Abstract
Since the liver drains antigens from the intestinal tract, and since the intestinal tract is a major site of viral replication, we examined the dynamics of liver macrophages (Kupffer cells) throughout SIV infection. Absolute numbers of Kupffer cells increased in the livers in acute infection, and in animals with AIDS. Significantly higher percentages of proliferating (BrdU+) Kupffer cells were detected in acute infection and in AIDS with similar trends in blood monocytes. Significantly higher percentages of apoptotic (AC3+) Kupffer cells were also found in acute and AIDS stages. However, productively infected cells were not detected in liver of 41/42 animals examined, despite abundant infected cells in gut and lymph nodes of all animals. Increased rates of Kupffer cell proliferation resulting in an increase in Kupffer cells without productive infection indicate SIV infection affects Kupffer cells, but the liver does not appear to be a major site of productive viral replication.
Keywords: liver, SIV, HIV, T cell, immunology, mucosal immunology
INTRODUCTION
Regardless of the route of transmission, the mucosal immune system in general, and the gastrointestinal system in particular, are central to the pathogenesis of HIV infection (Lackner et al., 2009). Several critical events in SIV/HIV pathogenesis including viral amplification, and CD4+ T cell destruction occur in the intestinal tract (Brenchley et al., 2004; Lackner et al., 2009; Mehandru et al., 2004; Veazey et al., 1998). Since the liver is the major draining organ for substances passing through the gut, it has a unique immunologic environment. The liver contains one of the largest populations of tissue macrophages (Crofton et al., 1978; Schmitt et al., 1990) and the specific distribution of liver macrophages (Kupffer cells) within the hepatic sinusoids allows them to be in close contact with circulating cells from blood. Housset et al, suggested infection of Kupffer cells (KC) may occur during primary HIV viremia (Housset et al., 1990a). Primary cultures of human KC have also been shown to be permissive for HIV infection (Schmitt et al., 1990). Moreover, HIV antigens or RNA have been detected in liver cells of HIV-infected individuals, but the percentage of virus positive cases, as well as the type and the number of virus-containing cells reported varies substantially (Cao et al., 1992; Hoda et al., 1991; Housset et al., 1990b; Housset et al., 1993). Further, studies by Hufert et al, (1993) suggested that KC isolated from livers of AIDS patients were only latently infected, and did not produce virus (Hufert et al., 1993). Thus whether infection of liver macrophages/Kupffer cells (KCs) occurs in vivo remains controversial (Hufert et al., 1993). Further, there is basically no information regarding the dynamics of lentivirus persistence or replication in the liver in HIV infection.
Lentiviruses can infect and replicate in non-dividing cells of monocyte/macrophage lineage (Verani et al., 2005). Macrophages contribute to innate immune responses to pathogens and are at the interface between innate and adaptive immunity. Thus, they play a central role in control of infection, either by secreting cytokines, directly destroying virus or infected cells, and/or activating either innate or adaptive immune responses. However, direct infection of macrophages with intracellular pathogens including HIV may impair their function and alter cytokine production, resulting in chronic inflammation and tissue damage. Unlike T cells, HIV infected macrophages appear resistant to the cytopathic effects of the virus, and the capacity of monocytes and macrophages to migrate to, and survive within tissues for years makes them potential major reservoirs for HIV-1 persistence (Herbein et al., 2002). Further, their role as APC and/or a source of chemoattractant cytokines for CD4 T cells may favor continual intercellular virus transmission. Therefore, monocytes/macrophages may play a dual role in HIV infection, contributing to both antiviral defenses and serving as targets for infection and persistence (Herbein et al., 2002).
The aim of this study was to examine and compare the kinetics of changes in absolute numbers of Kupffer cells in liver for comparison with monocytes in peripheral blood throughout the course of SIV infection. We also quantified the percentages of proliferating (BrdU+) and apoptotic (AC3+) Kupffer cells in liver by flow cytometry and performed in situ hybridization to quantify SIV-infected Kupffer cells throughout all stages of infection. Determining the kinetics of SIV infection and turnover of Kupffer cells in vivo may provide important information on the role of the liver in the pathogenesis of HIV infection.
Material and Methods
Animals, virus and BrdU
A total of 50 male and female rhesus macaques (Macaca mulatta) between 3–20 years of age housed at the Tulane National Primate Research Center were used to quantify Kupffer cells in liver, and monocytes in peripheral blood. Levels of plasma viremia and CD4+ T cell counts in blood are shown in Table 1. All animals were maintained in accordance with the standards of the American Association for Accreditation of Laboratory Animal Care, and all studies were reviewed and approved by the Tulane Institutional Animal Care and Use Committee.
Table 1.
Animals examined in this study
| Stage and animal# | Sex | Age | Days after SIV | Route of Inoc. | CD4 counts | Plasma viral load | Diagnosis |
|---|---|---|---|---|---|---|---|
| Control | |||||||
| AG71 | F | 11 | NA1 | NA1 | 796 | NA1 | NSL2 |
| BB01 | F | 13 | NA1 | NA1 | 2430 | NA1 | NSL2 |
| BE84 | F | 11 | NA1 | NA1 | 2454 | NA1 | NSL2 |
| CC10 | F | 15 | NA1 | NA1 | 1720 | NA1 | NSL2 |
| GN58 | F | 13 | NA1 | NA1 | 480 | NA1 | NSL2 |
| GN70 | F | 10 | NA1 | NA1 | 1091 | NA1 | NSL2 |
| GN74 | F | 13 | NA1 | NA1 | 585 | NA1 | NSL2 |
| N483 | F | 16 | NA1 | NA1 | 602 | NA1 | NSL2 |
|
| |||||||
| Acute | |||||||
| HI52 | F | 5 | 7 | IV3 | 664 | 3,700,600 | NSL2 |
| AV63 | F | 4 | 8 | IV3 | 463 | 24,310,900 | Lymphoid hyperplasia |
| BA57 | F | 14 | 8 | IV3 | 416 | 14,288,200 | Lymphoid hyperplasia |
| HI53 | F | 7 | 8 | IV3 | 325 | 3,555,700 | Lymphoid hyperplasia |
| T108 | F | 13 | 8 | IV3 | 653 | 57,314 | Lymphoid hyperplasia |
| BV13 | M | 3 | 8 | IV3 | 752 | 16,000,000 | Lymphoid hyperplasia |
| AV91 | M | 14 | 10 | IV3 | 472 | 157,190,000 | Lymphoid hyperplasia |
| L880 | M | 11 | 10 | IV3 | 212 | 7,000,000 | Lymphoid hyperplasia |
| C419 | M | 20 | 10 | IV3 | 383 | 11,000,000 | NSL2 |
| HI58 | F | 7 | 12 | IV3 | 651 | 8,496,000 | Lymphoid hyperplasia |
| HI63 | F | 7 | 12 | IV3 | 438 | 24,310,900 | Lymphoid hyperplasia |
| T139 | M | 7 | 13 | IV3 | 355 | 3,200,000 | Lymphoid hyperplasia |
| BA17 | M | 8 | 13 | IV3 | 416 | 11,000,000 | Lymphoid hyperplasia |
| M992 | F | 16 | 13 | IV3 | 227 | 34,949,800 | Lymphoid hyperplasia |
| AV85 | F | 8 | 21 | IV3 | 670 | 230,000 | Lymphoid hyperplasia |
| CB74 | F | 3 | 21 | IV3 | 992 | 2,100,000 | Lymphoid hyperplasia |
| BI58 | M | 3 | 21 | IV3 | 558 | 12,000,000 | Lymphoid hyperplasia |
| BN37 | M | 3 | 21 | IV3 | 542 | 340,000 | Lymphoid hyperplasia |
|
| |||||||
| Chronic | |||||||
| N998 | F | 10 | 180 | INVG4 | 584 | 427,600 | Lymphoid hyperplasia |
| R908 | F | 9 | 181 | INVG4 | 653 | 4,700 | Lymphoid hyperplasia |
| CF35 | M | 5 | 91 | IR5 | 546 | 121,680 | Lymphoid hyperplasia |
| BV74 | M | 5 | 63 | IR5 | 775 | 3,111 | Lymphoid hyperplasia |
| DI28 | M | 3 | 80 | IR5 | 810 | 3,064 | Lymphoid hyperplasia |
| DL31 | M | 3 | 128 | IR5 | 1332 | 54,500 | Lymphoid hyperplasia |
| DB53 | M | 5 | 77 | IR5 | 277 | 28,560 | Lymphoid hyperplasia |
| DE09 | M | 4 | 76 | IR5 | 1192 | 5,088 | Lymphoid hyperplasia |
| HG49 | F | 11 | 145 | INVG4 | 184 | 104,945 | Lymphoid hyperplasia |
| FK47 | M | 5 | 98 | IR5 | 459 | ND | Lymphoid hyperplasia |
| FD49 | M | 5 | 110 | IR5 | 318 | ND | Lymphoid hyperplasia |
| I553 | F | 11 | 646 | IV3 | 21 | 1,895,400 | AIDS; Pneumocystis inf. |
| R544 | F | 9 | 414 | INVG4 | 190 | 1,700,000 | AIDS: meningoencephalitis |
| AP53 | F | 6 | 63 | IV | 1127 | 4,100,000 | AIDS; bacterial septicemia |
| DJ18 | M | 7 | 173 | IV3 | 649 | ND | Lymphoid hyperplasia |
| HG45 | F | 14 | 148 | IV3 | 191 | 964,152 | AIDS; Pneumocystis inf. |
| HG58 | F | 11 | 283 | INVG4 | 351 | 7,074 | AIDS; encephalitis |
| HG56 | F | 10 | 152 | INVG4 | 339 | 19,522,700 | AIDS; Gsstric lymphoma |
| FA14 | F | 9 | 1068 | IV3 | 224 | 148,965 | AIDS; Pneumocystis inf. |
| HI68 | F | 10 | 155 | INVG4 | 22 | 8,076,200 | AIDS: CMV infection |
| BD78 | M | 4 | 205 | IV3 | 729 | 86,000 | AIDS; Vasculitis, thrombosis |
| BE65 | M | 6 | 1071 | IV3 | 288 | 480,000 | AIDS: M avium inf |
| T196 | M | 7 | 230 | IV3 | 270 | 750,000 | AIDS; Pneumocystis inf. |
| FE53 | M | 4 | 140 | IR5 | 469 | 343,459 | AIDS; hepatitis, gastroenteritis |
Not applicable
No significant lesions
Intravenous
Intravaginal
Intrarectal
Viremic but levels not determined at time of necropsy
Macaques were infected with SIVmac251 by the intravenous (IV) route for acute studies, and either the intra-vaginal (INVG) or intra-rectal (IR) route for chronic infection studies. Although it is conceivable some differences may have been attributed to different routes of inoculation, we elected to use intravenous infection especially in the early timepoints to ensure virus reached the liver in these early timepoints. Mucosal inoculation is relatively inefficient, and in very early infection (day 8, 10, etc.) plasma viremia often cannot be detected after mucosal exposure. However, the number of transmitted variants, and likely the numbers of viruses entering the host differs between animals intravenously and mucosally infected (Fennessey and Keele, 2013), and the effects this may have had on the effects observed on Kupffer cells here is not known.
For immunohistochemistry and quantitative image analysis of tissue sections, macaques were divided into four groups; controls, (uninfected, n=8); acute infection from 7–21 days post-inoculation (DPI; n=18); chronic, yet asymptomatic infection (n=7); and chronic infection with definitive signs of AIDS (n=17). For some analyses, the acute group was further divided into 7–10 DPI (n=9), 13 DPI (n=5), or 21 DPI (n=4).
For flow cytometry, livers were collected and processed immediately upon necropsy from 22 macaques, which were divided into three groups; controls (uninfected, n=8); acute (7–21 days post-inoculation, DPI (n=7); and AIDS (n=7). Acutely infected animals were euthanized for tissue collection on 7, 10, 13 and 21 DPI. Animals with AIDS were euthanized between 414–1071 DPI. For in vivo tracking of cell proliferation, animals were intra-peritoneally inoculated with bromodeoxy-uridine (BrdU) 24s prior to euthanasia and tissue collection.
Tissue collection and analysis
Whole blood samples were stained using a whole blood lysis protocol as previously described (Veazey et al., 2003). For analysis of liver leukocytes, single cell suspensions were prepared, using modifications of a previously described procedure for intestinal tissues (Veazey et al., 1997). Briefly, 3–5 gm liver tissue were minced with razor blades, and incubated with 1 mM EDTA in Hanks balanced salt solution for 30 min with rapid shaking (300 RPM) at 37° C, followed by 2 sequential 30 min incubations in RPMI containing 20U/ml collagenase (Type II, Sigma) with rapid shaking at 37° C. After each incubation, liver tissues were further disrupted by gently pipetting 5 to 10 times with a 16-g feeding needle, pelleted (400g, 7 min), and supernatants discarded and media replaced. At the end of these incubations, cell pellets were resuspended and filtered through nylon mesh and layered over a 35%/60% bilayer isotonic Percoll density gradient and centrifuged at 1000g for 30 min. The interface between the 35% and 60% Percoll layers were collected, washed, and adjusted to 107cells/ml. For flow cytometry, 100 μl aliquots (106 cells) were stained with appropriately diluted concentrations of monoclonal antibodies to CD68, CD163, and CD14, (BD Biosciences). Cells were then washed and fixed in 2% paraformaldehyde. For intracellular AC3 and BrdU staining, surface stained cells were washed and permeabilized with BD Cytofix/Ctoperm buffer followed by staining with activated caspase 3 (AC3) or a BrdU Flow Kit (BD Biosciences) according to the manufacturer’s instructions. Samples were acquired on FACS Aria flow cytometer (Becton Dickinson) within 24 hour of fixation. Data was analyzed with Flowjo software (Tree Star Inc.) At least 20,000 monocytes/macrophages were collected, and data was analyzed by gating through monocytes/macrophages (identified by back-gating on CD68) and then through cells of interest. Since CD68 is an intracellular lysosomal/endosomal-associated membrane glycoprotein highly expressed and specific for monocytes and tissue macrophages (Holness and Simmons, 1993), it was used as the major marker for identifying Kupffer cells by flow cytometry.
Quantitation of liver macrophages by Immunohistochemistry and flow cytometry
Five μm sections of paraffin-embedded liver tissues were stained for Ham56 and/or CD163 (macrophage markers) by immunohistochemistry (IHC) as previously described (Borda et al., 2008). Briefly, slides were fixed in xylene, rehydrated in alcohol gradients, and finally water. Antigen retrieval was performed by steam (>95°C) in 1X citrate buffer, pH 6.0 for 20 minutes, and slides were washed with 1X tris buffered saline (TBS) solution. A protein (DAKO Protein Blocker, Serum Free, Carpenteria, CAS) and peroxidase (Peroxidase Blocking Reagent, DAKO) block was performed. After TBS wash, slides were incubated with mouse anti-human Ham56 antibody (DAKO) or CD163 (clone 10D6, Novocastra Laboratories, Newcastle, UK) diluted in protein blocker for 60 minutes followed by TBS wash and amplification with a biotin free Peroxidase system with Mach3 probe and Polymer system (Biocare; Concord, CA) per manufacturer’s directions. As negative controls, serial sections were processed identically using equivalent concentrations of irrelevant primary antibodies of the same isotype, and with slides incubated without primary or secondary antibodies. For image analysis, Ham56+ macrophages were detected by Cytomation Liquid DAB substrate chromogen system (DAKO) after 5 min development. Tissues were washed overnight in TBS, coverslipped, and imaged using a 20X objective on a Leica DMLb microscope (Leica; Bannockburn, IL) with a Spot Insight color camera utilizing Spot Imaging Software (Diagnostic Instruments; Sterling Heights, MI). Each section was blindly examined and 10 random non-touching fields (each with an area of 0.28 mm2) were digitally imaged, and manually counted for Ham56+ cells (macrophages) and reported as mean cells/mm2. These absolute numbers of liver macrophages in sections (Ham56+) were then used to estimate the percentages of all other subsets detected in liver tissues by flow cytometry as described below.
Absolute numbers of Kupffer cells in liver were determined by both image analysis of Ham56+ cells in tissue sections as above, and flow cytometry. Cell suspensions of liver were surface stained with appropriately diluted directly conjugated monoclonal antibodies against CD68 or Activated Caspase 3 (AC3)-FITC, CCR5 or CD163-PE, CXCR4-PE-Cy5, HLA-DR-PE-Cy7, CD3-AL700, CD20-APC-Cy7, CD14 Pacific Blue, and CD4-Qdot655 for flow cytometry. For intracellular staining, surface stained cells were washed in dPBS/BSA, fixed, and permeabilized with BD Cytofix/Ctoperm buffer followed by intracellular staining for CD68-FITC and BrdU-APC. Cells were washed again with BD Perm/Wash buffer and samples were resuspended in BD stabilizing fixative buffer and acquired on FACS Aria flow cytometer (Becton Dickinson) within 24 hour of fixation. Isotype-matched controls for each fluorochrome were included in all experiments. The CD4-Qdot655 was provided by the NIH Nonhuman Primate Reagent Resource. All other antibodies and reagents were purchased from BD Biosciences Pharmingen (San Diego, CA). Stained samples were resuspended in BD Stabilizing Fixative (BD Biosciences) and stored in the dark at 4°C overnight and acquired on a BD FACS Calibur or FACSAria flow cytometer (Becton Dickinson) the next day. Data was analyzed with Flowjo software (Tree star, Ashland, OR). At least 20,000 lymphocytes were collected and data was analyzed by gating through lymphocytes and then through cells of interest. Percentages of CD68+ cells within the macrophage gate were determined by flow cytometry, and used to determine absolute counts in liver by comparison with immunohistochemistry results.
Cultured Kupffer cells from liver cell suspensions confirmed all Kupffer cells co-expressed CD68 and Ham56 (Fig. 1A). Thus, percentages of CD68+ Kupffer cell subsets in liver cell suspensions detected by flow cytometry were assumed equivalent to Ham56+ cells in liver sections and were used as the baseline to quantify further Kupffer cell subsets. For example, percentages of CD68+ cells co-expressing CD163, CD14, BrdU, or activated caspase 3 (AC3) detected by flow cytometry were multiplied by absolute numbers of Ham56+ cells measured in sections by IHC (above) to determine absolute changes in cells in liver sections and expressed as cells/mm2 liver tissue. In other words, if 5% of CD68+ cells expressed CD4 by flow cytometry, and there were 200 Ham56+ cells/mm2 liver section by IHC, then we could estimate that there were ten (5% of 200 cells) CD68+CD4+ cells per mm2 liver.
Figure 1.
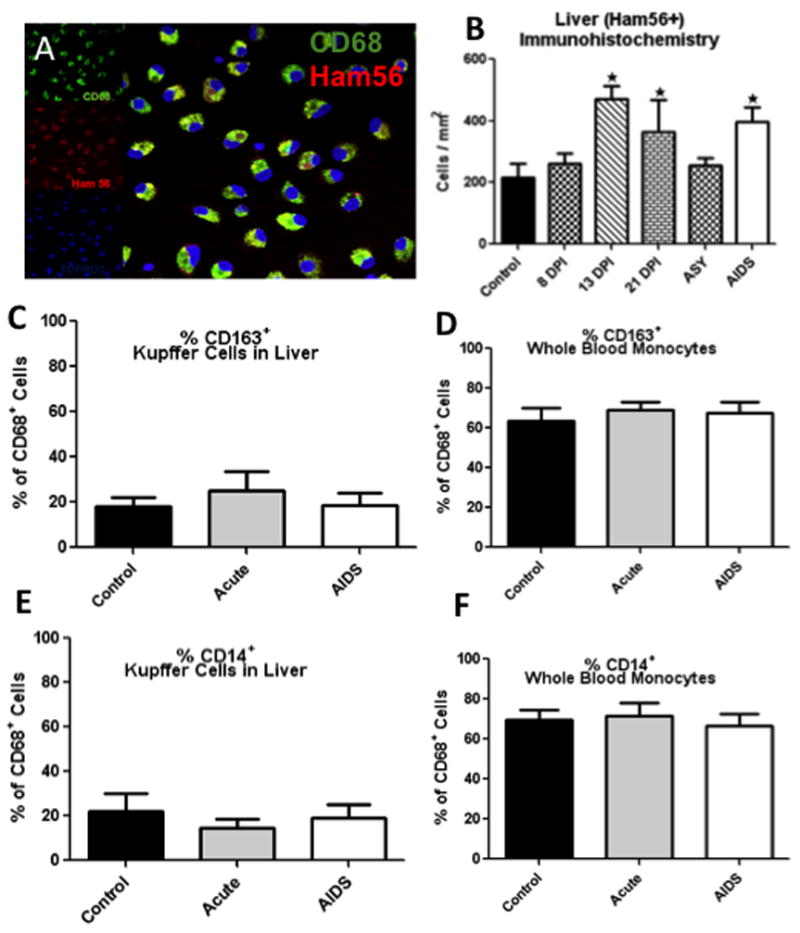
A: Triple-label confocal microscopy of liver macrophages (Kupffer cells) in vitro showing co-localization of CD68 (green) and Ham56 (red), indicating Kupffer cells co-express CD68 & Ham56 (CD68 with Alexa 488, green; Ham56 with Alexa 568, red; and cell nuclei with Topro3). B: Absolute numbers of Ham56+ Kupffer cells per/mm2 of liver in uninfected (control) and various stages of SIV infection as determined by immunohistochemistry for Ham56. Note significant increases in Ham56+ Kupffer cells per mm2 are detected after early SIV infection and in macaques with AIDS. *Indicates significant differences from controls (P<0.05). C–F: Percentages of CD68+ cells in the liver (C and E) and blood (D and F) co-expressing CD163 (C and D) or CD14 (E and F) in acute and chronic infection compared to controls. No significant differences in CD14 or CD163 expression were detected on CD68+ cells in liver or blood due to SIV infection (C–F).
Absolute numbers of monocytes in whole blood (WB) were determined using a complete blood count (CBC) performed on EDTA anti-coagulated blood collected prior to sacrifice on an ADVIA 120 hematology analyzer (Bayer, Terrytown, NY). Percentages of CD68+ macrophages within the WB monocyte gate determined by flow cytometry were multiplied by the absolute monocyte count from the CBC to obtain absolute numbers of CD68+ monocytes in blood. Percentages of CD68+ monocytes expressing BrdU and AC3 were similarly converted to absolute counts and expressed as 103 monocytes/μl blood.
SIV Immunohistochemistry
Five μm sections of paraffin-embedded liver tissues were stained for SIV gag protein using anti-P28 monoclonal antibody (Clone MX-0322, Microbix Biosystem Inc. Toronto, Ontario, Canada) by immunohistochemistry (IHC) as previously described (Borda et al., 2004). Briefly, slides were fixed in xylene, rehydrated in alcohol gradients, and finally water. Antigen retrieval was performed by steam (>95°C) in 1X citrate buffer, pH 6.0 for 20 minutes, and slides were washed with 1X tris buffered saline (TBS) solution. A protein (DAKO Protein Blocker, Serum Free, Carpenteria, CAS) and peroxidase (Peroxidase Blocking Reagent, DAKO) block was performed. After TBS wash, slides were incubated with mouse anti-P28 antibody (Microbix Biosystem Inc. Toronto, Ontario, Canada) diluted (1:20) in protein blocker for 60 minutes followed by TBS wash and amplification with a biotin free Peroxidase system (Biocare; Concord, CA) per manufacturer’s directions. P28+ cells were detected by Cytomation Liquid DAB substrate chromogen system (DAKO) after 5 min development.
SIV In situ Hybridization
SIV RNA In situ hybridization (ISH) was performed as previously described (Borda et al., 2004; Wang et al., 2007). Formalin-fixed, paraffin-embedded liver sections were de-paraffinized overnight at 60°C, then dehydrated by xylene washes followed by re-hydration in an alcohol series ending in a final distilled diethylpyrocarbonate (DEPC) water rinse. All solutions were made with DEPC treated water. Tissues were incubated in 0.2M HCl followed by a saline-sodium citrate (SSC) wash. Antigen retrieval by steam in 0.01M citrate buffer pH 6.0 was performed with a conventional microwave. A 60 minute pre-hybridization incubation was performed followed by an overnight incubation at 45°C in hybridization buffer with SIV RNA digoxigenin labeled probe (Lofstrand Labs Limited; Gaithersburg, MD) to detect SIV-infected cells. Short successive washes in SSC were conducted on day two. Excess SSC was removed and tissues were incubated in protein block (Serum Free Protein Blocker, DAKO; Carpenteria, CA). Slides were incubated overnight with appropriately diluted sheep Fab fragments of anti-digoxigenin antibody (Roche Diagnostics; Indianapolis, IN) in dark humidified chambers at 4°C. Two post-hybridization buffer washes were applied on day three. Controls included lymph node sections from known positives and negatives, and hybridization with digoxigenin-labeled sense RNA. The chromogen nitro blue tetrazolium chloride/5-Bromo-4-chloro-3-indolyl phosphate (NBT-BCIP) (Roche) was used to detect SIV infected cells. For RNA ISH, 3-hour NBT-BCIP incubation was performed. A blinded evaluation of random, non-touching fields per tissue was performed. Sections were imaged with a 20X objective using a Spot Insight color camera and Spot Imaging Software (Diagnostic Instruments; Sterling Heights, MI).
Confocal Microscopy
Confocal microscopy was performed using a Leica TCP SP2 confocal microscope equipped with three lasers (Leica Microsystems, Exton, PA). Image J64 (v1.6; NIH, Bethesda, MD) and Adobe Photoshop (CS5.1; Adobe, San Jose, CA) was used to assign colors to the four channels collected: HNPP/Fast Red, which fluoresces red when exposed to a 568-nm wavelength laser; Alexa 488 (Molecular probes) fluoresces green; Alexa 633 (Molecular Probes) appears blue; and the differential interference contrast (DIC) image is gray scale. The four channels were collected simultaneously. In some tissues the nuclei was stained with To-pro3 (nuclear marker, Molecular Probes) used at 1 μg/ml. Co-localization of antigens was demonstrated by the addition of colors as indicated in figure legends.
Statistics
Statistical analysis was performed with a non-parametric Mann Whitney U test using Graphpad Prism software (Graphpad software, San Diego, CA, USA). P values of ≤ 0.05 were considered significant. All data are expressed as means ± SEM
RESULTS
Kupffer cells increase in the liver during SIV infection
Liver sections from all 42 infected and 8 uninfected macaques were stained with mouse anti-human Ham56 antibody, which labels tissue macrophages. Positive cells were manually counted and quantified per mm2 of liver sections in a blinded fashion in 10 random fields for each section. Absolute numbers of Kupffer cells markedly and significantly increased in acutely infected macaques and in those with AIDS, with mean values of 471±41.6 (13 DPI), 365 ± 102.7 (21 DPI) and 397± 47.2 (AIDS) vs 217± 45.0 (controls) Ham56+ cells/mm2 liver (p≤0.002, 0.03 and 0.01 respectively) (Fig. 1B). Although there was a positive trend, numbers of Kupffer cells in the liver at 8 DPI were not significantly increased (262 ± 33.4). Interestingly the chronic, asymptomatic SIV-infected animals also did not show significant increases over controls, with means of 255 ± 24.9 in asymptomatic vs 217± 45.0 Ham56+ cells/mm2 in controls (Fig. 1B). There were also no significant changes in percentages of CD68+ cells co-expressing CD163 or CD14 in liver and blood in response to infection (Figs. 1C–F).
Changes in CD68+ Kupffer cells in liver and CD68+ monocytes in peripheral blood
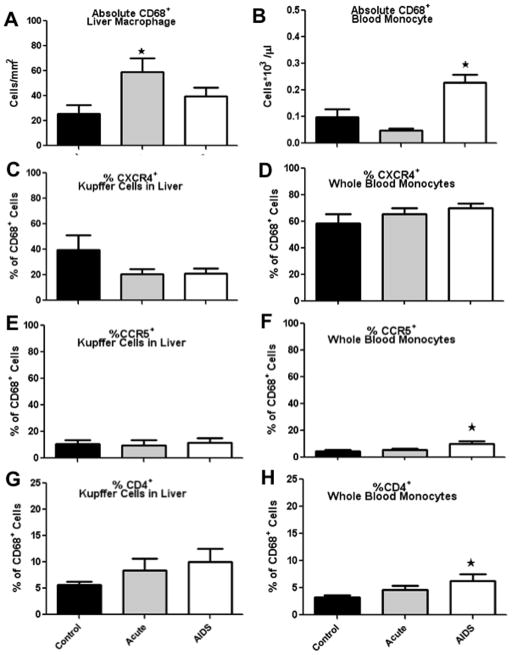
By flow cytometry, absolute numbers of CD68+ macrophages significantly increased in acutely infected macaques with a mean value of 26 (Control) vs 59 (acute) cells/mm2 of liver tissue (p≤0.0006). Absolute numbers of CD68+ macrophages in the AIDS group also increased from 26 (control) to 40 (AIDS) cells/mm2 of liver tissue, but these differences were not significant (Fig. 2A). Absolute numbers of CD68+ monocytes also significantly increased the blood in AIDS macaques with a mean value of 0.1 (Controls) vs 0.23 (AIDS) cells*103 /ul blood (p≤0.02), whereas absolute numbers of CD68+ monocytes slightly decreased in acute group (Fig. 2B). Co-expression of CCR5 and CXCR4 was also low on Kupffer cells (Figs 2C–F), similar to levels reported for intestinal macrophages (Meng et al., 2000; Shen et al., 2011). In fact, Kupffer cells had a phenotype resembling those reported for intestinal macrophages (Smith et al., 2005) including low levels of CCR5/CXCR4 (Fig. 2C, E), and lower levels of CD4 (Fig. 2G), and CD14 (Fig. 1E). There were no significant changes in CXCR4 or CCR5 expression on CD68+ monocyte/macrophages in blood or liver after SIV infection (Figs. 2C–F)
Figure 2.
Absolute numbers of CD68+ liver macrophages (Kupffer cells) (A) and CD68+ blood monocytes (B). Absolute numbers of monocytes in blood were determined by multiplying the percentages of CD68+ macrophages within the monocyte gate by absolute monocyte counts from the CBC analysis. Macrophage counts in liver were determined by multiplying absolute CD68+ cell counts in tissue sections by percentages of CD68+ cells detected by flow cytometry. *Indicates significant differences from controls (P<0.05). C–F: Percentages of CD68+ cells co-expressing CXCR4 (C, D) and CCR5 (E and F) are shown for liver macrophages (C and E) and blood monocytes (D and F) in acute infection and AIDS compared to controls.
Expression of CD163 and CD14 on Kupffer cells and monocytes
Expression of CD163 was examined in liver cell suspensions by flow cytometry and tissue sections by immunohistochemistry (Fig. 3A, B). Interestingly, while 60–70% of CD68+ monocytes in blood expressed CD163, only 20–30% of Kupffer cells expressed CD163 in liver cell suspensions. However, using double label immunohistochemistry for both Ham56 and CD163 on paraffin-embedded liver sections, we found the majority of cells were positive for both Ham56 and CD163 (Fig 3A). This suggested CD163 may have been shed upon tissue processing. Since CD163 is rapidly shed upon activation, and used as an in vivo marker of macrophage activation (Burdo et al., 2011; Zanni et al., 2012) surface expression on macrophages is likely very labile. The fact that cells were positive for both Ham56 and CD163 in situ, but not in cell suspensions, strongly suggests this discrepancy was an artifact associated with the cell isolation process. In addition, only 15–20% of liver Kupffer cells expressed CD14, whereas approximately 70–75% of blood monocytes express CD14 (Fig. 1E, F). Since CD14 is also often shed after activation, (Lien et al., 1998) we hypothesize that CD14 may be lost in tissue processing, or that Kupffer cells are simply more activated and express less CD14 than blood monocytes. Consistent with this, higher percentages of CD68+ Kupffer cells expressed CD4 in acute infection, and in animals with AIDS (Fig. 2G). However CD68+ blood monocytes exhibited higher expression of CD4 in the AIDS group only (Fig. 2H). This suggests CD4 may be upregulated on macrophages upon activation or expressed on newly recruited tissue macrophages, as previously suggested for intestinal macrophages (Smith et al., 2005).
Figure 3.
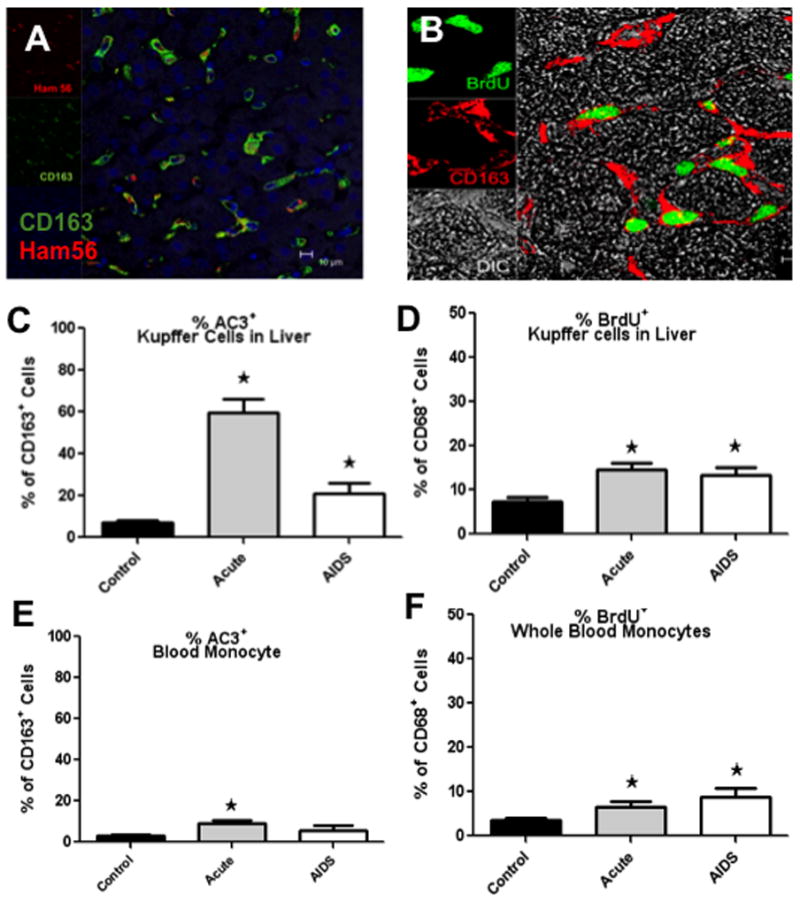
A, B: Confocal microscopy showing CD163+ and Ham56+ macrophages (A) and proliferating (BrdU+) macrophages in liver (B). Note the majority of the CD163+ Kupffer cells in the liver are Ham56+ and many are proliferating (BrdU+) during SIV infection. C–F: Percentages of apoptotic (AC3+) and proliferating (BrdU+) CD68+ liver macrophages (C, D) and blood (E, F). Note there are significant increases in the percentages of proliferating/ BrdU+ CD68+ Kupffer cells in liver and blood monocytes in both acute SIV infection and in macaques with AIDS. *Indicates significant differences from controls (P<0.05).
Proliferation and apoptosis of CD68+ Kupffer cells in liver and CD68+ monocytes in blood
In liver, percentages of CD68+ Kupffer cells in S-phase division (BrdU+) were significantly higher in acute infection and in AIDS compared to controls (p≤0.008 and p≤0.02 respectively) (Fig. 3D). Similarly, percentages of blood monocytes were significantly higher in acute infection and AIDS compared to controls in blood (p≤0.02 and p≤0.003 respectively) (Fig. 3F).
Interestingly, similar positive trends were observed in the percentages of CD163+ cells committed to apoptosis (AC3+) in the liver. Percentages were significantly higher in acutely infected, and AIDS macaques compared to controls (p≤0.0006 and p≤0.01 respectively) (Fig. 3C). Percentages of CD163+ AC3+ monocytes in the peripheral blood were also significantly higher in acute infection compared to controls (Fig. 3E). There was a slight increase in the percentage of CD163+ monocytes in the blood committed to apoptosis (AC3+) in AIDS compare to controls, but the difference was not significant (Fig. 3E).
SIV RNA In-situ hybridization in liver, jejunum and colon, mesenteric and axillary lymph node
To detect productively infected cells, both immunohistochemistry for SIV gag protein, and in-situ hybridization for SIV RNA was performed on liver of all 42 SIV infected macaques. However, SIV-infected cells were only detected in the liver of one animal with clinical AIDS (BE65) (Fig. 4A). Further, most infected cells in this macaque had a lymphocyte morphology, with very rare infected cells resembling Kupffer cells. Positive (Fig. 4B) and negative controls were used to confirm the sensitivity of both techniques. To confirm reactivity of the in situ probe, sensitivity, and infection of other lymphoid tissues in individual macaques, SIV in-situ hybridization was performed on four additional tissues including jejunum, colon, mesenteric and axillary lymph nodes of all 42 infected macaques. Viral RNA was detected in all four tissues of all animals during acute and AIDS stages confirming the animals had high numbers of infected cells in lymphoid and mucosal tissues, but not in the liver (data not shown). In acute infection, the highest numbers of virus-infected cells were detected in mesenteric lymph nodes and lamina propria of the jejunum, despite absence of viral RNA in liver tissue. In colon, most virus-infected cells were found in the organized lymphoid tissues (data not shown).
Figure 4.
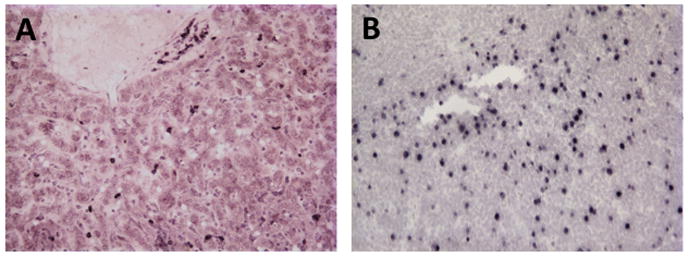
SIV In-situ hybridization for SIV RNA in liver of one macaque with AIDS (A). Note abundant infected cells (dark brown) mostly with a lymphocyte morphology. Productively infected cells (viral RNA) were only detected in the liver of this one animal from the 42 examined. Positive controls included lymph node sections from the same animal (B).
Discussion
Since the advent of anti-retroviral therapy (ART), liver disease has become the leading non-AIDS related cause of death in HIV patients (Price and Thio, 2010). However, the effects of HIV infection alone on the liver, especially in acute infection, are essentially unknown. Prior to the era of antiretroviral therapy (ART), the most common causes of liver dysfunction in HIV-infected patients were opportunistic infections and AIDS-related neoplasms (Price and Thio, 2010). Since ART, most descriptions of liver pathology are associated with ART, medications, alcohol use, or co-infection with various hepatitis viruses (Price and Thio, 2010). Thus, the effects of HIV infection alone on the liver are difficult to distinguish due to these confounding variables, making the SIV macaque model invaluable for examining the effects of retroviral infection alone on the liver.
The macrophage is a common target cell for essentially all lentiviral infections including HIV/SIV (Housset et al., 1993; Verani et al., 2005). Interestingly, recent studies have shown that increased monocyte/macrophage turnover is a key predictor of SIV disease progression and AIDS (Hasegawa et al., 2009). The Kupffer cells of the liver are the largest population of resident macrophages in the body and efficiently phagocytose translocated microbial products originating from the gut (Crofton et al., 1978). Here we show that absolute numbers of Ham56+ Kupffer cells markedly and significantly increased in the livers of SIV infected macaques by 13 and 21 DPI, and in animals with AIDS. Similar trends were observed by flow cytometry using CD68 to detect Kupffer cells in the liver and blood monocytes. There were significantly higher numbers of CD68+ Kupffer cells in liver of acutely infected macaques and higher numbers of CD68+ monocytes in blood of animals with AIDS. Moreover there were significantly higher percentages of proliferating (BrdU+) CD68+ Kupffer cells in the liver of acutely infected macaques and in animals with AIDS, and similar trends were observed in blood monocytes. There were also significantly higher percentages of CD163+ Kupffer cells in the liver committed to apoptosis (AC3+) during acute and AIDS stages of SIV infection. Increased percentages of CD163+ monocytes committed to apoptosis (AC3+) were detected in blood in acute infection. Combined, increased rates of Kupffer cell proliferation and apoptosis indicate there is marked turnover of monocytes/macrophages in the liver similar to what has been described in lymphoid organs (Hasegawa et al., 2009). However, in the liver this results in a net increased number of Kupffer cells at least during acute and AIDS stages of SIV infection. While numbers of Kupffer cells/mm2 in the liver may be increased, increased turnover may result in a smaller percentage of mature, fully functional Kupffer cells.
Localization of Kupffer cells within hepatic sinusoids allows contact with circulating cells in blood. Beach et al., suggested infection of Kupffer cells may occur during primary HIV viremia (Beach et al., 1992). Primary cultures of human Kupffer cells have been shown to be permissive for both HIV (Schmitt et al., 1990) and SIV (Schmitt et al., 1990). HIV antigens or RNA have been detected in liver cells of HIV- infected individuals, but the percentage of virus-positive cases, as well as the type and the number of virus-containing cells varies substantially (Cao et al., 1992; Hoda et al., 1991; Housset et al., 1990b; Housset et al., 1993; Hufert et al., 1993). Other studies suggest the liver may be a major organ for clearing SIV particles from the circulation (Zhang et al., 2002). However, Hufert et al, demonstrated that Kupffer cells isolated from livers of AIDS patients were only latently infected, and did not produce virus (Hufert et al., 1993), which is consistent with our RNA results.
In this study, both immunohistochemistry and SIV RNA In-situ hybridization detected infected cells in the liver of only one animal (BE65, AIDS animal), despite the fact viral RNA was detected in gut and lymphoid tissues of all SIV-infected animals examined using the same techniques. Other than an extended duration of time between infection and the onset of AIDS (1071 days) there was nothing unusual about this animal compared to the other infected animals (Table 1). This suggests the liver is not a major reservoir for SIV, at least not for replicating virus in vivo. Although we did detect increased apoptosis and proliferation indicating increased turnover, the net result was an increase in Kupffer cells throughout infection. This does not rule out the possibility that there may be low level infection (beyond the limits of detection by in situ hybridization) or latent infection of Kupffer cells in the liver. Since the liver is a highly vascularized tissue containing abundant peripheral blood cells within the sinusoids that could not be removed from these samples, we did not attempt RT-PCR for viral RNA or DNA on these samples, as we would not be able to discriminate infection of blood monocytes/lymphocytes and liver Kupffer cells in cell suspensions.
Prior studies have suggested the liver is a major site of viral clearance in early SIV infection (Zhang et al., 2002). However, we could not detect productively infected cells in the liver of macaques in early infection. Since the liver contains a vast network of sinusoids containing blood, previous studies using radiolabelled viruses may have overestimated the amount of virus actually within liver tissue versus the circulation. Alternatively, virus may be cleared through the liver in degradative pathways that do not allow viral integration and/or replication in Kupffer cells, which could result in bonafide clearance of virus in the liver, yet without productive infection. For example, Kupffer cells may attach and even phagocytize virions, yet destroy them before integration. Alternatively, Kupffer cells may inhibit inflammation, preventing viral replication within local lymphocytes or even autologous replication.
In summary, these data indicate that there is increased turnover of liver macrophages (Kupffer cells) resulting in a net increase in total numbers of Kupffer cells in response to SIV infection. However, these cells show no evidence of productive infection in vivo. Clearly, additional studies need to be performed in both SIV and HIV infection in vivo, but the data here suggest Kupffer cells proliferate in response to infection, and may play a major role in SIV clearance, without being productively infected.
Research Highlights.
Kupffer cells increase in the liver of SIV-infected macaques
Increased proliferation and apoptosis of Kupffer cells occurs in SIV infection
Productively infected cells are rarely detected in the liver
The liver is not a major site for SIV replication
Acknowledgments
We thank Julie Bruhn and Calvin Lanclos for flow cytometry support and Kelsi Rasmussen, Megan Gardner, Megan Watkins and Maury Duplantis for technical support. The CD4-Qdot655 reagent was provided by the NIH Nonhuman Primate Reagent Resource (R24 RR016001, N01 AI040101).
Financial support: This work was supported by NIH grants U19 AI076981, R01 AI084793, a Faculty Enhancement Grant from Tulane University, the National Center for Research Resources, and the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health through grant no. OD011104-51.
Footnotes
Potential conflicts of interest. Author certifies no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beach MJ, Meeks EL, Mimms LT, Vallari D, DuCharme L, Spelbring J, Taskar S, Schleicher JB, Krawczynski K, Bradley DW. Temporal relationships of hepatitis C virus RNA and antibody responses following experimental infection of chimpanzees. J Med Virol. 1992;36:226–237. doi: 10.1002/jmv.1890360314. [DOI] [PubMed] [Google Scholar]
- Borda JT, Alvarez X, Kondova I, Aye P, Simon MA, Desrosiers RC, Lackner AA. Cell tropism of simian immunodeficiency virus in culture is not predictive of in vivo tropism or pathogenesis. Am J Pathol. 2004;165:2111–2122. doi: 10.1016/S0002-9440(10)63261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borda JT, Alvarez X, Mohan M, Hasegawa A, Bernardino A, Jean S, Aye P, Lackner AA. CD163, a marker of perivascular macrophages, is up-regulated by microglia in simian immunodeficiency virus encephalitis after haptoglobin-hemoglobin complex stimulation and is suggestive of breakdown of the blood-brain barrier. Am J Pathol. 2008;172:725–737. doi: 10.2353/ajpath.2008.070848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, Rosenberg ES, Ellis RJ, Williams KC. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. 2011;204:154–163. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao YZ, Dieterich D, Thomas PA, Huang YX, Mirabile M, Ho DD. Identification and quantitation of HIV-1 in the liver of patients with AIDS. AIDS. 1992;6:65–70. doi: 10.1097/00002030-199201000-00008. [DOI] [PubMed] [Google Scholar]
- Crofton RW, Diesselhoff-den Dulk MM, van Furth R. The origin, kinetics, and characteristics of the Kupffer cells in the normal steady state. J Exp Med. 1978;148:1–17. doi: 10.1084/jem.148.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennessey CM, Keele BF. Using nonhuman primates to model HIV transmission. Curr Opin HIV AIDS. 2013;8:280–287. doi: 10.1097/COH.0b013e328361cfff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa A, Liu H, Ling B, Borda JT, Alvarez X, Sugimoto C, Vinet-Oliphant H, Kim WK, Williams KC, Ribeiro RM, Lackner AA, Veazey RS, Kuroda MJ. The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood. 2009;114:2917–2925. doi: 10.1182/blood-2009-02-204263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbein G, Coaquette A, Perez-Bercoff D, Pancino G. Macrophage activation and HIV infection: can the Trojan horse turn into a fortress? Current molecular medicine. 2002;2:723–738. doi: 10.2174/1566524023361844. [DOI] [PubMed] [Google Scholar]
- Hoda SA, White JE, Gerber MA. Immunohistochemical studies of human immunodeficiency virus-1 in liver tissues of patients with AIDS. Mod Pathol. 1991;4:578–581. [PubMed] [Google Scholar]
- Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood. 1993;81:1607–1613. [PubMed] [Google Scholar]
- Housset C, Boucher O, Girard PM, Leibowitch J, Saimot AG, Brechot C, Marche C. Immunohistochemical evidence for human immunodeficiency virus-1 infection of liver Kupffer cells. Hum Pathol. 1990a;21:404–408. doi: 10.1016/0046-8177(90)90202-g. [DOI] [PubMed] [Google Scholar]
- Housset C, Lamas E, Brechot C. Detection of HIV1 RNA and p24 antigen in HIV1-infected human liver. Res Virol. 1990b;141:153–159. doi: 10.1016/0923-2516(90)90017-d. [DOI] [PubMed] [Google Scholar]
- Housset C, Lamas E, Courgnaud V, Boucher O, Girard PM, Marche C, Brechot C. Presence of HIV-1 in human parenchymal and non-parenchymal liver cells in vivo. J Hepatol. 1993;19:252–258. doi: 10.1016/s0168-8278(05)80579-3. [DOI] [PubMed] [Google Scholar]
- Hufert FT, Schmitz J, Schreiber M, Schmitz H, Racz P, von Laer DD. Human Kupffer cells infected with HIV-1 in vivo. J Acquir Immune Defic Syndr. 1993;6:772–777. [PubMed] [Google Scholar]
- Lackner AA, Mohan M, Veazey RS. The gastrointestinal tract and AIDS pathogenesis. Gastroenterology. 2009;136:1965–1978. doi: 10.1053/j.gastro.2008.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien E, Aukrust P, Sundan A, Muller F, Froland SS, Espevik T. Elevated levels of serum-soluble CD14 in human immunodeficiency virus type 1 (HIV-1) infection: correlation to disease progression and clinical events. Blood. 1998;92:2084–2092. [PubMed] [Google Scholar]
- Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng G, Sellers MT, Mosteller-Barnum M, Rogers TS, Shaw GM, Smith PD. Lamina propria lymphocytes, not macrophages, express CCR5 and CXCR4 and are the likely target cell for human immunodeficiency virus type 1 in the intestinal mucosa. J Infect Dis. 2000;182:785–791. doi: 10.1086/315790. [DOI] [PubMed] [Google Scholar]
- Price JC, Thio CL. Liver disease in the HIV-infected individual. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2010;8:1002–1012. doi: 10.1016/j.cgh.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt MP, Steffan AM, Gendrault JL, Jaeck D, Royer C, Schweitzer C, Beyer C, Schmitt C, Aubertin AM, Kirn A. Multiplication of human immunodeficiency virus in primary cultures of human Kupffer cells--possible role of liver macrophage infection in the physiopathology of AIDS. Res Virol. 1990;141:143–152. doi: 10.1016/0923-2516(90)90016-c. [DOI] [PubMed] [Google Scholar]
- Shen R, Meng G, Ochsenbauer C, Clapham PR, Grams J, Novak L, Kappes JC, Smythies LE, Smith PD. Stromal down-regulation of macrophage CD4/CCR5 expression and NF-kappaB activation mediates HIV-1 non-permissiveness in intestinal macrophages. PLoS Pathog. 2011;7:e1002060. doi: 10.1371/journal.ppat.1002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PD, Ochsenbauer-Jambor C, Smythies LE. Intestinal macrophages: unique effector cells of the innate immune system. Immunol Rev. 2005;206:149–159. doi: 10.1111/j.0105-2896.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- Veazey R, Ling B, Pandrea I, McClure H, Lackner A, Marx P. Decreased CCR5 expression on CD4+ T cells of SIV-infected sooty mangabeys. AIDS Res Hum Retroviruses. 2003;19:227–233. doi: 10.1089/088922203763315731. [DOI] [PubMed] [Google Scholar]
- Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- Veazey RS, Rosenzweig M, Shvetz DE, Pauley DR, DeMaria M, Chalifoux LV, Johnson RP, Lackner AA. Characterization of gut-associated lymphoid tissue (GALT) of normal rhesus macaques. Clin Immunol Immunopathol. 1997;82:230–242. doi: 10.1006/clin.1996.4318. [DOI] [PubMed] [Google Scholar]
- Verani A, Gras G, Pancino G. Macrophages and HIV-1: dangerous liaisons. Mol Immunol. 2005;42:195–212. doi: 10.1016/j.molimm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Wang X, Rasmussen T, Pahar B, Poonia B, Alvarez X, Lackner AA, Veazey RS. Massive infection and loss of CD4+ T cells occurs in the intestinal tract of neonatal rhesus macaques in acute SIV infection. Blood. 2007;109:1174–1181. doi: 10.1182/blood-2006-04-015172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanni MV, Burdo TH, Makimura H, Williams KC, Grinspoon SK. Relationship between monocyte/macrophage activation marker soluble CD163 and insulin resistance in obese and normal-weight subjects. Clinical endocrinology. 2012;77:385–390. doi: 10.1111/j.1365-2265.2011.04284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Dailey PJ, Gettie A, Blanchard J, Ho DD. The liver is a major organ for clearing simian immunodeficiency virus in rhesus monkeys. J Virol. 2002;76:5271–5273. doi: 10.1128/JVI.76.10.5271-5273.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]