.109. [DOI] [PubMed] [Google Scholar]
- 18.Sy SK, Singh RP, Shilbayeh S, Zmeili R, Conrado D, Derendorf H. Influence of CYP3A5 6986A>G and ABCB1 3435C>T polymorphisms on adverse events associated with tacrolimus in Jordanian pediatric renal transplant patients. Clin Pharmacol Drug Dev. 2013;2(1):67–78. doi: 10.1002/cpdd.22. [DOI] [PubMed] [Google Scholar]
- 19.Shilbayeh S, Hazza I. Pediatric renal transplantation in the Jordanian population: the clinical outcome measures during long-term follow-up period. Pediatr Neonatol. 2012;53(1):24–33. doi: 10.1016/j.pedneo.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Bass RF. Stochastic processes. Cambridge: Cambridge University Press; 2011. [Google Scholar]
- 21.Lacroix BD, Lovern MR, Stockis A, Sargentini-Maier ML, Karlsson MO, Friberg LE. A pharmacodynamic Markov mixed-effects model for determining the effect of exposure to certolizumab pegol on the ACR20 score in patients with rheumatoid arthritis. Clin Pharmacol Ther. 2009;86(4):387–395. doi: 10.1038/clpt.2009.136. [DOI] [PubMed] [Google Scholar]
- 22.Ouellet D, Sutherland S, Wang T, Griffini P, Murthy V. First-time-in-human study with GSK1018921, a selective GlyT1 inhibitor: relationship between exposure and dizziness. Clin Pharmacol Ther. 2011;90(4):597–604. doi: 10.1038/clpt.2011.154. [DOI] [PubMed] [Google Scholar]
- 23.Bizzotto R, Zamuner S, Mezzalana E, De Nicolao G, Gomeni R, Hooker AC, et al. Multinomial logistic functions in Markov chain models of sleep architecture: internal and external validation and covariate analysis. AAPS J. 2011;13(3):445–463. doi: 10.1208/s12248-011-9287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng Z, Bao C, Zhao Y, Yi H, Xia L, Yu H, et al. Weighted Markov chains for forecasting and analysis in incidence of infectious diseases in Jiangsu Province, China. J Biomed Res. 2010;24(3):207–214. doi: 10.1016/S1674-8301(10)60030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy JN, Lajoie J, Zijenah LS, Barama A, Poirier C, Ward BJ, et al. CYP3A5 genetic polymorphisms in different ethnic populations. Drug Metab Dispos. 2005;33(7):884–887. doi: 10.1124/dmd.105.003822. [DOI] [PubMed] [Google Scholar]
- 26.Bhatnagar V, Garcia EP, O’Connor DT, Brophy VH, Alcaraz J, Richard E, et al. CYP3A4 and CYP3A5 polymorphisms and blood pressure response to amlodipine among African-American men and women with early hypertensive renal disease. Am J Nephrol. 2010;31(2):95–103. doi: 10.1159/000258688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao DN, Manjula G, Sailaja K, Surekha D, Raghunadharao D, Rajappa S, et al. Association of CYP3A5*3 polymorphism with development of acute leukemia. Indian J Hum Genet. 2011;17(3):175–178. doi: 10.4103/0971-6866.92098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho JH, Yoon YD, Park JY, Song EJ, Choi JY, Yoon SH, et al. Impact of cytochrome P450 3A and ATP-binding cassette subfamily B member 1 polymorphisms on tacrolimus dose-adjusted trough concentrations among Korean renal transplant recipients. Transplant Proc. 2012;44(1):109–114. doi: 10.1016/j.transproceed.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Zhao W, Elie V, Roussey G, Brochard K, Niaudet P, Leroy V, et al. Population pharmacokinetics and pharmacogenetics of tacrolimus in de novo pediatric kidney transplant recipients. Clin Pharmacol Ther. 2009;86(6):609–618. doi: 10.1038/clpt.2009.210. [DOI] [PubMed] [Google Scholar]
- 30.de Wildt SN, van Schaik RH, Soldin OP, Soldin SJ, Brojeni PY, van der Heiden IP, et al. The interactions of age, genetics, and disease severity on tacrolimus dosing requirements after pediatric kidney and liver transplantation. Eur J Clin Pharmacol. 2011;67(12):1231–1241. doi: 10.1007/s00228-011-1083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gijsen V, Mital S, van Schaik RH, Soldin OP, Soldin SJ, van der Heiden IP, et al. Age and CYP3A5 genotype affect tacrolimus dosing requirements after transplant in pediatric heart recipients. J Heart Lung Transplant. 2011;30(12):1352–1359. doi: 10.1016/j.healun.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JS, Aviles DH, Silverstein DM, Leblanc PL, Matti VV. Effect of age, ethnicity, and glucocorticoid use on tacrolimus pharmacokinetics in pediatric renal transplant patients. Pediatr Transplant. 2005;9(2):162–169. doi: 10.1111/j.1399-3046.2005.00263.x. [DOI] [PubMed] [Google Scholar]
- 33.Naesens M, Salvatierra O, Li L, Kambham N, Concepcion W, Sarwal M. Maturation of dose-corrected tacrolimus predose trough levels in pediatric kidney allograft recipients. Transplantation. 2008;85(8):1139–1145. doi: 10.1097/TP.0b013e31816b431a. [DOI] [PubMed] [Google Scholar]
- 34.Sy SK, Johnston JA, Class CF, Roberts EA, Kalow W, Tang BK. Mulitvariate cluster analysis of a human hepatic cytochrome P450 database. Eur J Clin Pharmacol. 2002;58(8):559–562. doi: 10.1007/s00228-002-0515-9. [DOI] [PubMed] [Google Scholar]
- 35.Sy SK, Ciaccia A, Li W, Roberts EA, Okey A, Kalow W, et al. Modeling of human hepatic CYP3A4 enzyme kinetics, protein, and mRNA indicates deviation from log-normal distribution in CYP3A4 gene expression. Eur J Clin Pharmacol. 2002;58(5):357–365. doi: 10.1007/s00228-002-0487-9. [DOI] [PubMed] [Google Scholar]
- 36.Montini G, Ujka F, Varagnolo C, Ghio L, Ginevri F, Murer L, et al. The pharmacokinetics and immunosuppressive response of tacrolimus in paediatric renal transplant recipients. Pediatr Nephrol. 2006;21(5):719–724. doi: 10.1007/s00467-006-0014-9. [DOI] [PubMed] [Google Scholar]
- 37.Yasuhara M, Hashida T, Toraguchi M, Hashimoto Y, Kimura M, Inui K, et al. Pharmacokinetics and pharmacodynamics of FK 506 in pediatric patients receiving living-related donor liver transplantations. Transplant Proc. 1995;27(1):1108–1110. [PubMed] [Google Scholar]
- 38.Wallemacq PE, Furlan V, Moller A, Schafer A, Stadler P, Firdaous I, et al. Pharmacokinetics of tacrolimus (FK506) in paediatric liver transplant recipients. Eur J Drug Metab Pharmacokinet. 1998;23(3):367–370. doi: 10.1007/BF03192295. [DOI] [PubMed] [Google Scholar]
- 39.Hines RN. Ontogeny of human hepatic cytochromes P450. J Biochem Mol Toxicol. 2007;21(4):169–175. doi: 10.1002/jbt.20179. [DOI] [PubMed] [Google Scholar]
- 40.de Wildt SN, Kearns GL, Leeder JS, van den Anker JN. Cytochrome P450 3A: ontogeny and drug disposition. Clin Pharmacokinet. 1999;37(6):485–505. doi: 10.2165/00003088-199937060-00004. [DOI] [PubMed] [Google Scholar]
- 41.Stevens JC, Hines RN, Gu C, Koukouritaki SB, Manro JR, Tandler PJ, et al. Developmental expression of the major human hepatic CYP3A enzymes. J Pharmacol Exp Ther. 2003;307(2):573–582. doi: 10.1124/jpet.103.054841. [DOI] [PubMed] [Google Scholar]
- 42.Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27(4):383–391. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 43.Dally H, Bartsch H, Jager B, Edler L, Schmezer P, Spiegelhalder B, et al. Genotype relationships in the CYP3A locus in Caucasians. Cancer Lett. 2004;207(1):95–99. doi: 10.1016/j.canlet.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 44.Gervasini G, Vizcaino S, Gasiba C, Carrillo JA, Benitez J. Differences in CYP3A5*3 genotype distribution and combinations with other polymorphisms between Spaniards and Other Caucasian populations. Ther Drug Monit. 2005;27(6):819–821. doi: 10.1097/01.ftd.0000186914.32038.a0. [DOI] [PubMed] [Google Scholar]
- 45.Sinues B, Vicente J, Fanlo A, Vasquez P, Medina JC, Mayayo E, et al. CYP3A5*3 and CYP3A4*1B allele distribution and genotype combinations: differences between Spaniards and Central Americans. Ther Drug Monit. 2007;29(4):412–416. doi: 10.1097/FTD.0b013e31811f390a. [DOI] [PubMed] [Google Scholar]
- 46.Ameyaw MM, Regateiro F, Li T, Liu X, Tariq M, Mobarek A, et al. MDR1 pharmacogenetics: frequency of the C3435T mutation in exon 26 is significantly influenced by ethnicity. Pharmacogenetics. 2001;11(3):217–221. doi: 10.1097/00008571-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Ostrovsky O, Nagler A, Korostishevsky M, Gazit E, Galski H. Genotype and allele frequencies of C3435T polymorphism of the MDR1 gene in various Jewish populations of Israel. Ther Drug Monit. 2004;26(6):679–684. doi: 10.1097/00007691-200412000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Santos PC, Soares RA, Santos DB, Nascimento RM, Coelho GL, Nicolau JC, et al. CYP2C19 and ABCB1 gene polymorphisms are differently distributed according to ethnicity in the Brazilian general population. BMC Med Genet. 2011;12:13. doi: 10.1186/1471-2350-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


