Abstract
RTS,S is a pre-erythrocytic malaria vaccine candidate antigen based on the circumsporozoite surface protein of Plasmodium falciparum fused to HBsAg, incorporating a novel Adjuvant System (AS02). The first field efficacy of RTS,S/AS02 against infection was demonstrated in a trial initiated in The Gambia in 1998. This paper presents the five-year safety and immunogenicity follow up of the 306 men who were enrolled in the original trial.
In the primary study men aged 18 to 45 years were randomized to receive either RTS,S/AS02 or rabies vaccine at 0, 1, 5 months followed by a booster dose at month 19. The subjects were observed for long term safety and immunogenicity continuously until month 58.
Of the 153 subjects in each group at enrollment, 80 (52%) subjects in the RTS,S/AS02 group and 83 (54%) subjects in the rabies group returned for the final long-term follow-up visit at month 58. The main reason for non-attendance at month 58 was migration (76% of all drop-outs). Nine subjects in the RTS,S/AS02 group and seven in the rabies group experienced serious adverse events (SAEs) over the 58 month surveillance period, of which seven had a fatal outcome (five RTS,S/AS02 and two rabies group). None of the SAEs with fatal outcome were attributed to the study vaccine. Anti-CS antibody persistence compared to control was observed for five years, although titres had waned from post-booster levels; similar responses in anti-HBs antibody persistence were observed in initially HBsAg seronegative subjects.
This study provides the first indication of the long-term safety and persistence of anti-CS and anti-HBs antibodies of the RTS,S vaccine candidate in combination with the novel AS02 Adjuvant System.
Keywords: malaria vaccine; RTS,S; five-year follow-up; long term persistence of anti-HBs; AS02 adjuvant system
Introduction
Malaria remains a major cause of human suffering and economic drain across sub-Saharan Africa.1 Today approximately 40% of the world’s population, mostly those living in the world’s poorest countries, are at risk of malaria. The World Health Organization (WHO) estimated that 1.27 million deaths worldwide were caused by malaria in 2002; over 90% of these deaths occurred in Africa.2 A vaccine is widely viewed as an essential part of the long-term strategy to control malaria, especially in Africa, where the climate and environment highly favor transmission.
The pre-erythrocytic malaria vaccine candidate RTS,S is being developed for the routine immunization of infants and children living in malaria-endemic areas. The RTS,S antigen consists of sequences of the circumsporozoite (CS) protein and hepatitis B surface antigen (HBsAg). It is administered with a novel Adjuvant System, AS02, which is proprietary oil in water emulsion with the immunostimulants MPL® and QS21.3
The first evidence of efficacy of RTS,S/AS02 under conditions of natural exposure in an African population came from a trial initiated in The Gambia in 1998. Vaccine efficacy after three doses, as determined by a time to infection analysis was measured to be 34% (95% CI: 8.0 to 53.0, p = 0.014) over a 15-week malaria transmission season.4 A booster dose of RTS,S/AS02 given one year later conferred 47% efficacy (95% CI: 4 to 71%, p = 0.037) over a 9-week malaria transmission season. These encouraging data resulted in subsequent evaluation in the paediatric population which found the vaccine to be effective against clinical malaria disease in children and infants.5,6 In this paper, we now report the five-year safety and immunogenicity follow-up of the 306 adult male recipients of RTS,S/AS02 or comparator who were enrolled in the original Gambian trial.
Results
Demographics
The demographic profile of the two groups of subjects at enrollment is shown in Table 1. The mean age at first vaccination was similar in both groups and at months 35, 46 and 58 for the ATP immunogenicity cohort; the drop-out rate was higher among younger age groups (data not shown).
Table 1. Demographics of the study groups at the start of surveillance (Total Cohort).
| Characteristics | RTS,S/AS02 N = 153 |
Rabies N = 153 |
Total N = 306 |
|
|---|---|---|---|---|
| Ethnicity | Mandinka | 147 (96.1%) | 144 (94.1%) | 291 (95.1%) |
| Other | 6 (3.9%) | 9 (5.9%) | 15 (4.9%) | |
| Age (years) | Mean [SD] | 26.4 [8.9] | 25.9 [9.0] | 26.2 [8.9] |
| Age category (years) |
17 to 19 | 55 (35.9%) | 58 (37.9%) | 113 (36.9%) |
| 20 to 24 | 24 (15.7%) | 31 (20.3%) | 55 (18.0%) | |
| 25 to 34 | 39 (25.5%) | 29 (19.0%) | 68 (22.2%) | |
| 35 to 40 | 20 (13.1%) | 19 (12.4%) | 39 (12.7%) | |
| 41 to 45 | 15 (9.8%) | 16 (10.5%) | 31 (10.1%) | |
| HBsAg Status | Positive | 22 (14.4%) | 28 (18.3%) | 50 (16.3%) |
| Negative | 131 (85.6%) | 125 (81.7%) | 256 (83.7%) |
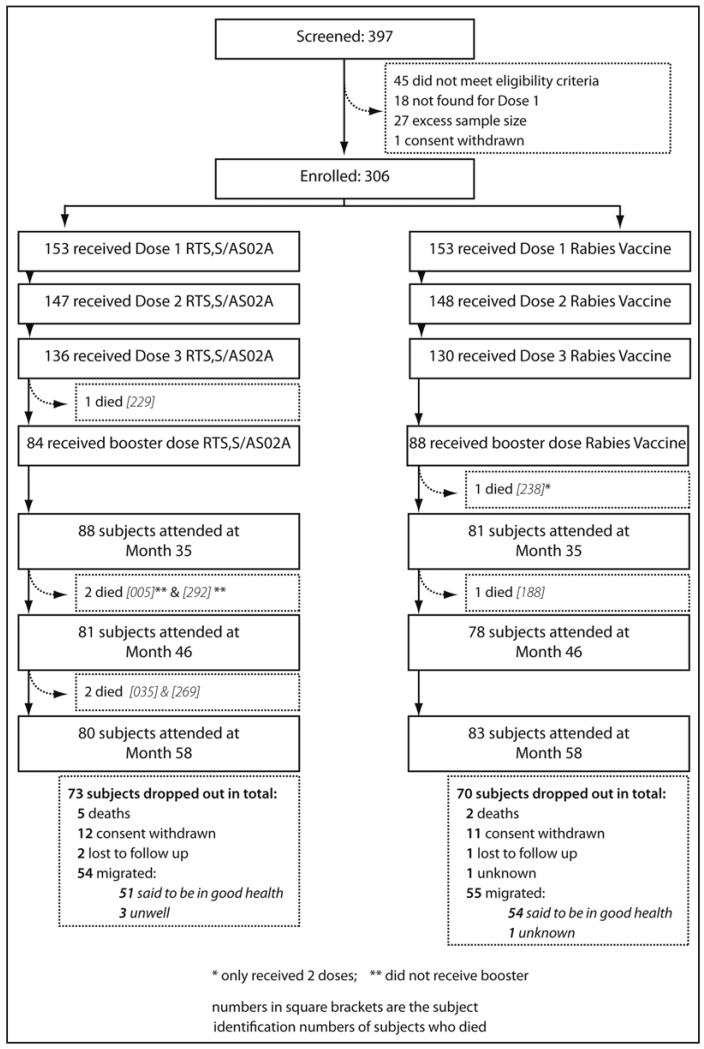
Of the 306 men enrolled, 295 (96%), 264 (86%) and 158 men (52%) received their second, third and fourth doses of vaccine respectively. In total, 163 subjects (80 RTS,S/AS02, 83 Rabies) returned for the final long-term follow-up visit at month 58. The main reason for not returning for the month 58 visit was migration away from the study area (109/143, 76.2% of all drop-outs) (Fig. 1). Health information was available from the subjects’ relatives for 108 of the 109 subjects who migrated from the study area.
Figure 1.
Consort diagram for flow of subjects in the study.
Safety follow-up
Of the 306 subjects who were enrolled in the primary study, 16 subjects (5.2%) (nine in the RTS,S/AS02 group and seven in the rabies group) experienced SAEs during the safety follow-up from first vaccination to month 58, of which seven subjects (2.3%) were reported as having SAEs with fatal outcome (5 RTS,S/AS02 and two rabies group). This translates into a mortality rate of approximately 9.3 (95% CI: 1.15 to17.53) per 1000 person years in the RTS,S/AS02 group and 3.7 (95% CI: −1.14 to 8.82) per 1000 person years in the control group. A cause was ascribed in four cases (all recipients of RTS,S/AS02): two subjects were infected with hepatitis B and died of hepatocellular carcinoma (subjects 229 and 292), one subject died of a lymphoproliferative malignancy (subject 35) and one subject died of pulmonary tuberculosis (subject 269). In the remaining three cases no cause of death could be attributed: one subject died whilst out of the country following a laparotomy for abdominal pain and no other details were available (rabies vaccine recipient; subject 238), one subject died after a long illness comprising chest and abdominal pain, generalized swelling and breathlessness for which he chose to seek traditional medical care (RTS,S/AS02 recipient; subject 5) and one subject died after a chronic illness of chest and abdominal pain for which a diagnosis was not made (rabies vaccine recipient; subject 188). All were judged to be not related to a study vaccine.
Fifty subjects were found to be HBsAg positive at screening (22 RTS,S/AS02 and 28 control vaccine). Of these one subject who received RTS,S/AS02 died (subject 292). Although subject 229, in the RTS,S/AS02 group, was infected with hepatitis B and died of hepatocellular carcinoma, while his HBsAg test at screening was negative; two subsequent tests were, however, positive.
At study end, relatives reported that three subjects (recipients of RTS,S/AS02) were ‘unwell’: two subjects suffered from tuberculosis, one combined with mental illness, and one subject suffered from “general body pain.” The health of one subject in the rabies vaccine group was unknown. All other subjects in both groups, who had migrated by the time of the final visit, were described as being “in good health” by their relatives.
Persistence of humoral immune response
Anti-CS (anti-R32LR) antibody response
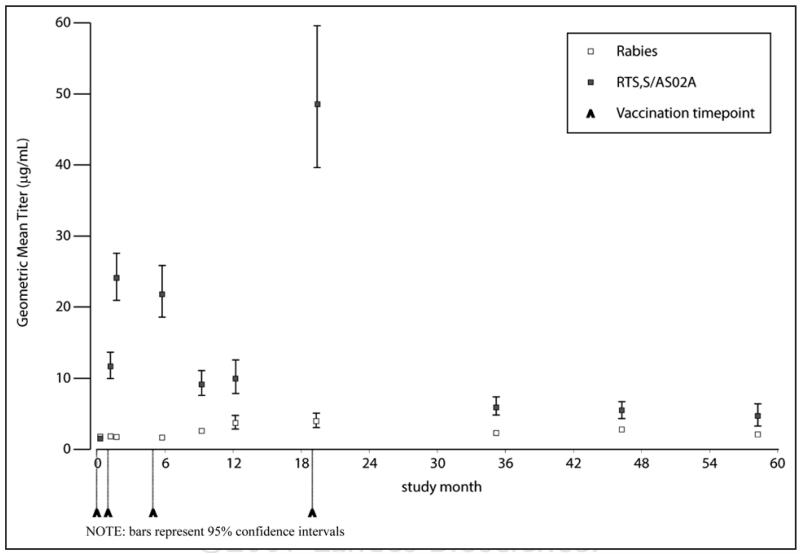
In the ATP cohort, following the primary vaccination course, anti-CS geometric mean titres (GMTs) were 21.8 μg/ml (95% CI: 18.4 to 25.8) in the RTS,S/AS02 group and 1.7 μg/ml (95% CI: 1.4–2.0) in the rabies group. A strong booster effect was observed two weeks after the fourth dose of RTS,S/AS02; GMTs were 45.4 μg/ml (95% CI: 35.8 to 57.7) compared to 4.5 μg/ml (95% CI: 3.4 to 6.0) in the rabies vaccine group. At month 58, GMTs were low but remained significantly higher among RTS,S/AS02 recipients compared to the rabies vaccine group; 4.7 μg/ml (95% CI: 3.5 to 6.4) versus 2.1 μg/ml (95% CI: 1.7 to 2.5) [difference: 2.6 μg/ml (95% CI: 0.3 to 4.8)]. The evolution of titres is shown in Figure 2.
Figure 2.
Evolution of anti-CS antibody titres over time (ATP Cohort).
Anti-HBs antibody response
In subjects who were HBsAg seronegative at baseline, after the primary immunization course of RTS,S/AS02 titres were 3160 mIU/ml and the proportion who were seroprotected had risen from 36% to 97% (Table 2). Titres rose further after the booster dose to 11737 mIU/ml. Titres and seroprotective levels fell at each successive annual measurement and at month 58 were 105.5 mIU/ml and 80%, respectively.
Table 2. Persistence of anti-HBs antibody response to month 58 in initially HBsAg seronegative subjects (ATP cohort).
| Vaccine group |
Timing | N | Seroprotected (%) |
GMT (mIU/ml) |
95% CI | |
|---|---|---|---|---|---|---|
| RTS,S/ AS02 |
Pre | 121 | 35.5 | 5.3 | 3.6 | 7.8 |
| Month 5.5* | 105 | 97.1 | 3159.8 | 2001.0 | 4989.6 | |
| Month 9 | 99 | 89.9 | 570.0 | 351.8 | 923.9 | |
| Month 19** | 47 | 100 | 11737.2 | 6284.5 | 21921.0 | |
| Month 35 | 48 | 89.6 | 393.4 | 204.8 | 755.9 | |
| Month 46 | 52 | 90.4 | 253.9 | 136.0 | 473.9 | |
| Month 58 | 39 | 79.5 | 105.5 | 50.3 | 221.1 | |
| Rabies | Pre | 119 | 26.1 | 4.4 | 3.0 | 6.4 |
| Month 5.5* | 101 | 35.6 | 6.1 | 4.1 | 9.2 | |
| Month 9 | 89 | 39.3 | 7.5 | 4.7 | 11.8 | |
| Month 19** | 51 | 52.9 | 11.6 | 6.7 | 20.1 | |
| Month 35 | 51 | 54.9 | 12.5 | 7.4 | 21.0 | |
| Month 46 | 54 | 40.7 | 8.1 | 4.8 | 13.8 | |
| Month 58 | 51 | 39.2 | 7.6 | 4.5 | 12.8 | |
Month 5.5 corresponds to post primary course
Month 19 corresponds to post booster. Seroprotection: subjects with anti-HBs titres ≥10 mIU/ml. GMT: geometric mean anti-HBs titres. 95% CI = 95% confidence intervals.
In subjects who were HBsAg positive at baseline, titres were 8.5 mIU/ml post RTS,S/AS02 primary immunization and 26.8 mIU/ml post booster. The proportion who was seroprotected rose from 0% prevaccination to 53% post primary vaccination; titres and seroprotective levels at month 58 were 1.7 mIU/ml and 0%, respectively.
Prevalence of parasitemia
The prevalence of parasitemia at each cross sectional survey at the end of each transmission season during the long-term follow-up period was low, but tended to be lower in recipients of RTS,S/AS02 than rabies vaccine [between group difference: Month 35, 6.7% (95% CI: −4.2 to 18.4); Month 46, 0% (95% CI: −5.9 to 5.9); Month 58, 14.5% (95% CI: −1.4 to 29.4)] (Table 3). The geometric mean density of the parasitaemia was similar in the two groups (data not shown).
Table 3. Prevalence of asexual Plasmodium falciparum parasitaemia at cross-sectional survey time points.
| Vaccine group | Timing | N | n | % | 95% CI | |
|---|---|---|---|---|---|---|
| RTS,S/AS02 | Month 35 | 56 | 3 | 5.4 | 1.1 | 14.9 |
| Month 46 | 61 | 0 | 0 | 0 | 5.9 | |
| Month 58 | 45 | 6 | 13.3 | 5.1 | 26.8 | |
| Rabies | Month 35 | 58 | 7 | 12.1 | 5.0 | 23.9 |
| Month 46 | 61 | 0 | 0 | 0 | 5.9 | |
| Month 58 | 58 | 16 | 27.6 | 16.7 | 40.9 | |
N = Total number of subjects tested for P. falciparum; n = Subjects tested positive for P. falciparum. 95% CI = 95% confidence intervals.
Discussion
This paper presents the first available long-term safety and immunogenicity data for the candidate RTS,S malaria vaccine combined with the novel Adjuvant System, AS02. During surveillance for a period up to five years, a similar frequency of SAEs was observed in recipients of RTS,S/AS02 and rabies vaccines. The incidence rate of SAEs with a fatal outcome occurring during the study were similar in the two groups [RTS,S/AS02: 9.3 (95% CI: 1.15 to17.53) per 1000 person years; control group: 3.7 (95% CI: −1.14 to 8.82) per 1000 person years]. The fatal SAEs reflected the morbidity patterns in the wider population of adult males, and none was attributed to the study vaccine. Additionally, in chronic carriers of HBsAg who received RTS,S/AS02, no safety issue was apparent. The number of deaths among the study population was similar to what would have been expected on the basis of data collected previously in Farafenni Demographic Surveillance Site, The Gambia; 4.9 deaths per 1,000 person years would have been expected in a group of men of similar age.7
Safety surveillance data were available for 53% of subjects in both groups at the month 58 study end visit; health status data were subsequently collected from relatives from a further 35% of subjects. Thus, this study provides follow-up data over a five-year period in a mobile population of adult males in a rural part of Africa.
Anti-CS antibody concentration persisted above the levels observed in the controls for five years, although titres had waned from post-booster levels. The clinical relevance of this long term anti-CS response, especially in adults in endemic lands, is uncertain at present.
Although the prevalence of parasitemia at the end of each transmission season over the five year follow-up was low, there was a trend towards lower prevalence in recipients of RTS,S/AS02 than of rabies vaccine, which is consistent with observations in young African children for up to 18 months.5,8
The subject population was from The Gambia, a country with a high prevalence of natural hepatitis B infection in those born before hepatitis vaccination was introduced, due to exposure to the hepatitis B virus in childhood or early adult life, and therefore the proportion of subjects with seroprotective levels of anti-HBs was high prior to vaccination (31%). In those subjects seronegative for HBsAg prior to vaccination, there was a good response induced by the primary course; antibody titres were 3160 mIU/ml and seroprotection rate was 97%. These results are high compared to those induced with licensed hepatitis B vaccines; lower responses have been observed in males and those over 30 years of age.9 In addition, a strong response to a booster dose was seen, suggesting good initial priming.
The observed decline in anti-HBs GMTs was rapid in the 16 months post booster dose of RTS,S/AS02, with a much slower decline in the subsequent 23 months. This is in line with descriptions of antibody kinetics associated with licensed vaccines; after booster administration there is an initial rapid decrease, followed by a more constant, slow decline.10-12 A similar pattern of anti-HBs titre decay observed in this study have been reported with both recombinant HB vaccines (Engerix-B™) and plasma-derived HB vaccines (HBVax™).10
Three years after the administration of the booster dose, the anti-HBs antibody titres were 106 mIU/ml and the seroprotection rate was 80%. Given the highly variable response to hepatitis B vaccines according to population, a trial directly comparing the RTS,S/AS02 to a licensed vaccine will be required to evaluate vaccine persistence.
This study presents safety data for over five years following vaccination with the RTS,S/AS02 candidate vaccine, thereby providing the first evidence of the long-term safety of the RTS,S vaccine candidate in combination with the novel AS02 Adjuvant System. The encouraging data obtained in the initial trial in this semi-immune adult population has led to subsequent efficacy trials of the RTS,S vaccine in children; follow-up of these children will provide further information about the long-term efficacy, safety and immunogenicity of this candidate vaccine.
Materials and Methods
Study design
The study consisted of three sequential phases. For the primary study, 306 adult males were randomized into one of two groups at a single centre in The Gambia to receive the candidate vaccine or control (rabies vaccine) in a double-blind manner. All enrolled subjects were healthy male adults from 18 to 45 years of age. Hepatitis B serologic status was not an inclusion/exclusion criterion for enrollment. Vaccines were administered intramuscularly to the deltoid. Three doses on a 0, 1, 5 month schedule were given during the dry season and the third dose coincided with the start of the malaria transmission season. Two weeks before administration of dose three, each subject was given three tablets of sulfadoxine/pyrimethamine to clear P. falciparum infections. Subjects were followed to determine vaccine efficacy against infection over the ensuing rainy season. This was followed by a booster phase, in which a single dose of RTS,S/AS02 or rabies, according to the original randomization, was given at month 19 before the next transmission season and subjects were followed for an additional three months over the wet season to estimate efficacy immediately after the booster. After the booster phase, the subjects were observed for long-term safety and immunogenicity continuously until month 58. Complete details of the study methodology pertaining to the primary and booster phases are available elsewhere.4
The study protocols, protocol amendments and the informed consent forms were reviewed and approved by the Gambia Government/MRC Joint Ethics Committee. The trials were conducted according to ICH Good Clinical Practice guidelines, and were monitored by GlaxoSmithKline Biologicals. Each of the three sequential stages required written consent from the volunteer, after explaining the risks and benefits of the study in his own language. In order to ensure that subjects who participated in the study were protected against rabies, cross-over immunization was done during the long-term follow-up period, whereby subjects in the malaria vaccine group received three doses of the rabies vaccine according to the recommended schedule of 0, 7 and 28 days, and the subjects in the rabies vaccine group received a further dose of rabies vaccine having received the primary vaccination not according to a recommended schedule.
Vaccines
The candidate RTS,S/AS02 vaccine used in this study was manufactured by GlaxoSmithKline Biologicals, Belgium and developed in collaboration with the Walter Reed Army Institute of Research. A 50 μg dose of RTS,S was administered with 0.5 ml of the proprietary Adjuvant System AS02. The comparator vaccine, human diploid cell rabies vaccine, was manufactured by Aventis Pasteur, Lyon, France.
Assessment of safety
After the booster phase, study subjects were followed for long term safety by documenting serious adverse events (SAEs) using passive surveillance; volunteers had 24-hour access to medical care provided by two study nurses and a physician based at the local health centre in Basse. Administration of the cross-over vaccination with rabies vaccine potentially unblinded trial staff and subjects, although as different staff were used for cross-over vaccinations, the field staff involved in long-term follow-up were unlikely to be aware of the subject’s vaccine group. Eligible volunteers were given a unique study number and a photographic identification card. Volunteers leaving the study site were instructed to carry their study photo identification card with them. This card explained in English that the individual was a volunteer in a long-term follow-up study. The card also explained that if the volunteer presented to any of the health facilities in the country for any medical condition, a written statement to that effect should be sent to the MRC Clinic in Basse. Completeness of safety data was maximized by active surveillance visits; in each year a visit occurred before and after the wet season (months 35, 40, 46, 52 and 58). Any major illness or admission to a health centre or hospital was recorded. In addition, if the subject had not been seen at study end (month 58), study field workers questioned the subject’s immediate family members with the aim of obtaining information about the subject’s health status and whereabouts.
Assessment of immunogenicity
After the primary and booster phases of the study venous blood samples of 5 ml were collected once annually at the end the malaria transmission season (months 35, 46 and 58) for the measurement of anti-CS and anti-HBs antibody titres, and for Giemsa-stained blood smears to detect asexual Plasmodium falciparum parasites. Antibody responses to P. falciparum CS repeats (anti R32LR antibodies) were assessed at the Walter Reed Army Institute of Research (WRAIR) Department of Immunology, USA by ELISA, using an assay cut-off of 1 μg/ml for defining seropositivity.13 Serological assays for Hepatitis B were performed at GSK Biologicals, Rixensart. For the measurement of anti-HBs antibodies, an EIA kit from Abbott Laboratories with a cut-off of 3.3 mIU/ml for seropositivity and 10 mIU/ml for seroprotection was used for the month 35, 46 and 58 samples. In the primary and booster phases of the study samples had been tested using an RIA kit from Abbott; comparability of the assay results was ascertained by re-testing the day 14 post-booster serum samples with the EIA [Kappa = 0.86 (95% CI 0.77 to 0.94)].
Statistical methods
The primary endpoint of the long term safety follow up was the occurrence of SAEs during the study period. Secondary endpoints were the anti-CS and anti-HBs antibodies at each blood sampling time point.
Datasets used
The analysis of safety included all subjects enrolled in the study for whom data were available.
The long-term according-to-protocol (ATP) cohort for immunogenicity included all evaluable subjects (i.e., those meeting all eligibility criteria and complying with the procedures defined in the protocol) who received all three doses of the primary vaccination and the booster dose, and for whom data concerning immunogenicity endpoint measures were available during the long-term follow-up.
Analysis of demographics
The mean age (plus range and standard deviation) of subjects at each long-term follow-up time point up to month 58 were calculated for each vaccine group.
Analysis of immunogenicity
Seropositivity for an anti-CS antibody titre was defined as a titre of >1 μg/ml. For anti-HBs antibody titre, seropositivity was defined as a titre ≥3.3 mIU/ml and seroprotective level as a titre ≥10 mIU/ml. The seroprotection rate for anti-HBs antibodies and their 95% confidence interval (CI) were tabulated at each time point. In addition, GMTs for anti-CS antibodies (μg/ml) and anti-HBs antibodies (mIU/ml) with 95% CI were calculated at each time point when a serology sample was taken.
Assay for the presence of HBsAg
Circulating hepatitis B surface antigen (HBsAg) was detected by reverse passive haemagglutination (Murex Diagnostics, UK).
Determination of parasitemia and parasite density
Cross-sectional surveys for the detection of asexual malarial parasites (trophozoites) were conducted at the end of the malaria transmission season at each long-term follow-up time point for which blood smears were performed. Thick blood films were air dried and stained with Giemsa. For each blood slide, 200 high power fields (HPF) were examined before a smear was declared negative. Parasite densities were recorded as the number of parasites per HPF; one parasite per HPF was assumed to indicate 500 parasites/μL.14
Acknowledgements
We thank the subjects who participated in this study, the local community for their invaluable assistance and the staff of the MRC Field Station at Basse.
GSK is grateful to the staff of the Malaria Project team at GSK, in particular Laurence Vigneron and Celia Barberousse. Sarah Benns, professional medical writer, helped with the writing of the document.
References
- 1.Breman JG. The ears of the hippopotamus: manifestations, determinants and estimates of the malaria burden. Am J Trop Med Hyg. 2001;64:1–11. doi: 10.4269/ajtmh.2001.64.1. [DOI] [PubMed] [Google Scholar]
- 2.WHO. http:/www.who.int/mediacentre/news/statements/2004/statement6/en. World Health Organisation, Geneva 2004.
- 3.Garçon N, Heppner DG, Cohen J. Development of RTS,S/AS02: a purified subunit-based malaria vaccine candidate formulated with a novel adjuvant. Expert Rev Vaccines. 2003;2:231–8. doi: 10.1586/14760584.2.2.231. [DOI] [PubMed] [Google Scholar]
- 4.Bojang KA, Milligan PJM, Pinder M, Vigneron L, Alloueche A, Kester KE, et al. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet. 2001;358:1927–34. doi: 10.1016/S0140-6736(01)06957-4. [DOI] [PubMed] [Google Scholar]
- 5.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Aide P, et al. Duration of protection with RTS,S/AS02 malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet. 2005;366:2012–8. doi: 10.1016/S0140-6736(05)67669-6. [DOI] [PubMed] [Google Scholar]
- 6.Aponte JJ, Aide P, Renom M, Mandomando I, Bassat Q, Sacarlal J, et al. Safety of the RTS,S/AS02D vaccine against Plasmodium falciparum infection in infants. A phase I/IIb trial in a highly endemic area in Mozambique. Lancet. 2007;370:1543–51. doi: 10.1016/S0140-6736(07)61542-6. [DOI] [PubMed] [Google Scholar]
- 7.Ratcliffe AA, Hill AG, Gomez P, Walraven G. Farafenni DSS, The Gambia. In: Population and Health in Developing Countries. IDRC; Ottawa: 2002. www.idrc.ca/en/ev-43025-201-1-DO_TOPIC.html. [Google Scholar]
- 8.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomized controlled trial. Lancet. 2004;364:1411–20. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 9.Assad S, Francis A. Over a decade of experience with a yeast recombinant hepatitis B vaccine. Vaccine. 1999;18:57–67. doi: 10.1016/s0264-410x(99)00179-6. [DOI] [PubMed] [Google Scholar]
- 10.Gesemann M, Scheiermann N. Quantification of hepatitis B vaccine-induced antibodies as a predictor of anti-HBs persistence. Vaccine. 1995;13:443–7. doi: 10.1016/0264-410x(94)00010-k. [DOI] [PubMed] [Google Scholar]
- 11.Jilg W, Schmidt M, Deinhardt F, Zachoval R. Hepatitis B vaccination: how long does protection last? Lancet. 1984;2:458. doi: 10.1016/s0140-6736(84)92926-x. [DOI] [PubMed] [Google Scholar]
- 12.Westmoreland D, Player V, Heap DC, Hammond A. Immunization against hepatitis B - what can we expect? Epid Infect. 1990;104:499–509. doi: 10.1017/s0950268800047506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: Immunization of adults. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5516a1.htm. [PubMed]
- 14.Greenwood BM, Armstrong JRM. Comparison of two simple methods for determining malaria parasite density. Trans Royal Society of Trop Med Hyg. 1991;85:186–8. doi: 10.1016/0035-9203(91)90015-q. [DOI] [PubMed] [Google Scholar]