Abstract
Application of sinusoidal electric fields (EFs) has been observed to affect cellular processes, including alignment, proliferation, and differentiation. In the present study, we applied low-frequency alternating current (AC) EFs to porcine neural progenitor cells (pNPCs) and investigated the effects on cell patterning, proliferation, and differentiation. pNPCs were grown directly on interdigitated electrodes (IDEs) localizing the EFs to a region accessible visually for fluorescence-based assays. Cultures of pNPCs were exposed to EFs (1 V/cm) of 1 Hz, 10 Hz, and 50 Hz for 3, 7, and 14 days and compared to control cultures. Immunocytochemistry was performed to evaluate the expression of neural markers. pNPCs grew uniformly with no evidence of alignment to the EFs and no change in cell numbers when compared with controls. Nestin expression was shown in all groups at 3 and 7 days, but not at 14 days. NG2 expression was low in all groups. Co-expression of glial fibrillary acidic protein (GFAP) and TUJ1 was significantly higher in the cultures exposed to 10- and 50-Hz EFs than the controls. In summary, sinusoidal AC EFs via IDEs did not alter the alignment and proliferation of pNPCs, but higher frequency stimulation appeared to delay differentiation into mature astrocytes.
Introduction
The potential for neural stem cells to produce regeneration in the central nervous system (CNS) has reawakened interest in their therapeutic potential (Louro and Pearse, 2008; Pollard and Conti, 2007; Sahni and Kessler, 2010; Sharp and Keirstead, 2009). However, translation of this potential into clinical reality is proving difficult (Duncan et al., 2008; Regenberg et al., 2009). Understanding the factors that can modulate the behavior of neural stem cells would facilitate the development of cellular-based therapies.
Endogenous electric currents generate local electric fields (EFs) in the developmental phase of various biological systems (Hotary and Robinson, 1992; Robinson, 1979). EFs can affect cellular processes, including microfilament reorganization, proliferation, differentiation, and apoptosis, by causing a redistribution of charged cell-surface receptors, altering cell shape, and increasing the release of signaling molecules such as intracellular calcium (Barnes, 1992; Cho et al., 1994, 1996; Haddad et al., 2007; Titushkin and Cho, 2009). Several studies have reported the effect of exogenous EFs applied to various stem cells in vitro. For example, exposure of human and mouse embryonic stem cells (ESCs) to EFs promotes cardiac differentiation through generation of reactive oxygen species (ROS) (Serena et al., 2009), and galvanotaxis occurs in adipose-derived stromal cells and human dermal fibroblasts when exposed to direct current (DC) EFs (Tandon et al., 2009; Guo et al., 2010; Hammerick et al., 2010). However, the response to EFs is diverse depending on cell type, developmental stage, and species. Moreover, the response can be altered by altering the cell culture media, culture dish materials, surface coating, or growth supplements (Feng et al., 2012; Rajnicek et al., 1998; Wood and Willits, 2009; Zhang et al., 2011).
Neuronal activity can be modulated by weak EFs, and it has been shown that EFs below 1 mV/mm have an effect on both single neurons and neuronal network responses (Francis et al., 2003; McCaig et al., 2005). When electric stimulation was applied to ESCs, differentiation into neuronal cell types was increased compared to the effect of growth factor application (Yamada et al., 2007). Many studies have shown that DC EFs guide neuronal growth cones in vitro (Ariza et al., 2010; McCaig 1986a, 1986b; Robinson and Cormie, 2008; Yao et al., 2011). Although less work has been done with alternating current (AC) EFs, their ability to produce asymmetric growth of neurites appears to be limited as a direct consequence of alternating the field (Ariza et al., 2010). However, in a study on hippocampal neural progenitor cells (NPCs), AC EFs did not affect cell viability and proliferation whereas DC EFs had a negative effect (Ariza et al., 2010), suggesting AC EFs might benefit cell survival. In addition, transformers can be used to alter applied voltages in AC systems easily, giving them an advantage over DC systems for clinical use (Zaghi et al., 2010). Recently, it was demonstrated that 1-Hz AC EFs enhanced viability and influenced the differentiation ratio of neural stem cells (Matos and Cicerone, 2010). These observations suggested that physiologically relevant AC EFs could be used as a tool to produce specific neural lineage modification and manipulation. However, there has been limited and contradictory research in this area (Ariza et al., 2010; Park et al., 2009; Yao et al., 2011).
In a previous experiment, we found that application of sinusoidal AC EFs via interdigitated electrodes (IDEs) increased intracellular calcium signaling in human adipose-derived stem cells (hASCs) (McCullen et al., 2010). McCullen et al. reported that the advantage of IDEs includes creation of EFs both parallel to and above the surface of the electrode that allows stimulation of the cultured cells in a highly reproducible, controlled, and quantifiable manner. Low voltages can be created to represent physiological EFs, and the fields are localized to the immediate vicinity of a particular electrode. In addition, IDEs allow direct observation of cell signaling pathways using fluorescent microscopy techniques. Our previous work showed that hASCs respond to AC EFs by increasing intracellular calcium and osteogenic differentiation; the most significant effect was seen using 1 V/cm for up to 14 days. This led us to hypothesize that AC EFs of 1 V/cm magnitude would influence the survival and differentiation of fetal porcine (p) NPCs. The present study was conducted to evaluate the effect of sinusoidal AC EFs applied at varying strengths and durations on pNPC alignment, proliferation, and differentiation.
Materials and Methods
Porcine neurosphere isolation and culture
Cerebrocortical tissue was obtained from 45-gestation-day-old fetuses of the Yucatan minipig. Porcine neurospheres were generated using a protocol based on previously reported neurosphere culture methods (Lim et al., 2010). Briefly, after euthanasia, brains were removed and placed into a balanced salt solution (Gibco-BRL, Gaithersburg, MD, USA) and then grossly dissected, minced, and enzymatically digested to produce single cells. Suspended single cells were plated on a noncoated, ultra-low-cluster six-well plate (Costar, Corning Inc., Corning, NY, USA) at a concentration of 1×106/well in NEP basal medium [Neurobasal™ medium (Gibco-BRL) supplemented with 2% B27 (Gibco-BRL), 1% N2 (Gibco-BRL) and 0.1% penicillin-streptomycin (Gibco-BRL)] with 10 ng/mL basic fibroblast growth factor (bFGF; Invitrogen, Carlsbad, CA, USA) and 100 ng/mL recombinant human epidermal growth factor (rhEGF; Invitogen). Once neurospheres had formed, half of the medium was replaced every 3–5 days. Passage was performed by mechanical dissociation of the neurospheres when their diameter reached 100–150 μm.
Characterization of pNPCs
pNPCs were characterized by immunocytochemical analysis. Neurospheres were collected and fixed with 4% paraformaldehyde (pH 7.4) for 30 min at room temperature (RT), then rinsed three times in phosphate-buffered saline (PBS; Gibco-BRL), permeabilized with 0.3% TritonX-100 for 15 min, and blocked with 10% normal goat serum for 1 h at RT. Spheres were then incubated overnight at 4°C with one of the following antibodies: Polyclonal rabbit-anti-GFAP (glial fibrillary acidic protein) (1:1000, Z0334, DAKO, Carpinteria, CA, USA), monoclonal mouse-anti-TUJ1 (TUJ1) (1:500, MMS435P, Covance Inc.), monoclonal mouse-anti-Nestin (nestin) (1:200, MAB5326, Millipore, Billerica, MA, USA), and monoclonal mouse-anti A2B5 (A2B5) (1:200, MAB312, Millipore). Cells were then incubated for 1 h with either goat-anti-rabbit or mouse Alexa 488, goat-anti-mouse Cy3-conjugated secondary antibody (1:1000, Invitrogen) and counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratory Inc., CA, USA). Immunostaining was visualized with a fluoromicroscope (AZ100 Macro/microscope Nikon, Japan).
Lineage differentiation was determined by culture of pNPCs in NEP basal medium from which bFGF and EGF had been withdrawn, and 3% fetal bovine serum (FBS) had been added (Gibco-BRL), a standard paradigm used to induce differentiation for neural stem cells (Gage et al., 1995; Reynolds and Weiss, 1992). Neurospheres were dissociated, triturated to a single-cell suspension, plated into the eight-chamber slide (Lab-Tek, Nalge Nunc Inc., Rochester, NY, USA), and then cultured in differentiation medium for 7 days. They were then assessed by immunocytochemistry using either A2B5, or nestin, or a combination of TUJ1 and GFAP, as described above.
EF stimulation via IDEs
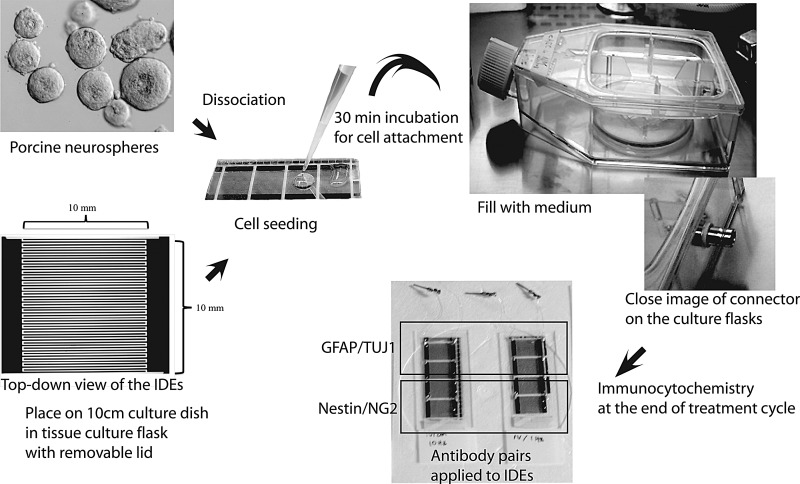
Electrical stimulation of pNPCs was performed using a previously designed setup that used tissue culture flasks with removable lids and custom-fabricated IDEs (McCullen et al., 2010). Briefly, IDEs were fabricated using a conventional ultraviolet (UV) lithography technique. IDEs consisted of two gold contact pads, in which each pad was connected to 25 electrode pairs. Both the electrode width and spacing between the electrodes was 100 μm, and the length of the electrode was 10 mm (Fig. 1). This provided a 10-×10-mm2 area for cell seeding and growth and for immunocytochemistry measurements. Four IDEs were embedded in each glass slide. Thus, each EF condition was replicated four times in a single culture flask, and each antibody pair was applied to two IDEs giving n=2 for each antibody pair. IDEs were coated with poly-l-lysine (Sigma, USA) to allow pNPC attachment. Platinum wires (gauge 36; California Fine Wire, Grover Beach, CA, USA) were attached to the end electrodes on the slide using silver epoxy and connected to the connectors on the culture flasks. Assembled electrode tissue culture flasks (Nalge Nunc Inc.) were cleaned with N2 gas and 70% ethanol before being sterilized with ethylene oxide (Andersen Sterilizers) before use. To generate EF strength of 1 V/cm, a voltage of 0.01V to the contact pads of the IDEs was applied using functional generator (Agilent 33220A 20MHz Function/Arbitrary Signal generator). With this applied voltage of 0.01 V and the diminutive spacing between the electrode fingers (0.01 cm), we calculated the EF strength as E (electric field)=Applied voltage (V)/electrode spacing (cm). IDEs produce an EF between the electrode fingers that also penetrates above and below the electrode. The EF is relatively constant above the plane of the IDE, until it begins to decay significantly ∼0.01 cm (one electrode spacing) above the plane of the electrode. pNPCs were seeded directly on the electrode, where the EF is essentially constant.
FIG. 1.
Diagram of the experimental design showing the appearance of the IDEs and the stages of research.
In previous work on electrical stimulation, EF amplitudes on the order of 0.1–10 V/cm have been identified as sufficient to produce an effect without damage, and frequencies of <15 Hz are commonly used, because aggregates of cells may act as a low-pass filter to the electrical signal. We chose the EF of 1 V/cm based on previous literature from other research groups, and our own experimentation in which we demonstrated that EF of this magnitude (1 V/cm) was sufficient in activating Ca2+ signaling pathways and affecting long-term growth and differentiation of human adipose-derived stem cells (McCullen et al., 2010).
Effects of EFs on pNPCs
pNPCs were used at passages 2 or 3 for all experiments. Neurospheres were dissociated mechanically and triturated to a single-cell suspension. IDEs were placed on 10-cm culture dishes in tissue culture flasks with removable lids and then cells were seeded at a density of 5×104cells/cm2 per field on the IDEs (Fig. 1). The flasks were incubated at RT for 30 min to allow cell attachment. Cells were cultured in NEP basal medium with 3% FBS. After 24 h in culture, pNPCs were assigned to treatment (exposure to 1 V/cm AC EFs) or control (no stimulation) groups. There were three treatment groups receiving 1 Hz, 10 Hz, and 50 Hz of stimulation, for 4 h a day, and each of these groups was further divided into three subgroups receiving stimulation for 3, 7, and 14 days, respectively, making a total of nine treatment groups and three control groups.
Immunocytochemistry was performed at the end of the treatment cycle to determine cell differentiation stage. Cell antigens were detected by double immunostaining using either nestin (to identify neural progenitor cells) and NG2 (to identify oligodendrocytes) (polyclonal rabbit anti-NG2, 1:200, AB5320, Millipore) or GFAP (to identify astrocytes) and TUJ1 (to identify neurons) for all groups. Cultures were fixed with 4% paraformaldehyde (pH 7.4) for 30 min at RT, rinsed three times in PBS, and blocked with 10% normal goat serum for 1 h at RT, and then primary and secondary antibodies were applied as described above. Experiments were replicated on four IDEs in a single culture flask (n=4), and each antibody pair (Nestin/NG2 or GFAP/TUJ1) was applied to two IDEs (Fig. 1). Immunostaining was detected using a fluoromicroscope (AZ100 Macro/microscope Nikon, Japan) with 20× magnification. Randomly selected magnification fields (n=10) were visualized on the two of IDE EFs for each group. The total number of cells (DAPI-counterstained nuclei) and the number expressing each antibody were counted. Immunoreactive cells were represented as nestin+/NG2+, nestin+/NG2− or nestin−/NG2+ cells, and GFAP+/TUJ1+, GFAP+/TUJ1− or GFAP−/TUJ1+ cells. Data were expressed as percentages of the total number of cells within the same magnification field and averaged for the 20 magnification fields counted (10 fields in two IDEs per antibody pair). Total cell number and the percentage expression of each antibody were compared between control and treatment groups.
Data analysis
Quantitative data were presented as means±standard deviations (SD). Data were tested for normal distribution using the Levene test. Cell numbers (total and immunoreactive cells) in each treatment group were compared using analysis of variance (ANOVA) and the Tukey post hoc test, with P<0.05 considered statistically significant (statistical software: IBM SPSS statistics 19® Inc.).
Results
pNPC characteristics
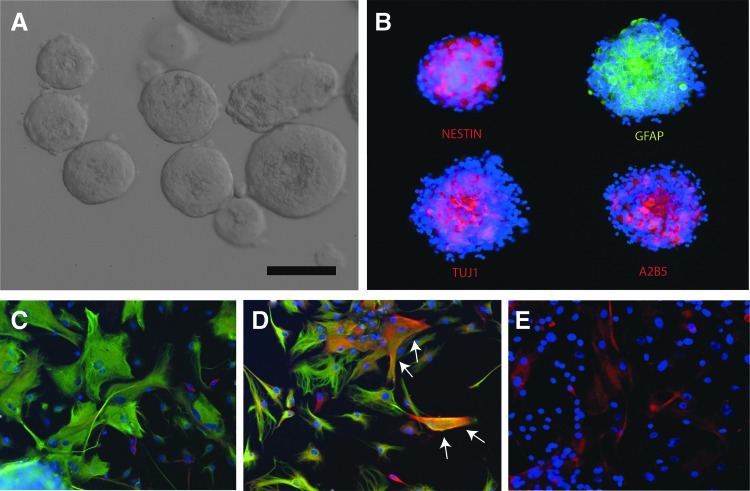
Neurospheres formed 3–5 days after seeding and grew to form spherical cellular aggregations in the medium containing growth factors epidermal growth factor (EGF) and bFGF (Fig. 2A). On immunocytochemistry, nestin was expressed throughout the cellular aggregates, and patchy expression of GFAP, TUJ1, and A2B5 was noted (Fig. 2B). After 7 days of differentiation, expression of all three lineage markers (GFAP, TUJ1, and A2B5) was observed (Fig. 2C–E).
FIG. 2.
Immunofluorescence characterization of pNPCs. (A) Phase-contrast photograph of cells isolated from porcine fetal brain. Neurospheres are seen. Scale bars, 100 μm. (B) Neurospheres labeled with antibodies as labeled. (C and D) Differentiated pNPCs were stained with TUJ1 (red), GFAP (green), and DAPI (blue). Cells were TUJ1+/GFAP−, TUJ1−/GFAP+, or TUJ1+/GFAP+. Arrows shows TUJ1+/GFAP+ cells. (E) Differentiated pNPCs were stained with A2B5 (red) and DAPI (blue). Color images available online at www.liebertpub.com/cell
Effect of EFs on pNPC viability and proliferation
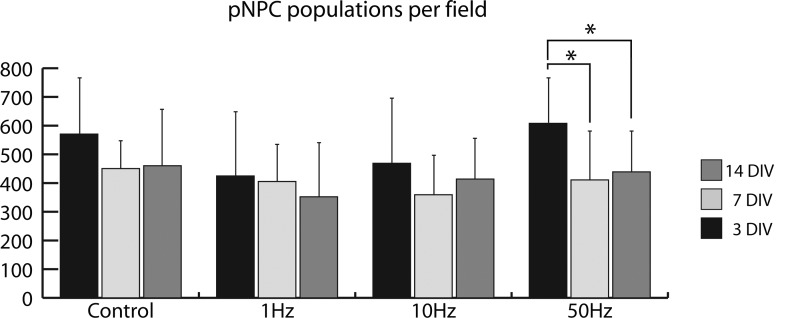
pNPCs attached to the IDEs coated with poly-l-lysine and grew well in all groups for the first 14 days in vitro (DIV). There was no significant difference in cell number between the control and treatment groups at any time point. The group exposed to 50 Hz, while it did not differ from the control group significantly, showed a significant decrease in cell numbers from 3 to 7 and 14 DIV (Fig. 3). Overall, EFs did not have a significant effect on cell survival and proliferation.
FIG. 3.
Effects of AC EFs on pNPC population. (*) p<0.05. DIV, days in vitro.
Effect of EFs on pNPC alignment and differentiation
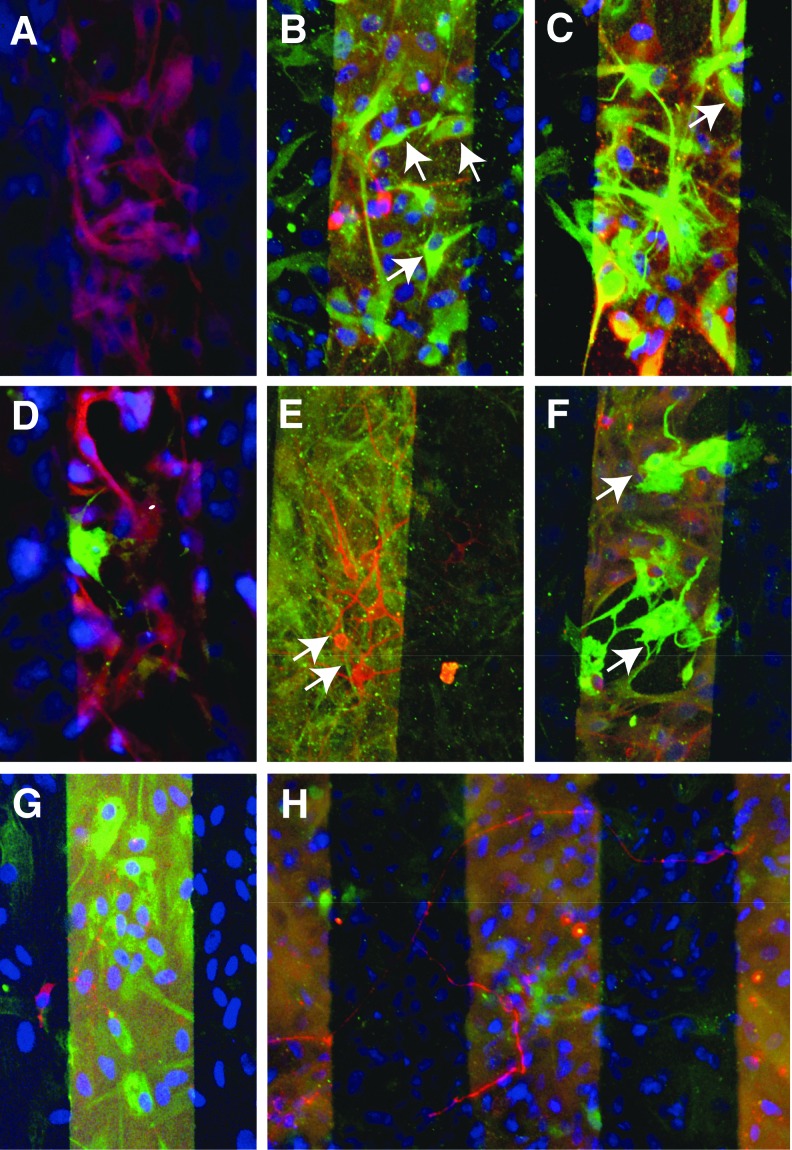
Specific patterns of alignment of the cells along the electrodes were not observed (Fig. 4). After 3 DIV, there was a population of cells with short bipolar processes typical of progenitor cells (Fig. 4B, C, arrows). After 7 and 14 DIV, the cells were predominantly polygonal with morphology typical of neurons (Fig. 4E, arrows) and astrocytes (Fig. 4F, arrows). Long GFAP−/TUJ1+ processes were observed at 14 DIV. However, these were oriented randomly with no obvious alignment with the IDEs (Fig. 4G, H).
FIG. 4.
Fluorescence image (magnification, 20×) of immunoreactive cells labeled with either nestin (red) and NG2 (green) (A and D), or TUJ1 (red) and GFAP (green) (B–C, E–F, G–H) and nuclei stained with DAPI (blue). (A) 1 Hz, 3 DIV; (B) 10 Hz, 3 DIV; (C) 50 Hz, 3 DIV; (D) 1 Hz, 7 DIV; (E) 10 Hz, 7 DIV; (F) 50 Hz, 7 DIV; (G–H) 10 Hz, 14 DIV. Color images available online at www.liebertpub.com/cell
In all groups, over 94% of cells expressed nestin at 3 and 7 DIV (Fig. 4A, D), whereas all nestin expression was lost at 14 DIV. Expression of NG2 was extremely low at all time points. Expression of nestin and NG2 is summarized in Table 1.
Table 1.
The Percentage of Immunoreactive Cells for Nestin and NG2
| Reactivity | Groups | 3 DIV | 7 DIV | 14 DIV |
|---|---|---|---|---|
| Nestin+/NG2−NPC | Control | 95.8±4.2 | 94.6±4.7 | 0.0±0.0 |
| 1 Hz | 96.6±3.8 | 97.4±2.5 | 0.0±0.0 | |
| 10 Hz | 98.2±1.7 | 96.0±4.2 | 0.0±0.0 | |
| 50 Hz | 96.3±4.3 | 95.9±3.4 | 0.0±0.0 | |
| Nestin−/NG2+(oligodendrocyte) | Control | 0.2±0.8 | 0.3±0.5 | 0.2±0.3 |
| 1 Hz | 0.1±0.1 | 0.2±0.3 | 0.1±0.3 | |
| 10 Hz | 0.0±0.1 | 0.1±0.3 | 0.7±0.2 | |
| 50 Hz | 0.0±0.0 | 0.0±0.1 | 0.1±0.2 | |
| Nestin+/NG2+ | Control | 0.0±0.1 | 0.0±0.1 | 0.0±0.0 |
| 1 Hz | 0.0±0.0 | 0.2±0.3 | 0.0±0.0 | |
| 10 Hz | 0.0±0.0 | 0.4±0.8 | 0.0±0.0 | |
| 50 Hz | 0.0±0.0 | 0.0±0.1 | 0.0±0.0 |
Values represent means±standard deviation (SD) (%). Control indicates cells that were grown without electric field stimulation.
DIV, days in vitro.
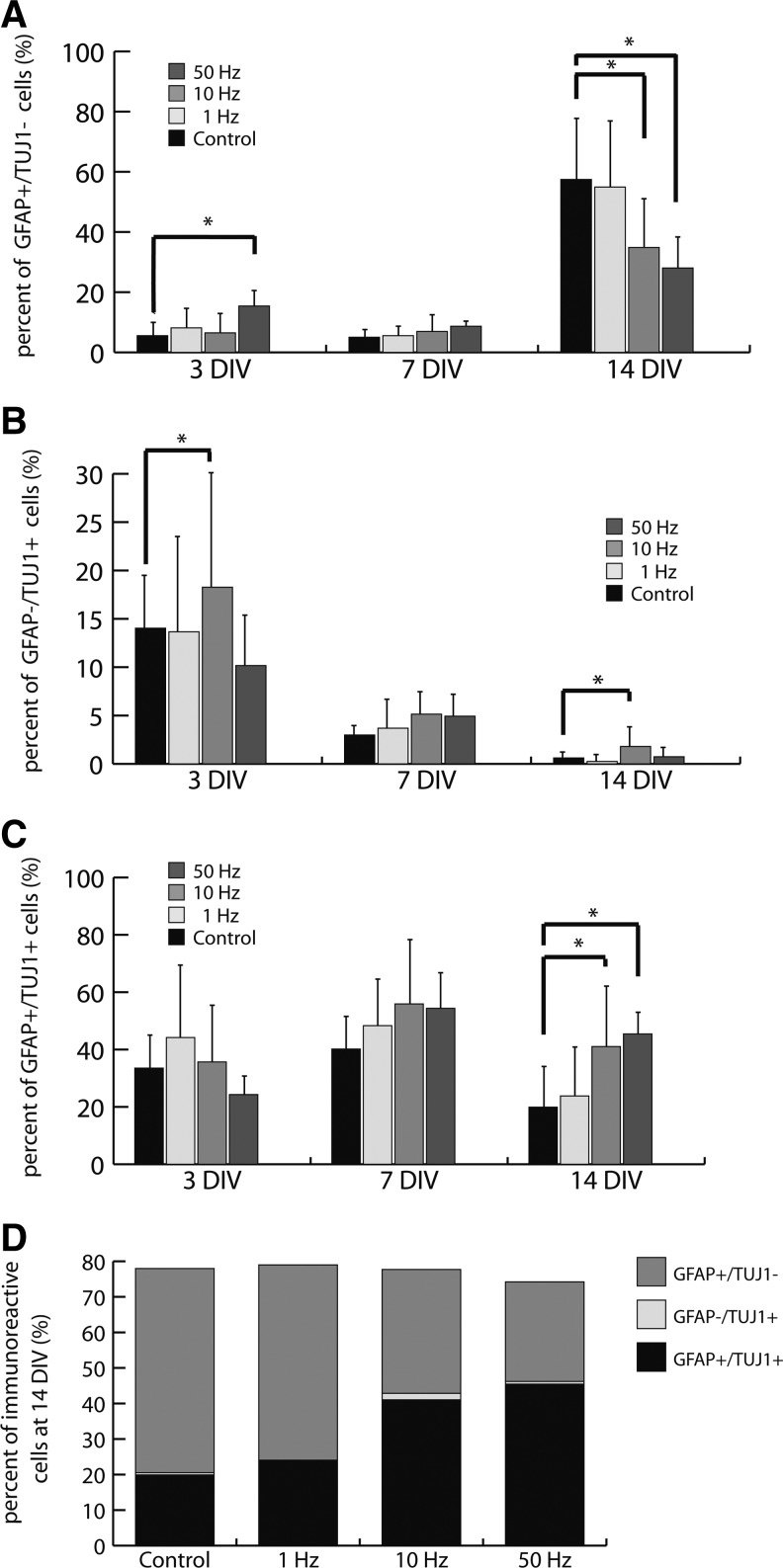
GFAP+/TUJ1− cells (astrocytes) were present at low levels at 3 and 7 DIV in all groups, whereas GFAP+/TUJ1− cell numbers increased in all groups by 14 DIV. There was no significant difference between the 1-Hz and control group, whereas there were significantly fewer GFAP+/TUJ1− cells in the 10- and 50-Hz groups at 14 DIV (Fig. 5A). GFAP−/TUJ1+ cells (neurons) were present at significantly higher levels in the 10-Hz stimulation group at 3 DIV, whereas GFAP−/TUJ1+ cell numbers decreased in all groups at 7 and 14 DIV (Fig. 5B). GFAP+/TUJ1+ cells were present in all groups at 3 and 7 DIV (Fig. 5C). However, at 14 DIV, although GFAP+/TUJ1+ cells decreased in all groups, there were significantly more GFAP+/TUJ1+ cells in the 10- and 50-Hz groups than the controls (P<0.05) (Fig. 5C, D). Expression of GFAP and TUJ1 is summarized in Table 2.
FIG. 5.
Effects of AC EFs on pNPC differentiation. (A) Percentage of GFAP+/TUJ1− cells after 3, 7, and 14 DIV under control, 1 Hz, 10 Hz, and 50 Hz AC stimulation at 1 V/cm (4 h/day). (B) Percentage of GFAP–/TUJ1+ cells after 3, 7, and 14 DIV under control, 1 Hz, 10 Hz, and 50 Hz at 1 V/cm stimulation (4 h/day). (C) Percentage of GFAP+/TUJ1+ cells after 3, 7, and 14 DIV under control, 1 Hz, 10 Hz, and 50 Hz at 1 V/cm stimulation (4 h/day). (D) Comparison of the ratio of TUJ1+/GFAP+ cells after 14 DIV in the different treatment groups. (*) Significance, p<0.05.
Table 2.
The Percentage of Immunoreactive Cells for GFAP and TUJ1
| Reactivity | Groups | 3 DIV | 7 DIV | 14 DIV |
|---|---|---|---|---|
| GFAP+/TUJ1−(astrocyte) | Control | 5.6±4.6 | 5.01±2.7 | 57.5±21.5 |
| 1 Hz | 8.1±6.7 | 5.5±3.4 | 54.9±23.5 | |
| 10 Hz | 6.5±6.8 | 6.9±6.7 | 34.9±17.3 | |
| 50 Hz | 15.4±5.4 | 8.7±1.9 | 28.0±11.0 | |
| GFAP−/TUJ1+(neuron) | Control | 14.0±5.5 | 3.0±1.0 | 0.6±0.6 |
| 1 Hz | 13.7±10.0 | 6.7±3.0 | 0.3±0.8 | |
| 10 Hz | 18.3±12.0 | 5.1±2.4 | 1.8±2.1 | |
| 50 Hz | 10.2±5.3 | 4.9±2.3 | 0.7±1.0 | |
| GFAP+/TUJ1+(immature neural lineage) | Control | 33.5±11.5 | 40.2±11.4 | 19.9±13.9 |
| 1 Hz | 44.2±25.3 | 48.3±16.5 | 23.8±17.3 | |
| 10 Hz | 35.7±19.8 | 55.9±22.6 | 41.0±21.3 | |
| 50 Hz | 24.3±6.4 | 54.4±12.4 | 45.5±7.2 |
Values represent means±standard deviation (SD) (%). Control indicates cells that were grown without electric field stimulation.
GFAP, glial fibrillary acidic protein; DIV, days in vitro.
Discussion
The purpose of this study was to determine whether 1-V/cm AC EFs applied at 1, 10, and 50 Hz using IDEs influence alignment, survival, proliferation, and differentiation of pNPCs. Our results showed that alignment, viability, and proliferation of pNPCs were not significantly affected by exposure to these EFs, although cell numbers showed a tendency to decline by 14 DIV. However, at 14 DIV, co-expression of GFAP and TUJ1, an indication of retention of a more immature status, was significantly higher in the cultures exposed to 10- and 50-Hz EFs than the controls.
pNPCs seeded on IDEs could be visualized clearly with fluoroscopy using immunostaining. They distributed uniformly with no specific alignment to the IDEs. This finding is consistent with our previous study showing the effect of AC EFs via IDEs on hASCs (McCullen et al., 2010). The influence of DC EFs on neuronal alignment and migration has been investigated extensively (Robinson and Cormie, 2008; Yao et al., 2011). Following application of DC EFs, neurites increase their growth rate toward the cathode and degenerate, retract, and reabsorb to move away from the anode (McCaig, 1986a, 1986b). In addition, DC EFs can guide migration of hippocampal neurons to the cathode, and the migration direction can be reversed by reversing the EFs (Yao et al., 2008). Study of the endogenous effects of AC EFs is more limited and controversial. One study showed that PC12 cell neurites increased in length after AC stimulation (Park et al., 2009). However, a more recent study of rat adult hippocampal NPCs suggested that AC EFs (46 mV/mm) had no influence on neurite growth or galvanotaxis (Ariza et al., 2010). Similar to the latter study, we found that pNPCs did not align to the AC 1 V/cm EFs at any of the stimulation rates tested. In DC EFs, membrane receptors, such as lectin and concanvalin A, accumulate asymmetrically facing the cathode and may activate signaling cascades in the cathode-facing side. Although the key receptor molecules linked to asymmetric distribution of intracellular Ca2+ are not yet known, changes in intracellular calcium signaling play an important role in EF-guided neuronal migration (Patel et al., 1982). Increases in intracellular calcium produce elevations of intracellular cyclic adenosine monophosphate (cAMP) via adenylate cyclase. As a related effect, cAMP activates protein kinase C–dependent kinase, and protein-kinase C–dependent kinase signals through small guanosine triphosphatases (GTPases) to regulate the dynamics of filamentous actin and microtubules, which steer growth cone migration (Henley et al., 2004; Yao et al., 2011). Generating asymmetric tension within the growth cone and asymmetric Ca2+ distribution may direct neuron migration (Yao et al., 2011). By culturing pNPCs directly on IDEs, the cells were exposed to very controlled AC EFs. This prevents asymmetric distribution of intracellular Ca2+, resulting in the apparently random distribution of pNPCs with no evidence of cell process alignment to the IDEs. Unlike a DC system, this system is not designed to evaluate directional growth along an electrical gradient, but rather to determine the effect of an AC EF on cell survival, proliferation, and differentiation.
Under the culture conditions evaluated, AC EFs did not appear to affect pNPC survival and proliferation. Cell numbers in all culture groups, including the controls, tended to decrease over time, and, although these changes were significant from 3 to 7 and 14 DIV in the 50-Hz treatment group, they were still not significantly different from the controls. These findings are in contrast to another study in which 1-Hz AC EFs significantly increased numbers of mouse NPCs (Matos and Cicerone, 2010). There could be many reasons for the difference in 1-Hz results between these two studies. The previous group used a cell suspension in alginate that was subjected to an EF that varied from 2 to 16 V over the alginate-embedded cultures (Matos and Cicerone, 2010). Thus, they used a higher minimum field that also had a gradient, and the cells were within a gel matrix. Further work on the size and distribution of EFs needs to be performed to optimize cell viability.
In a previous study, we demonstrated that AC sinusoidal EFs promoted osteogenic differentiation of hASCs related to dose-dependent increases in cytoplasmic calcium (McCullen et al., 2010). In hASCs, AC EFs of 1 V/cm and 1 Hz (4 h/day for 14 DIV) resulted in increased proliferation and extracellular mineral deposition. In pNPCs placed in culture medium that would result in differentiation, AC EFs did not affect the loss of nestin expression with time in culture. As is typical of differentiating NPCs, there was a much higher percentage of cells that are GFAP+/TUJ1− (a marker of glial lineage) than GFAP−/TUJ1+ (a neuronal marker) by 14 days. However, we found significantly increased numbers of cells exposed to AC EFs at 10 and 50 Hz co-expressed GFAP and TUJ1 by 14 DIV. These results suggest that AC EF exposure prolonged an immature stage after differentiation had begun. This could potentially be beneficial if using stem cells therapeutically because more immature cells may be able to integrate into the host more effectively than a fully differentiated cell.
The effects of EFs on differentiation are diverse, depending on the target cell and exposure conditions, and it is difficult to directly compare studies that use experimental parameters that differ in many aspects. In a study conducted by Chang et al., biphasic electric currents were found to promote both proliferation and neuronal differentiation of fetal mouse NPCs (Chang et al., 2011). Under control conditions, there was 6.7 times more glial than neuronal differentiation, but this ratio reduced to 3.6–4 in electric stimulation groups after 7 DIV. In another study, the differentiation of mouse ESCs specifically into the neuronal lineage was modulated when embryoid bodies were stimulated at several intensities via an electroporater by changing intracellular calcium signaling (Yamada et al., 2007). In particular, there were no differentiated glial cells by 10 DIV following this intervention. Piacentini et al. showed that 1 mT, 50-Hz electromagnetic fields produced neuronal differentiation of postnatal mouse NPCs via upregulation of Cav1-channel activity (Piacentini et al., 2008). In contrast, one study that investigated the effect of AC EFs on fetal mouse NPCs encapsulated in alginate hydrogel beads showed an enhanced propensity for astrocyte differentiation over neuronal differentiation using 1-Hz culture conditions by 14 DIV.
Clearly, the effect of EFs on survival, proliferation, and differentiation is affected by AC EFs, but the precise field strength and gradient, and AC stimulation rate appear to be critically important in promoting cell survival and proliferation and influencing differentiation. The IDE configuration presented here provides a precise and convenient method of controlling EFs and represents a powerful tool for ongoing investigation of the role of EFs in stem cell biology.
To summarize, the response of pNPCs to application of AC EFs was investigated using a custom planar IDE configuration and different stimulation frequencies. pNPCs were exposed to 1-Hz, 10-Hz, and 50-Hz EF treatments. Ten- and 50-Hz EFs appeared to decelerate cellular maturation, resulting in sustained GFAP+/TUJ1+ co-expression. Future experiments will investigate the mechanisms that result in deceleration of maturation of differentiating pNPCs when subjected to EF stimulation. Our results suggest that AC EFs can manipulate differentiation to produce a more immature cell that might prove valuable for future clinical use.
Acknowledgments
The authors thank Dr. Laura Clarke for guidance related to fabrication of interdigitated electrodes. This project was supported by a grant from the Center for Comparative Medicine and Translational Research, College of Veterinary Medicine, North Carolina State University, NIH/NIBIB1R03EB008790 (E.G.L.), and NSA/CBET1133427 (E.G.L).
A portion of this data was presented in abstract form at the Society for Neuroscience Forum (Chicago, October 17–21, 2009).
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Ariza C.A. Fleury A.T. Tormos C.J. Petruk V. Chawla S. Oh J. Sakaguchi D.S. Mallapragada S.K. The influence of electric fields on hippocampal neural progenitor cells. Stem Cell Rev. 2010;6:585–600. doi: 10.1007/s12015-010-9171-0. [DOI] [PubMed] [Google Scholar]
- Barnes F.S. Some engineering models for interactions of electric and magnetic fields with biological systems. Bioelectromagnetics. 1992;41:67–85. doi: 10.1002/bem.2250130708. [DOI] [PubMed] [Google Scholar]
- Chang K.A. Kim J.W. Kim J.A. Lee S.E. Kim S. Suh W.H. Kim H.S. Kwon S. Kim S.J. Suh Y.H. Biphasic electrical currents stimulation promotes both proliferation and differentiation of fetal neural stem cells. PLoS One. 2011;6:e18738. doi: 10.1371/journal.pone.0018738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M.R. Thatte H.S. Lee R.C. Golan D.E. Induced redistribution of cell surface receptors by alternating current electric fields. FASEB J. 1994;10:771–776. doi: 10.1096/fasebj.8.10.8050677. [DOI] [PubMed] [Google Scholar]
- Cho M.R. Thatte H.S. Lee R.C. Golan D.E. Reorganization of microfilament structure induced by ac electric fields. FASEB J. 1996;13:1552–1558. doi: 10.1096/fasebj.10.13.8940302. [DOI] [PubMed] [Google Scholar]
- Duncan I.D. Goldman S. Macklin W.B. Rao M. Weiner L.P. Reingold S.C. Stem cell therapy in multiple sclerosis: Promise and controversy. Mult. Scler. 2008;14:541–546. doi: 10.1177/1352458507087324. [DOI] [PubMed] [Google Scholar]
- Feng J.F. Liu J. Zhang X.Z. Zhang L. Jiang J.Y. Nolta J. Zhao M. Guided migration of neural stem cells derived from human embryonic stem cells by an electric field. Stem Cells. 2012;30:349–355. doi: 10.1002/stem.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis J.T. Gluckman B.J. Schiff S.J. Sensitivity of neurons to weak electric fields. J. Neurosci. 2003;23:7255–7261. doi: 10.1523/JNEUROSCI.23-19-07255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage F.H. Ray J. Fisher L.J. Isolation, characterization, and use of stem cells from the CNS. Annu. Rev. Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- Guo A. Song B. Reid B. Gu Y. Forrester J.V. Jahoda C.A. Zhao M. Effects of physiological electric fields on migration of human dermal fibroblasts. J. Invest. Dermatol. 2010;130:2320–2327. doi: 10.1038/jid.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad J.B. Obolensky A.G. Shinnick P. The biologic effects and the therapeutic mechanism of action of electric and electromagnetic field stimulation on bone and cartilage: New findings and a review of earlier work. J. Altern. Complement Med. 2007;13:485–490. doi: 10.1089/acm.2007.5270. [DOI] [PubMed] [Google Scholar]
- Hammerick K.E. Longaker M.T. Prinz F.B. In vitro effects of direct current electric fields on adipose-derived stromal cells. Biochem. Biophys. Res. Commun. 2010;397:12–17. doi: 10.1016/j.bbrc.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley J. Poo M.M. Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. 2004;14:320–330. doi: 10.1016/j.tcb.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotary K.B. Robinson K.R. Evidence of a role for endogenous electrical fields in chick embryo development. Development. 1992;114:985–996. doi: 10.1242/dev.114.4.985. [DOI] [PubMed] [Google Scholar]
- Lim J.H. Piedrahita J.A. Jackson L. Ghashghaei T. Olby N.J. Development of a model of sacrocaudal spinal cord injury in cloned Yucatan minipigs for cellular transplantation research. Cell. Reprogram. 2010;12:689–697. doi: 10.1089/cell.2010.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louro J. Pearse D.D. Stem and progenitor cell therapies: Recent progress for spinal cord injury repair. Neurol. Res. 2008;30:5–16. doi: 10.1179/174313208X284070. [DOI] [PubMed] [Google Scholar]
- Matos M.A. Cicerone M.T. Alternating current electric field effects on neural stem cell viability and differentiation. Biotechnol. Prog. 2010;26:664–670. doi: 10.1002/btpr.389. [DOI] [PubMed] [Google Scholar]
- McCaig C.D. Dynamic aspects of amphibian neurite growth and the effects of an applied electric field. J. Physiol. 1986a;375:55–69. doi: 10.1113/jphysiol.1986.sp016105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaig C.D. Electric fields, contact guidance and the direction of nerve growth. J. Embryol. Exp. Morphol. 1986b;94:245–255. [PubMed] [Google Scholar]
- McCaig C.D. Rajnicek A.M. Song B. Zhao M. Controlling cell behavior electrically: current views and future potential. Physiol. Rev. 2005;85:943–978. doi: 10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- McCullen S.D. McQuilling J.P. Grossfeld R.M. Lubischer J.L. Clarke L.I. Loboa E.G. Application of low-frequency alternating current electric fields via interdigitated electrodes: Effects on cellular viability, cytoplasmic calcium, and osteogenic differentiation of human adipose-derived stem cells. Tissue Eng. Part C Methods. 2010;6:1377–1386. doi: 10.1089/ten.tec.2009.0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.S. Park K. Moon H.T. Woo D.G. Yang H.N. Park K.H. Electrical pulsed stimulation of surfaces homogeneously coated with gold nanoparticles to induce neurite outgrowth of PC12 cells. Langmuir. 2009;25:451–457. doi: 10.1021/la8025683. [DOI] [PubMed] [Google Scholar]
- Patel N. Poo M.M. Orientation of neurite growth by extracellular electric fields. J. Neurosci. 1982;2:483–496. doi: 10.1523/JNEUROSCI.02-04-00483.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini R. Ripoli C. Mezzogori D. Azzena G.B. Grassi C. Extremely low-frequency electromagnetic fields promote in vitro neurogenesis via upregulation of Ca(v)1-channel activity. J. Cell Physiol. 2008;215:129–139. doi: 10.1002/jcp.21293. [DOI] [PubMed] [Google Scholar]
- Pollard S.M. Conti L. Investigating radial glia in vitro. Prog. Neurobiol. 2007;83:53–67. doi: 10.1016/j.pneurobio.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Rajnicek A.M. Robinson K.R. McCaig C.D. The direction of neurite growth in a weak DC electric field depends on the substratum: Contributions of adhesivity and net surface charge. Dev. Biol. 1998;203:412–423. doi: 10.1006/dbio.1998.9039. [DOI] [PubMed] [Google Scholar]
- Regenberg A. Mathews D.J. Blass D.M. Bok H. Coyle J.T. Duggan P. Faden R. Finkel J. Gearhart J.D. Hillis A. Hoke A. Johnson R. Johnston M. Kahn J. Kerr D. King P. Kurtzberg J. Liao S.M. McDonald J.W. McKhann G. Nelson K.B. Rao M. Siegel A.W. Smith K. Solter D. Song H. Sugarman J. Vescovi A. Young W. Greely H.T. Traystman R.J. The role of animal models in evaluating reasonable safety and efficacy for human trials of cell-based interventions for neurologic conditions. J. Cereb. Blood Flow Metab. 2009;29:1–9. doi: 10.1038/jcbfm.2008.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B.A. Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Robinson K.R. Electrical currents through full-grown and maturing Xenopus oocytes. Proc. Natl. Acad. Sci. USA. 1979;76:837–841. doi: 10.1073/pnas.76.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson K.R. Cormie P. Electric field effects on human spinal injury: Is there a basis in the in vitro studies? Dev. Neurobiol. 2008;68:274–280. doi: 10.1002/dneu.20570. [DOI] [PubMed] [Google Scholar]
- Sahni V. Kessler J.A. Stem cell therapies for spinal cord injury. Nat. Rev. Neurol. 2010;6:363–372. doi: 10.1038/nrneurol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp J. Keirstead H.S. Stem cell-based cell replacement strategies for the central nervous system. Neurosci. Lett. 2009;456:107–111. doi: 10.1016/j.neulet.2008.04.106. [DOI] [PubMed] [Google Scholar]
- Serena E. Figallo E. Tandon N. Cannizzaro C. Gerecht S. Elvassore N. Vunjak-Novakovic G. Electrical stimulation of human embryonic stem cells: Cardiac differentiation and the generation of reactive oxygen species. Exp. Cell Res. 2009;315:3611–3619. doi: 10.1016/j.yexcr.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon N. Goh B. Marsano A. Chao P.H. Montouri-Sorrentino C. Gimble J. Vunjak-Novakovic G. Alignment and elongation of human adipose-derived stem cells in response to direct-current electrical stimulation. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009:6517–6521. doi: 10.1109/IEMBS.2009.5333142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titushkin I. Cho M. Regulation of cell cytoskeleton and membrane mechanics by electric field: role of linker proteins. Biophys. J. 2009;96:717–728. doi: 10.1016/j.bpj.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M.D. Willits R.K. Applied electric field enhances DRG neurite growth: Influence of stimulation media, surface coating and growth supplements. J. Neural. Eng. 2009;6:46. doi: 10.1088/1741-2560/6/4/046003. [DOI] [PubMed] [Google Scholar]
- Yamada M. Tanemura K. Okada S. Iwanami A. Nakamura M. Mizuno H. Ozawa M. Ohyama-Goto R. Kitamura N. Kawano M. Tan-Takeuchi K. Ohtsuka C. Miyawaki A. Takashima A. Ogawa M. Toyama Y. Okano H. Kondo T. Electrical stimulation modulates fate determination of differentiating embryonic stem cells. Stem Cells. 2007;25:562–570. doi: 10.1634/stemcells.2006-0011. [DOI] [PubMed] [Google Scholar]
- Yao L. Shanley L. McCaig C. Zhao M. Small applied electric fields guide migration of hippocampal neurons. J. Cell. Physiol. 2008;216:527–535. doi: 10.1002/jcp.21431. [DOI] [PubMed] [Google Scholar]
- Yao L. Pandit A. Yao S. McCaig C.D. Electric field-guided neuron migration: a novel approach in neurogenesis. Tissue Eng. Part B Rev. 2011;17:143–153. doi: 10.1089/ten.TEB.2010.0561. [DOI] [PubMed] [Google Scholar]
- Zaghi S. Acar M. Hultgren B. Boggio P.S. Fregni F. Noninvasive brain stimulation with low-intensity electrical currents: Putative mechanisms of action for direct and alternating current stimulation. Neuroscientist. 2010;16:285–307. doi: 10.1177/1073858409336227. [DOI] [PubMed] [Google Scholar]
- Zhang J. Calafiore M. Zeng Q. Zhang X. Huang Y. Li R.A. Deng W. Zhao M. Electrically guiding migration of human induced pluripotent stem cells. Stem Cell Rev. 2011;7:987–996. doi: 10.1007/s12015-011-9247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]