Abstract
Deficits in working memory (WM) are a common consequence of pediatric traumatic brain injury (TBI) and are believed to contribute to difficulties in a range of cognitive and academic domains. Reduced integrity of the corpus callosum (CC) after TBI may disrupt the connectivity between bilateral frontoparietal neural networks underlying WM. In the present investigation, diffusion tensor imaging (DTI) tractography of eight callosal subregions (CC1–CC8) was examined in relation to measures of verbal and visuospatial WM in 74 children sustaining TBI and 49 typically developing comparison children. Relative to the comparison group, children with TBI demonstrated poorer visuospatial WM, but comparable verbal WM. Microstructure of the CC was significantly compromised in brain-injured children, with lower fractional anisotropy (FA) and higher axial and radial diffusivity metrics in all callosal subregions. In both groups of children, lower FA and/or higher radial diffusivity in callosal subregions connecting anterior and posterior parietal cortical regions predicted poorer verbal WM, whereas higher radial diffusivity in callosal subregions connecting anterior and posterior parietal, as well as temporal, cortical regions predicted poorer visuospatial WM. DTI metrics, especially radial diffusivity, in predictive callosal subregions accounted for significant variance in WM over and above remaining callosal subregions. Reduced microstructural integrity of the CC, particularly in subregions connecting parietal and temporal cortices, may act as a neuropathological mechanism contributing to long-term WM deficits. The future clinical use of neuroanatomical biomarkers may allow for the early identification of children at highest risk for WM deficits and earlier provision of interventions for these children.
Key words: axonal injury, cognitive function, diffusion tensor imaging, pediatric brain injury
Introduction
Traumatic brain injury (TBI) during childhood can result in long-term problems with academic achievement, as well as reduced cognitive, adaptive, and psychosocial functioning.1–4 Deficits in working memory (WM) are a common consequence of pediatric TBI5–10 and are believed to contribute to difficulties in a range of cognitive and academic domains, including discourse and reading comprehension, mathematics, complex learning, and reasoning.11–13
Neural networks in WM
WM is the capacity-limited ability to monitor, process, and maintain task-relevant information online to respond to immediate environmental demands.14–16 Functional neuroimaging studies in healthy individuals have identified bilateral frontoparietal cortical networks underlying both verbal and visuospatial WM. Frontal regions involved in WM include the rostral, ventrolateral, and dorsolateral prefrontal cortices, as well as the premotor cortex. In the parietal lobe, both inferior and superior parietal cortices are involved, especially in posterior parietal regions.17–20 Although the majority of functional neuroimaging research on WM has been conducted with adults, developmental studies suggest that children recruit similar cortical networks.21–26 With regard to white-matter pathways underlying WM, the few studies conducted in healthy individuals have identified the involvement of both intra- and interhemispheric tracts. In addition to the superior longitudinal fasciculi (the major white-matter pathways connecting frontal and parietal cortices),27–30 investigations of typically developing (TD) children have identified significant correlations between visual WM performance and development of white matter in left frontoparietal regions, as well as in the anterior corpus callosum (CC).31,32
Application of diffusion tensor imaging to TBI
The primary pathophysiological changes after TBI involve diffuse axonal degeneration and disconnection,33,34 often termed traumatic axonal injury (TAI). TAI is a progressive phenomenon evolving from focal axonal alteration to eventual axonal disconnection over several months after injury and appears to be the core pathology producing diffuse brain damage and associated cognitive and behavioral deficits after TBI.33,35
Diffusion tensor imaging (DTI) and tractography are increasingly being utilized to quantify the effects of TAI in vivo through examination of the orientation and magnitude of water diffusion in the brain.36,37 Metrics provided by DTI include fractional anisotropy (FA) and mean diffusivity, which is separable into axial and radial diffusivities. FA ranges from 0 to 1, where 0 represents maximal isotropic diffusion and 1 represents maximal anisotropic diffusion.38 FA is believed to index the integrity and degree of fiber organization39 and tends to be reduced after TBI, suggestive of demyelination and axonal degeneration.40 Axial diffusivity quantifies diffusion parallel to the principal axis of fibers, and radial diffusivity quantifies diffusion perpendicular to the principal axis. Higher diffusivities suggest less well-defined tissue organization, reduced myelination, and/or axonal pathology.38,41–43 Although the correlates of changes in different DTI metrics remain under investigation, recent studies suggest that FA and radial diffusivity, but not axial diffusivity, are significant predictors of post-traumatic changes in cognitive outcomes.44,45
WM and the CC after pediatric TBI
The CC, the largest commissural white-matter bundle in the human brain, is the main route for interhemispheric transfer of information and is implicated in a large number of cognitive processes.46–50 The CC is particularly vulnerable to injury in TBI.46,51,52 DTI studies have shown lower FA and higher diffusivity metrics in all callosal subregions, relative to TD comparison groups, after TBI in both children and adults at subacute and chronic stages of recovery.44,45,53–56 Given the proposed involvement of the CC in the bilateral frontoparietal neural networks underlying WM,31,32 and its particular vulnerability to injury, reduced microstructural integrity of the CC may act as a neuropathological mechanism, possibly contributing to long-term WM deficits after pediatric TBI.
The present study
The aim of the present investigation was to examine the relation between callosal microstructure and WM in children after TBI, relative to TD comparison children. DTI tractography of eight callosal subregions (CC1–CC8) was examined in relation to measures of verbal and visuospatial WM. We proposed the following hypotheses:
Children with TBI will demonstrate poorer verbal and visuospatial WM performance, relative to TD comparison children.
Children with TBI will have lower FA and higher axial and radial diffusivities in all callosal subregions, relative to TD comparison children.
Based on the evidence for the involvement of bilateral frontoparietal cortical networks in WM, lower FA and higher radial (but not axial) diffusivity in callosal subregion fibers connecting prefrontal (CC1), anterior frontal (CC2), anterior parietal (CC5), and posterior parietal (CC6) cortical regions will predict poorer verbal and visuospatial WM performance in children with TBI.
Methods
Participants
Participants included 74 children who sustained TBI and a TD comparison group composed of 23 children with orthopedic injuries and 26 healthy children. All children were 6–18 years of age at the time of participation. Children in the TBI group and those with orthopedic injuries were recruited from the Level 1 Pediatric Trauma Center at Children's Memorial Hermann Hospital in Houston, Texas. The TBI group was drawn from two prospective cohorts enrolled from 1994 to 1998 or from 2004 to 2007. Inclusionary criteria were (1) hospitalization for nonpenetrating injuries from vehicular accidents, falls, sports, or impact with blunt object and (2) English speaking or bilingual. Exclusionary criteria were (1) presumed inflicted neurotrauma from child abuse, (2) history of previous or subsequent TBI, (3) illegal immigrants or those residing outside the catchment area because of difficulty maintaining enrollment, and (4) history of major developmental or psychiatric disorder. The children with TBI sustained injuries between 0 and 15 years of age (mean [M], 9.7; standard deviation [SD], 3.7 years) and were evaluated from 5 months to 12 years postinjury (M, 30.0; SD, 38.1 months). Severity of TBI was classified using lowest postresuscitation Glasgow Coma Scale (GCS)57 scores and acute neuroimaging findings. Children with complicated mild TBI had GCS 13–15 with neuroimaging evidence of parenchymal injury (n=5). Children with moderate (n=15) and severe TBI (sTBI: n=54) had GCS scores from 9 to 12 and 3 to 8, respectively, with or without positive neuroimaging findings.
Children with orthopedic injuries had Abbreviated Injury Scores ≤4 (skeletal or body) and no history of head or brain injury. Healthy children without injuries were recruited from the community from fliers posted at libraries and from well-child clinics. Both of these groups also met exclusionary criteria 2–4. Comparisons of orthopedically injured and healthy children revealed no statistically significant differences (all p>0.05) in demographic variables, including age at test, gender, ethnicity, or socioeconomic status (SES), as estimated by Hollingshead's 4-Factor Index of Social Status58 or on any dependent variables, including both WM measures and the three DTI metrics at all CC subregions. Because the two comparison groups were demographically similar and comparable in WM performance and callosal microstructure, both samples of children were combined to form one TD comparison group (n=49).
Table 1 provides demographic and injury characteristics for the TBI and TD comparison groups. There were no statistically significant differences between the TBI and comparison groups in age at test (t(121)=1.93; p=0.057) or ethnicity (χ2(3)=7.53; p=0.057) or handedness (χ2(1)=0.30; p=0.583). SES was significantly lower in the TBI group (t(121)=2.16; p=0.033). In addition, the proportion of males to females was significantly greater in the TBI group (χ2(1)=7.31; p=0.007). The predominant mechanisms of injury differed between children with TBI and orthopedic injuries (χ2(6)=23.52; p<0.001), with higher proportions of motor vehicle accidents in the TBI group and higher proportions of sports/play injuries in the children with orthopedic injuries. As expected, Injury Severity Scores were significantly higher in children with TBI, relative to children with orthopedic injuries (t(92.61)=11.14; p<0.001.
Table 1.
Participant Characteristics of TBI and TD Comparison Groups
| Characteristics | TBI (n=74) | TD (n=49) |
|---|---|---|
| Years of age at test (M [SD]) | 12.2 (3.3) | 11.1 (3.0) |
| SES (M [SD])* | 37.4 (13.5) | 42.9 (14.4) |
| Gender (% male)* | 73 | 49 |
| Handedness (% RH dominant) | 86 | 90 |
| Ethnicity (%) | ||
| African American | 7 | 22 |
| Caucasian | 59 | 49 |
| Hispanic | 27 | 18 |
| Other or multiracial | 7 | 11 |
| Mechanism of injury (%)a* | ||
| Auto-pedestrian accident | 28 | 26 |
| Motor vehicle accident | 47 | 9 |
| Fall | 6 | 13 |
| High fall | 12 | 18 |
| Hit by falling object | 0 | 4 |
| Sports/play | 3 | 26 |
| Bicycle accident | 4 | 4 |
| Injury Severity Score (M [SD])a* | 21.5 (10.2) | 7.1 (2.5) |
| Lowest postresuscitation GCS (M [SD]) | ||
| Complicated mild | 13.6 (0.9) | — |
| Moderate | 10.9 (1.2) | — |
| Severe | 3.8 (1.7) | — |
| Days of impaired consciousness (M [SD]) | ||
| Complicated mild | 0 (0) | — |
| Moderate | 0.9 (1.8) | — |
| Severe | 7.6 (9.8) | — |
Mechanism of injury and Injury Severity Score in the typically developing group applies to orthopedically injured children only.
p<0.05.
GCS, Glasgow Coma Scale; M, mean; RH, right hand; SD, standard deviation; SES, socioeconomic status; TBI, traumatic brain injury; TD, typically developing.
In accord with the procedures established by the institutional review board of the University of Texas Health Science Center at Houston (Houston, TX), informed written consent was obtained from the parents or guardians of children who were eligible for the study. Oral assent was obtained for children who were 6 years of age. Children ages 7–11 years provided written assent, and written adolescent consent was obtained for children ages 12–18 years.
Procedure
All children underwent magnetic resonance imaging (MRI) of the brain and were individually administered one verbal and one visuospatial working memory task as part of a larger neuropsychological and academic assessment battery. WM performance was examined an average of 3.9 months (range, 0–7.2) after children's MRI scan.
Measures of WM
Children were administered two experimental WM tasks designed to have analogous task requirements while assessing the verbal and visuospatial modalities separately. Details of the tasks are also previously described in Gorman and colleagues.10
Category listening span dual-task (CLS-DT)
The CLS-DT, adapted from De Beni and colleagues,59–61 was administered as a measure of verbal WM. The CLS-DT is a dual task in which word strings of increasing number are read to the child and the child is required to (1) tap on the table after each string containing an animal name and (2) recall the last word from each string, in the correct order, at the end of each trial. The total number of correct trials (CLS-DT total correct) was analyzed.
Visuospatial span dual-task (VSS-DT)
The VSS-DT, adapted from Cornoldi and colleagues,61,62 was administered as a measure of visuospatial WM. The VSS-DT is composed of strings of contiguously touched positions in a 4×4 matrix of blocks presented in increasing number of strings. The child is required to (1) tap on the table after each string in which contiguously touched blocks form a straight line (horizontal, vertical, or diagonal) and (2) recall the last block touched from each string, in the correct order, at the end of each trial. The total number of correct trials (VSS-DT total correct) was analyzed.
Neuroimaging acquisition, processing, and tractography of the CC
Complete details of image acquisition, processing, and tractography of the CC are previously described in Hasan and colleagues.63 A high signal-to-noise ratio whole-brain DTI protocol at 3.0 T that was kept under 7 min was utilized. Diffusion-weighted data were collected axially (field of view, 240×240 mm; square matrix, 256×256 pixels) using 44 contiguous 3-mm axial sections.63 The diffusion sensitization of b-factor=1,000 sec/mm2 and the encoding scheme used 21 uniformly distributed directions.64 The DTI-derived rotationally invariant metrics examined in the present study included FA, axial diffusivity, and radial diffusivity.
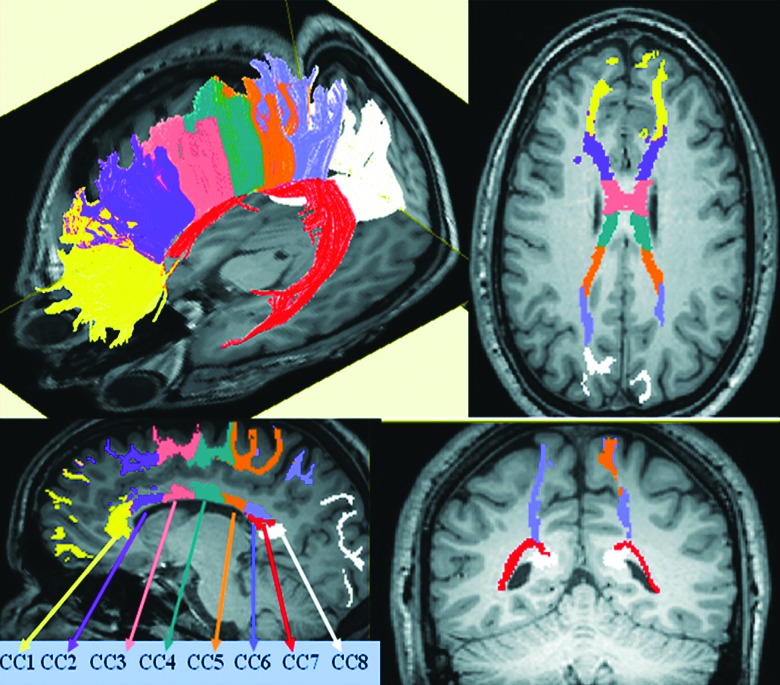
Deterministic compact white-matter fiber tracking was performed using DTI Studio software (Johns Hopkins University, Baltimore, MD; cmrm.med.jhmi.edu),65 based on the fiber assignment by continuous tracking algorithm,66 with a fractional anisotropy threshold of 0.2 for initial seeding and stopping and a principal eigenvector angle stopping threshold of 60 degrees. Commissural fibers traversing the CC were subdivided into eight subregions (CC1–CC8) by a slight modification of Witelson's seven subregions67 and the 10-sector method of Aboitiz and colleagues68 connecting homotopic regions. The rostrum and genu segments in the Witelson method correspond closely to CC1, which connects the left and right prefrontal cortex. Witelson's three mid-body segments and isthmus correspond to CC2 (anterior frontal cortex), CC3 (superior frontal cortex), CC4 (posterior frontal cortex), and CC5 (anterior parietal cortex), respectively, based on the cortical origin/termination of the fibers passing through these selected mid-sagittal areas. The splenium was further subdivided into three subregions based on the DTI evidence demonstrating that, unlike other subregions, the splenium is traversed by fibers that interconnect three different lobes of the brain: the parietal; temporal; and occipital lobes.69–71 Thus, CC6 connects the posterior parietal lobes, CC7 connects the temporal lobes, and CC8 connects the occipital lobes. Figure 1 contains an illustration of the eight callosal subregions tracked in a healthy individual from Hasan and colleagues.63
FIG. 1.
Visual representation of eight callosal subregions (CC1–CC8) tracked in a healthy individual. (Reprinted from Brain Research, Vol 1249, Khader M. Hasan, Arash Kamali, Amal Iftikhar, Larry A. Kramer, Andrew C. Papanicolaou, Jack M. Fletcher, Linda Ewing-Cobbs, Diffusion tensor tractography quantification of the human corpus callosum fiber pathways across the lifespan, pages 91–100, Copyright (2009), with permission from Elsevier.)
Because tracking criteria were held fixed, there were a few subjects for which some subregions did not complete tracking and, as a result, have missing data for these subregions. Of the 74 TBI children, data were missing for CC2–CC4 (n=1), CC5 (n=1), and for CC8 (n=1). Of the 49 comparison children, data were missing for CC6 (n=1), CC7 (n=2), and for CC8 (n=1). Tracking in these few cases may have failed to meet the tracking criteria as a result of the encounter of diffuse axonal injury or lesions in the TBI group. To retain power, children with missing subregion data were maintained in the analyses.
Statistical analyses
To test hypothesis 1, group differences in WM performance were examined using analysis of covariance (ANCOVA), with group as the between-subjects factor and dependent variables CLS-DT total correct and VSS-DT total correct. Covariates included SES and gender, to control for significant group differences, and age, to control for its effect on WM task performance. Given the wide range in age at injury in our TBI sample, we also examined the effect of age at injury on WM performance using Pearson's partial correlation analyses controlling for age at test.
To test hypothesis 2, a mixed-models approach to repeated-measures ANCOVA was used to examine group differences in callosal microstructure. The within-subjects factor was callosal subregion (CC1–CC8); the between-subjects factor was group. Dependent variables were FA, axial diffusivity, and radial diffusivity. Covariates included SES and gender, to control for significant group differences, and age, to control for its effect on the DTI metrics. Follow-up comparisons examined the simple main effect of group for each CC subregion. The Benjamini-Hochberg (B-H) method of correcting for multiple comparisons was used to control the false discovery rate while also protecting against type II error.72
To test hypothesis 3, hierarchically ordered regression analysis was used to examine the relations between each individual callosal subregion DTI metric, group, and WM performance. Models were tested using ordinary least squares and corrected for multiple comparisons using the B-H method. Dependent variables included CLS-DT total correct and VSS-DT total correct. The effects of FA, axial diffusivity, and radial diffusivity were modeled separately for each callosal subregion; 8 regions×3 DTI metrics=24 separate models. In step 1, demographic variables age, SES, and gender were entered into the model as covariates. Step 2 tested the effects of group, callosal subregion DTI metric, and their interaction.
Finally, as a post-hoc analysis of hypothesis 3, additional hierarchical regression analyses were performed to test whether callosal subregions were found to be significant predictors of WM when modeled individually would together account for significant variance in WM over and above remaining subregions. Because TBI results in diffuse TAI, it was of interest whether DTI metrics in significant callosal subregions accounted for relations with WM beyond global white-matter injury to the CC. In step 1, significant demographic variables from the a priori analysis were entered into the model as covariates. In step 2, all callosal subregions found not to be significant individual predictors of WM were together entered into the model. In step 3, callosal subregions found to be significant individual predictors of WM were together entered into the model.
Results
Group differences in WM
The effect of group on WM performance was examined using ANCOVA. Gender was trimmed from all analyses because it had no significant effect across models. Results revealed no significant group difference in verbal WM (F(1,119)=1.37; p=0.244), although the group means were in the expected direction with slightly lower performance in the TBI group (least square M±SD: TBI=9.94±4.73; TD comparison, 10.65±4.04). Verbal WM performance was higher with increasing age (F(1,119)=100.20; p<0.001) and SES (F(1,119)=13.30; p<0.001). On the visuospatial WM task, there was a significant effect of group (F(1,119)=7.00; p=0.009), with poorer performance in the TBI group (least square M±SD: TBI=10.74±4.71; TD comparison=12.47±4.61). Visuospatial WM performance was also higher with increasing age (F(1, 119)=86.04; p<0.001) and SES (F(1, 119)=9.44; p=0.003). Pearson's partial correlation analyses controlling for age at test revealed no significant correlations of age at injury with verbal (r=0.036; p=0.755) or visuospatial (r=0.194; p=0.092) WM.
Group differences in callosal microstructure
Mixed-models repeated-measures ANCOVA examined group differences in FA, axial, and radial diffusivity across the eight CC subregions. The group by age interaction was trimmed from all models because it was not significant for any DTI metric. In addition, both gender and SES were trimmed from all analyses because they had no significant effects across models. For all three DTI metrics, there was a significant interaction of group with CC subregion (FA: F(7,120)=3.16, p=0.004; axial: F(7,120)=3.09, p=0.005; radial: F(7,120)=2.11, p=0.047), indicating that the degree of group differences in DTI metrics varied across the eight CC subregions. Increasing age was associated with lower axial (F(1,120)=16.39; p<0.001) and radial diffusivity (F(1,120)=5.51; p=0.021), but was not significantly related to FA (F(1,120)=1.65; p=0.201). Results of simple main effect comparisons are displayed in Table 2. As hypothesized, children with TBI had significantly lower FA, and significantly higher axial and radial diffusivity after B-H correction, in all callosal subregions, relative to TD comparison children.
Table 2.
Simple Main Effect Comparisons of DTI Metrics across Eight Callosal Subregions by Group
| |
FA |
Axial diffusivity(10–3mm2/sec) |
Radial diffusivity(10–3mm2/sec) |
|||
|---|---|---|---|---|---|---|
| CC subregion | TBI | TD | TBI | TD | TBI | TD |
| CC1 | 0.52 (0.005) | 0.57 (0.006)* | 1.39 (0.007) | 1.34 (0.009)* | 0.56 (0.007) | 0.48 (0.009)* |
| CC2 | 0.50 (0.005) | 0.52 (0.006)* | 1.43 (0.011) | 1.33 (0.014)* | 0.60 (0.008) | 0.53 (0.009)* |
| CC3 | 0.52 (0.006) | 0.55 (0.007)* | 1.49 (0.015) | 1.41 (0.018)* | 0.60 (0.009) | 0.53 (0.012)* |
| CC4 | 0.52 (0.007) | 0.53 (0.008)* | 1.49 (0.013) | 1.41 (0.016)* | 0.61 (0.011) | 0.53 (0.014)* |
| CC5 | 0.48 (0.007) | 0.54 (0.009)* | 1.48 (0.013) | 1.39 (0.017)* | 0.65 (0.014) | 0.53 (0.017)* |
| CC6 | 0.52 (0.007) | 0.57 (0.008)* | 1.43 (0.011) | 1.38 (0.014)* | 0.58 (0.011) | 0.50 (0.013)* |
| CC7 | 0.54 (0.006) | 0.58 (0.008)* | 1.59 (0.012) | 1.51 (0.015)* | 0.61 (0.010) | 0.53 (0.013)* |
| CC8 | 0.60 (0.006) | 0.64 (0.007)* | 1.55 (0.009) | 1.52 (0.011)* | 0.52 (0.009) | 0.46 (0.011)* |
Values are least-square means and standard errors.
Significant following Benjamini-Hochberg correction.
CC, corpus callosum; FA, fractional anisotropy; DTI, diffusion tensor imaging; TBI, traumatic brain injury; TD, typically developing.
Relation of callosal microstructure to verbal WM
Results of hierarchical regression models predicting verbal WM are presented in Table 3. Gender was trimmed from all analyses because it had no significant effect across models. In step 1, age (t(120)=9.96; p<0.001) and SES (t(120)=3.99; p<0.001) were both significantly and positively associated with verbal WM performance (F(2,120)=63.13; p<0.001; R2=0.51). In step 2, the group and group by callosal subregion microstructure interaction terms were nonsignificant across all analyses and were therefore trimmed from the models. Following B-H correction, both lower FA and higher radial diffusivity in the callosal subregion connecting anterior parietal (CC5) cortical regions, and higher radial diffusivity in the callosal subregion connecting posterior parietal (CC6) cortical regions, were significantly predictive of lower verbal WM scores over and above demographic variables. All remaining subregions were nonsignificant. As hypothesized, axial diffusivity was not significantly related to verbal WM performance in any callosal subregion.
Table 3.
Individual Callosal Subregion DTI Metrics Predicting Verbal WM
| |
|
|
FA |
Radial diffusivity |
||
|---|---|---|---|---|---|---|
| CC subregion | Cortical termination | df | t | ΔR2 | t | ΔR2 |
| CC1 | Prefrontal | 122 | 1.41 | 0.008 | –1.01 | 0.004 |
| CC2 | Anterior frontal | 121 | 1.67 | 0.013 | –1.44 | 0.010 |
| CC3 | Superior frontal | 121 | 2.02 | 0.018 | –1.81 | 0.015 |
| CC4 | Posterior frontal | 121 | 2.38 | 0.024 | –1.97 | 0.017 |
| CC5 | Anterior parietal | 121 | 3.32 | 0.041* | –3.34 | 0.042* |
| CC6 | Posterior parietal | 121 | 2.45 | 0.024 | –2.80 | 0.031* |
| CC7 | Temporal | 120 | 2.32 | 0.022 | –2.35 | 0.022 |
| CC8 | Occipital | 121 | 1.80 | 0.015 | –1.87 | 0.016 |
ΔR2 is change in model R2 when the individual CC subregion FA or radial diffusivity was added to step 1 of the model containing age and SES. Degrees of freedom vary as a result of a few missing data points.
Significant following Benjamini-Hochberg correction.
CC, corpus callosum; DTI, diffusion tensor imaging; FA, fractional anisotropy.
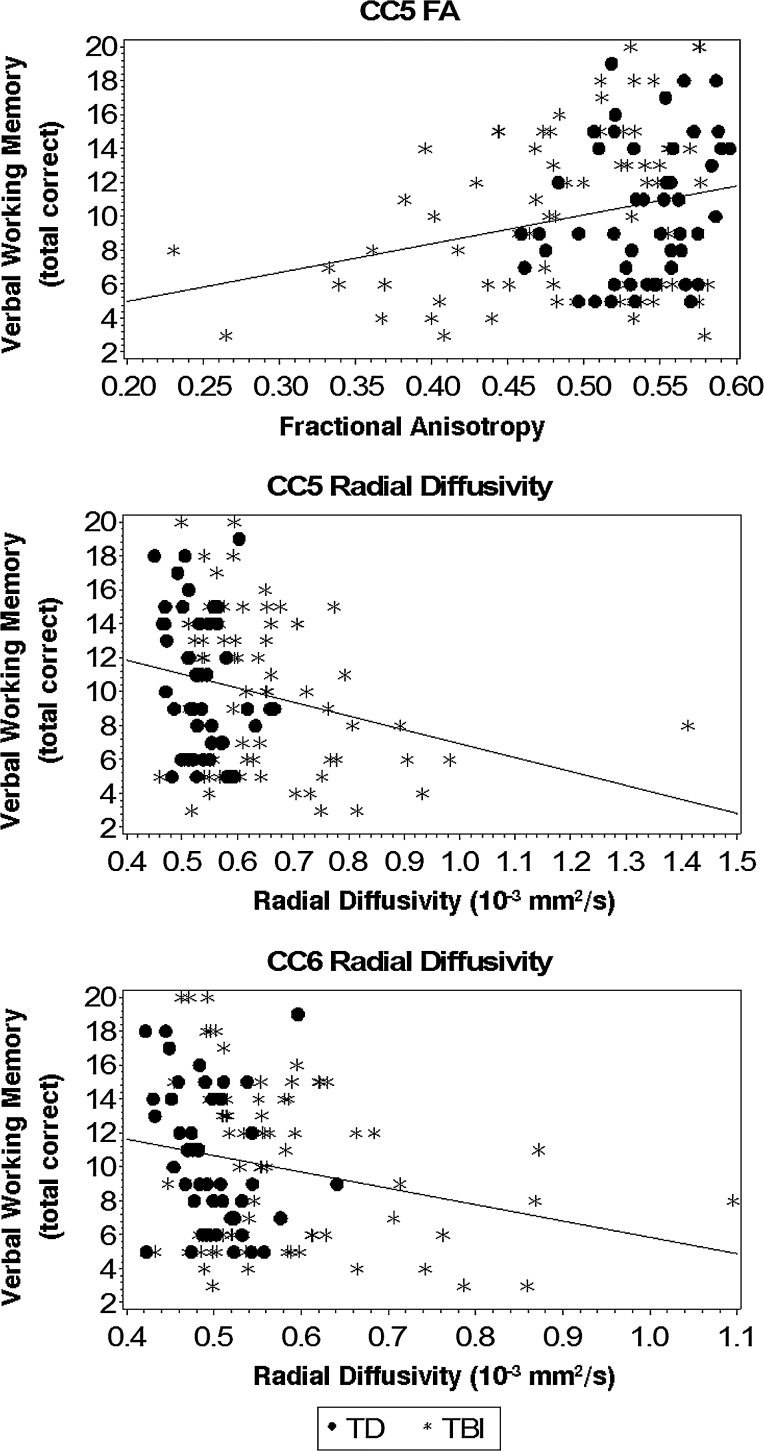
To test whether FA and radial diffusivity from callosal subregions connecting anterior parietal (CC5) and/or posterior parietal (CC6) cortical regions together accounted for significant variance in verbal WM over and above remaining callosal subregions, post-hoc hierarchical regression analyses were performed. As expected, neither FA (ΔR2=0.032; p=0.371) nor radial diffusivity (ΔR2=0.030; p=0.310) in callosal subregions that were not individually predictive of verbal WM in a priori analyses accounted for significant variance over and above demographic variables (R2=0.513; p<0.001) when entered together in step 2 of the models. In step 3, the addition of FA from the callosal subregion connecting anterior parietal (CC5) cortical regions to the model accounted for a statistically significant increase of 2.1% variance over and above remaining callosal subregions (p=0.041). The addition of radial diffusivity from callosal subregions connecting anterior parietal (CC5) and posterior parietal (CC6) cortical regions in step 3 accounted for a statistically significant increase of 2.9% variance in verbal WM over and above remaining callosal subregions (p=0.030). See Figure 2 for plots of verbal WM with FA and/or radial diffusivity from callosal subregions connecting anterior parietal (CC5) and posterior parietal (CC6) cortical regions.
FIG. 2.
Verbal working memory and callosal subregions connecting anterior parietal (CC5) and posterior parietal (CC6) cortical regions.
Relation of callosal microstructure to visuospatial WM
Results of hierarchical regression models predicting visuospatial WM are presented in Table 4. Gender was trimmed from all analyses because it had no significant effect across models. In step 1, age (t(120)=8.71; p<0.001) and SES (t(120)=3.65; p<0.001) were both significantly and positively associated with visuospatial WM performance (F(2,120)=49.00; p<0.001; R2=0.45). In step 2, the group and group by callosal subregion microstructure interaction terms were nonsignificant across all analyses and were therefore trimmed from the models. Following B-H correction, higher radial diffusivity in callosal subregions connecting anterior parietal (CC5), posterior parietal (CC6), and temporal (CC7) cortical regions were significantly predictive of lower visuospatial WM scores over and above demographic variables. Associations of lower FA in callosal subregions connecting anterior parietal (CC5) and posterior parietal (CC6) cortical regions with poorer visuospatial WM were notable trends (p=0.009 and 0.013, respectively), but failed to reach statistical significance following B-H correction. As hypothesized, axial diffusivity was not significantly related to visuospatial WM performance in any callosal subregion.
Table 4.
Individual Callosal Subregion DTI Metrics Predicting Visuospatial WM
| |
|
|
FA |
Radial diffusivity |
||
|---|---|---|---|---|---|---|
| CCsubregion | Corticaltermination | df | t | ΔR2 | t | ΔR2 |
| CC1 | Prefrontal | 122 | 2.12 | 0.020 | –2.15 | 0.020 |
| CC2 | Anterior frontal | 121 | 1.73 | 0.017 | –1.86 | 0.019 |
| CC3 | Superior frontal | 121 | 1.52 | 0.014 | –1.70 | 0.017 |
| CC4 | Posterior frontal | 121 | 1.67 | 0.016 | –1.37 | 0.012 |
| CC5 | Anterior parietal | 121 | 2.67 | 0.031 | –2.91 | 0.036* |
| CC6 | Posterior parietal | 121 | 2.51 | 0.026 | –2.61 | 0.028* |
| CC7 | Temporal | 120 | 1.80 | 0.015 | –2.76 | 0.034* |
| CC8 | Occipital | 121 | 1.85 | 0.018 | –1.95 | 0.019 |
ΔR2 is change in model R2 when the individual CC subregion FA or radial diffusivity was added to step 1 of the model containing age and SES. Degrees of freedom vary as a result of a few missing data points.
Significant following Benjamini-Hochberg correction.
CC, corpus callosum; DTI, diffusion tensor imaging; FA, fractional anisotropy.
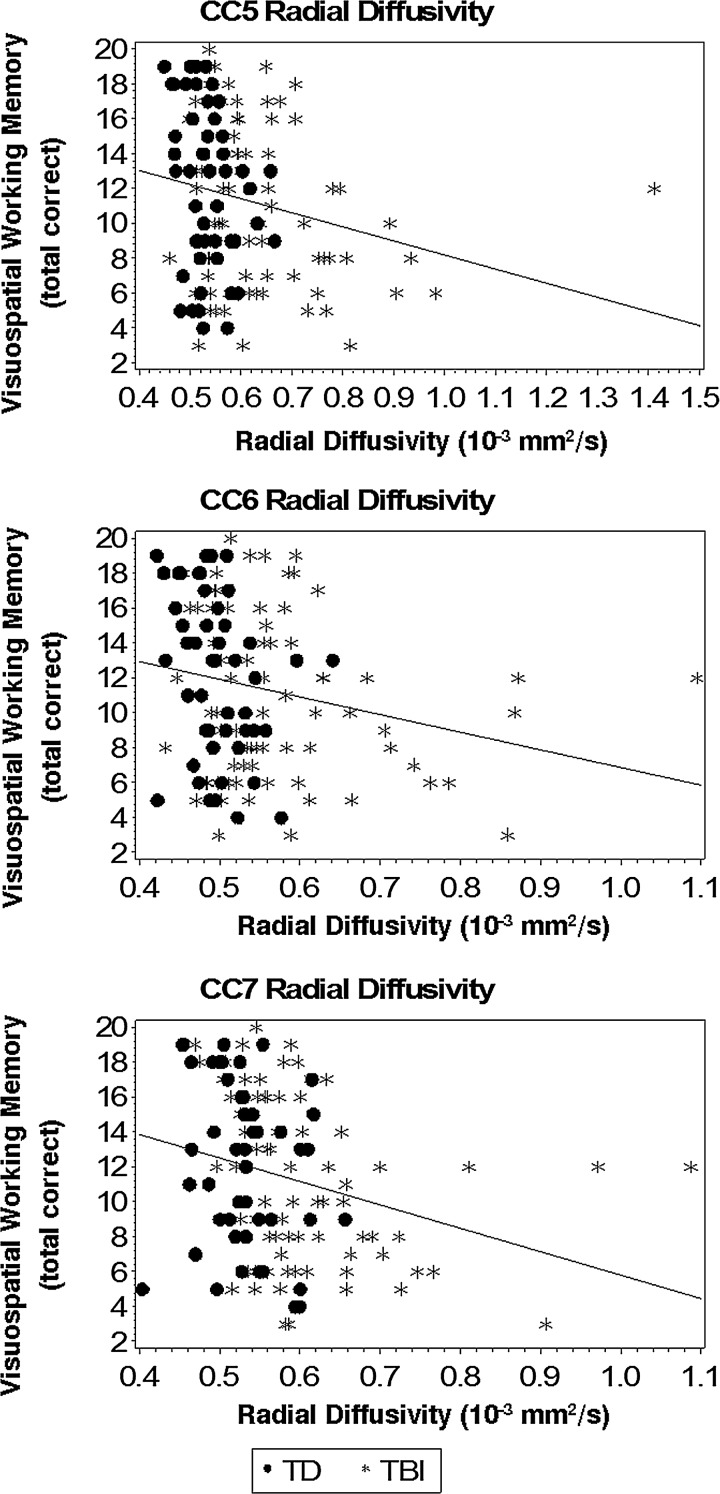
To test whether radial diffusivity from callosal subregions connecting anterior parietal (CC5), posterior parietal (CC6), and temporal (CC7) cortical regions together accounted for significant variance in visuospatial WM over and above remaining callosal subregions, post-hoc hierarchical regression analyses were performed. As expected, radial diffusivity (ΔR2=0.029; p=0.287) in callosal subregions that were not individually predictive of visuospatial WM in a priori analyses did not account for significant variance over and above demographic variables (R2=0.479; p<0.001) when entered together in step 2 of the model. In step 3, the addition of radial diffusivity from callosal subregions connecting anterior parietal (CC5), posterior parietal (CC6), and temporal (CC7) cortical regions accounted for a statistically significant increase of 4.2% variance in visuospatial WM over and above remaining callosal subregions (p=0.029). See Figure 3 for plots of visuospatial WM with radial diffusivity from callosal subregions connecting anterior parietal (CC5), posterior parietal (CC6), and temporal (CC7) cortical regions.
FIG. 3.
Visuospatial working memory and callosal subregions connecting anterior parietal (CC5), posterior parietal (CC6), and temporal (CC7) cortical regions.
Discussion
The present study investigated the relation between callosal microstructure and WM in children after TBI, relative to TD comparison children. Children sustaining TBI demonstrated poorer visuospatial WM, but comparable verbal WM. As expected, the microstructure of the CC was significantly compromised in brain-injured children, with results revealing lower FA and higher axial and radial diffusivity metrics in all callosal subregions. DTI metrics indexing microstructural organization and integrity of particular callosal subregions were associated with WM performance in both groups of children. Lower FA and higher radial diffusivity in callosal subregions connecting anterior and/or posterior parietal cortical regions predicted poorer verbal WM, with both FA and radial diffusivity in these subregions accounting for significant variance over and above remaining callosal subregions. Higher radial diffusivity in callosal subregions connecting anterior parietal, posterior parietal, and temporal cortical regions predicted poorer visuospatial WM and accounted for significant variance over and above remaining subregions. The results provide evidence that reduced microstructural integrity of the CC, particularly in subregions connecting parietal and temporal cortices, may act as a neuropathological mechanism contributing to long-term WM deficits after pediatric TBI.
Our finding of compromised white-matter integrity in all subregions of the CC is consistent with previous findings of reduced FA and increased diffusivity metrics in the CC of both children44,45,53 and adults54–56 after brain injury. Reduced integrity of the CC after TBI is believed to result from pathophysiological processes, including demyelination, expansion of extracellular space, possibly attributed to neuronal or glial loss, buildup of cellular debris from breakdown of axonal structure, and disordered microtubule arrangement.73–76 Our results are consistent with the building evidence suggesting that DTI of the CC may serve as an effective biomarker for the degree of TAI and potential cognitive dysfunction after traumatic injury to the brain.44,51,53,77–79
In addition to being a surrogate marker of general injury severity and outcome after TBI, increasing evidence suggests that reduced microstructural integrity of particular callosal subregions differentially predicts particular cognitive deficits. Reductions in processing speed have been associated with lower FA in the body and splenium of the CC after pediatric TBI.53,80 Impaired fine motor speed and bimanual coordination were associated with lower FA in splenial fibers, whereas impaired cognitive control of motor functions was associated with lower FA in callosal fibers connecting prefrontal, anterior parietal, and posterior parietal cortices in adults with TBI.81,82 Declarative memory impairment has been associated with posterior, but not anterior, callosal FA reductions in adult TBI.83 With regard to WM, in a case series of two pairs of twins discordant for sTBI sustained during childhood, poorer verbal WM was associated with lower mid-saggital-area FA in the rostral mid-body, whereas visuospatial WM was unrelated to callosal FA in any subregion.77 Poorer verbal WM was also associated with lower mid-sagittal-area FA in the splenium in a group of children with TBI.44 In adults with sTBI, whole-brain FA analysis revealed positive correlations between anterior and posterior callosal subregions with visual WM performance and functional activation patterns.83,84
The present study identified significant associations between verbal WM and integrity of callosal subregions connecting anterior and posterior parietal cortical regions, and between visuospatial WM and integrity of callosal subregions connecting anterior parietal, posterior parietal, and temporal cortical regions, across brain-injured and TD comparison children. In addition to using a larger sample than in previous pediatric studies, our results make a significant contribution because of our use of DTI tractography, rather than mid-sagittal-area DTI, allowing for stronger inferences regarding cortical terminations of callosal fibers and their effect on WM neural networks. Of particular importance, this approach enabled a more detailed examination of the splenium in relation to WM, revealing that the integrity of fibers traversing the splenium and terminating in the parietal and temporal cortices were predictive of WM, whereas splenial fibers connecting the occipital cortices were not.
Contrary to our hypothesis that integrity of callosal subregion fibers connecting both frontal and parietal cortical regions would predict poorer verbal and visuospatial WM, we found support only for associations with parietal CC subregions as well as the additional finding of an association between callosal fibers connecting temporal regions with visuospatial WM. This pattern of involvement of parietal, but not frontal, callosal subregions appears to be consistent with recent findings from our group examining WM storage capacity and central executive components of WM in children sustaining TBI.10 Based on the finding that increasing the central executive load on verbal and visuospatial WM tasks did not differentially affect performance of children with TBI, relative to TD comparison children, results suggested that decline in WM after pediatric TBI may result primarily from a general reduction in WM storage capacity, rather than a deficit in the central executive. Similar results have been reported in adult TBI.85 WM storage capacity is known to be associated with parietal cortex, whereas higher order executive control processes are associated with the prefrontal cortex,18,19 possibly explaining our pattern of results. The somewhat surprising association of callosal fibers connecting temporal regions with visuospatial WM is supported by evidence suggesting involvement of the (especially medial) temporal lobe in visual WM.86–88 Although the evidence in this area is building, additional research is needed to further elucidate the involvement of particular callosal subregions in neural networks supporting various neuropsychological functions to allow for prediction of deficits based on regional changes in callosal microstructure.
As hypothesized, both FA and radial diffusivity in particular callosal subregions predicted WM performance, whereas axial diffusivity was not significantly predictive. This pattern of relative sensitivity of DTI metrics in prediction of neuropsychological outcome after TBI is a somewhat consistent trend in the TBI literature,44,45 although it remains poorly understood.89 We found radial diffusivity to be the strongest predictor of WM performance, with radial diffusivity in particular callosal subregions accounting for significant variance over and above remaining callosal subregions for both verbal and visuospatial WM. These results suggest that radial diffusivity may be the most sensitive DTI biomarker for predicting poor neuropsychological outcome after TBI. The significance of radial diffusivity has been echoed in longitudinal studies in which increased radial, but not axial, diffusivity has accounted for reduced callosal FA over time since injury.82,90 Given evidence from animal studies suggesting that changes in axial diffusivity are associated with axonal pathology and changes in radial diffusivity are associated with pathology of myelin,42,91 the predictive value of radial diffusivity after TBI may point to changes in myelination as a primary mechanism leading to long-term neuropsychological impairment.82 Additional research is needed to determine the correlates of changes in DTI metrics over time after TBI and their relative value as biomarkers for long-term neuropsychological impairment.
WM performance in children with TBI did not appear to be related to age at injury. Despite some evidence suggesting that cognitive outcomes, including WM,92,93 may be worse in children acquiring brain injuries at younger ages,94,95 the relation between age at injury and cognitive outcome is complex. It is unclear why age at injury effects are observed in some studies of pediatric TBI but not others. Characteristics of the present study that may have precluded the detection of an age at injury effect might include an underrepresentation of children injured at very young ages and the wide range in time interval from injury to cognitive assessment. Future studies might use stronger statistical approaches, such as growth modeling, to better investigate whether cognitive functions within rapid stages of development at injury may be particularly vulnerable to disruption.
Some limitations of the present study include the cross-sectional design and the wide range of time interval from injury to study participation. Given the emerging evidence for changes in callosal microstructure over time since injury,80,89 as well as evidence that the level of impairment in WM after pediatric TBI also changes over time,6 future research should continue to characterize longitudinal changes in the CC and their relation to neuropsychological outcome after pediatric TBI. In addition, because fixed criteria were used for tractography of the CC, it is possible that missing data for callosal fibers that did not meet requirements for continuous tracking may be from the most severely injured children as a result of excessive lesions. Because data from the most severely injured callosal subregions may have been excluded, the results may not accurately characterize callosal microstructural relations with WM abilities in those children most likely to demonstrate long-term deficits. Future studies should employ systematic lesion analyses in relation to cognitive outcome to address this issue. In addition, diffusion imaging with higher spatial resolutions, multiple b-factors, or more diffusion orientations may also improve signal detection of aberrant or damaged white-matter pathways.
The present study provides evidence for the role of reduced callosal integrity as a neuropathological mechanism contributing to long-term deficits in WM after pediatric TBI. The findings highlight the important role of callosal white matter in neural networks underlying WM in both brain-injured and TD children. DTI of the CC may serve as a neuroanatomical biomarker for predicting WM deficits in children sustaining TBI. Given the particular vulnerability of the CC to damage in TBI, in combination with its primary role in the interhemispheric transfer of information, reduced integrity of the CC is a likely candidate for contributing to other cognitive difficulties after pediatric TBI as well. The future clinical use of neuroanatomical biomarkers may allow for the early identification of children at highest risk for cognitive difficulties and earlier provision of interventions for these children.
Acknowledgments
This work was funded by the National Institutes of Health (NIH-R01 NS046308 [awarded to L.E.C.] and NIH-R01 NS052505) and the Dunn Research Fund (to K.M.H.). The purchase of the 3.0 T MRI Clinical Scanner was partially funded by the NIH (grant no.: S10-RR19186; to Dr. Ponnada A. Narayana). The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting institutes. The authors thank Vipul Kumar Patel for helping in data acquisition.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ewing-Cobbs L. Prasad M.R. Kramer L. Cox C.S., Jr. Baumgartner J. Fletcher S. Mendez D. Barnes M. Zhang X. Swank P. Late intellectual and academic outcomes following traumatic brain injury sustained during early childhood. J. Neurosurg. 2006;105:287–296. doi: 10.3171/ped.2006.105.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ewing-Cobbs L. Barnes M. Fletcher J.M. Levin H.S. Swank P.R. Song J. Modeling of longitudinal academic achievement scores after pediatric traumatic brain injury. Dev. Neuropsychol. 2004;25:107–133. doi: 10.1080/87565641.2004.9651924. [DOI] [PubMed] [Google Scholar]
- 3.Fay T.B. Yeates K.O. Wade S.L. Drotar D. Stancin T. Taylor H.G. Predicting longitudinal patterns of functional deficits in children with traumatic brain injury. Neuropsychology. 2009;23:271–282. doi: 10.1037/a0014936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babikian T. Asarnow R. Neurocognitive outcomes and recovery after pediatric TBI: meta-analytic review of the literature. Neuropsychology. 2009;23:283–296. doi: 10.1037/a0015268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levin H.S. Hanten G. Chang C.C. Zhang L. Schachar R. Ewing-Cobbs L. Max J.E. Working memory after traumatic brain injury in children. Ann. Neurol. 2002;52:82–88. doi: 10.1002/ana.10252. [DOI] [PubMed] [Google Scholar]
- 6.Levin H.S. Hanten G. Zhang L. Swank P.R. Ewing-Cobbs L. Dennis M. Barnes M.A. Max J. Schachar R. Chapman S.B. Hunter J.V. Changes in working memory after traumatic brain injury in children. Neuropsychology. 2004;18:240–247. doi: 10.1037/0894-4105.18.2.240. [DOI] [PubMed] [Google Scholar]
- 7.Ewing-Cobbs L. Prasad M.R. Landry S.H. Kramer L. DeLeon R. Executive functions following traumatic brain injury in young children: a preliminary analysis. Dev. Neuropsychol. 2004;26:487–512. doi: 10.1207/s15326942dn2601_7. [DOI] [PubMed] [Google Scholar]
- 8.Sesma H.W. Slomine B.S. Ding R. McCarthy M.L. Executive functioning in the first year after pediatric traumatic brain injury. Pediatrics. 2008;121:e1686–e1695. doi: 10.1542/peds.2007-2461. [DOI] [PubMed] [Google Scholar]
- 9.Conklin H.M. Salorio C.F. Slomine B.S. Working memory performance following paediatric traumatic brain injury. Brain Inj. 2008;22:847–857. doi: 10.1080/02699050802403565. [DOI] [PubMed] [Google Scholar]
- 10.Gorman S. Barnes M.A. Swank P.R. Prasad M. Ewing-Cobbs L. The effects of pediatric traumatic brain injury on verbal and visual-spatial working memory. J. Int. Neuropsychol. Soc. 2011;18:29–38. doi: 10.1017/S1355617711001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daneman M. Carpenter P.A. Individual differences in working memory and reading. J. Verbal Learning Verbal Behav. 1980;19:450–466. [Google Scholar]
- 12.Engle R.W. Working memory capacity as executive attention. Curr. Dir. Psychol. Sci. 2002;11:19–23. [Google Scholar]
- 13.Gathercole S.E. Pickering S.J. Ambridge B. Wearing H. The structure of working memory from 4 to 15 years of age. Dev. Psychol. 2004;40:177–190. doi: 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- 14.Miyake A., editor; Shah P., editor. Models of Working Memory: Mechanisms of Active Maintenance and Control. Cambridge University Press; New York: 1999. [Google Scholar]
- 15.Baddeley A.D. Working memory: looking back and looking forward. Nat. Rev. Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- 16.Baddeley A.D. Logie R.H. Working memory: the multiple-component model. In: Miyake A., editor; Shah P., editor. Models of Working Memory: Mechanisms of Active Maintenance and Control. Cambridge University Press; New York: 1999. pp. 28–61. [Google Scholar]
- 17.Klingberg T. Development of a superior frontal-intraparietal network for visuo-spatial working memory. Neuropsychologia. 2006;44:2171–2177. doi: 10.1016/j.neuropsychologia.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Curtis C.E. D'Esposito M. Functional neuroimaging of working memory. In: Cabeza R., editor; Kingstone A., editor. Handbook of Functional Neuroimaging of Cognition. MIT Press; Cambridge, MA: 2006. pp. 269–306. [Google Scholar]
- 19.Wager T.D. Smith E.E. Neuroimaging studies of working memory: a meta-analysis. Cogn. Affect. Behav. Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 20.Owen A.M. McMillan K.M. Laird A.R. Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Hare E.D. Lu L.H. Houston S.M. Bookheimer S.Y. Sowell E.R. Neurodevelopmental changes in verbal working memory load-dependency: an fMRI investigation. Neuroimage. 2008;42:1678–1685. doi: 10.1016/j.neuroimage.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klingberg T. Forssberg H. Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J. Cogn. Neurosci. 2002;14:1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- 23.Kwon H. Reiss A.L. Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson C.A. Monk C.S. Lin J. Carver L.J. Thomas K.M. Truwit C.L. Functional neuroanatomy of spatial working memory in children. Dev. Psychol. 2000;36:109–116. doi: 10.1037//0012-1649.36.1.109. [DOI] [PubMed] [Google Scholar]
- 25.Thomas K.M. King S.W. Franzen P.L. Welsh T.F. Berkowitz A.L. Noll D.C. Birmaher V. Casey B.J. A developmental functional MRI study of spatial working memory. Neuroimage. 1999;10:327–338. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- 26.Casey B.J. Cohen J.D. Jezzard P. Turner R. Noll D.C. Trainor R.J. Giedd J. Kaysen D. Hertz-Pannier L. Rapoport J.L. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage. 1995;2:221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- 27.Burzynska A.Z. Nagel I.E. Preuschhof C. Li S.C. Lindenberger U. Backman L. Heekeren H.R. Microstructure of frontoparietal connections predicts cortical responsivity and working memory performance. Cereb. Cortex. 2011;21:2261–2271. doi: 10.1093/cercor/bhq293. [DOI] [PubMed] [Google Scholar]
- 28.Karlsgodt K.H. van Erp T.G. Poldrack R.A. Bearden C.E. Nuechterlein K.H. Cannon T.D. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol. Psychiatry. 2008;63:512–518. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 29.Charlton R.A. Barrick T.R. Lawes I.N. Markus H.S. Morris R.G. White matter pathways associated with working memory in normal aging. Cortex. 2010;46:474–489. doi: 10.1016/j.cortex.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Charlton R.A. Landau S. Schiavone F. Barrick T.R. Clark C.A. Markus H.S. Morris R.G. A structural equation modeling investigation of age-related variance in executive function and DTI measured white matter damage. Neurobiol. Aging. 2008;29:1547–1555. doi: 10.1016/j.neurobiolaging.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Nagy Z. Westerberg H. Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J. Cogn. Neurosci. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- 32.Olesen P.J. Nagy Z. Westerberg H. Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Brain Res. Cogn. Brain Res. 2003;18:48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Buki A. Povlishock J.T. All roads lead to disconnection?—traumatic axonal injury revisited. Acta Neurochir. 2006;148:181–194. doi: 10.1007/s00701-005-0674-4. [DOI] [PubMed] [Google Scholar]
- 34.Gennarelli T.A. Graham D.I. Neuropathology of the head injuries. Semin. Clin. Neuropsychiatry. 1998;3:160–175. [PubMed] [Google Scholar]
- 35.Kaplan G.B. Vasterling J.J. Vedak P.C. Brain-derived neurotrophic factor in traumatic brain injury, post-traumatic stress disorder, and their comorbid conditions: role in pathogenesis and treatment. Behav. Pharmacol. 2010;21:427–437. doi: 10.1097/FBP.0b013e32833d8bc9. [DOI] [PubMed] [Google Scholar]
- 36.Basser P.J. Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. Series B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 37.Eluvathingal T.J. Hasan K.M. Kramer L. Fletcher J.M. Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb. Cortex. 2007;17:2760–2768. doi: 10.1093/cercor/bhm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 39.Alexander A.L. Lee J.E. Lazar M. Field A.S. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Budde M.D. Janes L. Gold E. Turtzo L.C. Frank J.A. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain. 2011;134:2248–2260. doi: 10.1093/brain/awr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mac Donald C.L. Dikranian K. Song S.K. Bayly P.V. Holtzman D.M. Brody D.L. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp. Neurol. 2007;205:116–131. doi: 10.1016/j.expneurol.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song S.K. Sun S.W. Ramsbottom M.J. Chang C. Russell J. Cross A.H. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 43.Sun S.W. Liang H.F. Trinkaus K. Cross A.H. Armstrong R.C. Song S.K. Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn. Reson. Med. 2006;55:302–308. doi: 10.1002/mrm.20774. [DOI] [PubMed] [Google Scholar]
- 44.Ewing-Cobbs L. Prasad M.R. Swank P. Kramer L. Cox C.S., Jr. Fletcher J.M. Barnes M. Zhang X. Hasan K.M. Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. Neuroimage. 2008;42:1305–1315. doi: 10.1016/j.neuroimage.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levin H.S. Wilde E.A. Chu Z. Yallampalli R. Hanten G.R. Li X. Chia J. Vasquez A.C. Hunter J.V. Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. J. Head Trauma Rehabil. 2008;23:197–208. doi: 10.1097/01.HTR.0000327252.54128.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benavidez D.A. Fletcher J.M. Hannay H.J. Bland S.T. Caudle S.E. Mendelsohn D.B. Yeakley J. Brunder D.G. Harward H. Song J. Perachio N.A. Bruce D. Scheibel R.S. Lilly M.A. Verger-Maestre K. Levin H.S. Corpus callosum damage and interhemispheric transfer of information following closed head injury in children. Cortex. 1999;35:315–336. doi: 10.1016/s0010-9452(08)70803-7. [DOI] [PubMed] [Google Scholar]
- 47.Mathias J.L. Beall J.A. Bigler E.D. Neuropsychological and information processing deficits following mild traumatic brain injury. J. Int. Neuropsychol. Soc. 2004;10:286–297. doi: 10.1017/S1355617704102117. [DOI] [PubMed] [Google Scholar]
- 48.Pollmann S. Maertens M. von Cramon D.Y. Splenial lesions lead to supramodal target detection deficits. Neuropsychology. 2004;18:710–718. doi: 10.1037/0894-4105.18.4.710. [DOI] [PubMed] [Google Scholar]
- 49.Schulte T. Sullivan E.V. Muller-Oehring E.M. Adalsteinsson E. Pfefferbaum A. Corpus callosal microstructural integrity influences interhemispheric processing: a diffusion tensor imaging study. Cereb. Cortex. 2005;15:1384–1392. doi: 10.1093/cercor/bhi020. [DOI] [PubMed] [Google Scholar]
- 50.Zaidel E., editor; Iacoboni M., editor. The Parallel Brain: The Cognitive Neuroscience of the Corpus Callosum. MIT Press; Cambridge, MA: 2003. [Google Scholar]
- 51.Levin H.S. Benavidez D.A. Verger-Maestre K. Perachio N. Song J. Mendelsohn D.B. Fletcher J.M. Reduction of corpus callosum growth after severe traumatic brain injury in children. Neurology. 2000;54:647–653. doi: 10.1212/wnl.54.3.647. [DOI] [PubMed] [Google Scholar]
- 52.Mendelsohn D.B. Levin H.S. Harward H. Bruce D. Corpus callosum lesions after closed head injury in children: MRI, clinical features, and outcome. Neuroradiology. 1992;34:384–388. doi: 10.1007/BF00596495. [DOI] [PubMed] [Google Scholar]
- 53.Wilde E.A. Chu Z. Bigler E.D. Hunter J.V. Fearing M.A. Hanten G. Newsome M.R. Scheibel R.S. Li X. Levin H.S. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J. Neurotrauma. 2006;23:1412–1426. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- 54.Bendlin B.B. Ries M.L. Lazar M. Alexander A.L. Dempsey R.J. Rowley H.A. Sherman J.E. Johnson S.C. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. Neuroimage. 2008;42:503–514. doi: 10.1016/j.neuroimage.2008.04.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kraus M.F. Susmaras T. Caughlin B.P. Walker C.J. Sweeney J.A. Little D.M. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130:2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- 56.Salmond C.H. Menon D.K. Chatfield D.A. Williams G.B. Pena A. Sahakian B.J. Pickard J.D. Diffusion tensor imaging in chronic head injury survivors: correlations with learning and memory indices. Neuroimage. 2006;29:117–124. doi: 10.1016/j.neuroimage.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 57.Teasdale G. Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 58.Hollingshead A.B. Four Factor Index of Social Status. Hollingshead, A. B.; New Haven, CT: 1975. [Google Scholar]
- 59.De Beni R. Palladino P. Pazzaglia F. Cornoldi C. Increases in intrusion errors and working memory deficit of poor comprehenders. Q. J. Exp. Psychol. A. 1998;51:305–320. doi: 10.1080/713755761. [DOI] [PubMed] [Google Scholar]
- 60.Passolunghi M.C. Cornoldi C. De Liberto S. Working memory and intrusions of irrelevant information in a group of specific poor problem solvers. Mem. Cognit. 1999;27:779–790. doi: 10.3758/bf03198531. [DOI] [PubMed] [Google Scholar]
- 61.Cornoldi C. Marzocchi G.M. Belotti M. Caroli M.G. Meo T. Braga C. Working memory interference control deficit in children referred by teachers for ADHD symptoms. Child Neuropsychol. 2001;7:230–240. doi: 10.1076/chin.7.4.230.8735. [DOI] [PubMed] [Google Scholar]
- 62.Cornoldi C. Mammarella N. Intrusion errors in visuospatial working memory performance. Memory. 2006;14:176–188. doi: 10.1080/09658210544000033. [DOI] [PubMed] [Google Scholar]
- 63.Hasan K.M. Kamali A. Iftikhar A. Kramer L.A. Papanicolaou A.C. Fletcher J.M. Ewing-Cobbs L. Diffusion tensor tractography quantification of the human corpus callosum fiber pathways across the lifespan. Brain Res. 2009;1249:91–100. doi: 10.1016/j.brainres.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hasan K.M. A framework for quality control and parameter optimization in diffusion tensor imaging: theoretical analysis and validation. Magn. Reson. Imaging. 2007;25:1196–1202. doi: 10.1016/j.mri.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang H. van Zijl P.C. Kim J. Pearlson G.D. Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput. Methods Programs Biomed. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 66.Mori S. Crain B.J. Chacko V.P. van Zijl P.C. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 67.Witelson S.F. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain 112, Pt. 1989;3:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- 68.Aboitiz F. Scheibel A.B. Fisher R.S. Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- 69.Aboitiz F. Montiel J. One hundred million years of interhemispheric communication: the history of the corpus callosum. Braz. J. Med. Biol. Res. 2003;36:409–420. doi: 10.1590/s0100-879x2003000400002. [DOI] [PubMed] [Google Scholar]
- 70.Huang H. Zhang J. Jiang H. Wakana S. Poetscher L. Miller M.I. van Zijl P.C. Hillis A.E. Wytik R. Mori S. DTI tractography based parcellation of white matter: application to the mid-sagittal morphology of corpus callosum. Neuroimage. 2005;26:195–205. doi: 10.1016/j.neuroimage.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 71.Park H.J. Kim J.J. Lee S.K. Seok J.H. Chun J. Kim D.I. Lee J.D. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Hum. Brain Mapp. 2008;29:503–516. doi: 10.1002/hbm.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benjamini Y. Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B. 1995;57:289–300. [Google Scholar]
- 73.Moseley M. Bammer R. Illes J. Diffusion-tensor imaging of cognitive performance. Brain Cogn. 2002;50:396–413. doi: 10.1016/s0278-2626(02)00524-9. [DOI] [PubMed] [Google Scholar]
- 74.Pierpaoli C. Barnett A. Pajevic S. Chen R. Penix L.R. Virta A. Basser P. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13:1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- 75.Rugg-Gunn F.J. Symms M.R. Barker G.J. Greenwood R. Duncan J.S. Diffusion imaging shows abnormalities after blunt head trauma when conventional magnetic resonance imaging is normal. J. Neurol. Neurosurg. Psychiatry. 2001;70:530–533. doi: 10.1136/jnnp.70.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tasker R.C. Changes in white matter late after severe traumatic brain injury in childhood. Dev. Neurosci. 2006;28:302–308. doi: 10.1159/000094156. [DOI] [PubMed] [Google Scholar]
- 77.Ewing-Cobbs L. Hasan K.M. Prasad M.R. Kramer L. Bachevalier J. Corpus callosum diffusion anisotropy correlates with neuropsychological outcomes in twins disconcordant for traumatic brain injury. AJNR Am. J. Neuroradiol. 2006;27:879–881. [PMC free article] [PubMed] [Google Scholar]
- 78.Bigler E.D. Quantitative magnetic resonance imaging in traumatic brain injury. J. Head Trauma Rehabil. 2001;16:117–134. doi: 10.1097/00001199-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 79.Marquez de la Plata C.D. Yang F.G. Wang J.Y. Krishnan K. Bakhadirov K. Paliotta C. Aslan S. Devous M.D. Moore C. Harper C. McColl R. Munro Cullum C. Diaz-Arrastia R. Diffusion tensor imaging biomarkers for traumatic axonal injury: analysis of three analytic methods. J. Int. Neuropsychol. Soc. 2011;17:24–35. doi: 10.1017/S1355617710001189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu T.C. Wilde E.A. Bigler E.D. Li X. Merkley T.L. Yallampalli R. McCauley S.R. Schnelle K.P. Vasquez A.C. Chu Z. Hanten G. Hunter J.V. Levin H.S. Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Dev. Neurosci. 2010;32:361–373. doi: 10.1159/000317058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caeyenberghs K. Leemans A. Coxon J. Leunissen I. Drijkoningen D. Geurts M. Gooijers J. Michiels K. Sunaert S. Swinnen S.P. Bimanual coordination and corpus callosum microstructure in young adults with traumatic brain injury: a diffusion tensor imaging study. J. Neurotrauma. 2011;28:897–913. doi: 10.1089/neu.2010.1721. [DOI] [PubMed] [Google Scholar]
- 82.Farbota K.D. Bendlin B.B. Alexander A.L. Rowley H.A. Dempsey R.J. Johnson S.C. Longitudinal diffusion tensor imaging and neuropsychological correlates in traumatic brain injury patients. Front. Hum. Neurosci. 2012;6:160. doi: 10.3389/fnhum.2012.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Palacios E.M. Fernandez-Espejo D. Junque C. Sanchez-Carrion R. Roig T. Tormos J.M. Bargallo N. Vendrell P. Diffusion tensor imaging differences relate to memory deficits in diffuse traumatic brain injury. BMC Neurol. 2011;11:24. doi: 10.1186/1471-2377-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Palacios E.M. Sala-Llonch R. Junque C. Roig T. Tormos J.M. Bargallo N. Vendrell P. White matter integrity related to functional working memory networks in traumatic brain injury. Neurology. 2012;78:852–860. doi: 10.1212/WNL.0b013e31824c465a. [DOI] [PubMed] [Google Scholar]
- 85.Vallat-Azouvi C. Weber T. Legrand L. Azouvi P. Working memory after severe traumatic brain injury. J. Int. Neuropsychol. Soc. 2007;13:770–780. doi: 10.1017/S1355617707070993. [DOI] [PubMed] [Google Scholar]
- 86.Graham K.S. Barense M.D. Lee A.C. Going beyond LTM in the MTL: a synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 2010;48:831–853. doi: 10.1016/j.neuropsychologia.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 87.Ranganath C. Blumenfeld R.S. Doubts about double dissociations between short- and long-term memory. Trends Cogn. Sci. 2005;9:374–380. doi: 10.1016/j.tics.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 88.Ranganath C. D'Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31:865–873. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- 89.Wilde E.A. Ayoub K.W. Bigler E.D. Chu Z.D. Hunter J.V. Wu T.C. McCauley S.R. Levin H.S. Diffusion tensor imaging in moderate-to-severe pediatric traumatic brain injury: changes within an 18 month post-injury interval. Brain Imaging Behav. 2012;6:404–416. doi: 10.1007/s11682-012-9150-y. [DOI] [PubMed] [Google Scholar]
- 90.Sidaros A. Engberg A.W. Sidaros K. Liptrot M.G. Herning M. Petersen P. Paulson O.B. Jernigan T.L. Rostrup E. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain. 2008;131:559–572. doi: 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- 91.Song S.K. Sun S.W. Ju W.K. Lin S.J. Cross A.H. Neufeld A.H. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 92.Roncadin C. Guger S. Archibald J. Barnes M. Dennis M. Working memory after mild, moderate, or severe childhood closed head injury. Dev. Neuropsychol. 2004;25:21–36. doi: 10.1080/87565641.2004.9651920. [DOI] [PubMed] [Google Scholar]
- 93.Anderson V. Spencer-Smith M. Coleman L. Anderson P. Williams J. Greenham M. Leventer R.J. Jacobs R. Children's executive functions: are they poorer after very early brain insult. Neuropsychologia. 2010;48:2041–2050. doi: 10.1016/j.neuropsychologia.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 94.Anderson V. Jacobs R. Spencer-Smith M. Coleman L. Anderson P. Williams J. Greenham M. Leventer R. Does early age at brain insult predict worse outcome? Neuropsychological implications. J. Pediatr. Psychol. 2010;35:716–727. doi: 10.1093/jpepsy/jsp100. [DOI] [PubMed] [Google Scholar]
- 95.Anderson V. Catroppa C. Morse S. Haritou F. Rosenfeld J. Functional plasticity or vulnerability after early brain injury? Pediatrics. 2005;116:1374–1382. doi: 10.1542/peds.2004-1728. [DOI] [PubMed] [Google Scholar]