Abstract
The scope of this article is not to provide an exhaustive review of nuclear transfer research, because many authoritative reviews exist on the biological issues related to somatic and embryonic cell nuclear transfer. We shall instead provide an overview on the work done specifically on sheep and the value of this work on the greater nuclear transfer landscape.
Introduction
We can easily forgive a child who is afraid of the dark; the real tragedy of life is when men are afraid of the light.
—Plato
To begin to tell the story of nuclear transfer research—today oversimplified as “cloning”—one must go back to the middle of the 1950s and explain the driving force behind developing nuclear transplantation. It was only in the 1950s that experimental embryology tools reached the level of accuracy necessary to empower scientists to put into practice the “fantastic experiment” anticipated by Nobel Prize winner Hans Spemann in 1938 and his fellow developmental biologists. This fantastic experiment consisted of “fertilizing” an enucleated amphibian oocyte with a differentiated diploid nucleus (Spemann, 1938). The million-dollar question at the time was: How could a fertilized egg develop into a carefully organized organism having differentiated cells that were coping with everyday tasks? The differentiation theory in vogue at the time was that a specialized cell retained only the genes that empowered a cell to keep its specific function (interestingly, although the structure of DNA was not known then, the concept of an unknown entity responsible for a date character was already in use). Contrary to this theory, Spemann observed instead that the blastomeres that dislocated from the early salamander embryos developed into normal individuals (Spemann, 1938), suggesting that at least early in development there was no gene loss. The next logical question was: Does this phenomenon also apply to differentiated cells? The fantastic experiment could provide the final proof.
John Gurdon, working with Xenopus laevis, successfully carried out Spemann's experiment by employing a technique previously worked out by Briggs and King (1952) to produce viable frogs using nuclear transfer of tadpole intestinal epithelium cells (Gurdon and Uehlinger, 1966). Gurdon's seminal work demonstrated that cell differentiation takes place without gene loss (as a general rule, with few exceptions), and that cellular commitment might be reversible if the differentiated cells are exposed to the oocyte's cytoplasm, a phenomenon commonly defined to as “nuclear reprogramming.” Gurdon disproved the dogma demonstrating the totipotency of the genome, yet he established that cells taken from an adult individual cannot reverse their differentiated date through nuclear transfer, a dogma that endured until the end of the 20th century.
But a frog, Gurdon's animal model, is a frog, and a mammal is a mammal. Could these findings be extrapolated to mammals? Davor Solter and James McGrath said yes, that nuclear transfer could also be applied to mouse embryos (McGrath and Solter, 1984). Since their work in the 1984, there has been a crescendo of experiments with sheep as the primary model for nuclear transfer research. Contrary to the pioneering experiments in amphibians and mouse that addressed purely hypothesis-driven biological questions, the work done with sheep has pushed nuclear transfer into a more pragmatic framework. This is the amplification of the reproductive potential of selected genotypes by embryo multiplication though nuclear transfer of embryonic cells—essentially, translational research into farm animal breeding.
Embryonic Cloning
To understand this new direction of nuclear transfer research, it is important to recall the atmosphere of the early 1980s. Superovulation and embryo transfer were widely applied to maximize the reproductive potential of farm animals (Gearheart et al., 1989). Soon after, the first sheep embryos were manipulated to make monozygotic twins by blastomere separation (Willadsen, 1979) and blastocyst splitting (Shelton, 1988; Willadsen and Godke, 1984).
These earlier techniques were relatively easy to use, but had limited efficiency, hence the logical extension to multiply the embryos by nuclear transfer. Steen Willadsen's pioneering work in sheep—producing the first lambs by nuclear transfer of a blastomere into an enucleated oocyte (Willadsen, 1979)—laid the cornerstone for the further research. This was the heyday of cloning. The possibility of obtaining sets of clones from single embryos catalyzed a lot of research in other farm animals, with the sheep being a constant presence. Why sheep? Sheep are financially reasonable to purchase and maintain, and furthermore, are easy to handle. Riding this wave of sheep and other animal cloning, several private companies were established for the commercial exploitation of the technology (First, 1990; Wilmut and Campbell, 1992), but paradoxically, the more embryonic cloning work that was done, the more it became apparent how little we knew.
Search for the Best Cell Cycle Combination in Embryonic Cloning
Essentially, the fundamental rules of cell cycle stage compatibility, established two decades ago in cell fusion experiments (Johnson and Rao, 1970; Johnson et al., 1970), were being ignored. It was at this juncture that Keith Campbell first brought sheep and nuclear transfer together for the first time. Keith's contribution built upon the work on cell cycle compatibility between the oocyte's cytoplasm and the embryonic nucleus done previously by Lawrence Smith in Ian Wilmut's lab (Smith et al., 1988).
The data published in late 1980s on embryonic cloning of sheep and other large animals demonstrated our dominating ignorance of the basic concepts of cell cycle compatibility. Put simply, we did not know what we were doing. Not surprisingly then, we noted that shortly after nuclear transfer, the nuclear membrane disassembled into small vesicles and the chromatin compacted very rapidly, resulting in DNA fragmentation (Campbell et al., 1996a). These activities, which are normal events in a regular cell cycle, were devastating in nuclear transfer (Campbell et al., 1996a). In fact, oocytes in stage II of meiotic metaphase have high levels of maturation-promoting factor (MPF) (Procházka et al., 1989) and histone 1 phosphorylation activity (Pondaven et al., 1990). Mitotic, as well as meiotic, cytoplasm, hence including metaphase II, exerts a dominant effect on the other cell cycle stages. Because 90% of the blastomeres in an early embryo are in S phase, the breakdown of the nuclear envelope and condensation of chromatin induced by MPF in embryonic nuclei results in catastrophic DNA damage (Campbell et al., 1996a).
It was at this point that I joined Dr. Wilmut's group and collaborated with Keith Campbell. Strategies were then put forth to achieve cell cycle synchrony between the metaphase II cytoplast and the mitotic embryonic nuclei. These included: (1) Bringing the blastomere into M phase, compatible with the oocyte cell cycle stage; and (2) bringing the oocyte into S phase, compatible with the blastomere cell cycle stage.
The first approach, developed using mouse embryos (Otaegui et al., 1994), was quickly abandoned, because the long exposure of cytoskeleton inhibitors (e.g., nocodazole) that was required to stop all blastomeres in an early sheep embryo was deleterious to their further development (P. Loi, unpublished). The second method, which Keith Campbell passionately advocated, was developed further. The use of preactivated enucleated oocytes, first achieved in sheep, resulted in improved development to blastocyst-stage cloned embryos, compared to other methods (Campbell et al., 1994) (Fig 1). The preactivated cytoplast, called the “universal recipient,” was one of Keith's crowning achievements and surely the first example of nuclear transfer backed up by sound science and not blind empiricism.
FIG. 1.
(A and B) First cloned lambs produced using a “universal recipient.” (Reprinted from Campbell et al., 1994.)
While embryologists were all struggling to control the cell cycle in nuclear transfer embryos, other scientists started to dissect cell cycle regulators at the molecular level, particularly the cell division cyclin-dependent kinases (CDK). Soon after, the first inhibitors for CDK kinases, like roscovitine and olomoucine, were discovered (De Azevedo et al., 1997) and quickly adopted by embryologists (Moses and Masui, 1994). The “universal recipient” worked very well, yet two manipulation steps were required for its implementation, the second of which (the nuclear transfer), usually occurred late at night, making it impractical.
Then a paper published by Neal First's group further shed light on cell cycle control of nuclear transfer embryos. They found that the kinase inhibitor 6-(dimethylamino)purine (6-DMAP) promoted the direct transition from metaphase II to an interphase-like structure of artificially activated oocytes (Susko-Parris et al., 1994). Moreover, 6-DMAP appeared to also inhibit the kinases involved in the disassembly of the nuclear envelope (Susko-Parris et al., 1994). These two properties appeared to make 6-DMAP the magic ingredient to avoid nuclear envelope breakdown (NEBD) and its ensuing DNA damage in oocytes reconstructed with unsynchronized embryonic nuclei.
The empirical work confirmed the expectations. Embryonic nuclei retained an interphase-like nuclear structure following nuclear transfer, so that S phase was left undisturbed. The frequencies of development following in vivo culture were the highest ever recorded (Fig. 2), and, above all, the findings were highly reproducible (Loi et al., 1998): Large clones of embryonic cells were finally produced (Loi et al., 1997). The protocol, developed in sheep, was quickly adopted for other species and is still used for somatic cell nuclear transfer (SCNT).
FIG. 2.
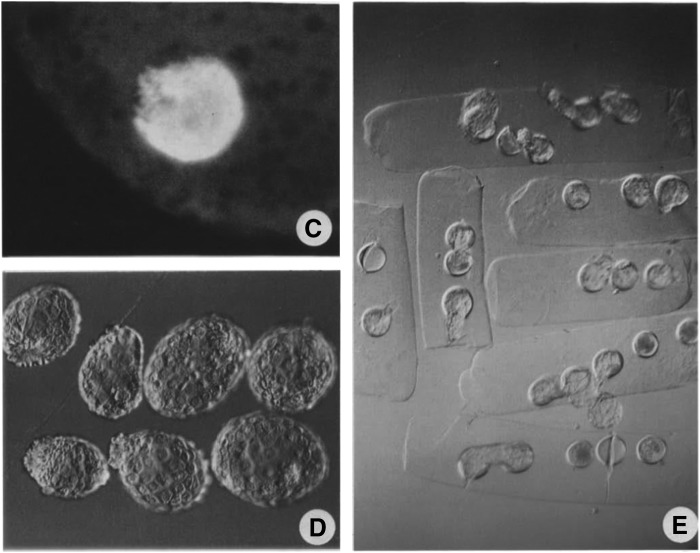
(C) Uninterrupted DNA synthesis of sheep embryonic nuclei transplanted in enucleated oocytes treated with 6-DMAP. (D and E) Cloned blastocysts derived from ionomycin and 6-DMAP-activated nuclear transfer embryos. (Reprinted from Loi et al., 1998.)
FIG. 3.
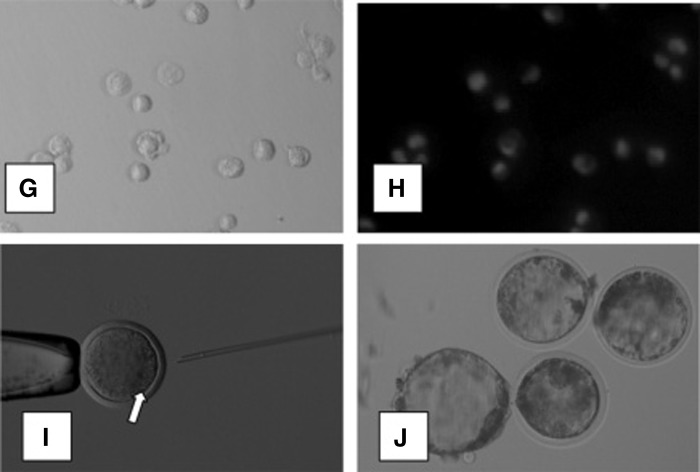
Lyophilized sheep somatic cells (granulosa cells, G and H) injected into enucleated oocytes (I) that developed into blastocysts (J). (Reprinted from Loi et al., 2007.)
The Path to Dolly
Once the cell cycle compatibility between the nucleus and the oocyte's cytoplasm was optimized, Keith and the Roslin group continued to shatter the traditional dogma established in amphibians. John Gurdon working with X. laevis established the concept of genome equivalence among all different cells, but also postulated the irreversibility of the differentiated state of a somatic cell taken from adult individuals. The Roslin group refuted the dogma stepwise, moving from embryonic cloning to embryonic/fetal cell cloning. The embryonic lines were established from cultured inner cell mass as an extra step in isolating embryonic stem cells (ESCs) from sheep embryos. Again, sheep proved to be central in this series of experiments. The phenotype of the embryonic cells was very fibroblast-like, indicating a certain degree of differentiation had occurred in vitro.
This sparked Keith Campbell's second intuition—that cell cycle synchrony was important—but additional “tricks” were needed to facilitate the nuclear reprogramming of even partially differentiated cells. The solution was to bring the nuclear donor cells into quiescence, or the G0 state, via serum starvation. G0 nuclei reduce all activities, causing transcription factors to detach from the chromatin, making it more accessible to the nuclear reprogramming machinery of the oocytes. His successful experiments resulted in producing two sheep clones from the embryonic cell line (Campbell et al., 1996b).
The achievement was extraordinary and boosted the enthusiasm of the team. Now they were ready to answer the biggest question yet: If fetal cells could be fully reprogrammed, why not somatic cells too? It was then only a matter of time before Dolly—a sheep produced from the nuclear transfer of a mammary gland cell of a Finn Dorset ewe (Wilmut et al., 1997)—was delivered in the fall of 1996. This was the first cloned mammal in history.
The breakthrough represented by Dolly is one of the major scientific achievements of the last century. It overturned the concept that the differentiated status of a somatic cell is irreversible. The mission of the team was completely fulfilled. Successful cloning by SCNT became a standard form of asexual reproduction that allowed for the “copying and pasting” of a selected genotype, and the opportunity to make, in theory, infinite numbers of it.
The potential applications of such revolutionary reproductive tools were enormous and at the same time frightening for a scientifically unprepared audience. SCNT heavily impacts breeding programs—the making of transgenic animals—and could also be a method of preserving rare/disappearing animal species (Campbell et al., 2001; Polejaeva and Campbell, 2000). The opportunity to multiply selected phenotypes has dramatic implications for animal production, with SCNT holding the potential to help meet the increasing worldwide demand for food. The impact of SCNT on animal production is already evident in the beef cattle industry, particularly in the United States and Japan, where SCNT has faced less resistance among consumers and met Food and Drug Administration (FDA) approval (Solomon et al., 2009).
As for SCNT and transgenic animals and the multiplication of rare animals, sheep have been again a major actor. The canonical way to make transgenic farm animals—where no ESCs have been isolated so far—has been the pronuclear injection of a few hundred copies of linearized plasmid of the selected gene into zygotes. Pronuclear injection works, but with low efficiency and unpredictable outcomes, given the uncontrollable integration of the new gene (Clark, 2002).
SCNT offers a standardized approach with reliable outcomes (100% of transgenic offspring) in cases where the somatic cells have been stably transformed with the transgene. The first transgenic cloned lambs expressing human Factor IX were produced by Keith's group using nuclear transfer (Schnieke et al., 1997), followed by the production of cloned lambs derived from fetal fibroblasts carrying the foreign human gene alpha1(I) procollagen (COL1A1) (McCreath et al., 2000). Soon after, the Roslin group produced the first gene knockout transgenic lamb, devoid of the endogenous prion gene, again by using SCNT of a genetically modified fibroblast cell line (Denning et al., 2001).
Keith dedicated all of his post-Dolly research efforts into optimizing SCNT, especially in addressing the enrichment of the oocyte's reprogramming potential using, among other factors, caffeine (Choi et al., 2010; Lee et al., 2010). The importance of caffeine in the reprogramming potential of the oocyte has been highlighted in the last SCNT study in humans finalized to derive ESCs from SCNT embryos (Tachibana et al., 2013).
Another biological issue linked to SCNT, particularly in its declination of interspecific SCNT (iSCNT), pursued by Keith, is the effect of mitochondria on the development of SCNT embryos. The effects of mitochondrial heteroplasmy in iSCNT is still under debate. A strategy to produce mitochondrial (mt) DNA homoplasmic clones, which avoids the negative effects of mtDNA heteroplasmy, has been successfully developed in sheep (Bowles et al., 2007). Sheep fetal fibroblasts (the cell line originally used in the report of Dolly) were treated with low doses of ethidium bromide in the culture medium, resulting in mtDNA depletion after 2 weeks. Normal lambs were derived from the nuclear transfer of these cells, providing the proof of principle of a standardized method to avoid mtDNA heteroplasmy in clones (Lee et al., 2010).
On the iSCNT front, somatic cells from mouflon, a partially endangered animal, were injected into sheep enucleated oocytes, which resulted in a surprisingly high number of blastocysts, that when transferred into presynchronized sheep established two pregnancies, one of which developed into the first cloned mouflon. This effort was the first evidence that iSCNT could be a feasible method for recovering or saving a threatened species (Loi et al., 2001).
It is true that many other animals, particularly mouse, are providing useful insights regarding nuclear reprogramming (Ogura et al., 2013); however, sheep still remain a central animal model for nuclear transfer research. A strong conviction I have developed since the beginning of SCNT, underpinned by the invariably low efficiency of cloned offspring in all species, is the need to manipulate the chromatin structure heavily to render it more compliant with the reprogramming engines available in the oocyte. To test how far we can push it, I have denatured somatic cells (granulosa cells) by heating them at 75°C for half an hour. Surprisingly, the nucleus of heated cells was fully reprogrammed until the birth of normal cloned lambs upon nuclear transfer (Loi et al., 2002). The next logical undertaking is the use of lyophilized somatic cells (granulosa cells and lymphocytes) for nuclear transfer. Again, dry nuclei were reactivated by the oocyte and began to direct embryonic development until the blastocyst stage (Loi et al., 2008).
These most recent breakthroughs are important because: (1) They prove that radical approaches, even those incompatible with cell viability, might be applied to the somatic nucleus to reduce/abolish the “resistance to reprogramming” (Pasque et al., 2011); (2) the fact that nonviable cells can be suitable nuclear donors has inspired the possibility of bringing back extinct species. This venture, termed “de-extinction” in a recent conference organized by Scientific American, aims to restore long-extinct species, including the woolly mammoth, the passenger pigeon, and others (www.ted.com/tedx/events/7650).
More realistically, we would like to apply this research more pragmatically to preserve living, but endangered, species. The ability to store cell lines in a dry, lyophilized state, which we and then others (Das et al., 2010; Ono et al., 2008; Wakayama and Yanagimachi, 1998) have proved feasible, allows as to establish biobanks with zero maintenance costs, where specimens can be preserved until a time when SCNT will be reliable and safe.
The sheep has been a primary actor in nuclear transfer research and still remains a unique model for basic research, especially now that its genome has been annotated (Pierre Taberlet, personal communication). The lack of the knowledge of the sheep sequences so far has impaired mechanistic research in sheep, as normally carried out in mouse, whose genome has been sequenced long ago.
Concluding Remarks
The production of the first animals through transfer of a somatic cell into enucleated oocytes was the final step in a scientific enterprise spanning two centuries. Normally, when a major breakthrough is established and confirmed, the entire field benefits from it. In the case of cloning with somatic cells, this has not been the case. The implications of the technique sparked heated ethical discussions and a media-fuelled outcry that cloning might be extended to humans, which dampened nascent SCNT efforts, particularly in Europe. As a result, in many European Union countries, SCNT experiments were not welcomed. In Italy, for instance, the ban established by the Minister of Health lasted for 5 years. Even now, there are rumors at the EU level that SCNT should be no longer funded on the basis of animal welfare concerns. Up until his death in late 2012, Keith Campbell had been a passionate defender of cloning for animal breeding/transgenic research, a view that all the first-moment “cloners” share without reservation.
But the most important aspect of Dolly's legacy goes far beyond reproductive cloning. Although it was implicit from Gurdon's studies that the differentiated nucleus transplanted into the enucleated oocytes acquired a condition of totipotency, it was only after Dolly and the almost contemporary isolation of human ESCs (Thomson et al., 1998) that scientists and then clinicians began to foresee the huge potential for alternative medicine.
Robert Lanza was one of the first to coin the term “therapeutic cloning” (Lanza et al., 1999), where nuclear reprogramming and stem cell properties could be exploited in tandem to combat diseases untreatable with the available medical practices. In essence, somatic cells taken from a patient carrying a degenerative or otherwise debilitating disease are transplanted into an enucleated human oocyte. The resulting embryo is not allowed to develop further than the blastocyst stage but is used instead to establish ESCs. The patient-derived stem cells are then induced to differentiate into the cell type that is defective in the patient and are then transplanted back into the patient, elegantly avoiding immunological rejection (Lanza et al., 1999).
The prospect of developing a revolutionary “patient-derived cell therapy” for curing untreatable diseases has become one of the hottest issues in science, with some of the world's foremost research institutions involved in the task. A wealth of data has been published on embryonic and adult (mesenchymal) stem cells, their biology, and and their transdifferentiation potential, with this research culminating in the ethically flawless induced pluripotent stem cells (iPSCs) (Takahashi and Yamanaka, 2006). It is hard to say whether iPSCs would have been discovered without SCNT; while they may very have, Dolly undoubtedly triggered all fields of cell transplantation studies.
In 2012, the Nobel Prize in Physiology of Medicine was conferred jointly on Sir John Gurdon, for the canonical “old” nuclear transfer method of inducing nuclear reprogramming, and to Shinya Yamanaka, for the “new” one, induced pluripotency (Takahashi and Yamanaka, 2006; Takahashi et al., 2007). Yet, the long-awaited development of patient-derived stem cells has just been achieved by canonical “old”nuclear transfer (Tachibana et al., 2013): Back to the future?
Acknowledgments
Our Laboratory received funding from the European Research Council under the European Community Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no. 210103; GP: PRIN 2007, no. 2007MY2M92 to G.P. P.L. acknowledges the support of the EU FP7-KBBE2009-3 Programme, project no. 244356, NextGene; PRIN MIUR founding (protocol no. 2009JE3CHM); Project “GenHome.” Funds from the Bank Foundation Tercas (Teramo, Italy) are also acknowledged. The Laboratory members are part of the COST action FA1201 Epiconcept “Epigenetics and Periconception Environment.”
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Briggs R. King T.J. Transplantation of living nuclei from blastula cells into enucleated frog eggs. Proc. Natl. Acad. Sci. USA. 1952;38:455–463. doi: 10.1073/pnas.38.5.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles E.J. Lee J.H. Alberio R. Lloyd R.E. Stekel D. Campbell K.H. St. John J.C. Contrasting effects of in vitro fertilization and nuclear transfer on the expression of mtDNA replication factors. Genetics. 2007;176:1511–1526. doi: 10.1534/genetics.106.070177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K.H.S. Loi P. Cappai P. Wilmut I. Improved development to blastocyst of ovine nuclear transfer embryos reconstructed during the presumptive S-phase of enucleated activated oocytes. Biol. Reprod. 1994;50:1385–1393. doi: 10.1095/biolreprod50.6.1385. [DOI] [PubMed] [Google Scholar]
- Campbell K.H. Loi P. Otaegui P.J. Wilmut I. Cell cycle co-ordination in embryo cloning by nuclear transfer. Rev. Reprod. 1996a;1:40–46. doi: 10.1530/ror.0.0010040. [DOI] [PubMed] [Google Scholar]
- Campbell K.H. McWhir J. Ritchie W.A. Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996b;380:64–66. doi: 10.1038/380064a0. [DOI] [PubMed] [Google Scholar]
- Campbell K.H. Alberio R. Lee J.H. Ritchie W.A. Nuclear transfer in practice. Cloning Stem Cells. 2001;3:201–208. doi: 10.1089/15362300152725927. [DOI] [PubMed] [Google Scholar]
- Choi I. Lee J.H. Fisher P. Campbell K.H. Caffeine treatment of ovine cytoplasts regulates gene expression and foetal development of embryos produced by somatic cell nuclear transfer. Mol. Reprod. Dev. 2010;77:876–887. doi: 10.1002/mrd.21230. [DOI] [PubMed] [Google Scholar]
- Clark A.J. Generation of transgenic livestock by pronuclear injection. Methods Mol. Biol. 2002;180:273–287. doi: 10.1385/1-59259-178-7:273. [DOI] [PubMed] [Google Scholar]
- Das Z.C. Gupta M.K. Uhm S.J. Lee H.T. Lyophilized somatic cells direct embryonic development after whole cell intracytoplasmic injection into pig oocytes. Cryobiology. 2010;61:220–224. doi: 10.1016/j.cryobiol.2010.07.007. [DOI] [PubMed] [Google Scholar]
- De Azevedo W.F. Leclerc S. Meijer L. Havlicek L. Strnad M. Kim S.H. Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine. Eur. J. Biochem. 1997;243:518–526. doi: 10.1111/j.1432-1033.1997.0518a.x. [DOI] [PubMed] [Google Scholar]
- Denning C. Burl S. Ainslie A. Bracken J. Dinnyes A. Fletcher J. King T. Ritchie M. Ritchie W.A. Rollo M. de Sousa P. Travers A. Wilmut I. Clark A.J. Deletion of the alpha(1,3)galactosyl transferase (GGTA1) gene and the prion protein (PrP) gene in sheep. Nat. Biotechnol. 2001;19:559–562. doi: 10.1038/89313. [DOI] [PubMed] [Google Scholar]
- First N.L. New animal breeding techniques and their application. J. Reprod. Fertil. Suppl. 1990;41:3–14. [PubMed] [Google Scholar]
- Gearheart W.W. Smith C. Teepker G. Multiple ovulation and embryo manipulation in the improvement of beef cattle: relative theoretical rates of genetic change. J Anim. Sci. 1989;67:2863–2871. doi: 10.2527/jas1989.67112863x. [DOI] [PubMed] [Google Scholar]
- Gurdon J.B. Uehlinger V. “Fertile” intestine nuclei. Nature. 1966;210:1240–1241. doi: 10.1038/2101240a0. [DOI] [PubMed] [Google Scholar]
- Johnson R.T. Rao P.N. Mammalian cell fusion: Induction of premature chromosome condensation in interphase nuclei. Nature. 1970;226:717–722. doi: 10.1038/226717a0. [DOI] [PubMed] [Google Scholar]
- Johnson R.T. Rao P.N. Hughes H.D. Mammalian cell fusion. III. A HeLa cell inducer of premature chromosome condensation active in cells from a variety of species. J. Cell. Physiol. 1970;76:151–158. doi: 10.1002/jcp.1040760204. [DOI] [PubMed] [Google Scholar]
- Lanza R.P. Cibelli J.B. West M.D. Human therapeutic cloning. Nat. Med. 1999;5:975–977. doi: 10.1038/12404. [DOI] [PubMed] [Google Scholar]
- Lee J.H. Peters A. Fisher P. Bowles E.J. St. John J.C. Campbell K.H. Generation of mtDNA homoplasmic cloned lambs. Cell. Reprogram. 2010;12:347–355. doi: 10.1089/cell.2009.0096. [DOI] [PubMed] [Google Scholar]
- Loi P. Boyazoglu S. Gallus M. Ledda S. Naitana S. Wilmut I. Cappai P. Casu S. Embryo cloning in sheep: Work in progress. Theriogenology. 1997;48:1–10. doi: 10.1016/S0093-691X(97)00187-8. [DOI] [PubMed] [Google Scholar]
- Loi P. Ledda S. Fulka J., Jr. Cappai P. Moor R.M. Development of parthenogenetic and cloned ovine embryos: Effect of activation protocols. Biol. Reprod. 1998;58:1177–1187. doi: 10.1095/biolreprod58.5.1177. [DOI] [PubMed] [Google Scholar]
- Loi P. Ptak G. Fulka J., Jr. Cappai P. Clinton M. Genetic rescue of an endangered mammal by cross-species nuclear transfer using post-mortem somatic cells. Nat. Biotechnol. 2001;19:962–964. doi: 10.1038/nbt1001-962. [DOI] [PubMed] [Google Scholar]
- Loi P. Clinton M. Barboni B. Fulka J., Jr. Cappai P. Feil R. Moor R.M. Ptak G. Nuclei of nonviable ovine somatic cells develop into lambs after nuclear transplantation. Biol. Reprod. 2002;67:126–132. doi: 10.1095/biolreprod67.1.126. [DOI] [PubMed] [Google Scholar]
- Loi P. Matsukawa K. Ptak G. Clinton M. Fulka J., Jr. Nathan Y. Arav A. Freeze-dried somatic cells direct embryonic development after nuclear transfer. PLoS One. 2008;3:e2978. doi: 10.1371/journal.pone.0002978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreath K.J. Howcroft J. Campbell K.H. Colman A. Schnieke A.E. Kind A.J. Production of gene-targeted sheep by nuclear transfer from cultured somatic cells. Nature. 2000;405:1066–1069. doi: 10.1038/35016604. [DOI] [PubMed] [Google Scholar]
- McGrath J. Solter D. Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell. 1984;37:179–183. doi: 10.1016/0092-8674(84)90313-1. [DOI] [PubMed] [Google Scholar]
- Moses R.M. Masui Y. Enhancement of mouse egg activation by the kinase inhibitor, 6-dimethylaminopurine (6-DMAP) J. Exp. Zool. 1994;270:211–218. doi: 10.1002/jez.1402700210. [DOI] [PubMed] [Google Scholar]
- Ogura A. Inoue K. Wakayama T. Recent advancements in cloning by somatic ell nuclear transfer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;368:20110329. doi: 10.1098/rstb.2011.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T. Mizutani E. Li C. Wakayama T. Nuclear transfer preserves the nuclear genome of freeze-dried mouse cells. J. Reprod. Dev. 2008;54:486–491. doi: 10.1262/jrd.20112. [DOI] [PubMed] [Google Scholar]
- Otaegui P.J. O'Neill G.T. Campbell K.H.S. Wilmut I. Transfer of nuclei from 8-cell stage mouse embryos following use of nocodazole to control the cell cycle. Mol. Reprod. Dev. 1994;39:147–152. doi: 10.1002/mrd.1080390205. [DOI] [PubMed] [Google Scholar]
- Pasque V. Jullien J. Miyamoto K. Halley-Stott R.P. Gurdon J.B. Epigenetic factors influencing resistance to nuclear reprogramming. Trends Genet. 2011;27:516–525. doi: 10.1016/j.tig.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polejaeva I.A. Campbell K.H. New advances in somatic cell nuclear transfer: application in transgenesis. Theriogenology. 2000;53:117–126. doi: 10.1016/s0093-691x(99)00245-9. [DOI] [PubMed] [Google Scholar]
- Pondaven P. Meijer L. Beach D. Activation of M-phase-specific histone H1 kinase by modification of the phosphorylation of its p34cdc2 and cyclin components. Genes Dev. 1990;4:9–17. doi: 10.1101/gad.4.1.9. [DOI] [PubMed] [Google Scholar]
- Procházka R. Motlík J. Fulka J. Activity of maturation promoting factor in pig oocytes after microinjection and serial transfer of maturing cytoplasm. Cell Differ. Dev. 1989;27:175–181. doi: 10.1016/0922-3371(89)90698-9. [DOI] [PubMed] [Google Scholar]
- Schnieke A.E. Kind A.J. Ritchie W.A. Mycock K. Scott A.R. Ritchie M. Wilmut I. Colman A. Campbell K.H. Human factor IX transgenic sheep produced by transfer of nuclei from transfected fetal fibroblasts. Science. 1997;278:2130–2133. doi: 10.1126/science.278.5346.2130. [DOI] [PubMed] [Google Scholar]
- Shelton J.N. Embryo manipulation in research and animal production. Aust. J. Biol. Sci. 1988;41:117–132. [PubMed] [Google Scholar]
- Smith L.C. Wilmut I. Hunter R.H.F. Influence of cell cycle stage at nuclear transplantation on the development in vitro of mouse embryos. J. Reprod. Fertil. 1988;84:619–624. doi: 10.1530/jrf.0.0840619. [DOI] [PubMed] [Google Scholar]
- Solomon L.M. Noll R.C. Mordkoff D.S. Murphy P. Rolerson M. A brave new beef: The US Food and Drug Administration's review of the safety of cloned animal products. Gend. Med. 2009;6:402–409. doi: 10.1016/j.genm.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Spemann H. Hafner Publishing Company; New York: 1938. Embryonic Development and Induction; pp. 210–211. [Google Scholar]
- Susko-Parris J.L. Leibfreid-Rutledge M.L. Northey D.L. Schutzkus V. First N.L. Inhibition of protein kinases after an induced calcium transient causes transition of bovine oocytes to embryonic cycles without meiotic completion. Dev. Biol. 1994;166:729–739. doi: 10.1006/dbio.1994.1351. [DOI] [PubMed] [Google Scholar]
- Tachibana M. Amato P. Sparman M. Gutierrez N.M. Tippner-Hedges R. Ma H. Kang E. Fulati A. Lee H.S. Sritanaudomchai H. Masterson K. Larson J. Eaton D. Sadler-Fredd K. Battaglia D. Lee D. Wu D. Jensen J. Patton P. Gokhale S. Stouffer R.L. Wolf D. Mitalipov S. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153:1228–1238. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K. Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K. Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;30(131):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Thomson J.A. Itskovitz-Eldor J. Shapiro S.S. Waknitz M.A. Swiergiel J.J. Marshall V.S. Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Wakayama T. Yanagimachi R. Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nat. Biotechnol. 1998;16:639–641. doi: 10.1038/nbt0798-639. [DOI] [PubMed] [Google Scholar]
- Willadsen S.M. A method for culture of micromanipulated sheep embryos and its use to produce monozygotic twins. Nature. 1979;25(277):298–300. doi: 10.1038/277298a0. [DOI] [PubMed] [Google Scholar]
- Willadsen S.M. Godke R.A. A simple procedure for the production of identical sheep twins. Vet. Rec. 1984;114:240–243. doi: 10.1136/vr.114.10.240. [DOI] [PubMed] [Google Scholar]
- Wilmut I. Campbell K.H.S. Embryo multiplication in livestock: present procedures and the potential for improvement. In: Gandolfi F., editor; Lauria A., editor. Embryonic Development and Manipulation in Animal Development. Trends in Research and Applications. Portland Press; London: 1992. pp. 135–145. [Google Scholar]
- Wilmut I. Schnieke A.E. McWhir J. Kind A.J. Campbell K.H.S. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]