Abstract
Aims
The pathogenesis of cardiac allograft vasculopathy (CAV) remains complex and may involve multiple mechanisms. We tested the hypothesis that the multilayer (ML) appearance, an intravascular ultrasound (IVUS) finding suggestive of repetitive thrombosis, is associated with plaque progression in heart transplant (HTx) recipients.
Methods and results
Our study population consisted of 132 HTx recipients undergoing at least two grayscale and virtual histology (VH)-IVUS examinations. A retrospective serial analysis was performed between the first (baseline) and the last (follow-up) IVUS data during a median follow-up of 3.0 years. The subjects were divided into two groups based on the presence of the ML appearance on the baseline IVUS. At baseline, subjects with ML appearance (n = 38) had a longer time elapsed since transplant, larger vessel volume, and larger plaque volume than those without (n = 94) (all P < 0.01). Intraluminal thrombi and plaque ruptures were identified only in subjects with ML appearance (P < 0.01 vs. those without). More subjects with ML appearance at baseline developed subsequent ML formation compared with those without [21 (55%) vs. 22 (23%), P < 0.01] during follow-up. There was an increase in plaque volume, necrotic core volume, and dense calcium volume in subjects with ML appearance (all P < 0.01 vs. those without). Multivariable linear regression analysis showed that ML appearance was a potential predictor of plaque progression (regression coefficient 0.28, 95% CI 0.10–0.45, P < 0.01).
Conclusions
The current study demonstrates that a finding of ML appearance, indicative of repeated episodes of mural thrombosis, is not infrequent in asymptomatic HTx recipients and possibly contributes to progression of CAV.
Keywords: Cardiac allograft vasculopathy, Thrombosis, Plaque progression, Intravascular ultrasound
Introduction
Cardiac allograft vasculopathy (CAV) is the major cause of long-term mortality after heart transplant (HTx).1 Cardiac allograft vasculopathy has heterogeneous pathologic features characterized by vascular wall inflammation, fibrous intimal thickening, and atherosclerosis.2 Concentric fibrous intimal hyperplasia along the entire length of the affected arteries often leads to underestimation of disease in coronary angiography. Therefore, at our medical centre, intravascular ultrasound (IVUS) imaging has been incorporated into routine post-transplant surveillance coronary angiography. A previous serial IVUS study demonstrated that progression of intimal thickening occurs mostly during the first year followed by a slower rate of progression over time.3 However, such studies were performed in early post-transplant years during the limited follow-up period <2 years. Thus, there is a lack of information on the longitudinal natural history of CAV. In this study, we evaluated serial changes in coronary artery lesion morphology in HTx patients over a long period of time, using both grayscale and radiofrequency data, known as virtual histology (VH)-IVUS.
Methods
Patient population
In the Mayo HTx programme, IVUS imaging of the left anterior descending coronary artery (LAD) has been performed in HTx recipients in conjunction with routine annual coronary angiography for surveillance of CAV. Between the years 2006 and 2011, among 251 recipients undergoing coronary angiography, 214 who received IVUS examinations were screened for eligibility for a retrospective analysis of serial IVUS examinations. Patients were included if they underwent at least two VH-IVUS examinations ≥12 months apart. Thus, of 214 subjects, 43 without follow-up IVUS, 33 without serial VH data, and 1 whose serial IVUS examinations were not apart ≥12 months were not included. Subjects were excluded if they had IVUS images with inadequate quality (n = 2), lesions with severe calcifications limiting the quantitative assessment (n = 1), and lesions with previous percutaneous coronary intervention (n = 2). Finally, 132 patients were enrolled in this study. For patients with more than two IVUS evaluations during the study period, the first (baseline) and the last (follow-up) IVUS data were used for analysis. All laboratory tests were performed at baseline as part of clinical routine examinations. The study protocol was approved by the institutional review board, and all participants gave written informed consent.
Coronary angiography
Based on the International Society of Heart and Lung Transplantation (ISHLT) guidelines,3 CAV was classified by coronary angiography as ISHLT CAV0 (not significant), CAV1 (mild), CAV2 (moderate), and CAV3 (severe).
Intravascular ultrasound image acquisition and analyses
Virtual histology -intravascular ultrasound examinations were performed using a 20 MHz, 2.9 F phased-array IVUS catheter (Eagle Eye Gold, Volcano Corporation, Rancho Cordova, CA, USA). After intracoronary administration of nitroglycerin, the transducer was placed at a distal portion of the LAD using the fiduciary side branch as the starting point, where the luminal diameter exceeded 2 mm and an imaging run was performed back to the coronary ostium at 0.5 mm/s. Three- to four-matched coronary segments of the LAD were determined from the images acquired at baseline and follow-up studies on the basis of the fiducial location of distal and proximal major side branches. The length of the segment was assessed as the distance between these two landmarks. The electrocardiogram-gated grayscale IVUS images and radiofrequency signals were acquired at the peak of the R wave. Offline volumetric reconstructions and analyses were performed side by side by two experienced investigators in a blinded manner using pcVH version 2.2 or Volcano Image Analysis Software V3.1 (Volcano Corporation). In case of disagreement, the observers reanalysed the IVUS image and reached a consensus on the diagnosis. Manual contour detection of both lumen and the outer vessel wall border was performed for each frame (median interslice distance 0.36 mm). For each coronary segment, quantitative volumetric analysis was performed and plaque volume (vessel volume-lumen volume) and percent plaque burden (plaque volume/vessel volume × 100) were determined. All volumetric data were divided by segment length to compensate for the different segment length of each examined artery and were shown as a volume index. The segment with the largest change in plaque volume index from baseline to follow-up in any matched site was used for the analysis in each patient (segment length 13.5 ± 4.8 mm).
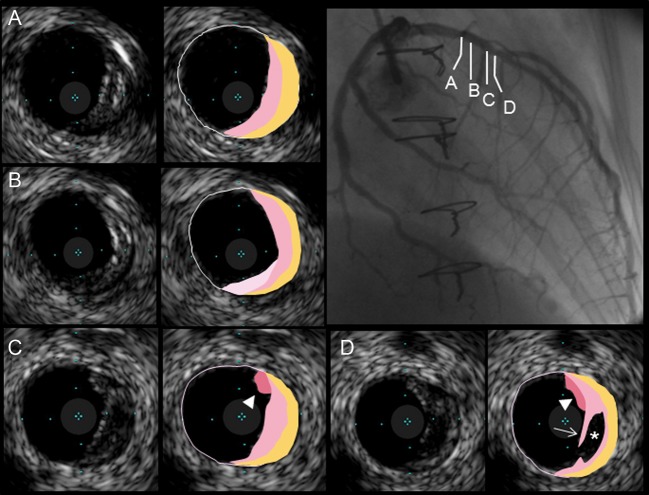
Multilayer (ML) appearance was defined as multiple layers with distinct echogenicity at baseline4,5 (Figures 1 and 2). When one or more superficial layers with distinct echogenicity were superimposed on a preexisting plaque during follow-up, the finding was defined as ML formation (Figure 2). Intraluminal thrombus was defined as an intraluminal mass with a layered, lobulated or pedunculated appearance with speckling at baseline IVUS examination.6 Plaque ruptures are characterized by a cavity communicating with the lumen with an overlying residual fibrous cap fragment (Figure 1). When plaque rupture was assumed to have occurred during follow-up, it was defined as subsequent plaque rupture (Figure 3).
Figure 1.
A representative case with multilayer appearance. Eccentric plaque with multilayer appearance was identified longitudinally in the left anterior descending artery. Intraluminal thrombi (arrowheads) and a ruptured cavity (asterisk) with residual fibrous cap (arrow).
Figure 2.
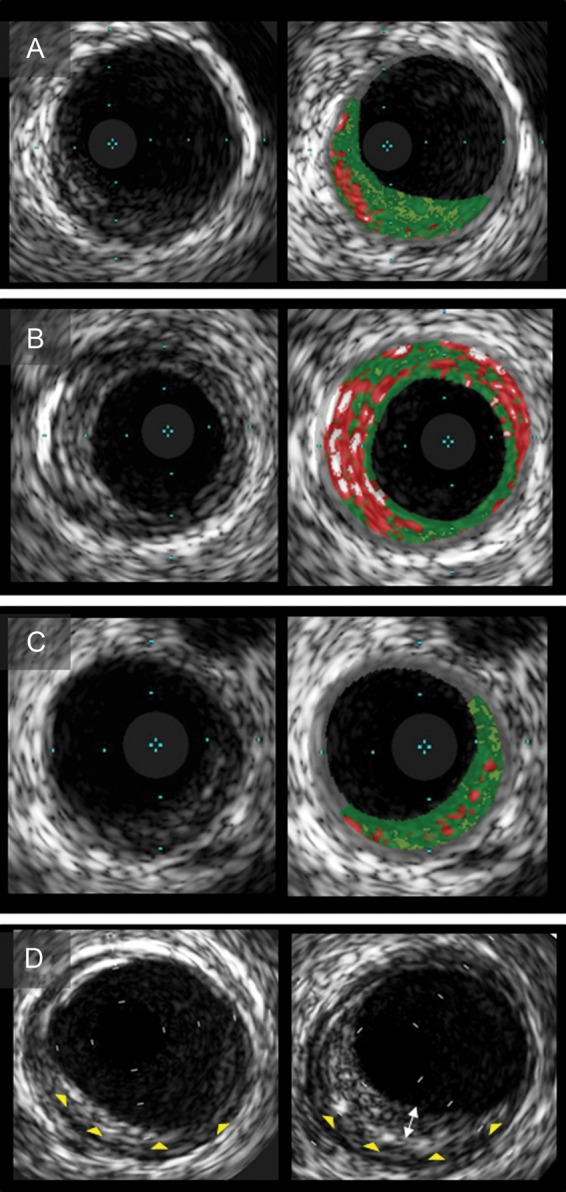
Intravascular ultrasound images of plaques with multiple layers. Grayscale intravascular ultrasound demonstrated that multilayer appearance consisted of stratified layers with distinct echogenicity (A–C). Corresponding virtual histology-intravascular ultrasound images show a single (A), multiple layers of necrotic core (B), and plaque without necrotic core layer (C). (D) Multilayer formation during follow-up. A new layer (bidirectional arrow) was superimposed on the preexisting layer (arrow heads). Intravascular ultrasound indicates intravascular ultrasound.
Figure 3.
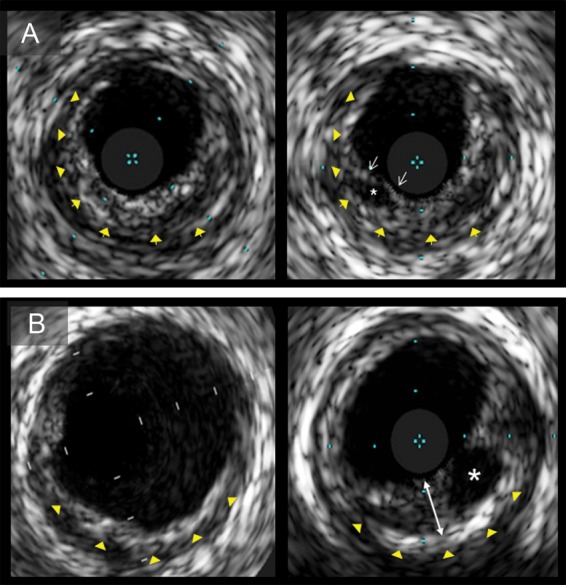
Grayscale intravascular ultrasound images of plaque ruptures. (A) Plaque with multilayer appearance (left) had subsequent plaque rupture in the superficial layer during follow-up (right) (yellow arrowheads show the border between superficial and deep layers; white arrows, fibrous cap; and asterisk, ruptured cavity). (B) Follow-up intravascular ultrasound image (right) demonstrates a rupture cavity in a newly formed layer (bidirectional arrow) on top of the preexistent layer at baseline (left; yellow arrowheads). Intravascular ultrasound indicates intravascular ultrasound.
To assess plaque composition, absolute amounts and percentage of plaque volume of VH-IVUS parameters (red for necrotic core, white for dense calcium, green for fibrous, and green-yellow for fibrofatty)7,8 were measured for each segment. Plaques with multiple layers were classified as fibroatheroma if they had a confluent necrotic core of >10% of plaque area for three consecutive frames; otherwise, they were defined as plaques without a necrotic core layer. Fibroatheromas were subclassified as a single or multiple layers of necrotic core9 (Figure 2).
Statistical analysis
Variables were expressed as mean ± SD, median [interquartile range (IQR)], or counts (percentage). Comparisons between two groups were performed using Student's t, Mann–Whitney U, or chi-square test as appropriate. We previously reported interobserver variability for VH compositional data in transplant recipients.10 Inter- and intra-observer agreement for ML appearance, intraluminal thrombus, plaque rupture and multiple necrotic core layers were assessed by the kappa statistics using random samples of 30 vessels. In addition, in order to evaluate multicentre interobserver agreement of ML appearance, all 132 segments were analysed by two independent external core laboratories (Cardiovascular Research Foundation and Wakayama Medical University) without knowledge of the patients' demographic or the investigator's interpretation of the IVUS. A Cochran–Armitage test was used for ordered categorical variables. The Wilcoxon-signed rank test was used to compare the changes of IVUS parameters between baseline and follow-up. Correlations between continuous variables were assessed by Spearman correlation analysis. Multivariable linear regression analysis was performed to assess adjusted association with change in plaque volume during follow-up. To compensate for variations in follow-up period, the change of plaque volume index were normalized for the length of follow-up period (mm3/mm/year).11 All clinical, angiographic, and IVUS parameters for change in plaque volume index per year were evaluated using univariate linear regression analysis. Recipient age, male sex, time since transplant, plaque volume index and variables with a P value of <0.20 on the univariate assessment were entered into a multivariable model. Statistical analyses were performed using JMP version 9.0 (SAS Institute, Inc., Cary, NC, USA).
Results
Baseline characteristics
At baseline, 38 (29%) showed a finding of ML appearance and 94 (71%) did not. Baseline characteristics are shown in Table 1. On coronary angiography, 12 (32%) subjects were graded as CAV 0, 20 (53%) as CAV 1, 4 (11%) as CAV 2, and 2 (5%) as CAV 3 in the ML appearance (+) group, whereas 73 (78%) graded as CAV 0, 20 (21%) as CAV 1, 1 (1%) as CAV 2, and none (0%) as CAV 3 in the ML appearance (−) group (P < 0.001). Of note, no ML appearance, thrombus or plaque rupture was suspected by angiography.
Table 1.
Baseline characteristics
| All | ML appearance (−) | ML appearance (+) | P value | |
|---|---|---|---|---|
| Number of recipients, n | 132 | 94 | 38 | |
| Male sex, n (%) | 96 (73) | 64 (68) | 32 (84) | 0.060 |
| Recipient age, years | 54.2 ± 12.6 | 52.4 ± 1.4 | 58.6 ± 1.6 | 0.004 |
| Donor age, years | 31.8 ± 13.6 | 28.5 ± 12.7 | 40.5 ± 12.2 | <0.001 |
| Allograft ischaemic time, min | 173 ± 49 | 172 ± 53 | 174 ± 37 | 0.855 |
| Cytomegalovirus viremia, n (%) | 15 (11) | 10 (11) | 5 (13) | 0.680 |
| Treatment episode for rejection, n (%) | 17 (13) | 11 (12) | 6 (16) | 0.403 |
| Time since transplant, years | 3.0 (0.2–7.3) | 2.0 (0.2–5.3) | 6.5 (2.0–9.2) | <0.001 |
| Pre-transplant diagnosis, n (%) | ||||
| Ischaemic cardiomyopathy | 33 (25) | 19 (20) | 14 (37) | 0.122 |
| Non-ischaemic cardiomyopathy | 79 (60) | 59 (63) | 20 (53) | |
| Others | 20 (15) | 16 (17) | 4 (11) | |
| Comorbidities | ||||
| Body mass index, kg/m2 | 26.9 ± 5.3 | 28.5 ± 5.7 | 26.3 ± 5.0 | 0.042 |
| Diabetes mellitus, n (%) | 38 (29) | 23 (24) | 15 (39) | 0.085 |
| Fasting glucose, mg/dL | 107 ± 25 | 108 ± 41 | 113 ± 26 | 0.387 |
| Current smoking, n (%) | 5 (4) | 5 (5) | 0 (0) | 0.147 |
| Hypertension, n (%) | 112 (85) | 75 (78) | 37 (97) | 0.014 |
| SBP (mmHg) | 122 ± 15 | 121 ± 14 | 124 ± 16 | 0.287 |
| DBP (mmHg) | 77 ± 10 | 77 ± 10 | 77 ± 10 | 0.934 |
| Dyslipidemia, n (%) | 109 (83) | 74 (80) | 35 (92) | 0.082 |
| Total cholesterol, mg/dL | 194 ± 49 | 199 ± 51 | 181 ± 40 | 0.029 |
| Triglyceride, mg/dL | 164 ± 78 | 164 ± 82 | 165 ± 67 | 0.969 |
| HDL cholesterol, mg/dL | 56 ± 19 | 58 ± 19 | 50 ± 15 | 0.014 |
| LDL cholesterol, mg/dL | 106 ± 38 | 109 ± 39 | 98 ± 35 | 0.116 |
| Serum creatinine, mg/dL | 1.5 ± 0.8 | 1.5 ± 0.9 | 1.4 ± 0.3 | 0.729 |
| GFR, mL/min/1.73m2 | 53 ± 16 | 54 ± 17 | 52 ± 15 | 0.558 |
| Medication | ||||
| Aspirin, n (%) | 42 (32) | 23 (24) | 19 (50) | 0.004 |
| ACE inhibitors, n (%) | 53 (40) | 35 (37) | 18 (47) | 0.282 |
| Calcium channel blocker, n (%) | 34 (26) | 28 (30) | 6 (16) | 0.096 |
| Beta blocker, n (%) | 24 (18) | 14 (15) | 10 (26) | 0.123 |
| Statin, n (%) | 115 (87) | 81 (86) | 34 (89) | 0.608 |
| Sirolimus, n (%) | 52 (39) | 36 (38) | 16 (42) | 0.685 |
| Cyclosporine, n (%) | 68 (52) | 47 (50) | 21 (55) | 0.584 |
| Tacrolimus, n (%) | 23 (17) | 20 (21) | 3 (8) | 0.067 |
| Azathioprine, n (%) | 35 (27) | 23 (24) | 12 (32) | 0.402 |
| Mycophenolate mofetil, n (%) | 89 (67) | 66 (70) | 23 (61) | 0.282 |
Data are expressed as mean ± SD, median (interquartile range) or n (%).
ACE indicates angiotensin-converting enzyme; DBP, diastolic blood pressure; GFR, glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ML, multilayer; and SBP, systolic blood pressure.
Grayscale intravascular ultrasound findings
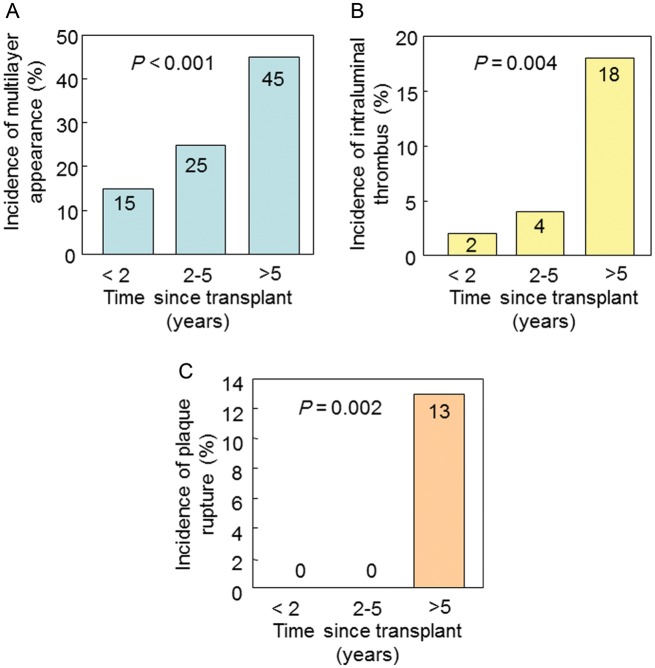
Grayscale IVUS findings are summarized in Table 2. Subjects with ML appearance had a greater vessel volume and plaque volume. The findings of ML appearance were more often identified in lesions with greater plaque burden (P < 0.001, Figure A1A). A positive relationship was observed between the time since transplant and plaque volume index at baseline (Spearman's r = 0.36, P < 0.001). Intriguingly, intraluminal thrombi (n = 11) and plaque ruptures (n = 7) were observed only in subjects with ML appearance. Moreover, all ruptured cavities were identified in the luminal surface of multiple layers (Figure 1D). When patients were categorized according to the time since transplant [<2 years (n = 53), 2–5 years (n = 28), and >5 years (n = 53)], the incidence of ML appearance, intraluminal thrombus, and plaque rupture increased with time since transplant (Figure 4).
Table 2.
Grayscale intravascular ultrasound findings
| ML appearance (−) | ML appearance (+) | P value | ||
|---|---|---|---|---|
| Baseline | ||||
| Vessel volume, mm3/mm | 15.3 (12.9–20.4) | 17.8 (16.1–22.8) | 0.006 | |
| Lumen volume, mm3/mm | 12.3 (9.2–15.4) | 11.3 (8.9–14.5) | 0.296 | |
| Plaque volume, mm3/mm | 3.7 (2.7–5.0) | 7.2 (5.6–8.7) | <0.001 | |
| Plaque burden, % | 22.3 (17.9–30.2) | 38.1 (30.5–44.6) | <0.001 | |
| Incidence of intraluminal thrombus | 0 (0) | 11 (29) | <0.001 | |
| Incidence of plaque rupture | 0 (0) | 7 (18) | <0.001 | |
| One finding | ||||
| Intraluminal thrombus | 0 (0) | 9 (24) | ||
| Plaque rupture | 0 (0) | 5 (13) | ||
| Two findings | ||||
| Intraluminal thrombus + plaque rupture | 0 (0) | 2 (5) | ||
| No other two findings (intraluminal thrombus and plaque rupture) | 94 (100) | 22 (58) | ||
| Follow-up | ||||
| Follow-up duration, years | 2.9 (2.0–4.0) | 3.1 (2.0–4.0) | 0.624 | |
| Vessel volume, mm3/mm | 15.5 (13.3–20.2) | 17.0 (15.5–22.5) | 0.019 | |
| Lumen volume, mm3/mm | 11.7 (8.7–14.0) | 8.8 (6.6–12.4)** | 0.004 | |
| Plaque volume index, mm3/mm | 4.5 (2.9–5.8)** | 8.1 (6.7–11.0)** | <0.001 | |
| Plaque burden, % | 26.2 (19.8–35.4)* | 47.7 (39.7–54.6)** | <0.001 | |
| Incidence of ML formation | 22 (23) | 21 (55) | <0.001 | |
| Incidence of intraluminal thrombus formation | 4 (4) | 6 (16) | 0.023 | |
| Incidence of subsequent rupture | 1 (1) | 6 (16) | 0.002 | |
| One finding | ||||
| ML formation | 17 (18) | 13 (45) | ||
| Intraluminal thrombus formation | 0 (0) | 1 (3) | ||
| Subsequent rupture | 0 (0) | 0 (0) | ||
| Two findings | ||||
| Intraluminal thrombus formation + subsequent rupture | 0 (0) | 1 (3) | ||
| ML formation + intraluminal thrombus formation | 4 (4) | 3 (8) | ||
| ML formation + subsequent rupture | 1 (1) | 4 (11) | ||
| All three findings (ML formation + subsequent rupture + intraluminal thrombus formation) | 0 (0) | 1 (3) | ||
| Change between baseline and follow-up | ||||
| Δ Vessel volume, mm3/mm | 0.4 (−1.0–1.8) | −0.3 (−1.6–0.7) | 0.190 | |
| Δ Lumen volume, mm3/mm | 0.05 (−2.5–1.2) | −1.5 (−4.2–0.1) | 0.016 | |
| Δ Plaque volume, mm3/mm | 0.2 (−0.3–1.4) | 1.0 (0.1–3.5) | 0.005 | |
Data are expressed as median (interquartile range) or number (%). ML indicates multilayer.
*P < 0.01, **P < 0.001 vs. baseline.
Figure 4.
Incidence of complex lesion morphology at baseline. When patients were categorized according to the time since transplant [<2 years (n = 53), 2–5 years (n = 28), and >5 years (n = 53)], the incidence of ML appearance (A), intraluminal thrombus (B), and plaque rupture (C) increased with time since transplant.
After a median follow-up of 3.0 (IQR: 2.0–4.0) years, subjects in both groups showed increases in plaque volume. When the change from baseline was compared between the two groups, there was an increase in plaque volume in subjects with ML appearance, but not in vessel volume, signifying a decrease in lumen volume. The change in plaque volume index correlated with follow-up duration (Spearman's r = 0.223, P = 0.010) in overall subjects. Nevertheless, the change in plaque volume index was related neither to the baseline plaque volume nor to the time since transplant (Figure A2), implying that the plaque volume increased in a linear manner with time after transplantation. During follow-up, subjects with ML appearance had more ML formations, intraluminal thrombus formations, and subsequent plaque ruptures. More than half of lesions with rapid progression (>10% increase in plaque volume per year) revealed ML formation generated from segments both with and without ML appearance at baseline (P < 0.001, Figure A1B).
All intraluminal thrombus formations were observed in segments with multiple layers (both ML appearance and ML formation). Of seven lesions with subsequent ruptures, three ruptured cavities were identified in preexisting multiple layers and four in the subsequently formed layers (Figure 3).
Virtual histology-intravascular ultrasound findings
Plaque compositions are shown in Table 3. At baseline, subjects with ML appearance had greater percentage of necrotic core and dense calcium compared with those without. At follow-up, the plaque volume increased in all compositions [except fibrofatty tissue in the ML appearance (+) group]. When the change from baseline was compared between the two groups, necrotic core and dense calcium volume increased in subjects with ML appearance compared with those without (Figure 5). Of 38 subjects with ML appearance at baseline, 22 (58%) had a single and 9 (24%) had multiple layers of necrotic core. Likewise, of 43 subjects with ML formation during follow-up, 23 (53%) revealed a single and 17 (40%) multiple layers of necrotic core.
Table 3.
Virtual histology intravascular ultrasound findings
| ML appearance (−) | ML appearance (+) | P value | |
|---|---|---|---|
| Baseline | |||
| Necrotic core, mm3/mm | 0.0 (0.0–0.2) | 0.5 (0.2–1.2) | <0.001 |
| Dense calcium, mm3/mm | 0.0 (0.0–0.1) | 0.2 (0.0–0.5) | <0.001 |
| Fibrous, mm3/mm | 0.1 (0.0–0.8) | 1.9 (1.2–2.8) | <0.001 |
| Fibrofatty, mm3/mm | 0.0 (0.0–0.2) | 0.2 (0.1–0.5) | <0.001 |
| Necrotic core, % | 9.6 (1.2–18.3) | 22.5 (9.9–27.5) | <0.001 |
| Dense calcium, % | 1.3 (0–6.6) | 6.7 (1.9–17.5) | <0.001 |
| Fibrous, % | 64.1 (44.5–78.4) | 60.1 (49.3–68.3) | 0.282 |
| Fibrofatty, % | 6.6 (1.5–14.6) | 7.0 (3.4–14.3) | 0.338 |
| Follow-up | |||
| Necrotic core, mm3/mm | 0.1 (0.0–0.4)*** | 1.3 (0.7–2.0)*** | <0.001 |
| Dense calcium, mm3/mm | 0.0 (0.0–0.1)* | 0.7 (0.2–1.1)*** | <0.001 |
| Fibrous, mm3/mm | 0.7 (0.0–1.6)*** | 2.6 (1.3–3.7)* | <0.001 |
| Fibrofatty, mm3/mm | 0.1 (0.0–0.2)* | 0.3 (0.1–0.5) | <0.001 |
| Necrotic core, % | 11.5 (2.0–20.1) | 26.4 (21.5–31.5)*** | <0.001 |
| Dense calcium, % | 0.9 (0–5.7) | 12.6 (5.6–23.9)** | <0.001 |
| Fibrous, % | 68.7 (43.1–77.0) | 51.2 (40.0–63.2)** | 0.002 |
| Fibrofatty, % | 6.5 (2.2–10.5)* | 6.2 (4.1–9.4) | 0.669 |
| Change between baseline and follow-up | |||
| Δ Necrotic core volume, mm3/mm | 0.0 (0.0–0.2) | 0.5 (0.2–1.2) | <0.001 |
| Δ Dense calcium volume, mm3/mm | 0.0 (0.0–0.0) | 0.2 (0.0–0.7) | <0.001 |
| Δ Fibrous volume, mm3/mm | 0.1 (0.0–0.7) | 0.4 (-0.5–1.7) | 0.494 |
| Δ Fibrofatty volume, mm3/mm | 0.0 (0.0–0.1) | 0.0 (−0.1–0.2) | 0.636 |
Data are expressed as median (interquartile range). ML indicates multilayer.
*P < 0.05, **P < 0.01, ***P < 0.001 vs. baseline.
Figure 5.
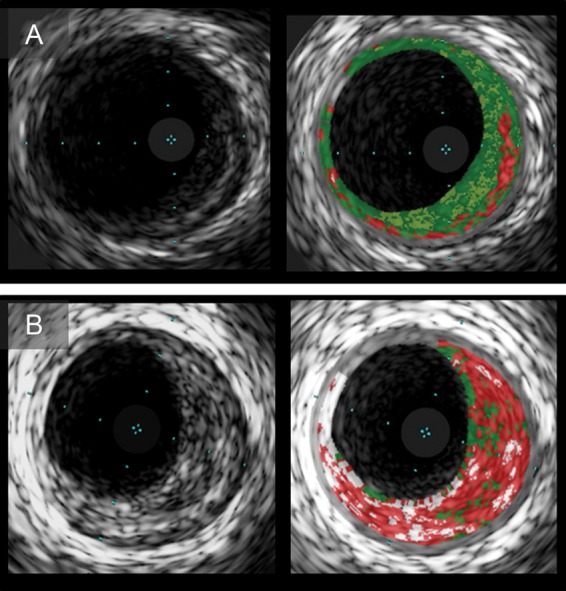
Serial intravascular ultrasound images of plaques with multilayer appearance. Plaque with multilayer appearance at baseline (A) had subsequent multilayer formation at follow-up (B). Virtual histology-intravascular ultrasound images show a single layer of necrotic core (A) and multiple layers (B).
Multivariable linear regression analysis
Patients with ML appearance at baseline imaging had a higher rate of change in plaque volume [covariance adjusted mean 0.28 mm3/mm/year, 95% CI (0.10, 0.45), P = 0.003] than those without ML (Table A1).
Interobserver and intra-observer agreement
Inter- and intra-observer agreement for ML appearance, intraluminal thrombus, plaque rupture and multiple necrotic core layers were 0.85; 0.92, 0.78; 0.84, 0.78; 0.84, and 0.80; 0.93, respectively. Likewise, the interobserver agreement for ML appearance between the one onsite reader and the two offsite readers (offsite readers 1 and 2) was 0.73 (onsite reader vs. offsite reader 1), 0.71 (onsite reader vs. offsite reader 2), and 0.64 (offsite reader 1 vs. offsite reader 2), respectively (Table A2).
Discussion
The current serial IVUS study demonstrated that a substantial number of asymptomatic HTx recipients had lesions with complex lesion morphology, such as multiple layers, intraluminal thrombi, and plaque ruptures. Furthermore, this study implies that recurrent episodes of coronary thrombosis, presenting as ML appearance, may mediate the progression of CAV.
Multiple layers are often indicative of repetitive, periodically occurring asymptomatic thrombus formation.12–14 Post-mortem studies for native atherosclerosis demonstrated healed plaque ruptures and erosions with multiple layers of distinct tissue components.12 ML appearance identified by cross-sectional IVUS imaging has been interpreted as mural thrombus.4,6 To our knowledge, this is the first longitudinal IVUS study, demonstrating multiple layers not only at a single time point (ML appearance) but also longitudinally (ML formation). The present serial IVUS study demonstrated that lesions with ML formation exhibited new inner layers with distinct echogenicity overlaying preexisting outer layers. This observation could be highly indicative of repeated episodes of mural thrombosis.
A previous autopsy study in 64 allograft hearts showed that 19 arteries had recent or organized luminal thrombi.15 Another postmortem study demonstrated coronary thrombi in 81% of transplanted hearts with CAV, and notably, most of the thrombi (78%) were non-occlusive mural thrombi,16 consistent with our observations. The current study extends these previous in vitro observations and confirmed these findings in vivo, and suggests that coronary thrombosis might occur more frequently in CAV than previously suspected.
Plaque composition of multiple layers may differ between plaque rupture and erosion. Immunohistological analyses of CAV17 demonstrated that mural thrombi were stratified on discontinuous or absent endothelial layers without atheromatous lesions, suggesting plaque erosion as a possible underlying mechanism in CAV. In this study, in contrast to the histological analysis, most plaques with multiple layers exhibited a single or multiple layers of necrotic core, implying healed plaque rupture. These seemingly disparate results need to be interpreted with caution because of the paucity of data comparing VH-IVUS imaging with histology in various degrees of thrombus organization. Further studies are warranted to determine the underlying mechanisms of multiple layers in CAV.
In the current study, segments with ML appearance were more likely to increase in plaque volume and have more subsequent ML formation. Insufficient endothelial cell ingrowth over the surface of the thrombus14 may predispose to recurrent thrombosis. Growth factors from platelets in mural thrombus18 and repetitive ruptures13 may cause plaque expansion due to smooth muscle cell proliferation. Thus, several episodes of mural thrombosis and subsequent fibrotic organization may contribute to plaque progression in CAV.
The present study revealed that 9% of subjects had plaque ruptures during the study period. While the precise mechanisms leading to plaque rupture in CAV remain uncertain, several factors can be entertained. Mechanical stress such as blood pressure19 and plaque stress20 may lead to interfacial debonding between inner and outer plaque layers due to the large mismatch in hardness of thrombus layers with different degrees of organization. Plaque destabilization of advanced lesions is another plausible mechanism. Atheromatous plaques were identified histologically in longer surviving allografts21 and even in paediatric recipients.15 In addition, organized thrombi contribute to the progression to advanced atherosclerotic plaques,22 consistent with our findings that necrotic core and dense calcium were the major components of increased plaque volume.
A previous study from our group10 showed that plaques with increased inflammatory burden assessed by VH-IVUS are associated with progression of CAV. The current study showing the relation of multiple layers to plaque progression extends the previous observation and raises a possibility that specific plaque characteristics may contribute to plaque progression in CAV.
The association of the IVUS finding of multiple layers with myocardial damage is of concern. In the current study, 7 (18%) of 38 recipients with ML appearance at baseline had regional wall motion abnormalities. Six of them had a segmental wall motion abnormality in the perfusion territory corresponding to the LAD. The other one had in a different region. Of 22 recipients who developed ML formation during follow-up without ML appearance at baseline, 2 (9%) acquired a new wall motion abnormality in the corresponding region to the LAD. There is a possibility that myocardial damage can be attributed to repeated thrombotic episodes to some extent. As the present study was not designed to determine the impact of specific IVUS findings on cardiac function, further studies are needed.
It should be mentioned that more subjects with ML appearance were on aspirin therapy at baseline. Extensive coronary artery stenosis in these subjects would affect physicians' clinical decisions to use aspirin.
Given complex lesion morphology and their contribution to plaque progression, the current study may call attention to the possibility that pharmacologic agents targeting clotting or coagulation cascade may attenuate the progression of CAV.
Limitations
The present study has several potential limitations. As this study cohort consisted of recipients undergoing repeated routine IVUS examinations, this may introduce a selection bias. Moreover, plaque characterization using VH-IVUS in HTx patients has not been validated. In this study, IVUS examinations were performed only in the LAD, not in the entire coronary arteries. Therefore, the findings in this study may reflect the disease process in a limited number of coronary sites in each patient. In addition, multivariable linear regression analysis was performed based on the assumption that plaque progression was linear over time. However, natural course of plaque progression during long-term follow-up in the same individual is uncertain due to insufficient data. Finally, since this is a single-centre study involving a small sample, the results are subject to the shortcomings inherent to all single-centre studies, and the results and conclusions of this study should be regarded as exploratory, provocative, and hypothesis-generating. A study of larger patient populations from various centres with an independent core IVUS laboratory is warranted to confirm the results.
Conclusions
In conclusion, our observations demonstrate that a finding of ML appearance, which may be indicative of repeated episodes of mural thrombosis, is not infrequent in asymptomatic cardiac transplant recipients. These findings may contribute to progression of CAV. The current study gives new insight into the potential role of coronary thrombosis in plaque progression in CAV.
Funding
The work was supported by the National Institute of Health (NIH Grants HL-92954 and AG-31750 to A.L., and NIH Grants DK-73608, HL-77131, and HL-085307 to L.O.L.).
Conflict of interest: none declared.
Appendix 1
Figure A1.
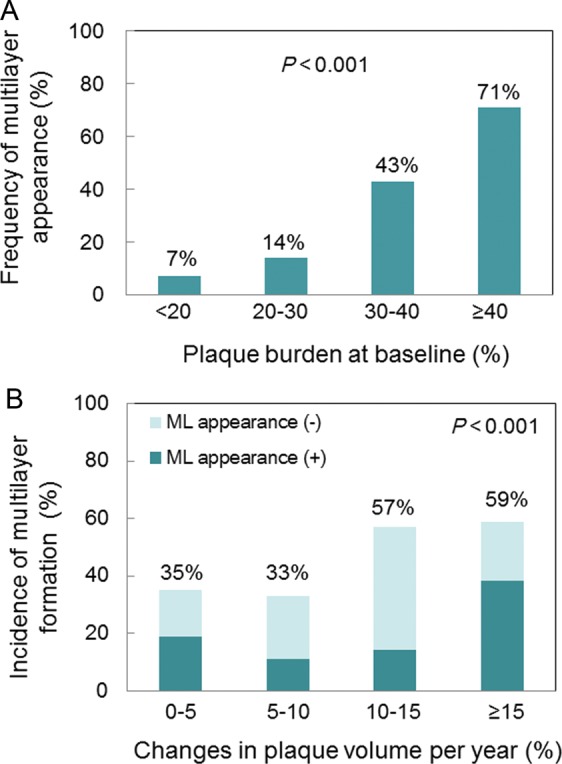
(A) Frequency of multilayer appearance according to plaque burden at baseline. Multilayer appearance was more frequently identified in lesions with greater plaque burden. (B) Incidence of multilayer formation during follow-up according to percentage changes in plaque volume per year. Multiple layer formation was more often identified in segments with more rapid progression, and in those both with multilayer appearance at baseline and without. Comparisons were made by a Cochran–Armitage trend test.
Appendix 2
Figure A2.
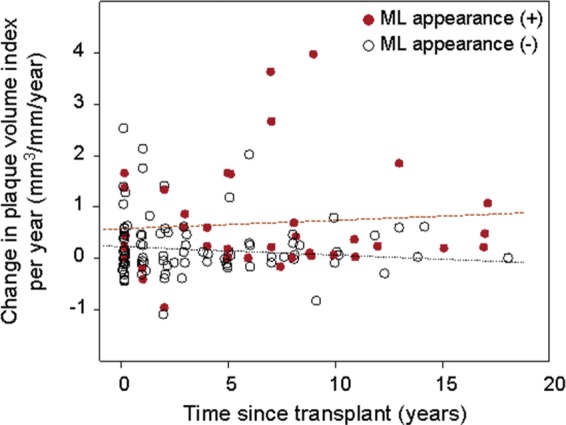
Scatter plot of change in plaque volume per year (y-axis) vs. time since transplant (x-axis). Overall, there was no relationship between the rate of change in plaque volume and the time since transplant (Spearman's r = 0.085, P = 0.333). Closed circle indicates subjects with ML appearance at baseline; and open circle, those without ML appearance.
Appendix 3
Table A1.
Multivariable predictors of plaque volume increase per year
| Multivariable predictors | Regression coefficient | Standard error | 95% CI |
P value | |
|---|---|---|---|---|---|
| Intercept | 0.02 | 0.69 | −1.34 | 1.38 | 0.976 |
| Recipient age (per 10 year increase) | −0.02 | 0.05 | −0.12 | 0.09 | 0.748 |
| Male sex | 0.06 | 0.07 | −0.09 | 0.20 | 0.459 |
| Time since transplant | 0.01 | 0.01 | −0.02 | 0.04 | 0.582 |
| Treatment episode for rejection | 0.20 | 0.09 | 0.01 | 0.38 | 0.037 |
| Current smoking | 0.38 | 0.17 | 0.04 | 0.71 | 0.027 |
| Aspirin use | 0.09 | 0.07 | −0.05 | 0.23 | 0.199 |
| Calcium channel blocker use | 0.12 | 0.08 | −0.03 | 0.26 | 0.120 |
| SBP (per 10 mmHg increase) | 0.10 | 0.05 | 0.01 | 0.19 | 0.029 |
| ML appearance | 0.28 | 0.09 | 0.10 | 0.45 | 0.003 |
| Plaque volume index | −0.05 | 0.03 | −0.02 | 0.01 | 0.102 |
The adjusted R2 value was 0.179. Age was entered into a multivariable model as (age-50)/10, so that the parameter estimate for age reflects the expected change per 10 year increase and the intercept reflects the expected mean for a 50-year-old with all other covariates set to zero. The lumen volume index (P = 0.040 on univariate analysis) and vessel volume index (P = 0.018 on univariate analysis) were not included in the multivariable model due to multicollinearity with plaque volume index. CI, confidence interval; ML, multilayer; and SBP, systolic blood pressure.
Appendix 4
Table A2.
Interobserver variability of multilayer appearance
| Onsite reader | Offsite reader 1 | Offsite reader 2 | Cases (N = 132) |
|---|---|---|---|
| No | No | No | 87 |
| No | No | Yes | 2 |
| No | Yes | No | 3 |
| No | Yes | Yes | 0 |
| Yes | No | No | 6 |
| Yes | No | Yes | 5 |
| Yes | Yes | No | 6 |
| Yes | Yes | Yes | 23 |
Onsite vs. Offsite reader 1: κ = 0.73 (SE = 0.07, 95% CI, 0.60–0.86). Onsite vs. Offsite reader 2: κ = 0.71 (SE = 0.07, 95% CI, 0.57–0.84). Offsite reader 1 vs. Offsite reader 2: κ = 0.64 (SE = 0.08, 95% CI, 0.48–0.79). SE indicates standard error.
References
- 1.Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, Kirk R, Rahmel AO, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: twenty-eighth Adult Heart Transplant Report—2011. J Heart Lung Transplant. 2011;30:1078–1094. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Rahmani M, Cruz RP, Granville DJ, McManus BM. Allograft vasculopathy versus atherosclerosis. Circ Res. 2006;99:801–815. doi: 10.1161/01.RES.0000246086.93555.f3. [DOI] [PubMed] [Google Scholar]
- 3.Yeung AC, Davis SF, Hauptman PJ, Kobashigawa JA, Miller LW, Valantine HA, Ventura HO, Wiedermann J, Wilensky R. Incidence and progression of transplant coronary artery disease over 1 year: results of a multicenter trial with use of intravascular ultrasound. Multicenter Intravascular Ultrasound Transplant Study Group. J Heart Lung Transplant. 1995;14:S215–S220. [PubMed] [Google Scholar]
- 4.Kearney P, Erbel R, Rupprecht HJ, Ge J, Koch L, Voigtländer T, Stähr P, Görge G, Meyer J. Differences in the morphology of unstable and stable coronary lesions and their impact on the mechanisms of angioplasty. An in vivo study with intravascular ultrasound. Eur Heart J. 1996;17:721–730. doi: 10.1093/oxfordjournals.eurheartj.a014939. [DOI] [PubMed] [Google Scholar]
- 5.Deftereos S, Giannopoulos G, Kossyvakis C, Pyrgakis VN. Virtual histology. Hellenic J Cardiol. 2010;51:235–244. [PubMed] [Google Scholar]
- 6.Kotani J, Mintz GS, Rai PB, Pappas CK, Gevorkian N, Bui AB, Pichard AD, Satler LF, Suddath WO, Waksman R, Laird JR, Jr, Kent KM, Weissman NJ. Intravascular ultrasound assessment of angiographic filling defects in native coronary arteries: do they always contain thrombi? J Am Coll Cardiol. 2004;44:2087–2089. doi: 10.1016/j.jacc.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Nasu K, Tsuchikane E, Katoh O, Vince DG, Virmani R, Surmely JF, Murata A, Takeda Y, Ito T, Ehara M, Matsubara T, Terashima M, Suzuki T. Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. J Am Coll Cardiol. 2006;47:2405–2412. doi: 10.1016/j.jacc.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 8.Diethrich EB, Pauliina Margolis M, Reid DB, Burke A, Ramaiah V, Rodriguez-Lopez JA, Wheatley G, Olsen D, Virmani R. Virtual histology intravascular ultrasound assessment of carotid artery disease: the Carotid Artery Plaque Virtual Histology Evaluation (CAPITAL) study. J Endovasc Ther. 2007;14:676–686. doi: 10.1177/152660280701400512. [DOI] [PubMed] [Google Scholar]
- 9.Maehara A, Cristea E, Mintz GS, Lansky AJ, Dressler O, Biro S, Templin B, Virmani R, de Bruyne B, Serruys PW, Stone GW. Definitions and methodology for the grayscale and radiofrequency intravascular ultrasound and coronary angiographic analyses. JACC Cardiovasc Imaging. 2012;5:S1–S9. doi: 10.1016/j.jcmg.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Raichlin E, Bae JH, Kushwaha SS, Lennon RJ, Prasad A, Rihal CS, Lerman A. Inflammatory burden of cardiac allograft coronary atherosclerotic plaque is associated with early recurrent cellular rejection and predicts a higher risk of vasculopathy progression. J Am Coll Cardiol. 2009;53:1279–1286. doi: 10.1016/j.jacc.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann M, von Birgelen C, Mintz GS, Stoel MG, Eggebrecht H, Wieneke H, Fahy M, Neumann T, van der Palen J, Louwerenburg HW, Verhorst PM, Erbel R. Relation between lipoprotein(a) and fibrinogen and serial intravascular ultrasound plaque progression in left main coronary arteries. J Am Coll Cardiol. 2006;48:446–452. doi: 10.1016/j.jacc.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 12.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 13.Burke AP, Kolodgie FD, Farb A, Weber DK, Malcom GT, Smialek J, Virmani R. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103:934–940. doi: 10.1161/01.cir.103.7.934. [DOI] [PubMed] [Google Scholar]
- 14.Tavora F, Li L, Ripple M, Fowler D, Burke A. Morphologic characteristic of coronary artery disease, with emphasis on thromboses, in patients younger than 40 years of age. Patholog Res Int. 2010;2010:628247. doi: 10.4061/2010/628247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu WH, Palatnik K, Fishbein GA, Lai C, Levi DS, Perens G, Alejos J, Kobashigawa J, Fishbein MC. Diverse morphologic manifestations of cardiac allograft vasculopathy: a pathologic study of 64 allograft hearts. J Heart Lung Transplant. 2011;30:1044–1050. doi: 10.1016/j.healun.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Arbustini E, Dal Bello B, Morbini P, Gavazzi A, Specchia G, Vigano M. Multiple coronary thrombosis and allograft vascular disease. Transplant Proc. 1998;30:1922–1924. doi: 10.1016/s0041-1345(98)00526-0. [DOI] [PubMed] [Google Scholar]
- 17.Arbustini E, Dal Bello B, Morbini P, Gavazzi A, Specchia G, Viganò M. Immunohistochemical characterization of coronary thrombi in allograft vascular disease. Transplantation. 2000;69:1095–1101. doi: 10.1097/00007890-200003270-00013. [DOI] [PubMed] [Google Scholar]
- 18.Häyry P, Yilmaz S. Role of growth factors in graft vessel disease. Transplant Proc. 1995;27:2066–2067. [PubMed] [Google Scholar]
- 19.Hoeks AP, Reesink KD, Hermeling E, Reneman RS. Local blood pressure rather than shear stress should be blamed for plaque rupture. J Am Coll Cardiol. 2008;52:1107–1108. doi: 10.1016/j.jacc.2008.04.071. [DOI] [PubMed] [Google Scholar]
- 20.Li ZY, Howarth SP, Tang T, Gillard JH. How critical is fibrous cap thickness to carotid plaque stability? A flow-plaque interaction model. Stroke. 2006;37:1195–1199. doi: 10.1161/01.STR.0000217331.61083.3b. [DOI] [PubMed] [Google Scholar]
- 21.Johnson DE, Gao SZ, Schroeder JS, DeCampli WM, Billingham ME. The spectrum of coronary artery pathologic findings in human cardiac allografts. J Heart Transplant. 1989;8:349–359. [PubMed] [Google Scholar]
- 22.Roberts WC, Buja LM. The frequency and significance of coronary arterial thrombi and other observations in fatal acute myocardial infarction: a study of 107 necropsy patients. Am J Med. 1972;52:425–443. doi: 10.1016/0002-9343(72)90033-2. [DOI] [PubMed] [Google Scholar]