Abstract
DNA binding factors are essential for regulating gene expression. CTCF and cohesin are DNA binding factors with central roles in chromatin organization and gene expression. We determined the sites of CTCF and cohesin binding to DNA in mouse brain, genome wide and in an allele-specific manner with high read-depth ChIP-seq. By comparing our results with existing data for mouse liver and embryonic stem (ES) cells, we investigated the tissue specificity of CTCF binding sites. ES cells have fewer unique CTCF binding sites occupied than liver and brain, consistent with a ground-state pattern of CTCF binding that is elaborated during differentiation. CTCF binding sites without the canonical consensus motif were highly tissue specific. In brain, a third of CTCF and cohesin binding sites coincide, consistent with the potential for many interactions between cohesin and CTCF but also many instances of independent action. In the context of genomic imprinting, CTCF and/or cohesin bind to a majority but not all differentially methylated regions, with preferential binding to the unmethylated parental allele. Whether the parental allele-specific methylation was established in the parental germlines or post-fertilization in the embryo is not a determinant in CTCF or cohesin binding. These findings link CTCF and cohesin with the control regions of a subset of imprinted genes, supporting the notion that imprinting control is mechanistically diverse.
DNA sequences that control transcription are frequently located in the noncoding portion of the mammalian genome (The ENCODE Project Consortium 2012). These elements can act over long distances (Noonan and McCallion 2010). The identification of these control elements is important for elucidating human genetic disease since genome-wide association studies regularly point to noncoding regions as candidates in disease etiology (Manolio 2010). One of the proteins that contributes to the regulation of gene expression across the genome is CTCF (CCCTC-binding factor), a protein with 11 zinc fingers (Filippova et al. 1996) and multiple regulatory functions (Ohlsson 2001; Gaszner and Felsenfeld 2006). CTCF can act as an insulator by blocking interactions between enhancers and promoters (Bell et al. 1999), it can directly regulate chromosomal interactions (Yusufzai and Felsenfeld 2004; Hadjur et al. 2009), and it can act as an enhancer of transcription (Kuzmin et al. 2005). CTCF binds regions of DNA with high sequence specificity and is sensitive to DNA methylation, having a lower binding affinity for methylated DNA (Mukhopadhyay et al. 2004). The canonical consensus binding motif of CTCF and the sites of CTCF binding are evolutionarily conserved between mammals and birds (Martin et al. 2011; Schmidt et al. 2012). In vitro assays have shown that CTCF can use different combinations of its zinc fingers to bind to distinct DNA sequences (Filippova et al. 1996). CTCF interacts with a variety of other factors. In particular, the cohesin complex, best known for its role in mediating sister-chromatid cohesion during cell division, has been found to frequently colocalize with CTCF during interphase (Parelho et al. 2008; Rubio et al. 2008; Wendt et al. 2008; Xiao et al. 2011) with consequences for gene expression. At specific loci, cohesin is required for cell-type-specific long-range chromosomal interactions in cis during cellular differentiation (Hadjur et al. 2009).
Genomic imprinting refers to the parental allele-specific transcription of a subset of genes in mammals and flowering plants (Reik and Walter 2001; da Rocha et al. 2008). Roughly 140 transcripts are known to be imprinted in mammals (Schulz et al. 2008). Imprinting is controlled by epigenetic modifications that differ between the two parental genomes, including differences in DNA methylation (Li et al. 1993). Imprinted genes can occur in large, coordinately regulated clusters exemplified by the Gnas locus (Peters and Williamson 2007); they can form small domains such as the Mcts2/H13 locus (Wood et al. 2008) that are comprised of only two genes (McCole and Oakey 2008), or they can be singletons like Impact (Hagiwara et al. 1997). In all cases, their parental allele-specific expression is ultimately due to an imprinting control region (ICR), a region of DNA that is differentially methylated between the parental alleles. The parental allele-specific methylation of a differentially methylated region (DMR) is in most cases the consequence of the sex-specific epigenetic reprogramming of the parental germ cells (Edwards and Ferguson-Smith 2007; Bartolomei 2009). In addition, DMRs are actively protected from post-fertilization epigenetic reprogramming (Quenneville et al. 2012) and, thus, persist into adulthood. In some cases, the parental allele-specific methylation of a DMR is set up post-fertilization during early embryogenesis (somatic DMRs) (Kobayashi et al. 2012). DMRs with a direct germline origin are referred to as germline DMRs (gDMRs). Disruption of imprinted gene expression after deletion of a DMR is considered evidence that the latter functions as an ICR. There are 22 well-established gDMRs in mouse, of which 19 are maternally methylated and three are paternally methylated. Many mechanisms exist to “translate” allele-specific methylation into differential gene expression, including differential protein binding (Lewis and Reik 2006).
CTCF has long been associated with genomic imprinting due to its selective binding of the unmethylated maternal allele of the Igf2/H19 ICR resulting in parent-of-origin-specific expression of Igf2 and H19 in mouse and human (Bell and Felsenfeld 2000; Hark et al. 2000; Kanduri et al. 2000; Fedoriw et al. 2004; Szabo et al. 2004). CTCF has been studied at several other imprinted loci, and it binds the unmethylated allele at the gDMRs of Rasgrf1, Peg13, Kcnq1ot1 (Yoon et al. 2005; Fitzpatrick et al. 2007; Singh et al. 2011), and Grb10 (Hikichi et al. 2003; Mukhopadhyay et al. 2004). CTCF-mediated regulation is postulated to be one of two major control mechanisms operating at ICRs (Lewis and Reik 2006; Kim et al. 2009). Cohesin also has been linked to imprinting through its association with CTCF at the H19/Igf2 and Kcnq1ot1 DMRs (Stedman et al. 2008; Lin et al. 2011), and a role for cohesin in the allele-specific organization of higher-order chromatin has been proposed (Nativio et al. 2009). Here we present the first comprehensive analysis of allele-specific CTCF and cohesin binding at all known DMRs in a single tissue, providing an unbiased assessment of the extent to which CTCF and cohesin are involved in imprinting control.
Genome-wide ChIP-seq in mouse ES cells (Chen et al. 2008; Kagey et al. 2010) and human cells (Kim et al. 2007b) has shown that CTCF and cohesin bind tens of thousands of discrete sites across the genome, and CTCF binding is enriched in and near genes, consistent with a role in the control of gene expression. Mouse embryonic stem (ES) cell data identify CTCF and cohesin binding at the gDMRs of Peg13, Zim2 (Peg3), Peg10, Grb10, and Mest but not at the H19/Igf2 ICR, even though CTCF is known to be important for imprinting regulation at this domain. Imprinting is dispensable in ES cells, where loss of imprinting frequently occurs without affecting viability in culture (Kim et al. 2007a; Rugg-Gunn et al. 2007; Frost et al. 2011). The same is true for Dnmt1−/−, Dnmt3a−/−, Dnmt3b−/− triple knockout mouse ES cells that consequently lack all DNA methylation imprints but yet are viable (Tsumura et al. 2006). In contrast, a differentiated tissue where imprinting plays an important role is the brain (Davies et al. 2007), and this is supported by multiple lines of evidence. Firstly, the human imprinting disorders Prader-Willi syndrome and Angelman syndrome present with behavioral and neurodevelopmental phenotypes (Cassidy et al. 2000; Lossie et al. 2001; Williams et al. 2006); secondly, of the ∼140 imprinted gene transcripts in the mouse, more than 50 are expressed in brain (Wilkins 2008); thirdly, the disruption of certain mouse imprinted genes, including Peg3 (Li et al. 1999), Mest (Lefebvre et al. 1998), Nesp55 (Plagge et al. 2005), and Grb10 (Garfield et al. 2011), results in behavioral phenotypes; finally, genome-wide allele-specific studies of transcription in mouse brain suggest that this tissue is a focus for imprinted gene expression (Gregg et al. 2010a,b; DeVeale et al. 2012).
Our analyses of CTCF and cohesin binding in mouse brain are based on ChIP-seq data of high quality and an order of magnitude higher read depth than existing data. The use of reciprocal inter-subspecies hybrid mice enabled independent interrogation of the parental alleles in terms of CTCF and cohesin binding in unprecedented detail. We examined postnatal day 21 (P21) mouse brain, a time point in development shortly after the growth spurt in neurogenesis that occurs in the first 2 wk of postnatal development (Lyck et al. 2007). In the adult mouse brain, ∼56% of cells are neurons and 44% are nonneuronal cells (Fu et al. 2012). Neurons and the principle type of nonneuronal cells, the macroglia, both derive from the neuroepithelium. These data are representative of adult rather than immature brain cell types and are unaffected by long-term aging effects.
Results
We demonstrate that in mouse brain, CTCF and cohesin each bind to ∼50,000 sites in the genome, with ∼27,000 sites bound by both factors, indicative of CTCF and cohesin acting throughout the genome both in concert as well as independently. Genes are enriched for CTCF binding sites, while intergenic regions are depleted. The binding sites are highly enriched for the canonical consensus binding motif. CTCF binding sites are relatively hypomethylated, both in the CpG and non-CpG sequence context. Parental allele-specific CTCF binding is rare, with most sites at or near imprinted loci. However, a majority but not all DMRs are bound by CTCF (or cohesin), and the binding is not necessarily allele specific. The Magel2/Peg12 imprinted locus is unique in the genome, comprising a cluster of eight allele-specific CTCF binding sites. Comprehensive expression profiling in mouse brain of genes near allele-specific CTCF binding sites not previously associated with imprinting did not reveal novel imprinted genes. No allele-specific cohesin binding sites of genome-wide significance were found, although at allele-specific CTCF binding sites, there is a trend for cohesin to bind the same allele.
Deep ChIP-seq for CTCF and cohesin to detect parental allele-specific binding
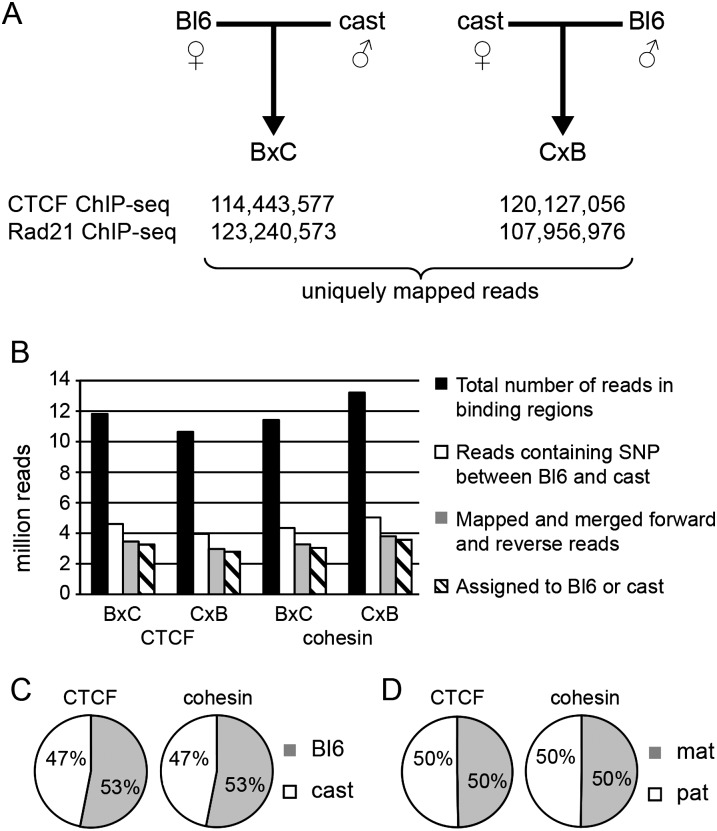
Sites of CTCF and cohesin binding to DNA were determined genome wide in whole P21 mouse brain by chromatin immunoprecipitation (ChIP) using antibodies specific to CTCF and the RAD21 cohesin subunit followed by high-throughput sequencing (ChIP-seq). The mice were the offspring of crosses between C57BL/6 (Bl6) females and Mus musculus castaneus (cast) males (B × C), and vice versa (C × B) (Fig. 1A). We generated 235 million and 231 million high-quality and uniquely mapping sequence reads for CTCF and cohesin, respectively (Fig. 1A). The percentage of reads representing clonal duplication was below 6.2% for all samples (Supplemental Fig. S1). Duplicate reads were excluded from further analysis, and regions of CTCF and RAD21 binding were identified using USeq and assigned to either the Bl6 or cast genome based on known SNPs (Fig. 1B; Supplemental Fig. S2; Supplemental Table S1; Keane et al. 2011; Yalcin et al. 2011).
Figure 1.
(A) ChIP-seq was performed for CTCF and cohesin (RAD21) on P21 brain in B × C and C × B F1 hybrid animals. The experimental design and number of uniquely mapped reads taken forward for further analysis are shown. (B) Regions of CTCF and RAD21 binding were identified using the Useq, and regions identified with a FDR of <13 were considered significant and were tested for parent-of-origin-specific binding. (Black bar) The number of reads for each experiment that fell at, or within ±500 bp of a binding region; (white bar) indicates reads in binding regions that aligned over a SNP between C57BL/6 (Bl6) and Mus m. castaneus (cast); (gray bar) the number of reads after the paired reads are considered together and the best-quality read is used to map the read to Bl6 or cast. (Hatched bar) The final number of reads assigned. There was a consistent bias toward the reference sequence (C); however, this effect was eliminated after we combined B × C and C × B reads (D).
A systematic read mapping bias toward the reference Bl6 genome was observed, consistent with a Bl6 allele read in a polymorphic region being more likely to align for both CTCF and cohesin. However, our use of reciprocal crosses prevented parental allele-specific binding being confounded: There was no overall bias toward either of the parental alleles when the reads generated from both reciprocal crosses were considered together (Fig. 1C,D).
CTCF and cohesin binding in mouse brain
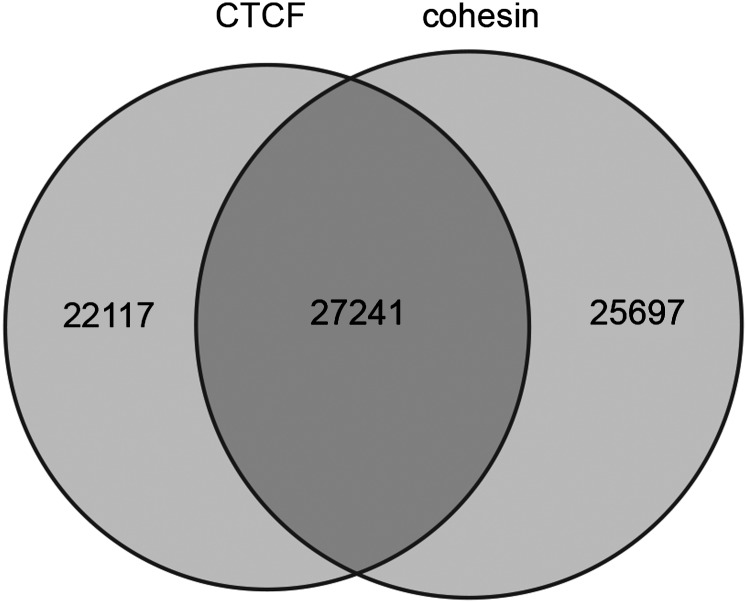
Genome wide, we detected 49,358 CTCF and 52,938 cohesin binding sites with a high degree of statistical confidence. Of these, 27,241 sites were bound by both CTCF and cohesin, accounting for 55.3% of the CTCF and 51.5% of the cohesin binding sites, respectively (Fig. 2). This is consistent with previous studies that show both independent and coordinated roles for these factors (Wendt et al. 2008; Lin et al. 2011).
Figure 2.
Overlap of the 49,358 CTCF and 52,938 cohesin binding regions in mouse brain. This demonstrates that just over half of CTCF (55%) and cohesin (51%) binding sites are shared, suggesting both independent and combinatorial functions for CTCF and cohesin in the 3-wk mouse brain.
CTCF binds to regions containing the canonical consensus motif
CTCF binds to a specific DNA sequence motif in ES cells (Chen et al. 2008) and liver (Schmidt et al. 2012). To search for CTCF binding motifs in brain, we applied the MEME de novo motif-finding tool to the sequences of all CTCF binding regions in P21 mouse brain (Fig. 3A). The most significant motif (P = 2.9 × 10−199) is highly similar to the published CTCF binding motif (Chen et al. 2008; Schmidt et al. 2010, 2012). To ensure the consistency of the comparison, we repeated the MEME analysis using identical parameters on CTCF binding regions previously identified using ChIP-seq in ES cells and liver (Chen et al. 2008; Schmidt et al. 2012). Again, the canonical motif was identified as the most significant motif in both ES cells (P = 7.4 × 10−924) and liver (P = 1.4 × 10−367). All three motifs display a high degree of sequence homology, particularly at the core 12-bp sequence at the center of the identified motifs (Fig. 3A).
Figure 3.
CTCF binding analysis. (A) MEME motif finder was executed on the CTCF binding locations identified by ChIP-seq in brain and compared with motifs identified using previously published ES cells and liver binding locations. Each data set found the canonical motif with high degrees of certainty. (B) The level of cytosine methylation within CTCF binding sites in the brain was compared with that across the whole genome using data from Xie et al. (2012). In both CpG and non-CpG context cytosine methylation, cytosines within CTCF binding sites are methylated less than those outside CTCF binding sites (χ2 contingency table tests, P < 0.001 for CpG and non-CpG context), confirming that CTCF prefers to bind to unmethylated DNA. (C) Genomic locations of CTCF binding are normalized to the proportion of the genome that constitutes each location (represented by the red line). This was considered for all CTCF peaks called with an FDR < 13 and separately for the 116 regions where CTCF binding was seen on one parental allele only (regions identified with a P < 0.001). CTCF is significantly enriched at genic regions, but depleted in distal intergenic regions. Parent-of-origin-specific CTCF binding locations are similar but show that binding is depleted in introns but not in intergenic regions.
CTCF binding sites are hypomethylated
The preference of CTCF to bind unmethylated DNA was confirmed by assessing the level of cytosine methylation at CTCF binding sites in brain. Using genome-wide bisulfite-sequencing (BS-seq) data for adult mouse brain (Xie et al. 2012), we compared the overall genome-wide level of methylation at cytosine residues, separately for CpG dinucleotides and non-CpG cytosines, with the portion of the genome corresponding to regions of CTCF binding. We found that methylation at CpG dinucleotides appears to have a greater influence on CTCF binding than non-CpG methylation. Genome wide, 60.8% of CpGs are methylated in the mouse brain, in contrast to 51.9% of CpGs in regions of CTCF binding. Non-CpG methylation also is less frequent in regions of CTCF binding (2.1%) compared with the genome-wide level (2.5%) (Fig. 3B). These differences are statistically significant (χ2 test, P < 1 × 10−6).
CTCF preferentially binds near genes
We explored the genome-wide location of both CTCF binding regions and parent-of-origin-specific CTCF binding regions using the cis-regulatory element annotation (CEAS) tool (Shin et al. 2009). CTCF binding is particularly enriched in regions up to ±3 kb upstream of and downstream from genes, but is depleted in intergenic regions (Fig. 3C). This is consistent with the insulator function of CTCF and, more generally, its involvement in controlling gene expression. When we limited our analysis to parent-of-origin-specific CTCF binding sites, we found the results to be similar. However, intronic regions appeared to be slightly underrepresented and intergenic regions slightly overrepresented relative to the distribution of all CTCF binding sites (Fig. 3C). Given the small number of parent-of-origin-specific CTCF binding sites, these differences are likely due to chance.
Noncanonical CTCF binding sites are tissue specific
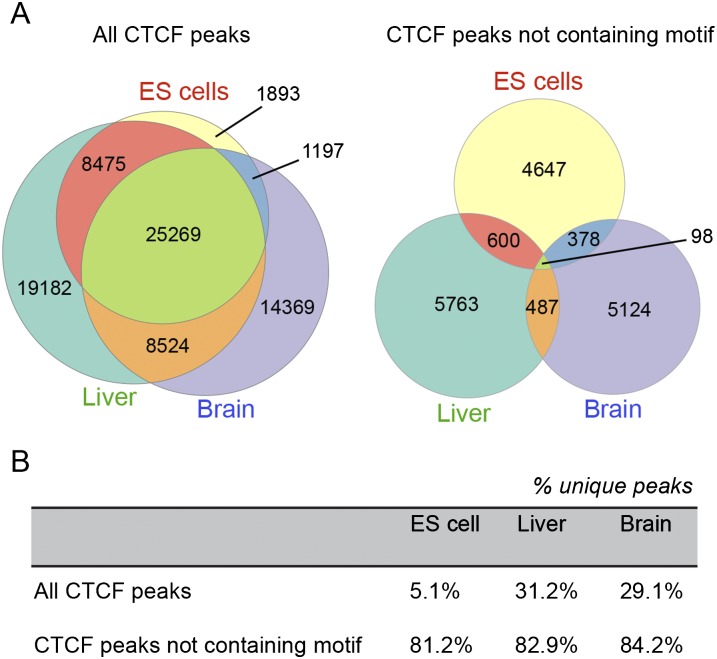
We compared the locations of CTCF binding sites in P21 brain with those reported for mouse ES cells and liver (Chen et al. 2008; Schmidt et al. 2012). The incidence of overlap between sites reported in different studies increases with increasing the peak size used for the comparison. Beyond a certain peak size, increases in overlap are mostly due to chance. Therefore, we iteratively increased peak size and compared the incidence of overlap between sites in ES cells, liver, and brain with randomized site locations. Beyond a peak size of 1 kb, increases in the incidence of overlaps were likely due to chance (Supplemental Fig. S3). For a common peak size of 1 kb, 32.0% of all binding sites were shared between ES cells, brain, and liver, suggesting that they are invariant during differentiation and regardless of cell type (Fig. 4A). Only 1893 binding sites were occupied exclusively in ES cells (5.1% of ES-cell binding sites), suggesting that most CTCF binding sites in ES cells represent a ground state that is added to during differentiation, with few binding sites being characteristic of pluripotency per se. In differentiated tissues, 29.1% of brain and 31.2% of liver binding sites are unique to the respective tissue. These analyses were repeated using alternative CTCF ChIP-seq data from ES cells and liver (Shen et al. 2012), producing similar results, even though significantly fewer binding sites were identified in these studies because of limited read depth and quality (Supplemental Fig. S4). We hypothesize that the canonical consensus CTCF binding motif may be at the core of binding sites that are largely invariant with respect to cell type, concordant with other findings (Essien et al. 2009). We restricted the above overlap analysis to CTCF binding sites that lack the canonical binding motif. There was a large reduction in the number of binding sites shared between tissue types (Fig. 4A), with most binding sites now being tissue specific: 84.2% of binding sites in brain that lack the canonical motif were brain specific, and similarly for ES cells (81.2%) and liver (82.9%) (Fig. 4B). These results suggest that CTCF binding to tissue-specific sites may involve other consensus motifs recognized by cofactors or tissue-specific conformations of the 11 zinc finger domains of CTCF itself.
Figure 4.
(A) Proportional Venn diagrams comparing coincidence of CTCF binding sites between ES cells, liver, and brain demonstrate significant overlap of CTCF binding in these tissues, Coincident binding was also considered after the removal of binding regions containing the consensus CTCF motif; overlap of CTCF binding in the absence of the consensus motif was much lower than when all binding sites were considered. (B) The percentages of shared peaks for each tissue type for all peaks and for nonmotif peaks.
Parent-of-origin-specific CTCF and/or cohesin binding is limited to specific DMRs
We systematically investigated the binding of CTCF and cohesin at or near 22 known well-characterized mouse gDMRs (Table 1) associated with imprinted gene expression (most of which are classified as ICRs). Of the 22 gDMRs (Table 1), 19 have a CTCF and/or cohesin binding site in brain within 2.5 kb. Of these sites, 12 are bound by both CTCF and cohesin, three by CTCF alone and four by cohesin alone. gDMRs with both CTCF and cohesin binding sites within 2 kb formed two categories: those where CTCF and cohesin colocalized precisely at the gDMR (eight regions), and a further four regions where binding occurred near but not over the gDMR and CTCF and cohesin each bound distinct sites (Table 1). Where the two factors are precisely colocalized on the DNA, cohesin binding is probably linked mechanistically to CTCF, while this is less likely at gDMRs where binding sites do not coincide.
Table 1.
Gene names for imprinted regions are listed with corresponding gDMR positions

For gDMRs where genome-wide significant (P < 1 × 10−6) parent-of-origin-specific binding events for CTCF (Table 1) were detected (H19/Igf2, Mest, Peg13, and Zim2 [Peg3]), binding occurred as expected on the unmethylated allele (Table 1). The Mest and Zim2 gDMRs were not previously known to bind CTCF in a parent-of-origin-specific manner. In addition, the 95% confidence intervals (Supplemental Fig. S5; Supplemental Table S2) for parent-of-origin-specific binding showed a trend toward preferential binding of CTCF to the unmethylated alleles of the Grb10, Mcts2, Cdh15, Nespos, Zrsr1, Peg10, and Meg3/Dlk1 gDMRs. We considered CTCF binding to be completely biallelic if the 95% confidence interval for the maternal-over-paternal read ratio was between 0.35 and 0.65 and spanned 0.5. This was the case for the Inpp5f_v2 and Plagl1 gDMRs. At H19/Igf2 and Peg13, parent-of-origin-specific binding of cohesin was detected but was not genome-wide significant after Bonferroni multiple testing correction (Table 1). The overall pattern of the 95% confidence intervals for the ratio of maternal-to-paternal reads for CTCF and cohesin suggests that in comparison to CTCF, cohesin binding is less biased toward the unmethylated parental allele (Supplemental Fig. S5; Supplemental Table S2). This is consistent with increased recruitment of cohesin to sites bound by CTCF.
Parent-of-origin-specific CTCF and cohesin binding at gDMRs could only be tested where a Bl6-cast SNP is within the bound region (Table 1). In addition, CTCF and cohesin peaks did not always overlap perfectly so that for some gDMRs, a SNP was informative for one factor but not the other. Another limitation for the detection of parent-of-origin-specific binding arose when a SNP was located at the periphery of the respectively bound region where fewer reads align and the statistical power of the binomial test was diminished. For CTCF, these limitations applied in particular to the Grb10, Mcts2, Nnat, Nespos, Zrsr1, Impact, and Peg10 gDMRs. For cohesin, the above limitations applied to half of the cohesin-bound gDMRs (Supplemental Table S2). The results for CTCF support the notion that it plays a central role in imprinting control at several loci. This is in contrast to cohesin, in particular, four gDMRs (Gnas-exon1A, Igf2r-air, Kcnq1ot1, and Snurf/Snrpn) were bound by cohesin but not by CTCF; here binding was not parental allele specific. Cohesin binding independently of CTCF is not unprecedented (Schmidt et al. 2012), and there is evidence that it is more generally involved in transcriptional activation (Kagey et al. 2010).
Genome-wide, parental allele-specific binding of CTCF and cohesin is rare and mostly restricted to imprinted loci
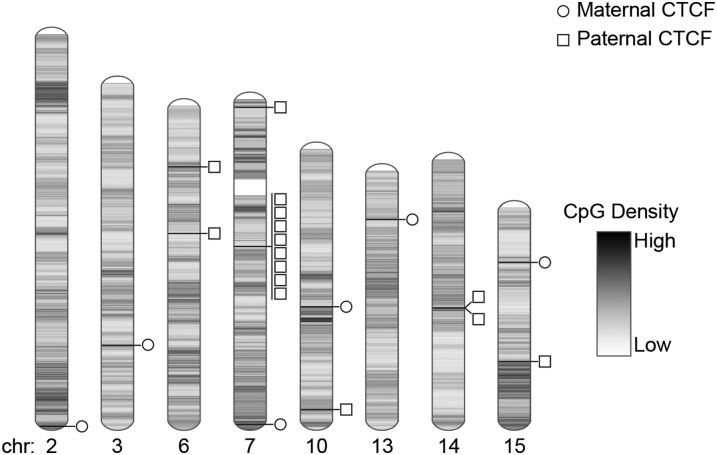
CTCF binding efficiency to methylated DNA is reduced compared with unmethylated DNA (Mukhopadhyay et al. 2004) explaining parental allele-specific binding at the H19/Igf2 and other gDMRs. If CTCF and cohesin are exerting a key regulatory role at several imprinted loci, then genome wide, other occurrences of parental allele-specific CTCF and/or cohesin binding may identify novel DMRs and imprinted genes. Four known ICRs—H19/Igf2, Peg13, Zim2 (Peg3), and Mest—met the genome-wide significance threshold for parental allele-specific CTCF binding providing proof of principle. Only an additional 17 regions reached genome-wide significance (Fig. 5; Supplemental Fig. S3; Table 2). Eight of these sites clustered in a 250-kb region on chromosome 7 at the Peg12/Magel2 imprinted domain (Fig. 6). A further four sites were within 6 Mb of other known imprinted regions. Two more are 30 kb apart on chromosome 14 (Fig. 5). Many chromosomes were devoid of parental allele-specific CTCF binding, and no cohesin binding regions were detected at genome-wide significance (Supplemental Table S3). Of all 21 genome-wide significant parental allele-specific CTCF binding sites, six were on the maternal and 15 on the paternal allele. We tested all gene transcripts at or near these sites not previously reported as imprinted for parental allele-specific expression in mouse brain. Many showed a complex organization of transcripts (Supplemental Fig. S6), but none were imprinted (Supplemental Table S4).
Figure 5.
Chromosomal location of genome-wide significant parent-of-origin-specific CTCF binding regions. Where CTCF is bound on the maternally inherited allele, this is illustrated with a circle; where CTCF is bound on the paternally inherited allele, this is illustrated with a square. Only chromosomes where parent-of-origin-specific binding was seen are shown. CpG density is indicated.
Table 2.
21 regions were identified where CTCF binds to one allele in a parent-of-origin-specific manner

Figure 6.
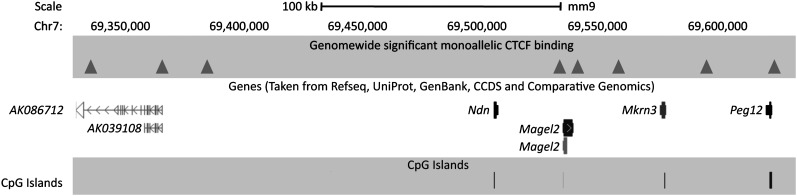
Multiple parent-of-origin-specific CTCF binding sites are observed on the paternal allele at the Magel2/Peg12 locus. (Triangles) Paternally bound CTCF binding sites. Genes and CpG islands are indicated. This region represents a unique example in the mouse genome of CTCF bound only on the paternal allele at eight regions in close proximity. This figure was adapted from the UCSC Genome Browser.
Eight sites of parental allele-specific CTCF binding at the Peg12/Magel2 imprinted domain (Fig. 6) bound CTCF on the paternal allele, indicating maternal methylation. We assayed methylation of the CpG island at the promoter of Magel2, which is in close proximity to two CTCF sites and maternally methylated (Supplemental Fig. S7). This is confirmation of the parental allele-specific methylation of the region recently reported (Xie et al. 2012). The Magel2 DMR is likely somatic and established post-fertilization that is supported by genome-wide methylation data in oocytes (Smallwood et al. 2011). In addition, Dnmt3L−/+ 8.5 days postcoitum (dpc) embryos are unchanged at the Magel2 promoter relative to wild type and are unmethylated (Proudhon et al. 2012). This suggests that the maternal allele-specific methylation at the Magel2 promoter, and presumably the other sites of paternal allele-specific CTCF binding in the domain, is established post-implantation and/or is brain specific. The regulation of the imprinted domain comprising Ndn, Magel2, Mkrn3, and Peg12 deserves further study since the human orthologs of Ndn, Magel2, and Mkrn3 are in the region associated with Prader-Willi syndrome (Lee and Wevrick 2000), with patients displaying a notable range of neurological symptoms. Given this extensive investigation of parental allele-specific CTCF binding, we predict that there are few additional DMRs in the adult mouse brain bound by CTCF.
Validation of CTCF and cohesin binding at specific loci
Quantitative assays for H19/Igf2, Peg10, Nap1l5, Nnat, and Grb10 DMR validated that the ChIP-seq data (Supplemental Fig. S8A,B) results were in agreement (Table 1) with the exception of CTCF binding at Nnat and cohesin binding at Peg10. Both are borderline cases. Using qPCR to detect CTCF binding at the Nnat DMR resulted in P = 0.08, just above our cutoff for binding, At Peg10, RAD21 binding was detected by qPCR, but no peak was identified by ChIP-seq. When the stringency of the ChIP-seq peak detection is relaxed, two RAD21 binding regions ∼1 kb either side of the qPCR regions are detected (Supplemental Fig. S8C).
Validation of parental allele-specific binding
To validate allele-specific binding we pyrosequenced ChIP'd mouse brain from reciprocal crosses. We selected three representative DMRs, based on the ChIP-seq results: Inpp5f_v2, where biallelic CTCF binding was detected; Mest, where we detected paternal allele-specific binding; and Peg10, where the CTCF binding site did not meet the significance threshold for allele-specific binding but where the 95% confidence interval was suggestive of CTCF binding on the paternal allele. These results agreed with our ChIP-seq data (Supplemental Fig. S9): Inpp5f_v2 does not deviate from the expected 50:50 allelic ratio (P = 0.3214), Mest shows paternal binding (P = 0.0017), and Peg10 shows a bias toward enrichment of the paternal allele (P = 0.0813).
CTCF and cohesin binding at somatic DMRs
A set of 23 known somatic and novel putative somatic DMR coordinates has recently been defined by whole-genome bisulfite sequencing (BS-seq) in mouse brain (Xie et al. 2012). We evaluated CTCF and cohesin binding in the somatic DMRs identified in this study (Supplemental Table S5). We found 13 instances of CTCF binding, two of which were parental allele specific (P < 1 × 10−6) and 14 instances of cohesin binding, none of which were parental allele specific. All parental allele-specific binding involved the unmethylated allele. Overall, the results for somatic DMRs (Supplemental Table S6) are in close agreement with the results for gDMRs (Table 1) so that the origin of a DMR, germline versus somatic, is not a determinant of CTCF and/or cohesin involvement in the regulation of imprinting.
Discussion
CTCF and cohesin act synergistically and independently in mouse brain and show tissue-specific distributions compared with undifferentiated cells
Several studies have examined the colocalization of CTCF with cohesin (Chen et al. 2008; Parelho et al. 2008; Rubio et al. 2008; Kagey et al. 2010; Schmidt et al. 2012), and CTCF physically associates with cohesin via the Stag1 (Scc3/SA1) subunit in human cells (Wendt et al. 2008). Here 55% of CTCF binding sites overlap with cohesin binding, and the remaining sites binding independently (Fig. 2), indicating that CTCF fulfils a role independent from as well as in combination with cohesin in brain. This supports the idea that different functions may be a result of the context of CTCF binding (Gaszner and Felsenfeld 2006), and it is possible that coordinate binding of cohesin may influence CTCF. Cohesin is involved in tissue-specific transcriptional control (Faure et al. 2012) and associated with the Mediator complex, which has a role in transcriptional activation (Taatjes 2012). Studies have shown a link between cohesin, the Mediator complex, transcription, and chromatin looping (Kagey et al. 2010). We report that 51% of cohesin sites in brain are not coincident with CTCF, consistent with CTCF not being required for the loading of cohesin onto DNA (Rubio et al. 2008).
This comparison of CTCF binding in ES cells, liver, and brain reveals more unique CTCF binding sites in differentiated cells than in ES cells, suggesting tissue-specific CTCF binding in the specification and/or maintenance of differentiated tissue. We observe a significant overlap in CTCF binding between tissues (Fig. 4A), consistent with studies reporting highly conserved CTCF binding between cell types (Kim et al. 2007b).
CTCF binding
In brain, CTCF binds to unmethylated regions of DNA, usually to the canonical CTCF motif (Chen et al. 2008; Schmidt et al. 2010, 2012). CTCF binding outside this motif is much more tissue specific, and there is little overlap between tissues (Fig. 4A). This is consistent with evidence from CTCF knockout mouse studies, which exhibit embryonic lethality prior to implantation (Splinter et al. 2006). We found that the canonical consensus binding motif is most frequent at CTCF binding sites shared between ES cells, brain, and liver; thus, it is associated with invariant binding during differentiation. CTCF binding appears overrepresented just up or downstream from gene bodies with a paucity in distal intergenic regions, unsurprising given the known role of CTCF in gene expression (Bell et al. 1999; Cuddapah et al. 2009). CTCF mediates long-range chromosomal interactions genome wide in cis and in trans in ES cells (Handoko et al. 2011), and we provide additional evidence genome wide that CTCF is an insulator at or near gene coding regions by binding to noncoding DNA.
Cytosine methylation at CTCF binding regions
Cytosine methylation in both a CpG and non-CpG context is reduced in regions of CTCF binding compared with the level observed genome wide, consistent with published data (Mukhopadhyay et al. 2004). Interestingly the canonical motif lacks CpG dinucleotides, suggesting that methylation of DNA in the motif does not preclude CTCF binding, but surrounding methylation is important. The canonical motif may not function alone, but in concert with another region of DNA ∼20 bp downstream, suggesting that CTCF interaction with DNA is not limited to the 20-mer motifs (Schmidt et al. 2012).
Parent-of-origin-specific CTCF and cohesin binding
CTCF and cohesin bind at numerous imprinting control regions and other DMRs as previously detected, but not systematically tested. The presence of CTCF and cohesin together at 12 imprinting associated gDMRs in brain (Table 1) is consistent with a regulatory role for these proteins at imprinted loci. Studies using 3C and 4C have shown that several imprinted domains are physically clustered (Sandhu et al. 2009), in part because CTCF (Botta et al. 2010) and cohesin (Murrell et al. 2004; Nativio et al. 2009) form loops that contribute to three-dimensional (3D) nuclear architecture (Phillips and Corces 2009). The CTCF and cohesin binding (Table 1) supports the idea of three types of imprinting mechanisms: CTCF dependent, CTCF/cohesin mediated, and CTCF/cohesin independent.
CTCF and cohesin bind at somatic DMRs, suggesting a role for them here. Parental allele-specific binding of CTCF together with cohesin regulates allele-specific expression in somatic cells (Lin et al. 2011), while cohesin binding alone may be involved in the transcriptional regulation of imprinted gene expression generally. CTCF and cohesin are likely to have distinct functions in different cell types at a subset of targets (Lin et al. 2011), and findings in mouse brain support the idea that these proteins play a role in imprinting at some loci and at others they act more generally.
These data provide a resource for interrogating the roles of CTCF and cohesin and point to a role at more imprinted loci than was previously appreciated, although further functional studies would be needed to confirm this. Three gDMRs do not bind either factor, illustrating the heterogeneous nature of gDMRs as a group of regulatory regions. For example, the four imprinted retrogene/host gene pairs Mcts2/H13, Nap1l5/Herc3, Inpp5f/Inpp5f_v2, and Zrsr1/Commd1 share several sequence-based and genomic context–related features (Wood et al. 2007). Since within this group, Mcts2 binds both CTCF and cohesin together, Nap1l5 binds neither CTCF nor cohesin, Inpp5f_v2 binds CTCF on both parental alleles equally, and Zrsr1 binds both CTCF and cohesin, but they do not colocalize, this suggests no consistent mechanism for imprinting control despite the other shared features.
CTCF binding profiles vary between different tissues. We show that many CTCF binding sites are shared between ES cells and differentiated tissues and that this type of invariant CTCF binding is associated with the canonical CTCF motif. CTCF binding in the absence of the canonical motif is associated with tissue-specific CTCF binding.
Methods
Chromatin immunoprecipitation
Chromatin from whole tissue was isolated, sonicated, and immunoprecipitated for ChIP-seq library preparation according to Supplemental Methods 1.
Next-generation sequencing
Library preparation
DNA enriched through ChIP was quantified using the Qubit (Invitrogen) and Quant-iT dsDNA high-sensitivity assay kit (Invitrogen:Q32854) and was sized using the Agilent Bioanalyzer with a High Sensitivity DNA Bioanalyzer kit (5067-4626). DNA was fragmented to a size appropriate for the library preparation step using the Covaris S220, samples were sheared over two cycles: 5% duty cycle, 3 intensity, 200 cycles per burst, and time of 65 sec. DNA from ChIPs performed on chromatin extracted from two mice was pooled.
ChIP-seq libraries were prepared using the Illumina ChIP-seq library preparation kit (IP-102-1001) and the NEBNext ChIP-seq library preparation kit (E6240). Libraries were sized and quantified using an Agilent Bioanalyzer and a High Sensitivity Kit (5067-4626).
ChIP-seq data analysis
Sequence reads were aligned to the mouse reference genome (mm9) using Novoalign (v. 2.01.13; http://www.novocraft.com/). USeq (Nix et al. 2008) was used to identify mean peak shift separately for CTCF and cohesin reads using only the first of each pair-end matched read. Peaks were identified using peak shifts and window sizes of 138 bp and 144 bp for CTCF and cohesin, respectively, and a False Discovery Rate (FDR) of 95% (Supplemental Table S2A). A subset of peaks was obtained to a false discovery ratio of 5% (Phred-scaled FDR 13) expanded by 500 bp upstream and downstream and overlapping peaks merged prior to further analysis. Refer to Supplemental Table S2A for the number of raw reads that pass a quality control map in a CTCF or cohesin binding region.
Parental allele-specific binding analysis
Parental allele-specific binding was assessed by binomial testing, using a custom bioinformatics pipeline. For performance reasons, only reads of interest, which overlapped the previously identified CTCF or RAD21 binding sites and a SNP between the two parental strains, were extracted from the SAM files and used for subsequent analysis.
Individual reads were assigned to one of the parental alleles using a custom Perl script, using the SAMtools Perl library. Each read was mapped as either derived from the reference sequence (Bl6) or from the cast allele on the basis of a SNP between the parental strains. If more than one SNP was present, the SNP with the best quality of read sequence was used. Reads were only considered for subsequent analysis if the Phred-scaled alignment mapping quality exceeded 50 and the base call quality at the SNP used for mapping of the read exceeded 20.
Paired reads were mapped to parental strains separately and merged. Because paired reads are not independent data points, when they were in disagreement (<1%) the read pair was assigned on the basis of the best SNP in either of the two reads.
Assigned reads were converted to maternally or paternally derived, and data from both B × C and C × B reciprocal crosses were merged for the CTCF and RAD21 data sets independently. Counts of maternal and paternal reads were obtained on a per-region basis using MySQL. Binding regions were only tested for parent-of-origin-specific expression if three or more reads could be mapped.
Parental allele-specific binding was assessed using a two-sided binomial test (implemented in R) of the maternal-versus-paternal allelic read counts. Regions were sorted by P-value score using MySQL. The genome-wide significance of P-values was assessed by means of Bonferroni correction. UCSC BED tracks were prepared at different cutoffs with maternal/paternal annotation.
Peak intersections
All subsequent bioinformatic analyses were performed on expanded regions unless otherwise specified. CTCF peak intersections between ES cell, brain, and liver data were performed using an optimized peak size of 1 kb for all data sets. ES cell data were converted to mm9 using the UCSC liftOver tool. Peak overlap counts were obtained used the BEDTools intersectBed command. For each intersection, counts of the intersecting peaks were calculated in both possible ways, and the peaks count reported was the mean of the two measurements. For intersections of more than two data sets, only one of the possible configurations of intersections was examined; the same configuration was used for all analyses.
Identification of non-motif-containing peaks
Peaks that did not contain the CTCF motif were identified using the FIMO tool from the MEME suite (Grant et al. 2011). Peak sequences were obtained using the UCSC Genome Browser table tool in FASTA format with repeat sequences masked. The CTCF motif identified from the brain data set was used throughout, and the threshold for detection was set to 10−3. Custom UNIX shell scripts were used to extract the coordinates of the peaks from the FIMO output.
Motif finding
Motif finding was performed using MEME (Bailey et al. 2009) on binding regions using default MEME parameters. For the brain data, the best subwindow coordinates were used.
Genomic distribution of peaks
The CEAS tool (Shin et al. 2009) was used to assess the genomic distribution of unexpanded CTCF binding regions, and unexpanded parent-of-origin-specific CTCF binding sites were detected with a P < 0.001. Relative abundance was normalized to the proportion of the genome represented by each genomic region. For the CEAS analysis, parent-of-origin-specific CTCF expanded regions were assigned back to their original constituent unexpanded peaks using bedmap (Neph et al. 2012).
Quantitative PCR validation of CTCF and cohesin ChIP-seq
These assays are detailed in Supplemental Methods 1.
Validation of parent-of-origin-specific binding using pyrosequencing
Chromatin was extracted from four biological replicates, two B × C and two C × B P21 brains. Pyrosequencing validated CTCF binding at three regions (Supplemental Table S6). ChIP was performed as for ChIP-seq. Maternal-to-paternal proportions were assigned using SNPs between Bl6 and cast. Allelic proportions were normalized to input DNA, which represents a 50:50 ratio of maternal-to-paternal reads. Using the normalized maternal proportion, a two-sided t-test against a 0.5 null proportion was performed.
Testing for imprinted expression
Transcripts were tested for allele-specific expression using PCR followed by Sanger sequencing (using the primers in Supplemental Table S7).
Bisulfite mutagenesis
Genomic DNA from B × C and C × B intercross mouse brain tissue was converted using the Zymo EZ DNA Methylation-Direct Kit (D5020). Amplified regions of interest were ligated into pGEM-T Easy (Promega:A1360), transformed into competent Escherichia coli, and sequenced. Primers were designed with MethPrimer (For:GTGTTTGTTGAGAGTTGTTGAGAGA; Rev: ACCAAACAACCATAAAAACCTACAA) (Li et al. 2002).
Data access
Primary sequencing data have been deposited in the NCBI Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE35140.
Acknowledgments
We thank Dr. Michael Cowley and Heba Saadeh for their critical reading of this manuscript and Dr. Sabrina Böhm for competent cells. We thank Dr. Deborah Bourc'his for providing methylation data from Dnmt3L+/− embryos. We thank Seth Seegobin and Dr. Jennifer Mollon for discussions about the value of statistical methodologies for qPCR and pyrosequencing analyses, for discussions on confidence intervals and t-tests, and Dr. Michael Weale for discussions about genic enrichment measurements for binding proteins. We acknowledge funding by the Wellcome Trust (Grant number 084358/Z/07/Z to R.J.O.), the Philip Harris prize studentship fund (R.B.M.), the British Heart Foundation studentship FS/08/051/25748 and latterly the MRC (to R.J.O. supporting A.R.P.), the BBSRC Doctoral Training Grant (S.H.), the RCUK fellowship program (R.S.), and King's College London for a partial PhD studentship (N.B.). We acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's & St Thomas' NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust. Their support is through a partial PhD studentship (N.B.), access to the high-performance computing cluster through Don Lokuadassuriyage (Systems Administrator), and services provided by the genomics core facility (from Mudassa Mirza and Dr. Efterpi Papouli) including high-throughput sequencing and access to equipment. We also thank Dr. Matt Arno and Dr. Estibaliz Aldecoa-Otalora Astarloa from the KCL genomic facility, and Dr. Charles Mein from the Barts and the London Genome Centre for pyrosequencing support.
Author contributions: A.R.P. carried out the ChIP-seq experimental work, made the majority of the data acquisition, performed some of the analyses, and drafted part of the paper. N.B. performed the majority of the bioinformatic analyses, made the tables and figures, and revised some of the paper. R.B.M. contributed to the conception and initial experimental design and to initial technique development and some bioinformatics and drafted part of the paper. S.H. performed the bisulfite analysis. S.M.M. performed the allele-specific assays. R.S. contributed to the conception and design of the experiments and bioinformatic analyses and wrote part of the paper. R.J.O. contributed to the conception and design of the experiments and wrote part of the paper. All authors contributed to the final version of the paper.
Footnotes
[Supplemental material is available for this article.]
Article published online before print. Article, supplemental material, and publication date are at http://www.genome.org/cgi/doi/10.1101/gr.150136.112.
References
- Bailey TL, Bodén M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. 2009. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res 37: W202–W208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomei M 2009. Genomic imprinting: Employing and avoiding epigenetic processes. Genes Dev 23: 2124–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A, Felsenfeld G 2000. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature 405: 482–485 [DOI] [PubMed] [Google Scholar]
- Bell A, West A, Felsenfeld G 1999. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell 98: 387–396 [DOI] [PubMed] [Google Scholar]
- Botta M, Haider S, Leung IX, Lio P, Mozziconacci J 2010. Intra- and inter-chromosomal interactions correlate with CTCF binding genome wide. Mol Syst Biol 6: 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy S, Dykens E, Williams C 2000. Prader-Willi and Angelman syndromes: Sister imprinted disorders. Am J Med Genet 97: 136–146 [DOI] [PubMed] [Google Scholar]
- Chen X, Xu H, Yuan P, Fang F, Huss M, Vega V, Wong E, Orlov Y, Zhang W, Jiang J, et al. 2008. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133: 1106–1117 [DOI] [PubMed] [Google Scholar]
- Cuddapah S, Jothi R, Schones DE, Roh TY, Cui K, Zhao K 2009. Global analysis of the insulator binding protein CTCF in chromatin barrier regions reveals demarcation of active and repressive domains. Genome Res 19: 24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rocha S, Edwards C, Ito M, Ogata T, Ferguson-Smith A 2008. Genomic imprinting at the mammalian Dlk1–Dio3 domain. Trends Genet 24: 306–316 [DOI] [PubMed] [Google Scholar]
- Davies W, Isles A, Humby T, Wilkinson L 2007. What are imprinted genes doing in the brain? Epigenetics 2: 201–206 [DOI] [PubMed] [Google Scholar]
- DeVeale B, van der Kooy D, Babak T 2012. Critical evaluation of imprinted gene expression by RNA-seq: A new perspective. PLoS Genet 8: e1002600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C, Ferguson-Smith A 2007. Mechanisms regulating imprinted genes in clusters. Curr Opin Cell Biol 19: 281–289 [DOI] [PubMed] [Google Scholar]
- doi: 10.1038/nature11247. The ENCODE Project Consortium. 2012. An integrated encyclopedia of DNA elements in the human genome. Nature489: 57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essien K, Vigneau S, Apreleva S, Singh LN, Bartolomei MS, Hannenhalli S 2009. CTCF binding site classes exhibit distinct evolutionary, genomic, epigenomic and transcriptomic features. Genome Biol 10: R131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure AJ, Schmidt D, Watt S, Schwalie PC, Wilson MD, Xu H, Ramsay RG, Odom DT, Flicek P 2012. Cohesin regulates tissue-specific expression by stabilizing highly occupied cis-regulatory modules. Genome Res 22: 2163–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoriw A, Stein P, Svoboda P, Schultz R, Bartolomei M 2004. Transgenic RNAi reveals essential function for CTCF in H19 gene imprinting. Science 303: 238–240 [DOI] [PubMed] [Google Scholar]
- Filippova GN, Fagerlie S, Klenova EM, Myers C, Dehner Y, Goodwin G, Neiman PE, Collins SJ, Lobanenkov VV 1996. An exceptionally conserved transcriptional repressor, CTCF, employs different combinations of zinc fingers to bind diverged promoter sequences of avian and mammalian c-myc oncogenes. Mol Cell Biol 16: 2802–2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick G, Pugacheva E, Shin J-Y, Abdullaev Z, Yang Y, Khatod K, Lobanenkov V, Higgins M 2007. Allele-specific binding of CTCF to the multipartite imprinting control region KvDMR1. Mol Cell Biol 27: 2636–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost J, Monk D, Moschidou D, Guillot PV, Stanier P, Minger SL, Fisk NM, Moore HD, Moore GE 2011. The effects of culture on genomic imprinting profiles in human embryonic and fetal mesenchymal stem cells. Epigenetics 6: 52–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Rusznak Z, Herculano-Houzel S, Watson C, Paxinos G 2012. Cellular composition characterizing postnatal development and maturation of the mouse brain and spinal cord. Brain Struct Funct doi: 10.1007/s00429-012-0462-x [DOI] [PubMed] [Google Scholar]
- Garfield AS, Cowley M, Smith FM, Moorwood K, Stewart-Cox JE, Gilroy K, Baker S, Xia J, Dalley JW, Hurst LD, et al. 2011. Distinct physiological and behavioural functions for parental alleles of imprinted Grb10. Nature 469: 534–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaszner M, Felsenfeld G 2006. Insulators: Exploiting transcriptional and epigenetic mechanisms. Nat Rev Genet 7: 703–713 [DOI] [PubMed] [Google Scholar]
- Grant CE, Bailey TL, Noble WS. 2011. FIMO: Scanning for occurrences of a given motif. Bioinformatics 27: 1017–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C, Zhang J, Butler J, Haig D, Dulac C 2010a. Sex-specific parent-of-origin allelic expression in the mouse brain. Science 329: 682–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C, Zhang J, Weissbourd B, Luo S, Schroth G, Haig D, Dulac C 2010b. High-resolution analysis of parent-of-origin allelic expression in the mouse brain. Science 329: 643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjur S, Williams L, Ryan N, Cobb B, Sexton T, Fraser P, Fisher A, Merkenschlager M 2009. Cohesins form chromosomal cis-interactions at the developmentally regulated IFNG locus. Nature 460: 410–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara Y, Hirai M, Nishiyama K, Kanazawa I, Ueda T, Sakaki Y, Ito T 1997. Screening for imprinted genes by allelic message display: Identification of a paternally expressed gene impact on mouse chromosome 18. Proc Natl Acad Sci 94: 9249–9254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handoko L, Xu H, Li G, Ngan CY, Chew E, Schnapp M, Lee CW, Ye C, Ping JL, Mulawadi F, et al. 2011. CTCF-mediated functional chromatin interactome in pluripotent cells. Nat Genet 43: 630–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hark A, Schoenherr C, Katz D, Ingram R, Levorse J, Tilghman S 2000. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature 405: 486–489 [DOI] [PubMed] [Google Scholar]
- Hikichi T, Kohda T, Kaneko-Ishino T, Ishino F 2003. Imprinting regulation of the murine Meg1/Grb10 and human GRB10 genes; roles of brain-specific promoters and mouse-specific CTCF-binding sites. Nucleic Acids Res 31: 1398–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagey M, Newman J, Bilodeau S, Zhan Y, Orlando D, van Berkum N, Ebmeier C, Goossens J, Rahl P, Levine S, et al. 2010. Mediator and cohesin connect gene expression and chromatin architecture. Nature 467: 430–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanduri C, Pant V, Loukinov D, Pugacheva E, Qi C-F, Wolffe A, Ohlsson R, Lobanenkov V 2000. Functional association of CTCF with the insulator upstream of the H19 gene is parent of origin-specific and methylation-sensitive. Curr Biol 10: 853–856 [DOI] [PubMed] [Google Scholar]
- Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, Heger A, Agam A, Slater G, Goodson M, et al. 2011. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 477: 289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KP, Thurston A, Mummery C, Ward-van Oostwaard D, Priddle H, Allegrucci C, Denning C, Young L 2007a. Gene-specific vulnerability to imprinting variability in human embryonic stem cell lines. Genome Res 17: 1731–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Abdullaev ZK, Smith AD, Ching KA, Loukinov DI, Green RD, Zhang MQ, Lobanenkov VV, Ren B 2007b. Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell 128: 1231–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Samaranayake M, Pradhan S 2009. Epigenetic mechanisms in mammals. Cell Mol Life Sci 66: 596–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Sakurai T, Sato S, Nakabayashi K, Hata K, Kono T 2012. Imprinted DNA methylation reprogramming during early mouse embryogenesis at the Gpr1–Zdbf2 locus is linked to long cis-intergenic transcription. FEBS Lett 586: 827–833 [DOI] [PubMed] [Google Scholar]
- Kuzmin I, Geil L, Gibson L, Cavinato T, Loukinov D, Lobanenkov V, Lerman MI 2005. Transcriptional regulator CTCF controls human interleukin 1 receptor-associated kinase 2 promoter. J Mol Biol 346: 411–422 [DOI] [PubMed] [Google Scholar]
- Lee S, Wevrick R 2000. Identification of novel imprinted transcripts in the Prader-Willi syndrome and Angelman syndrome deletion region: Further evidence for regional imprinting control. Am J Hum Genet 66: 848–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre L, Viville S, Barton S, Ishino F, Keverne E, Surani A 1998. Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene Mest. Nat Genet 20: 163–169 [DOI] [PubMed] [Google Scholar]
- Lewis A, Reik W 2006. How imprinting centres work. Cytogenet Genome Res 113: 81–89 [DOI] [PubMed] [Google Scholar]
- Li LC, Dahiya R. 2002. MethPrimer: Designing primers for methylation PCRs. Bioinformatics 18: 1427–1431. [DOI] [PubMed] [Google Scholar]
- Li E, Beard C, Jaenisch R 1993. Role for DNA methylation in genomic imprinting. Nature 366: 362–365 [DOI] [PubMed] [Google Scholar]
- Li LL, Keverne EB, Aparicio SA, Ishino F, Barton SC, Surani MA 1999. Regulation of maternal behavior and offspring growth by paternally expressed Peg3. Science 284: 330–334 [DOI] [PubMed] [Google Scholar]
- Lin S, Ferguson-Smith A, Schultz R, Bartolomei M 2011. Nonallelic transcriptional roles of CTCF and cohesins at imprinted loci. Mol Cell Biol 31: 3094–3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossie AC, Whitney MM, Amidon D, Dong HJ, Chen P, Theriaque D, Hutson A, Nicholls RD, Zori RT, Williams CA, et al. 2001. Distinct phenotypes distinguish the molecular classes of Angelman syndrome. J Med Genet 38: 834–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyck L, Kroigard T, Finsen B 2007. Unbiased cell quantification reveals a continued increase in the number of neocortical neurones during early post-natal development in mice. Eur J Neurosci 26: 1749–1764 [DOI] [PubMed] [Google Scholar]
- Manolio TA 2010. Genomewide association studies and assessment of the risk of disease. N Engl J Med 363: 166–176 [DOI] [PubMed] [Google Scholar]
- Martin D, Pantoja C, Fernandez Minan A, Valdes-Quezada C, Molto E, Matesanz F, Bogdanovic O, de la Calle-Mustienes E, Dominguez O, Taher L, et al. 2011. Genome-wide CTCF distribution in vertebrates defines equivalent sites that aid the identification of disease-associated genes. Nat Struct Mol Biol 18: 708–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCole R, Oakey R 2008. Unwitting hosts fall victim to imprinting. Epigenetics 3: 258–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay R, Yu W, Whitehead J, Xu J, Lezcano M, Pack S, Kanduri C, Kanduri M, Ginjala V, Vostrov A, et al. 2004. The binding sites for the chromatin insulator protein CTCF map to DNA methylation-free domains genome-wide. Genome Res 14: 1594–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell A, Heeson S, Reik W 2004. Interaction between differentially methylated regions partitions the imprinted genes Igf2 and H19 into parent-specific chromatin loops. Nat Genet 36: 889–893 [DOI] [PubMed] [Google Scholar]
- Nativio R, Wendt K, Ito Y, Huddleston J, Uribe-Lewis S, Woodfine K, Krueger C, Reik W, Peters J-M, Murrell A 2009. Cohesin is required for higher-order chromatin conformation at the imprinted IGF2-H19 locus. PLoS Genet 5: e1000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neph S, Kuehn MS, Reynolds AP, Haugen E, Thurman RE, Johnson AK, Rynes E, Maurano MT, Vierstra J, Thomas S, et al. 2012. BEDOPS: High-performance genomic feature operations. Bioinformatics 28: 1919–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nix D, Courdy S, Boucher K 2008. Empirical methods for controlling false positives and estimating confidence in ChIP-seq peaks. BMC Bioinformatics 9: 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan JP, McCallion AS 2010. Genomics of long-range regulatory elements. Annu Rev Genomics Hum Genet 11: 1–23 [DOI] [PubMed] [Google Scholar]
- Ohlsson R 2001. CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends Genet 17: 520–527 [DOI] [PubMed] [Google Scholar]
- Parelho V, Hadjur S, Spivakov M, Leleu M, Sauer S, Gregson H, Jarmuz A, Canzonetta C, Webster Z, Nesterova T, et al. 2008. Cohesins functionally associate with CTCF on mammalian chromosome arms. Cell 132: 422–433 [DOI] [PubMed] [Google Scholar]
- Peters J, Williamson C 2007. Control of imprinting at the Gnas cluster. Epigenetics 2: 207–213 [DOI] [PubMed] [Google Scholar]
- Phillips JE, Corces VG 2009. CTCF: Master weaver of the genome. Cell 137: 1194–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagge A, Isles A, Gordon E, Humby T, Dean W, Gritsch S, Fischer-Colbrie R, Wilkinson L, Kelsey G 2005. Imprinted Nesp55 influences behavioral reactivity to novel environments. Mol Cell Biol 25: 3019–3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudhon C, Duffie R, Ajjan S, Cowley M, Iranzo J, Carbajosa G, Saadeh H, Holland ML, Oakey RJ, Rakyan VK, et al. 2012. Protection against de novo methylation is instrumental in maintaining parent-of-origin methylation inherited from the gametes. Mol Cell 47: 909–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenneville S, Verde G, Corsinotti A, Kapopoulou A, Jakobsson J, Offner S, Baglivo I, Pedone PV, Grimaldi G, Riccio A, et al. 2012. In embryonic stem cells, ZFP57/KAP1 recognize a methylated hexanucleotide to affect chromatin and DNA methylation of imprinting control regions. Mol Cell 44: 361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W, Walter J 2001. Genomic imprinting: Parental influence on the genome. Nat Rev Genet 2: 21–32 [DOI] [PubMed] [Google Scholar]
- Rubio E, Reiss D, Welcsh P, Disteche C, Filippova G, Baliga N, Aebersold R, Ranish J, Krumm A 2008. CTCF physically links cohesin to chromatin. Proc Natl Acad Sci 105: 8309–8314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg-Gunn PJ, Ferguson-Smith AC, Pedersen RA 2007. Status of genomic imprinting in human embryonic stem cells as revealed by a large cohort of independently derived and maintained lines. Hum Mol Genet 16: R243–R251 [DOI] [PubMed] [Google Scholar]
- Sandhu KS, Shi C, Sjolinder M, Zhao Z, Gondor A, Liu L, Tiwari VK, Guibert S, Emilsson L, Imreh MP, et al. 2009. Nonallelic transvection of multiple imprinted loci is organized by the H19 imprinting control region during germline development. Genes Dev 23: 2598–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Schwalie P, Ross-Innes C, Hurtado A, Brown G, Carroll J, Flicek P, Odom D 2010. A CTCF-independent role for cohesin in tissue-specific transcription. Genome Res 20: 578–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Schwalie PC, Wilson MD, Ballester B, Goncalves A, Kutter C, Brown GD, Marshall A, Flicek P, Odom DT 2012. Waves of retrotransposon expansion remodel genome organization and CTCF binding in multiple mammalian lineages. Cell 148: 335–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Woodfine K, Menheniott TR, Bourc'his D, Bestor T, Oakey RJ 2008. WAMIDEX: A web atlas of murine genomic imprinting and differential expression. Epigenetics 3: 89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Yue F, McCleary DF, Ye Z, Edsall L, Kuan S, Wagner U, Dixon J, Lee L, Lobanenkov VV, et al. 2012. A map of the cis-regulatory sequences in the mouse genome. Nature 488: 116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Liu T, Manrai AK, Liu XS 2009. CEAS: Cis-regulatory element annotation system. Bioinformatics 25: 2605–2606 [DOI] [PubMed] [Google Scholar]
- Singh P, Wu X, Lee D-H, Li A, Rauch T, Pfeifer G, Mann J, Szabo P 2011. Chromosome-wide analysis of parental allele-specific chromatin and DNA methylation. Mol Cell Biol 31: 1757–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood SA, Tomizawa S, Krueger F, Ruf N, Carli N, Segonds-Pichon A, Sato S, Hata K, Andrews SR, Kelsey G 2011. Dynamic CpG island methylation landscape in oocytes and preimplantation embryos. Nat Genet 43: 811–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splinter E, Heath H, Kooren J, Palstra RJ, Klous P, Grosveld F, Galjart N, de Laat W 2006. CTCF mediates long-range chromatin looping and local histone modification in the β-globin locus. Genes Dev 20: 2349–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman W, Kang H, Lin S, Kissil J, Bartolomei M, Lieberman P 2008. Cohesins localize with CTCF at the KSHV latency control region and at cellular c-myc and H19/Igf2 insulators. EMBO J 27: 654–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo P, Pfeifer G, Mann J 2004. Parent-of-origin-specific binding of nuclear hormone receptor complexes in the H19-Igf2 imprinting control region. Mol Cell Biol 24: 4858–4868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taatjes DJ 2012. The human Mediator complex: A versatile, genome-wide regulator of transcription. Trends Biochem Sci 35: 315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumura A, Hayakawa T, Kumaki Y, Takebayashi S, Sakaue M, Matsuoka C, Shimotohno K, Ishikawa F, Li E, Ueda HR, et al. 2006. Maintenance of self-renewal ability of mouse embryonic stem cells in the absence of DNA methyltransferases Dnmt1, Dnmt3a and Dnmt3b. Genes Cells 11: 805–814 [DOI] [PubMed] [Google Scholar]
- Wendt K, Yoshida K, Itoh T, Bando M, Koch B, Schirghuber E, Tsutsumi S, Nagae G, Ishihara K, Mishiro T, et al. 2008. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature 451: 796–801 [DOI] [PubMed] [Google Scholar]
- Wilkins J. 2008. Genomic imprinting. Landes Bioscience/Springer, Austin, TX. [Google Scholar]
- Williams C, Beaudet A, Clayton-Smith J, Knoll J, Kyllerman M, Laan L, Magenis E, Moncla A, Schinzel A, Summers J, et al. 2006. Angelman syndrome 2005: Updated consensus for diagnostic criteria. Am J Med Genet 140A: 413–418 [DOI] [PubMed] [Google Scholar]
- Wood AJ, Roberts RG, Monk D, Moore GE, Schulz R, Oakey RJ 2007. A screen for retrotransposed imprinted genes reveals an association between X chromosome homology and maternal germ-line methylation. PLoS Genet 3: e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AJ, Schulz R, Woodfine K, Koltowska K, Beechey CV, Peters J, Bourc'his D, Oakey RJ 2008. Regulation of alternative polyadenylation by genomic imprinting. Genes Dev 22: 1141–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T, Wallace J, Felsenfeld G 2011. Specific sites in the C terminus of CTCF interact with the SA2 subunit of the cohesin complex and are required for cohesin-dependent insulation activity. Mol Cell Biol 31: 2174–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Barr CL, Kim A, Yue F, Young Lee A, Eubanks J, Dempster EL, Ren B 2012. Base-resolution analyses of sequence and parent-of-origin dependent DNA methylation in the mouse genome. Cell 148: 816–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin B, Wong K, Agam A, Goodson M, Keane TM, Gan X, Nellaker C, Goodstadt L, Nicod J, Bhomra A, et al. 2011. Sequence-based characterization of structural variation in the mouse genome. Nature 477: 326–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon B, Herman H, Hu B, Park Y, Lindroth A, Bell A, West A, Chang Y, Stablewski A, Piel J, et al. 2005. Rasgrf1 imprinting is regulated by a CTCF-dependent methylation-sensitive enhancer blocker. Mol Cell Biol 25: 11184–11190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusufzai TM, Felsenfeld G 2004. The 5′-HS4 chicken β-globin insulator is a CTCF-dependent nuclear matrix-associated element. Proc Natl Acad Sci 101: 8620–8624 [DOI] [PMC free article] [PubMed] [Google Scholar]