Abstract
Background
Our laboratory has previously demonstrated that Deferoxamine (DFO) promotes angiogenesis and bone repair in the setting of radiation therapy (XRT) coupled with Distraction Osteogenesis (DO). However, clinically relevant effects of deferoxamine administration on union rate, micro-Computed Tomography (μCT) and biomechanical parameters are unknown. We posit that administration of deferoxamine will increase union rate, mineralization, and strength of the regenerate in an irradiated DO model.
Materials and Methods
Sprague Dawley rats were randomized into three groups; DO-Control, DO-XRT, and DO-XRT-DFO. All animals underwent an osteotomy and DO across a 5.1mm distraction gap. Irradiated animals received 35Gy human-equivalent XRT 2 weeks prior to surgery and deferoxamine was injected postoperatively in the regenerate site of treatment animals. Animals were sacrificed at postoperative day 40 and mandibles harvested to determine rates of bony union as well as μCT and biomechanical parameters.
Results
Compared to irradiated mandibles, deferoxamine-treated mandibles exhibited higher union rate (11% vs. 92%, respectively). Across μCT and biomechanical parameters, we observed significant diminutions with administration of XRT while deferoxamine therapy resulted in significant restorations to levels of controls, with select metrics exhibiting significant increases even beyond controls.
Conclusion
Our data confirm that deferoxamine restores clinically relevant metrics of bony union and μCT and biomechanical parameters in a model of irradiated DO in the murine mandible. Our findings support a potential use for deferoxamine in treatment protocols to allow predictable and reliable use of DO as a viable reconstructive option in patients with head and neck cancer.
Level of Evidence
Animal study, not gradable for level of evidence.
Introduction
Head and neck cancer (HNC) accounted for over 52,000 new cases and 11,000 deaths in 2012.1 The preponderance of patients with HNC require tumor extirpation with subsequent reconstruction aimed at functional and aesthetic restoration. In addition, radiation therapy (XRT) is often a necessary component of the treatment regimen. But despite its well-intentioned therapeutic strategy, impediments of XRT include inhibition of bony healing and degradation of biomechanical properties, consequences that can lead to debilitating long-term morbidities such as pathologic fracture and non-union.2-7 As such, patients are vulnerable to functional and aesthetic impairments of the mandible and other components of the craniofacial skeleton. These impairments compromise the ability to eat, to communicate, and to engage in social interactions, all of which can have a profoundly negative impact on a patient’s quality of life.
Distraction osteogenesis (DO) is an operative technique in which regenerate bone is created through the separation of opposing osteogenic fronts. It is a powerful reconstructive modality with a variety of applications in craniofacial surgery, including operative management of micrognathia, mandibular hypoplasia, and craniosynostosis.8-11 However, in an irradiated field with resultant compromises in bone healing, angiogenesis, and biomechanical properties, XRT renders DO virtually incapable of producing consistent, viable, and efficacious reconstructive options.
Deferoxamine (DFO) is an iron chelator in clinical use for the treatment of transfusion-related iron overload.12 In addition, recent animal model studies reveal that prolyl-hydroxylase inhibitors such as deferoxamine act as pro-angiogenic factors via activation of the hypoxia inducible factor 1-alpha (HIF-1a) pathway and subsequent up-regulation of vascular endothelial growth factor (VEGF).13-15 Specifically, Wan et al. demonstrated the unique ability of deferoxamine to augment bony mineralization metrics in a murine model of femoral DO via the bolstering of local vascular supply. However, investigations into coupling pharmacological agents with DO as a method to remediate XRT-induced injury to bone have been limited in scope.
Given that deferoxamine is already on formulary, it is a drug that has the ability to quickly translate meaningful experimental findings into the clinical arena. Our intent is to explore whether deferoxamine can mitigate the negative sequelae of radiation therapy and allow for the inclusion of DO as a viable, consistent reconstructive option in the surgeon’s armamentarium for the management of irradiated bone, where surgical approaches are otherwise limited to costly and complication-prone free flap operations.
We aim to demonstrate that administration of deferoxamine in an irradiated mandibular model of DO will alleviate XRT-induced compromise of the clinically relevant metrics of biomechanics, mineralization, and bony union.
Materials and Methods
Animal experimentation was conducted in accordance with the guidelines published in the Guide for the Care and Use of Laboratory Animals: Eighth Edition. Protocols were approved by the University of Michigan’s Committee for the Utilization and Care of Animals (UCUCA) prior to implementation. Group sizes were determined prior to experimentation with the use of nQuery Advisor 7.0 software. Under the assumption that the data would be evaluated using a general linear model with associated analysis of variance with a desired power of 0.80 with a difference between groups of one standard deviation, we required at least five animals per group. Due to the addition of radiotherapy and biomechanical testing of compromised bone, we cautiously increased group sizes accordingly.
Male Sprague Dawley rats were randomized into three groups: Group 1, DO-Control (n=10); Group 2, DO-XRT (n=9); and Group 3, DO-XRT-DFO (n=12). All animals underwent a left hemi-mandible osteotomy procedure with placement of an external distraction device as previously described.16,17 Prior to the operative procedure, Group 2 and 3 left hemi-mandibles were irradiated using a Philips RT250 orthovoltage unit (250 kV X-rays, 15 mA; Kimtron Medical, Woodbury, CT), and administered a 35Gy human-equivalent dose of radiation in 5 fractions. This protocol produced the normalized equivalent dose of XRT that a human mandible is subjected to during treatment for HNC.18 Animals were subsequently allowed a 2-week recovery period between receiving XRT administration and undergoing the osteotomy procedure; this recovery period mimics the temporal gap between radiation and subsequent operative intervention for head and neck cancer patients. Group 3 animals were administered 5 separate 300 μL doses of deferoxamine, every other day, beginning on post-operative day (POD) 4. The timing and dose of deferoxamine administration was derived from prior studies investigating the use of deferoxamine in animal models and peak periods of angiogenesis in bone healing.13,14,19 Distraction took place twice daily from POD 4 through POD 12 to achieve a 5.1mm distraction gap. Animals were allowed to recover, were cared for, and then sacrificed at POD 40 when left hemi-mandibles were harvested en bloc for further micro-computed tomography (μCT), biomechanical, and union analysis.
Bony union was determined clinically and defined as solid bony bridging and an absence of motion across the DO gap. μCT images were obtained using 80 kVp, 80 mA and 1100 ms exposures. 392 projections were taken at a 45-micron voxel size for bone analysis. GE’s Microview 2.2 software was used to generate our Region of Interest (ROI). The ROI included the regenerate 5.1 mm posterior to the 3rd molar with the incisor root excluded, from which we derived the metrics of bone volume fraction (BVF), bone mineral density (BMD), tissue mineral content (TMC), and bone mineral content (BMC).20
After imaging, mandibles were potted and loaded to failure in uniaxial monotonic tension at 0.5 mm/s using a servohydraulic 858 Minibiox II testing machine (MTS Systems Corporation; Eden Prairie, MN). Crosshead displacement was recorded by using an external linear variable differential transducer (LVDT; Lucas Schavitts, Hampton, VA), and load data was collected with a 100-lb load cell (Sensotec, Columbus, OH). Data was sampled at 200 Hz on a TestStar system (TestStar IIs System version 2.4; MTS Systems Corporation). Load-displacement curves were analyzed for yield load, ultimate load, and failure load using custom computational code (MATLAB 7.11; Mathworks Inc., Natick, MA).
All statistical analysis was conducted using SPSS version 20 software (IBM, Armonk, NY). All variables were compared using ANOVA with Tukey’s post hoc method. All data was presented as the means plus standard deviation. Statistical significance is defined at p < 0.05.
Results
Gross Bony Union
After dissection, DO-Control hemi-mandibles demonstrated complete bony union across the distraction gap in 10 out of 10 specimens (100%). Bony regeneration across the distraction gap for DO-XRT hemi-mandibles was substantially diminished with only 1 out of 9 (11%) hemi-mandibles demonstrating bony union. With deferoxamine treatment, we observed an improvement in union compared to XRT-treated specimens, as 11 of 12 (92%) hemi-mandibles in the DO-XRT-DFO group demonstrated formation of bony union across the distraction gap. In addition to gross observation, this union formation was further appreciated using three-dimensional μCT reconstruction (Figure 1).
Figure 1.
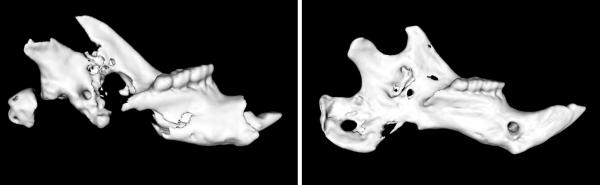
Three-dimensional μCT images of a DO-XRT left-hemimandible (left image) and a DO-XRT-DFO left-hemimandible (right image). The incisor, the anterior-most portion of the mandible, is to the right in both images. Note the difference in regenerate union for the distraction gap posterior to the molars. The more robust regenerate is seen in the irradiated hemi-mandible treated with deferoxamine.
μCT
Administration of XRT resulted in a diminution of radiomorphometric parameters when comparing DO-Control to DO-XRT hemi-mandibles. Specifically, there was a significant reduction in BMC (102%, p=0.001), TMC (57%, p=0.001), BVF (36%, p=0.001), and BMD (36%, p=.001) upon radiation administration. Utilizing DFO treatment, we observed significant restoration from DO-XRT values for BMC (92%, p=0.002), TMC (135%, p=0.001), BVF (90%, p=0.001) and BMD (89%, p=0.001). BMC and TMC were restored to levels not significantly different from DO-Controls (Figure 2). In addition to these restorations from radiation levels to control levels, we observed restorations beyond control levels for BVF (21%,p=0.003) and BMD (21%, p=0.005) (Figure 3) when comparing DO-Control to DO-XRT-DFO mandibles.
Figure 2.
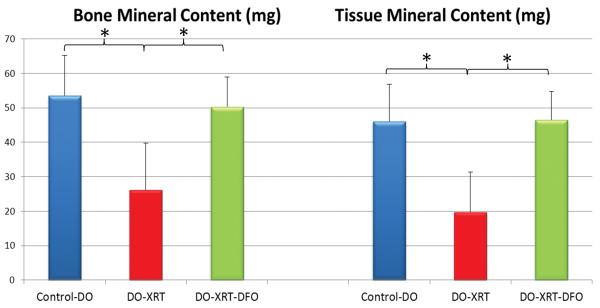
Graphical representation of Bone Mineral Content (BMC) and Tissue Mineral Content (TMC) μCT parameters. There are significant increases in both BMC and TMC for DO-XRT-DFO specimens compared to DO-XRT specimens. No significant difference was seen for either BMC or TMC between DO-XRT-DFO specimens and DO-Controls.
Figure 3.
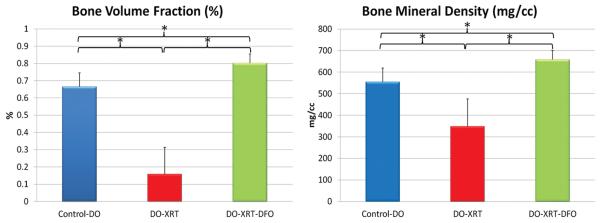
Graphical representation of Bone Volume Fraction (BVF) and Bone Mineral Density (BMD) μCT parameters. There are significant improvements above DO-XRT levels in the DO-XRT-DFO group. There are also significant increases in BVF and BMD above DO-Controls for the DO-XRT-DFO specimens.
Biomechanical Tension Testing
Biomechanical tension testing demonstrated overall trends similar to those of μCT with respect to the diminution of properties after radiation and subsequent restoration with the addition of deferoxamine. We observed significant reductions when comparing DO-XRT to DO-Control specimens for ultimate load (56%, p=0.015) and failure load (64%, p=0.026) with a trending decrease in yield load (49%, p=0.09). When comparing DO-XRT-DFO mandibles to DO-XRT mandibles, we noted significant increases in biomechanical parameters for ultimate load (205%, p=0.001), failure load (312%, p=0.001) and yield load (216%, p=0.001). Ultimate load and failure load were restored to levels not significantly different from control. For yield load, the trending decrease was not only reversed from XRT levels, but also bolstered significantly beyond control in the DO-XRT-DFO group (59%, p=0.026) (Figure 4).
Figure 4.
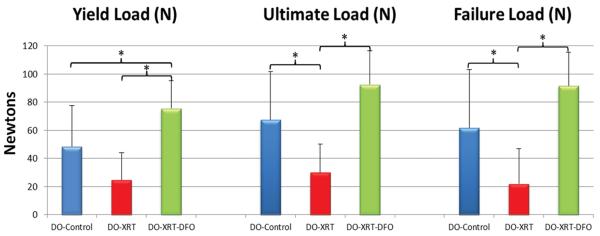
Results for Biomechanical Testing (BMT) analysis illustrating significant increases in yield load, ultimate load, and failure load for DO-XRT-DFO hemi-mandibles compared with DO-XRT specimens. No significant difference is noted for failure load and ultimate load between DO-Controls and DO-XRT-DFO specimens. However, there is a significant increase for yield load above DO-Control levels for DO-XRT-DFO hemi-mandibles.
Discussion
Distraction Osteogenesis (DO) is a metabolically demanding reconstructive process heavily dependent on adequate local blood supply.19 A crucial adverse consequence of XRT administration is the diminution of local vascularity, which renders DO an untenable therapeutic modality in the setting of irradiated bone.21-23 Previous investigations from our laboratory examining DO in an irradiated murine mandible demonstrate that DO alone is not a viable option for restoring vascularity and subsequent biomechanical function.24 Further studies utilizing the angiogenic properties of deferoxamine in concert with DO in an irradiated setting were successful in showing significant increases for vascular number and vascular density metrics compared to irradiated-DO specimens.25 On a cellular level, we have obtained in vitro data that demonstrate the ability of deferoxamine to remediate XRT-induced impediments to vessel formation in a human umbilical vein endothelial cell assay. This in vitro data provide a strong cellular and mechanistic foundation for our translational work in this animal model.26 For our current study, we sought to build upon these results by specifically analyzing the metrics of bone quality and strength through micro-computed tomography (μCT) and uniaxial biomechanical tension testing to obtain clinically relevant data.
Previous examination of the angiogenic properties of deferoxamine has demonstrated a propensity to bolster vascularity at fracture and distraction sites, thereby improving metrics of bone quality as measured by μCT parameters.13,14 As DO uniquely demonstrates a robust angiogenic response in the first few days, this period is an ideal time-point for drug delivery to combat untoward effects of XRT on distraction site vascularity and subsequent healing outcomes. We hypothesize that the detrimental effects of radiation on distraction regenerate bone quality, bone strength, and rate of successful bony union will be remediated through the administration of deferoxamine injection during the distraction process.
Our results suggest that deferoxamine is a promising potential pharmacological means to make DO a feasible reconstructive option in irradiated bone. The data that supports this contention are three fold; first, we observed an 81% increase in bony union rate with the use of deferoxamine treatment, providing a tangible clinical link between increased angiogenesis and superior healing outcomes. Secondly, we observed remediation in select μCT parameters, indicating that this increased rate in union formation was accompanied by better-mineralized bone. Specifically, we observed significant restorations to control levels with the use of deferoxamine for metrics indicative of callus mineralization, like BMC and TMC, and even restorations beyond control for metrics reflecting callus structure, like BVF and BMD. We were intrigued by this bolstering of certain metrics above control, which might be the result of increased nutrient and blood supply secondary to combining deferoxamine with distraction osteogenesis, two therapies with angiogenic effects. Lastly, we observed biomechanical results congruent with our μCT findings, demonstrating the efficacy of deferoxamine to not only improve union rate and mineralization, but to functionally increase the strength of the regenerate.
Biomechanical analysis is particularly pertinent to our investigation. It is considered the gold standard for measuring regenerate integrity and a logical means by which to assess the functional quality of bone healing. The particular form of biomechanical analysis undertaken, uniaxial tension testing, is a unique method central to our evaluation of mandibular distraction and thus best-suited for assessing the mechanics and quality of the regenerate. In terms of specific results, we observed a significant restoration to control levels with the use of deferoxamine for failure load, defined as the maximum load resulting in partial or complete failure of the regenerate. For yield load, defined as the point where elastic properties become plastic and cannot revert back to their original architecture, we observed a restoration beyond control level with the use of deferoxamine therapy, indicating a clinically meaningful restoration in bone quality. By increasing the threshold where irreversible damage occurs in bone, deferoxamine provides an important pharmacological means to provide a functional regenerate in distraction osteogenesis of irradiated bone.24,27
Our study has some limitations worthy of discussion. Firstly, we utilize one dosage of deferoxamine at one time-point for drug delivery, though the dosage and timing are calculated based on previous investigations utilizing deferoxamine in animal models and previously reported peak periods of angiogenesis in distraction osteogenesis.13,14,19 Specific-dose studies and time-point studies could offer a means to further the evaluation of this promising therapy. Clinically, we anticipate utilizing a localized deferoxamine injection in proximity to the distraction gap after both radiotherapy and operative intervention have occurred. The specific dose and rate need to be further studied, and based on currently accepted administration practices. Secondly, this study is limited by its lack of histological analysis that could potentially provide further validation to the use of deferoxamine in irradiated DO. However, as biomechanical testing and histological analysis are both destructive metrics, we could not use both of these techniques in the same cohort of animals; we chose to pursue clinically-relevant biomechanical data before investigating the efficacy of deferoxamine on a cellular level. We plan to investigate this latter, promising avenue of research in future studies. Lastly, and most pertinent in terms of patient safety, the oncogenic properties of deferoxamine need to be further elucidated. While select studies demonstrate an anti-tumorigenic property of deferoxamine against particular cancer types, any angiogenic therapy must be rigorously tested and demonstrate a lack of tumor growth or recurrence associated with therapy.28-32 We therefore advocate that more investigation be undertaken before implementing deferoxamine into any treatment regimen for patients with HNC.
Conclusions
In conclusion, the corrosive effects of radiation currently preclude the utilization of DO as a reconstructive option in the clinical setting. This study has demonstrated the ability of deferoxamine to reverse the scourge of XRT-induced consequences of the clinically relevant metrics of bony union, callus mineralization, and biomechanical testing. We believe that the utilization of deferoxamine in the setting of irradiated DO is an attractive option and a potentially viable tool to aid in the reconstruction and treatment regimens for patients with head and neck cancer.
Acknowledgements
Funding was provided by NIH RO1 CA 12587-01 to S. R. Buchman. The authors would like to thank Charles Roehm for fabrication of fixator devices, Joseph Perosky and Dr. Ken Kozloff for assistance with μCT and biomechanical data collection and analysis.
Funding supported by the following grant: “Optimization of Bone Regeneration in the Irradiated Mandible”, NIH-R01#CA 125187-01, PI: Steven R. Buchman.
Footnotes
Financial Disclosure and Products
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society . Cancer Facts & Figures 2012. American Cancer Society; Atlanta: 2012. [Google Scholar]
- 2.Coletti D, Ord RA. Treatment rationale for pathological fractures of the mandible: a series of 44 fractures. Int J Oral Maxillofac Surg. 2008;37(3):215–222. doi: 10.1016/j.ijom.2007.09.176. [DOI] [PubMed] [Google Scholar]
- 3.Brown RK, Pelker RR, Friedlaender GE, Peschel RE, Panjabi MM. Post fracture irradiation effects on the biomechanical and histologic parameters of fracture healing. J Orthop Res. 1991;9:876–882. doi: 10.1002/jor.1100090614. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsson M, Albrektsson T, Turesson I. Dynamics of irradiation injury to bone tissue: A vital microscopic investigation. Acta Radiol Oncol. 1985;24:343–350. doi: 10.3109/02841868509136063. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsson MG, Jonsson AK, Albrektsson TO, Turesson IE. Short- and long-term effects of irradiation on bone regeneration. Plast Reconstr Surg. 1985;76:841–850. doi: 10.1097/00006534-198512000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Sugimoto M, Takahashi S, Toguchida J, Kotoura Y, Shibamoto T, Yamamuro T. Changes in bone after high-dose irradiation: Biomechanics and histomorphology. J Bone Joint Surg (Br.) 1991;73:492–497. doi: 10.1302/0301-620X.73B3.1670456. [DOI] [PubMed] [Google Scholar]
- 7.Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41(5):283–8. doi: 10.1016/0278-2391(83)90294-x. [DOI] [PubMed] [Google Scholar]
- 8.Toth BA, Kim JW, Chin M, Cedars M. Distraction osteogenesis and its application to the midface and bony orbit in craniosynostosis syndromes. J Craniofac Surg. 1998;9(2):100–13. doi: 10.1097/00001665-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Mofid MM, Manson PN, Robertson BC, Tufaro AP, Elias JJ, Vander Kolk CA. Distraction osteogenesis: a review of 3278 cases. Plast Reconstr Surg. 2001;108(5):1103–14. doi: 10.1097/00006534-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Warren SM, Fong KD, Chen CM, et al. Tools and techniques for craniofacial tissue engineering. Tissue Eng. 2003;9(2):187–200. 278. doi: 10.1089/107632703764664666. [DOI] [PubMed] [Google Scholar]
- 11.Yannas IV, Mistry AS, Mikos AG. Tissue engineering strategies for bone regeneration. Adv Biochem Eng Biotechnol. 2005;94:1–22. doi: 10.1007/b99997. [DOI] [PubMed] [Google Scholar]
- 12.Brittenham GM. Iron-chelating therapy for transfusional iron overload. N Engl J Med. 2011;364:146–156. doi: 10.1056/NEJMct1004810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Street J, Bao M, deGuzman L, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan C, Gilbert SR, Cao X, et al. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc Natl Acad Sci USA. 2008;105:686–691. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen X, Wan C, Ramaswamy G, et al. Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. J Orthop Res. 2009;27:1298–1305. doi: 10.1002/jor.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchman SR, Ignelzi MA, Jr, Radu C, et al. A unique rodent model of distraction osteogenesis of the mandible. Ann Plast Surg. 2002;49:511–519. doi: 10.1097/00000637-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Tong L, Buchman SR, Ignelzi MA, Jr, Rhee S, Goldstein SA. Focal adhesion kinase expression during mandibular distraction osteogenesis: evidence for mechanotransduction. Plast Reconstr Surg. 2003 Jan;111(1):211–22. doi: 10.1097/01.PRS.0000033180.01581.9A. discussion 223-4. [DOI] [PubMed] [Google Scholar]
- 18.Tchanque-Fossuo CN, Monson LA, Farberg AS, Donneys A, Deshpande SS, Razdolsky ER, Halonen NR, Goldstein SA, Buchman SR. Dose-response effect of human equivalent radiation in the murine mandible. Plast Reconstr Surg. 2011;128(5):480e–487e. doi: 10.1097/PRS.0b013e31822b67ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AI-Aql ZS, Alagl S, Graves DT, Gerstenfeld LC, Einhorn T. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res. 2008;87(2):107–118. doi: 10.1177/154405910808700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 21.Cao X, Wu X, Frassica D, Yu B, Pang L, Xian L, Wan M, Lei W, Armour M, Tryggestad E, Wong J, Wen CY, Lu WW, Frassica FJ. Irradiation induces bone injury by damaging bone marrow microenvironment for stem cells. Proc Natl Acad Sci USA. 2011;108(4):1609–1614. doi: 10.1073/pnas.1015350108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie XT, et al. Experimental study of radiation effect on the mandibular microvasculature of the guinea pig. Chin J Dent Res. 1998;1(2):46–51. [PubMed] [Google Scholar]
- 23.Deshpande SS, Donneys A, Farberg AS, Tchanque-Fossuo CN, Zehtabzadeh AJ, Buchman SR. Quantification and characterization of radiation-induced changes to mandibular vascularity using micro-computed tomography. Plast Reconstr Surg. 2010;125(6):40. doi: 10.1097/SAP.0b013e318255a57d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz DA, Jamali AM, Kakwan MS, et al. Biomechanical assessment of regenerate integrity in irradiated mandibular distraction osteogenesis. Plast Reconstr Surg. 2009;123(2 Suppl):114S–22S. doi: 10.1097/PRS.0b013e318191c5d2. [DOI] [PubMed] [Google Scholar]
- 25.Farberg AS, Jing XL, Monson LA, Donneys A, Tchanque-Fossuo CN, Deshpande SS, Buchman SR. Deferoxamine reverses radiation induced hypovascularity during bone regeneration and repair in the murine mandible. Bone. 2012;50(5):1184–7. doi: 10.1016/j.bone.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donneys A, Weiss DM, Deshpande SS, Ahsan S, Tchanque-Fossuo CN, Sarhaddi D, Levi B, Goldstein SA, Buchman SR. Localized deferoxamine injection augments vascularity and improves bony union in pathologic fracture healing after radiotherapy. Bone. 2013;52(1):318–25. doi: 10.1016/j.bone.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner CH, Burr DB. Basic biomechanical measurements of bone: A tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 28.Blatt J, Taylor SR, Stitely S. Mechanism of antineuroblastoma activity of deferoxamine in vitro. J Lab Clin Med. 1988;112:433. [PubMed] [Google Scholar]
- 29.Hann HWL, Stahlhut MW, Hann CL. Effect of iron and desferoxamine on cell growth and in vitro ferritin synthesis in human hepatoma cell lines. Hepatology. 1990;11:566–569. doi: 10.1002/hep.1840110407. [DOI] [PubMed] [Google Scholar]
- 30.Hann HWL, Stahlhut MW, Rubin R, Maddrey WC. Antitumor effect of deferoxamine in human hepatocellular carcinoma growing in athymic nude mice. Cancer. 1992;70:2051. doi: 10.1002/1097-0142(19921015)70:8<2051::aid-cncr2820700806>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 31.Donfrancesco A, Deb G, Dominici C, Pileggi D, Castello MA, Helson L. Effects of a single course of deferoxamine in neuroblastoma patients. Cancer Res. 1990;50:4929–4930. [PubMed] [Google Scholar]
- 32.Kulp KS, Vulliet PR. Mimosine blocks cell cycle progression by chelating iron in asynchronous human breast cancer cells. Toxicol. Appl. Pharmacol. 1996;139:356–364. doi: 10.1006/taap.1996.0176. [DOI] [PubMed] [Google Scholar]


