Abstract
Recent interest in mild traumatic brain injury (mTBI) has increased the recognition that repetitive mTBI occurring within the sports and military settings can exacerbate the adverse consequences of the initial injury. While multiple studies have recently reported the pathological, metabolic, and functional changes associated with repetitive mTBI, no consideration has been given to the development of therapeutic approaches to attenuate these abnormalities. In this study, we used the model of repetitive impact acceleration insult previously reported by our laboratory to cause no initial structural and functional changes, yet evoke dramatic change following second insult of the same intensity. Using this model, we employed established neuroprotective agents including FK506 and hypothermia that were administered 1 h after the second insult. Following either therapeutic intervention, changes of cerebral vascular reactivity to acetylcholine were assessed through a cranial window. Following the completion of the vascular studies, the animals were prepared to access the numbers of amyloid precursor protein (APP) positive axons, a marker of axonal damage. Following repetitive injury, cerebral vascular reactivity was dramatically preserved by either therapeutic intervention or the combination thereof compared to control group in which no intervention was employed. Similarly, APP density was significantly lower in the therapeutic intervention group compared in controls. Although the individual use of FK506 or hypothermia exerted significant protection, no additive benefit was found when both therapies were combined. In sum, the current study demonstrates that the exacerbated pathophysiological changes associated with repetitive mTBI can be therapeutically targeted.
Key words: FK506, hypothermia, repetitive traumatic brain injury, therapeutic intervention
Introduction
Traumatic brain injury (TBI) remains a major national and international health care problem. Although historically most have focused on the pathobiological features of severe TBI, contemporary interest has focused on the consequences of mild TBI (mTBI), particularly as it relates to those injuries occurring within both the professional and amateur sports settings.1–4 This interest also has been accelerated by the finding of mTBI following multiple forms of blast or military-associated injury.5–6 It is also recognized that some mTBIs are associated with persistent morbidity and there is now increased recognition that repetitive mild brain injuries may be associated with an exacerbated burden of disease.7–11 These premises have been supported in part, by limited human studies examining the chronic sequelae of repeated injury, as well as those examining a subset of patients who manifest the secondary impact syndrome.1,12–17 These clinical studies are further supported by comparable observation made in laboratory studies examining repetitive injury in various animal TBI model systems.18–24 In such animal studies, multiple investigators have shown that repetitive injuries administered over hours to days post initial insult result in increased morbidity together with exacerbated brain parenchymal structural change reflected in increased synaptic loss, altered brain metabolism, impaired vascular function and an increased burden of traumatically-induced axonal injury.10–24 While the majority of experimental studies focusing on repetitive injuries have been framed in the context of an exacerbation of pathological change seen following the initial insult, more recent research by Fujita and colleagues10 also has shown that even repeat injuries in which the initial insult elicits no structural or functional change can be associated with an unmasking of dramatic structural and functional change following a second insult of comparable severity. This dramatic structural and functional damage occurred when the injury was administered within a specific temporal framework, with the caveat that if the intervals between the repetitive injuries are increased, the potential for second insult dramatically decreases or is eliminated altogether.
Despite our progress in the understanding of the pathobiology associated with repetitive brain injury and the issues relevant to insult severity and time between insults, there has been virtually no consideration of the use of therapeutic approaches to attenuate the damaging consequences of repetitive injury, particularly when these therapies are administered over a relatively delayed time course following the primary traumatic insult. These issues are of more than academic interest as the identification of potentially protective therapies would have obvious preclinical relevance, setting the stage for clinical studies in brain-injured patients.
To this end, we evaluated in the current investigation, the axonal and cerebral microvascular changes, which we have previously linked to mTBI and their enhanced//exacerbated responses following repetitive injury.10,25–31 In these studies, we employed mild repetitive injury to determine if established neuroprotective agents exert benefit when administered in a delayed time frame following a repetitive brain insult of comparative severity. We utilized FK506 (Tacrolimus) and moderate hypothermia as well as a combination of both agents given at a delayed time point following the second/repetitive injury.25–31 These agents were chosen based upon our previous studies demonstrating that via their inhibition of various calcineurin-mediated, oxygen-radical linked and/or metabolically-regulated cascades, these therapeutic approaches significantly attenuate the axonal and vascular damage associated with TBI.25–31 In the current study, we determined that the dramatically exacerbated burden of axonal and microvascular change found following repetitive mild injury was significantly attenuated through the use of either delayed FK506 administration or hypothermia alone. However, no enhanced benefit was found when both therapies were combined.
Methods
Experimental design
All experimental procedures were performed using a protocol approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University. Five adult male Spargue-Dawley rats were assigned to each group. Animals were housed in individual cages on a 12-h light/dark cycle with free access to water and food.
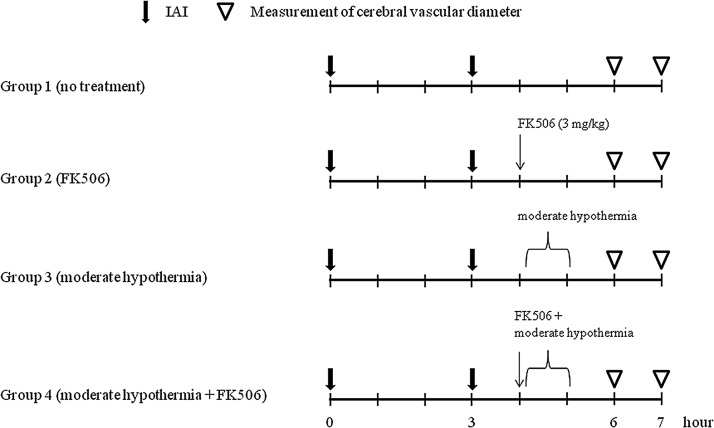
In this study, we investigated the effect of two therapeutic interventions employed after repetitive mild impact acceleration injury (IAI). In Group 1, which constituted the control group, animals were exposed to repetitive mild IAI without intervention. In the other groups, therapeutic interventions, included FK506 administration (Group 2), moderate hypothermia (32°C - 33°C; Group 3), and the combination of these two agents (Group 4) initiated 1 h after the second insult. Both pial vascular function and the burden of intraparenchymal axonal damage were quantitatively assessed. In this study, the animals were arbitrarily divided into the four groups specified above, with each group containing five animals each (Fig. 1). In these studies, two animals in which the vasoreactivity could not be assessed due to either brain swelling or sudden death caused by airway obstruction were replaced.
FIG. 1.
This chart shows the time course for each experimental group. All animals were subjected to repetitive impact acceleration insult at a 3-h interval. Therapeutic intervention was administered 1 h after the second insult. Vascular reactivity to acetylcholine was assessed at 3 and 4 h after the second insult. IAI, impact acceleration insult.
Group 1: Repetitive mild IAI without intervention
A 450 g-brass weight was dropped from a height of 1.0 meter previously recognized to produce a mild impact.10 Based on our previous studies, we considered this injury to be mild due to the fact that when used as a singular insult, the injury evoked no overt change in the brain parenchyma, eliciting neither contusion nor intraparenchymal hemorrhage. Further, although being struck, immunocytochemical analyses failed to reveal any APP positive/damaged axons, while functional assessments of the pial vasculature failed to reveal any change in vascular reactivity.10 Collectively, the absence of structural or functional change involving features long associated with TBI was considered to be consistent with mTBI. The only caveat was our inability to perform early behavioral assessments due to the use of barbituates which precluded our full characterization of this injury as mild.10 Following the induction of this initial mild insult another 1.0 meter insult was employed 3 h later to mimic repetitive injury. During all procedures basic physiological parameters including blood gases and blood pressure were monitored and controlled at a constant level. After the second insult, a cranial window was installed and cerebral vascular reactivity to acetylcholine (ACh) was evaluated 3 and 4 h after the second insult.
Group 2: Repetitive mild IAI with FK506 administration
Animals were exposed to the same repetitive mild IAI used in Group 1. Again, basic physiological parameters were monitored and controlled at a constant level throughout the experimental process. One hour after the second insult, FK506 (3 mg/kg) was slowly administered via a femoral venous line. Cerebral vascular reactivity to ACh was evaluated at the same time points as in Group 1.
Group 3: Repetitive mild IAI with moderate hypothermia
Animals were exposed to the same repetitive mild IAI used in Group 1 and again basic physiological parameters with the exception of the rectal and temporalis temperatures were maintained at a constant level throughout all experimental procedures. One hour after the second insult, moderate hypothermia was induced by placing an ice pack under animal's body. Rectal temperature was maintained at between 32 and 33°C for 1 h. As described in a previous report,32 rewarming rate was adjusted not to exceed at 1°C per 20 minutes. Cerebral vascular reactivity to ACh was evaluated at the same time points as in Group 1.
Group 4: Repetitive mild IAI with combinational therapy employing both FK506 administration and moderate hypothermia
Animals were exposed to the same repetitive mild IAI used in Group 1. Basic physiological parameters with the exception of the rectal and temporalis temperatures were maintained at a constant level throughout the experimental process. One hour after the second insult, FK506 (3 mg/kg) was slowly administered via the femoral venous line, and at the same time, moderate hypothermia was induced. Rectal temperature was maintained at between 32 and 33°C for 1 h, and rewarming rate was adjusted not to exceed at 1°C per 20 min. Cerebral vascular reactivity to ACh was evaluated at the same time points as in Group 1.
General preparation
After initial intraperitoneal anesthesia with sodium pentobarbital at 60–65 mg/kg, the femoral artery was cannulated with a PE50 catheter (Becton Deckinson, Spark, MD) for continuous monitoring of arterial blood pressure (PowerLab, AD Instruments, Colorado Springs, CO) and periodic collection of blood samples for determination of arterial oxygen pressure (PaO2), arterial carbon dioxide pressure (PaCO2), and pH values (Stat Profile® pHOx, Nova biomedical, Waltham, MA). Then, the femoral vein was cannulated with a PE50 catheter for administration of medication. After completion of the tracheotomy, the animals were mechanically ventilated (Harvard Apparatus, Holliston, MA) on room air. The resting PaCO2 was maintained at a constant level, between 35 and 40 mm Hg, by adjusting the rate and/or volume of respirator. Rectal temperature was controlled at 37°C with a heat lamp and/or a heating pad throughout the experiment except during the hypothermic period. Post-injury pancronium bromide (3 mg/kg) was administered intravenously to produce muscular blockade after the second impact.
Repetitive mild impact acceleration injury
Consistent with previous reports,10,30 an incision was made to expose the skull between the coronal and lambdoid suture. After cleaning and drying the bone surface, a 10-mm circular stainless steel helmet was fixed on the midline skull between bregma and lambda with dental acrylic. Animals were placed prone on the foam bed and head was placed under a 2.5-m Plexiglas tube, with the metallic disc centered under the lower end of the tube. A belt was fastened over the animal to prevent the rat from falling after the induction of trauma. A 450-g brass weight within the tube was dropped from a height of 1.0 meter onto the metallic disc. After hitting the metal disc, the rats were quickly removed from the foam bed and placed on the respirator. A second insult was performed employing the same level 3 h later.
Visualization and assessment of the cerebral vascular reactivity
After completing repetitive mild IAI, the metal disc was removed. A rectangular 2×4 mm2 craniotomy was made on the left side of parietal bone. The underlying dura was cut and peeled off from surface of the brain. Next, a cranial window was placed over the craniotomy and fixed in place with bone wax and cemented with dental acrylic. As previously described,10,30,31 the window consisted of a cover glass and metallic frame with three outlets, that permitted infusion of various agents. Two of the outlets served as inflow and outflow paths for the perfusion and clearance of the employed vasoactive agents, while the free end of the other outlet was set at a predetermined height to maintain an intercranial pressure of 5 mm Hg. The space under the cranial window was filled with artificial cerebrospinal fluid, with its pH adjusted to approximately 7.35 by equilibration with 6% O2 and 6% CO2 gas mixture balanced with N2. Next, the pial microcirculation was visualized and pial arteriolar diameters were measured using a Vickers image-splitting device (Vickers Instruments, Maiden, MA). Typically, in each window preparation a minimum of four arteriolar segments were evaluated. The vasodilator ACh was used in two different concentrations to assess vascular responses after topical application via the cranial window. Acethylcholine (Sigma, St Louis, MO) is, well known to elicit endothelial-dependent vasodilatation.33 It was dissolved in artificial cerebrospinal fluid to achieve final concentration of 10−7 and 10−5 mol/L. After application, ACh was allowed to remain in place for 2 to 4 minutes. Vascular reactivity to ACh was expressed as a percent change from the baseline diameter at each measurement time.
Tissue preparation
To evaluate the burden of axonal damage following repetitive mild IAI, we analyzed the medullospinal junction which we have previously shown to contain significant axonal injury in this animal model system.10 At 4 h following the second insult, and after the evaluation of the cerebral vascular reactivity, animals were euthanized. The animals were then immediately perfused with 1 L of saline and fixed with 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 mol/L Milloning's phosphate buffer. Each brain was divided into cortex and brain stem. The brain stem was sagittally blocked into 4 mm sections containing the corticospinal tract. For tissue sectioning, the brain stem was attached on a metal plate with cyanoacrylate. Brain stem was sagittally sectioned at 40 μm in 0.1 mol/L phosphate buffer using a vibratome (Leica Biosystems, St. Louis, MO). Sagittal sections were serially collected in alternating wells with each well containing adjacent sections. Systematic uniform sampling of sagittal sections was initiated from a random starting well, with every third section collected for a total of 20 sections per animal.
Immunocytochemistry for axonal damage
Brain stem sections were processed for visualization of antibody targeting the amyloid precursor protein (APP), a marker of impaired axonal transport and axonal damage, using a previously reported protocol.34 In brief, the sections were reacted with 0.3% H2O2 in phosphate-buffered saline (PBS) for 30 min to block endogenous peroxidase and microwaved twice in citric acid buffer while maintaining a 45°C maximum temperature for 5 min. The sections were then allowed to cool for 20 min. The sections were preincubated for 1 h in 10% normal goat serum (NGS) with 0.2% Triton X in PBS and then incubated for 18 h with the rabbit anti-β-APP (Invitrogen, Carlsbad, CA) diluted 1:700 in 1% NGS in PBS. Next, the sections were incubated for 1 h with biotinylated goat anti-rabbit immunoglobulin G (IgG) (Vector Laboratories Inc., Burlingame, CA) diluted 1:1000 in 1% NGS in PBS. The reaction product was visualized by incubation for 1 h in avidin-biotinylated enzyme complex (Vectastain® ABC kit, Vector Laboratories Inc.), followed by 0.05% diaminobenzidene, 0.01% H2O2, and 0.3% imidazole in 0.1 mol/L sodium phosphate buffer for 15 minutes. The sections were mounted on 0.5% gelatin-corted glass slides, serially dehydrated, and coverslipped.
Quantitative analysis of the axonal damage
After completion of APP immunocytochemical procedures, the slides were transferred in a blinded manner, to an Eclipse 800 microscope (Nikon, Tokyo, Japan), interfaced using a computer-assisted imaging system DP Controller, version 3.2 (Olympus Corporation, Tokyo, Japan). Consistent regions of medulla at the medullospinal junction were enlarged to a magnification of 10x and saved as a tagged image file format. Based on our previous experience,29 the image was viewed on a monitor using image analysis software, IPLab, version 3.7 (BD Biosciences Bioimaging, Rockville, MD) and changed to gray scale. The APP-immunoreactive axonal profiles were outlined and overlaid with cyan color to suppress background immunoreactivity. The sampling area in the medullospinal junction was delineated by a rectangle measuring 500×200 μm2 that was superimposed over the specified region. Within these rectangles the number of damaged APP-immunoreactive axonal profiles that exceed 0.968 μm2 in size was then counted. This number was expressed as the density of damaged axons per unit area. For the corticospinal tract, eight alternate serial sections from the same tissue block were analyzed in this fashion, together with the use of investigator blinding.
General statistical analysis
Statistical analysis was performed using the statistical software PASW Statistics 17.0 (SPSS Inc., Chicago, IL). All data were presented as mean±standard error of the mean (SEM). The physiological parameters and laboratory data, which were normally distributed, were analyzed by one-way analysis of variance. When a significant difference was found, differences among time points in the same group and among groups at each time point were determined by using Bonferroni correction and Tukey's test, respectively. The data of vascular reactivity to ACh and axonal injury were analyzed by Kruskal-Wallis test because these data were not normally distributed, and post hoc multiple comparisons were made by Mann-Whitney U test and Bonferroni correction. A value of P<0.05 was considered to be statistically significant.
Results
General physiological findings
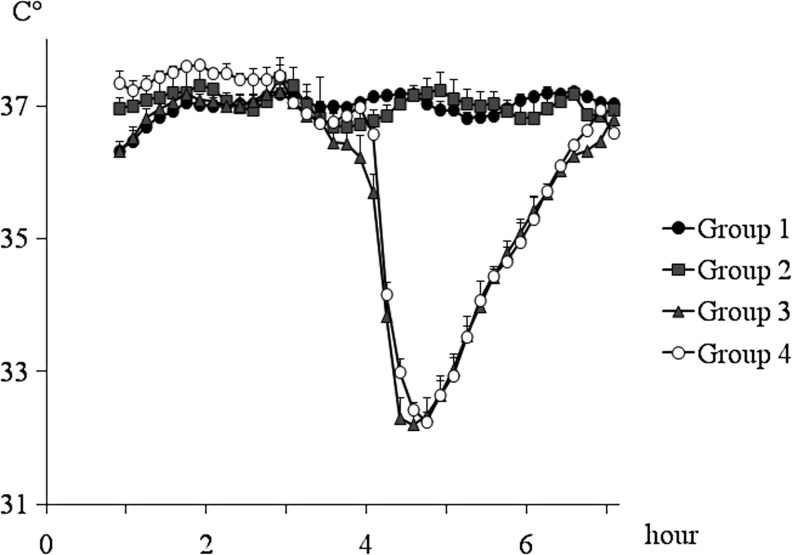
A total of 20 rats were included in this study. There were no significant differences in baseline body weight and hematocrit between groups. Table 1 shows time course measurements of mean arterial pressure (MAP) and blood gas analysis. There were no significant differences in these physiological parameters, including MAP, PaO2, PaCO2, and pH value in each measurement time, except for pH value in Group 2 at 1 and 3 h after the first insult versus Group 1 (p=0.010 and 0.042, respectively). The rectal temperature was maintained at approximately 37°C throughout the experiment except during the use of therapeutic hypothermia (Fig. 2). During the induction of therapeutic hypothermia, the rectal and temporalis temperatures dropped quickly, within 10–20 min to reach at between 32 and 33°C. There were no significant differences in rectal temperature during the hypothermic period between these two groups. Rewarming rates in hypothermia groups were less than 1°C/20 min and were not significantly different between groups.
Table 1.
Changes of Physiological Parameters after the First Insult
|
Time after the first insult | |||
|---|---|---|---|
| Group | 1 hour | 3 hours | 6 hours |
| MAP | |||
| 1 | 110±3 | 108±2 | 101±3 |
| 2 | 105±10 | 97±9 | 99±5 |
| 3 | 115±4 | 105±4 | 115±5 |
| 4 | 117±6 | 104±6 | 106±7 |
| PaO2 | |||
| 1 | 98±3 | 78±5 | 80±6 |
| 2 | 80±6 | 71±5 | 78±5 |
| 3 | 84±5 | 79±7 | 69±2 |
| 4 | 76±7 | 75±4 | 91±15 |
| PaCO2 | |||
| 1 | 35±1 | 37±1 | 37±2 |
| 2 | 39±1 | 39±1 | 41±2 |
| 3 | 39±2 | 39±1 | 40±1 |
| 4 | 38±2 | 39±2 | 39±0 |
| pH | |||
| 1 | 7.48±0.01 | 7.46±0.01 | 7.46±0.02 |
| 2 | 7.42±0.01* | 7.40±0.02* | 7.43±0.01 |
| 3 | 7.45±0.01 | 7.43±0.00 | 7.40±0.01 |
| 4 | 7.45±0.01 | 7.43±0.02 | 7.42±0.01 |
Values are expressed as the mean±standard error of the mean (*significant difference at p<0.05 vs. Group 1, one-way analysis of variance).
MAP, mean arterial pressure; PaO2, partial arterial oxygen pressure; PaCO2, partial arterial carbon dioxide pressure.
FIG. 2.
This figure illustrates the changes of the mean rectal temperatures throughout the duration of the study. A parallel temperature response was seen with the use of a temporalis muscle probe, with the caveat that this temperature slightly trailed the rectal temperature by approximately 0.5°C. The data points represent 10-min intervals. Values are expressed as mean±standard error of the mean.
Comparison of cerebral vascular reactivity after repetitive mild impact acceleration insult in the FK506, moderate hypothermia, and combinational therapy groups
Cerebral vascular responses to ACh were evaluated at 3 and 4 h following the second insult using two different ACh concentrations of 10−7 M and 10−5 M (Fig. 3). The vascular response was expressed as the percent change from the baseline diameter at each measurement time point. In Group 1, where animals were exposed to repetitive mild IAI without treatment, cerebral vascular response to ACh was abolished completely (10−7 M ACh, 1.3±0.9 and 0.05±0.5% at 3 and 4 h following the second insult, respectively; 10−5 M ACh, 0.9±0.8 and 0.5±0.8% at 3 and 4 h following the second insult, respectively). In Group 2, using FK506 at 1 h following the second insult, the cerebral vascular response was significantly preserved at both ACh concentrations of 10−7 M (6.4±0.7 and 6.2±0.5% at 3 and 4 h following the second insult, respectively) and 10−5 M (11.8±0.7 and 11.6±0.9% at 3 and 4 h following the second insult, respectively). Similarly, significant preservation of the cerebral vascular response was observed in Group 3 (10−7 M ACh, 8.7±0.7 and 7.3±1.5% at 3 and 4 h following the second insult, respectively; 10−5 M ACh, 15.3±1.0 and 13.5±1.8% at 3 and 4 h following the second insult, respectively). In Group 4, using combinational therapy, the vascular response was also preserved at both ACh concentrations of 10−7 M (7.7±0.9 and 8.9±1.1% at 3 and 4 h following the second insult, respectively) and of 10−5 M (15.2±1.1 and 16.7±1.2% at 3 and 4 h following the second insult, respectively). In these therapeutic intervention groups (Group 2, Group 3, and Group 4), all values of the cerebral vascular response were significantly higher than those in Group 1 (p<0.001). While there was no significant difference at 10−7 M ACh among therapeutic intervention groups, with 10−5 M ACh, the cerebral vascular responses in Group 3 and Group 4 were significantly higher than that in Group 2 at 3 h following the second insult (p=0.002 and 0.002, respectively), and at 4 h following the second insult, the cerebral vascular response in Group 4 was also significantly higher than that in Group 2 (p=0.004; Fig. 3).
FIG. 3.
This bar graph shows cerebral vascular reactivity to 10−7M and 10−5M acetylcholine (ACh) at 3 and 4 h after the second insult. The percent changes from baseline cerebral vascular diameter following exposure to ACh were compared between groups in each measurement time point. In Group 1, without treatment group, cerebral vascular responses were significantly lower than those in the other groups at all measurement points. Partial preservation of cerebral vascular response to ACh was observed in all therapeutic intervention groups (Group 2, Group 3, and Group 4). While there were no significant differences between Group 2, Group 3, and Group 4 at ACh concentration of 10−7M, the cerebral vascular response at 10−5M ACh in Group 2 was significantly lower than in Group 3 and Group 4 at 3 h after the second insult, as well as in Group 4 at 4 h after the second insult (*significant difference at p<0.001; **significant difference at p<0.005). Statistical differences were analyzed by the Kruskal-Wallis test, followed by the Bonferroni test for multiple comparisons. All values are expressed as mean±standard error of the mean. ACh, acetylcholine.
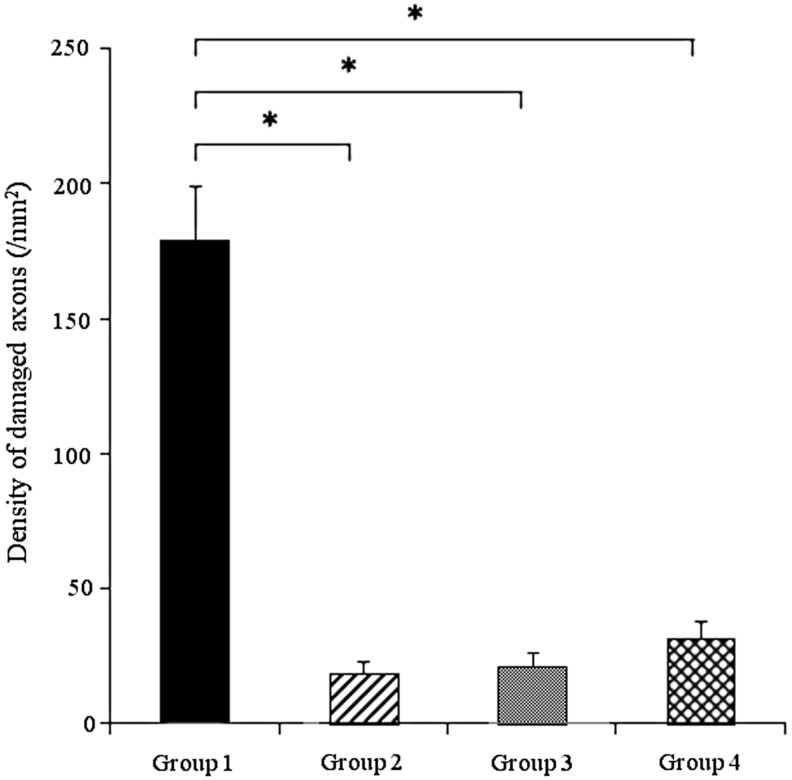
Evaluation of axonal injury after repetitive mild impact acceleration insult
Figure 4 illustrates the comparison of the damaged axon density in medullospinal junction between Group 1, Group 2, Group 3, and Group 4. Numerous APP positive axonal profiles were observed in Group 1 (179.3±20 /mm2), a finding consistent with previous reports.10 Compared with Group 1, the APP density observed in Group 2, Group 3, and Group 4 were significantly reduced (19±4.2 /mm2, 21±5.6 /mm2, 32±6.6 /mm2, respectively; p<0.001 vs. Group 1), demonstrating that these therapeutic interventions provided significant axonal protection. There were no significant differences between the therapeutic intervention groups (Group 2, Group 3, and Group 4), suggesting that the combinational therapy of both FK506 and moderate hypothermia did not exert any additive effect.
FIG. 4.
This bar graph shows mean density of amyloid precursor protein (APP)-immunoreactive damaged axons in the medullospinal tract at 4 h following the second insult. Significantly increased APP density was observed in Group 1. While APP density in Group 2, Group 3, and Group 4 were significantly lower than that in Group 1, there were no significant differences between therapeutic intervention groups (Group 2, Group 3, and Group 4). All values represent the mean±standard error of the mean. Statistical differences were analyzed by the Kruskal-Wallis test, followed by the Bonferroni test for multiple comparisons. *Significant difference at p<0.001.
Discussion
In the current communication, we revisit our previous studies using repetitive brain injury to assess the potential therapeutic targeting of two distinct pathologies associated with these repetitive injuries.10 Specifically, using an initial impact level of a severity previously reported to exert no structural or functional vascular change, we again found that when the same insult was administered within 3 h of the initial insult dramatic axonal and vascular perturbation followed. As reported previously,10 the level of injury used (a 1.0 m impact weight drop injury) following initial impact elicited neither structural or functional change and it was not associated with any evidence of contusion or intraparenchymal hemorrhage. Our current finding that the repetitive injury administered within a relatively close temporal time frame generated significant axonal and vascular damage is consistent with previous observations.10 However, importantly, in the current communication, we also demonstrate that this axonal and vascular damage could be dramatically attenuated and/or eliminated by the use of delayed administration of either FK506 or hypothermia following the second traumatic insult. While these findings are consistent with previous descriptions of their efficacy when used in the early time frames following a singular brain injury, our current findings suggest a significantly extended window of opportunity for their use, particularly in the context of repetitive impacts of low intensity. Our findings that both the delayed administration of FK506 and the delayed use of hypothermia were protective in terms of axonal and vascular change is intriguing as both approaches achieved efficacy when used as late as 4 h following the initial insult which in the context of experimental TBI, is a relatively delayed window of therapeutic opportunity. As with the initial descriptions of the FK506's therapeutic potential, its precise mode of action is unknown. This is particularly so in the context of the current study where the drug proved efficacious when administered after a repetitive injury. As noted previously, the initial insult used in these studies was not capable of evoking primary axonal nor vascular damage. In terms of the observed axonal response, it is most likely that the FK506 targets that axonal pathological change that evolves following the second traumatic insult. In this context, it is conceivable that via its modulation of calcineurin, FK506 could impact upon cytoskeletal change which could preserve axonal transport and integrity.35 Alternatively, recent studies have shown that calcineurin-mediated pathways may mediate the translocation of BAD and Bcl-x to the mitochondria which, in turn, affords mitochondrial protection by precluding mitochondrial permeability transition and thereby maintains the mitochondrial integrity needed to preserve local axolemmal membrane pumps and the axon cylinder.36,37 Similarly, while the axonal protection provided by hypothermia is also not well understood, it is appreciated that the use of early posttraumatic hypothermia following an uncomplicated primary TBI does exert neuroprotection on the axonal front. It is assumed that the hypothermia slows down and/or reverses the metabolic processes initiated by the initial traumatic episode and thereby exerts axonal protection.38–41 The current study's observation that the combinational approach using both FK506 and hypothermia exerts no added benefits was not unexpected in that previous work from our lab, using a singular traumatic episode, suggested that this approach did not result in increased protection.30
In terms of the observed vascular responses our findings also are consistent with previous protection described following repetitive traumatic insult.10 Our current finding that FK506 was protective even when given 4 h after the initial insult is intriguing and again is consistent with the observations made in relation to the observed axonal protection. Mechanistically, the vascular protective effects of FK506 are not well appreciated; however, recent studies have shown that FK506 can suppress adhesion molecule expression in vascular endothelial cells and/or nitric oxide synthase in vascular smooth muscle cells, perhaps partially explaining its mode of action.42,43 Similarly, the use of posttraumatic hypothermia also has shown to provide protection in an uncomplicated TBI; however, its benefits following repetitive TBI as observed in the current study, have not been previously described. Again, mechanistically, its mode of action remains to be determined yet, it is assumed to exert its protective effect through multiple pathways including, but not limited to, metabolic suppression, the suppression of oxygen-free radicals, and/or the attenuation of lipid peroxidation.38–41 As noted above, the finding that the combination of hypothermia and FK506 exerted no synergistic effect was not unanticipated given previous reports from our lab that its use following an uncomplicated primary TBI similarly failed to provide additive effects.
In sum, the current communication demonstrates the damaging consequences of repetitive brain injury while also demonstrating, in part, that the use of delayed FK506 treatment, as well as hypothermia, can dramatically attenuate and/or reverse these damaging consequences in terms of the axonal and microvascular change. While the implications of these studies for the clinical setting must be approached with caution, they may suggest that in those situations of repeat injury associated with potentially increased morbidity, therapeutic interventions such as these used in the current study may have long-term benefit. This may be particularly so in the context of a more select population of patients manifesting a second impact syndrome in which neurovascular paralysis and vascular engorgement transitions to increased morbidity and/or mortality.15 Thus, although repetitive injury can be associated with significant pathophysiological change, it could be potentially targeted by therapeutic interventions to reverse its damaging consequences.
Acknowledgments
The authors would like to thank Ms. Lynn Davis and Mrs. Susan Walker for their technical assistance. This study was supported by NIH grants NS077657 and NS047463.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kelly J.P. Nichols J.S. Filley C.M. Lillehei K.O. Rubinstein D. Kleinschmidt-DeMasters B.K. Concussion in sports; Guidelines for the prevention of catastrophic outcome. JAMA. 1991;266:2867–2869. doi: 10.1001/jama.266.20.2867. [DOI] [PubMed] [Google Scholar]
- 2.Boden P.B. Tacchetti R.L. Cantu R.C. Knowles S.B. Mueller F.O. Catastrophic head injuries in high school and college football players. Am. J. Sports Med. 2007;35:1075–1081. doi: 10.1177/0363546507299239. [DOI] [PubMed] [Google Scholar]
- 3.Watjen N.M. Pichelmann M.A. Atkinson J.L.D. Second impact syndrome: Concussion and second injury brain complications. Am. Coll. Surg. 2010;211:553–557. doi: 10.1016/j.jamcollsurg.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Len T.K. Neary J.P. Asmundson G.J.G. Godman D.G. Bjornson B. Bhambhani Y.N. Cerebrovascular reactivity impairment after sport-induced concussion. Med. Sci. Sports Exerc. 2011;43:2241–2248. doi: 10.1249/MSS.0b013e3182249539. [DOI] [PubMed] [Google Scholar]
- 5.McDonald C.L. Johnson A.M. Cooper D. Nelson E.C. Werner N.J. Shimony J.S. Synder A.Z. Raichle M.E. Witherow J.R. Fang R. Flaherty S.F. Brody D.L. Detection of blast-related traumatic brain injury in U.S. military personnel. N. Engl. J. Med. 2011;364:2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Omalu B. Hammers J.L. Bailes J. Hamilton R.L. Kamboh M.I. Webster G. Fitzsimmonz RP. Chronic traumatic encephalopathy in an Iraqi war veteran with posttraumatic disorder who committed suicide. Neurosurg. Focus. 2011;31(5):E3. doi: 10.3171/2011.9.FOCUS11178. [DOI] [PubMed] [Google Scholar]
- 7.Collins M.W. Lovell M.R. Iverson G.L. Cantu R.C. Maroon J.C. Field M. Cummulative effects of concussion in high school athletes. Neurosurgery. 2002;51:1175–1181. doi: 10.1097/00006123-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Moser R.S. Schatz P. Jordan B.D. Prolonged effects of concussion in high school athletes. Neurosurgery. 2005;57:300–306. doi: 10.1227/01.neu.0000166663.98616.e4. [DOI] [PubMed] [Google Scholar]
- 9.Byard R.W. Vink R. The second impact syndrome. Forensic Sci. Med. Pathol. 2009;5:36–38. doi: 10.1007/s12024-008-9063-7. [DOI] [PubMed] [Google Scholar]
- 10.Fujita M. Wei E.P. Povlishock J.T. Intensity- and Interval-specific repetitive traumatic brain injury can evoke both axonal and microvascular damage. J. Neurotrauma. 2012;10:2172–2180. doi: 10.1089/neu.2012.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCrory P. Davis G. Makdissi M. Second impact syndrome or cerebral swelling after sporting head injury. Curr. Sports Med. Rep. 2012;11:21–23. doi: 10.1249/JSR.0b013e3182423bfd. [DOI] [PubMed] [Google Scholar]
- 12.Saunders R.L. Harbaugh R.E. The second impact catastrophic contact-sports head trauma. JAMA. 1984;252:538–539. [PubMed] [Google Scholar]
- 13.Giza C.C. Hovda D.A. The neurometabolic cascade of concussion. J. Athl. Train. 2001;36:228–235. [PMC free article] [PubMed] [Google Scholar]
- 14.McCrory P. Johnston K.M. Mohtadi N.G. Meeuwisse W. Evidence-based review of sport-rerated concussion. Basic Science. 2001;11:160–165. doi: 10.1097/00042752-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Cantu R.C. Gean A.D. Second-impact syndrome and a small subdural hematoma: an uncommon catastrophic result of repetitive head injury with a characteristic imaging appearance. J. Neurotrauma. 2010;27:1557–1564. doi: 10.1089/neu.2010.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schatz P. Moser R.S. Covassin T. Karpf R. Early indications of enduring symptoms in high school athletes with multiple previous concussions. Neurosurgery. 2011;68:1562–1567. doi: 10.1227/NEU.0b013e31820e382e. [DOI] [PubMed] [Google Scholar]
- 17.Baugh C.M. Stamm J.M. Riley D.O. Gavett B.E. Shenton M.E. Lin A. Nowinski C.J. Cantu R.C. McKee A.C. Stern R.A. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 2012;6:244–254. doi: 10.1007/s11682-012-9164-5. [DOI] [PubMed] [Google Scholar]
- 18.Allen G.V. Gerami D. Esser M.J. Conditioning effects of repetitive mild neurotrauma on motor function in an animal model of focal brain injury. Neuroscience. 2000;99:93–105. doi: 10.1016/s0306-4522(00)00185-8. [DOI] [PubMed] [Google Scholar]
- 19.Laurer H. Bareyre F.M. Lee V.M.Y.C. Trojanowski J.Q. Longhi L. Hoover R. Saatman K.E. Raghupathi R. Hoshino S. Grady M.S. McIntosh T.K. Mild head injury increasing the brain's vulnerability to a second concussive impact. J. Neurosurg. 2001;95:859–870. doi: 10.3171/jns.2001.95.5.0859. [DOI] [PubMed] [Google Scholar]
- 20.Longhi L. Saatman K.E. Fujimoto S. Raghupathi R. Meaney D.F. Davis J. McMillan A. Conte V. Laurer H.L. Stein S. Stocchetti N. McIntosh T.K. Temporal window of vulnerability to repetitive experimental concussive brain injury. Neurosurgery. 2005;56:364–374. doi: 10.1227/01.neu.0000149008.73513.44. [DOI] [PubMed] [Google Scholar]
- 21.Huh J.W. Widing A.G. Raghupathi R. Repetitive mild non-concussive brain trauma in immature rats exacerbates traumatic axonal injury and axonal calpain activation: a preliminary report. J. Neurotrauma. 2007;24:15–27. doi: 10.1089/neu.2006.0072. [DOI] [PubMed] [Google Scholar]
- 22.Prins M.L. Hales A. Reger M. Giza C.C. Hovda D.A. Repeat traumatic brain injury in the juvenile rats is associated with increased axonal injury and cognitive impairments. Dev. Neurosci. 2010;32:510–518. doi: 10.1159/000316800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shitaka Y. Tran H.T. Bennett R.E. Sanchez L. Levy M.A. Dikranian K. Brody D.L. Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J. Neuropathol. Exp. Neurol. 2011;70:551–567. doi: 10.1097/NEN.0b013e31821f891f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prins M.L. Alexander D. Giza C.C. Hovda D.A. Repeated mild traumatic brain injury: mechanisms of cerebral vulnerability. J. Neurotrauma. 2013;30:30–38. doi: 10.1089/neu.2012.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singeleton R.H. Stone J.R. Okonkwo D.O. Pellicane A.J. Povlishock J.T. The immunophilin ligand FK506 attenuates axonal injury in an impact-acceleration model of traumatic brain injury. J. Neurotrauma. 2001;18:607–614. doi: 10.1089/089771501750291846. [DOI] [PubMed] [Google Scholar]
- 26.Suehiro E. Singeleton R.H. Stone J.R. Povlishock J.T. The immunophilin ligand FK506 attenuates axonal damage associated with rapid rewarming following posttraumatic hypothermia. Exp. Neurol. 2001;172:199–210. doi: 10.1006/exnr.2001.7765. [DOI] [PubMed] [Google Scholar]
- 27.Ueda Y. Wei E.P. Kontos H.A. Suehiro E. Povlishock J.T. Effect of delayed, prolonged hypothermia on the pial vascular response after traumatic brain injury in rats. J Neurotrauma. 2003;99:899–906. doi: 10.3171/jns.2003.99.5.0899. [DOI] [PubMed] [Google Scholar]
- 28.Baranova A.I. Wei E.P. Ueda Y. Sholley M.M. Kontos H.A. Povlishock J.T. Cerebral vascular responsiveness after experimental traumatic brain injury: the beneficial effects of delayed hypothermia combined with superoxide dismutase administration. J. Neurotrauma. 2008;109:502–509. doi: 10.3171/JNS/2008/109/9/0502. [DOI] [PubMed] [Google Scholar]
- 29.Gao G. Oda Y. Wei E.P. Povlishock J.T. The adverse pial arteriolar and axonal consequences of traumatic brain injury complicated by hypoxia and their therapeutic modulation with hypothermia in rat. J. Cereb. Blood Flow Metab. 2010;30:628–637. doi: 10.1038/jcbfm.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujita M. Oda Y. Wei E.P. Povlishock J.T. The combination of either Tempol or FK506 with delayed hypothermia: implications for traumatically induced microvascular and axonal protection. J. Neurotrauma. 2011;28:1209–1218. doi: 10.1089/neu.2011.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oda Y. Gao G. Wei E.P. Povlishock J.T. Combinational therapy using hypothermia and the immunophilin ligand FK506 to target altered pial arteriolar reactivity, axonal damage, and blood-brain barrier dysfunction after traumatic brain injury in rat. J. Cereb. Blood Flow Metab. 2011;31:1143–1154. doi: 10.1038/jcbfm.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suehiro E. Ueda Y. Wei E.P. Komtos H.A. Povlishock J.T. Posttraumatic hypothermia followed by slow rewarming protects the cerebral microcirculation. J. Neurotrauma. 2003;20:381–390. doi: 10.1089/089771503765172336. [DOI] [PubMed] [Google Scholar]
- 33.Kontos H.A. Wei E.P. Marshall J.J. In vivo bioassay of endothelium-derived relaxing factor. Am J Physiol. 1988;255:H1259–H1262. doi: 10.1152/ajpheart.1988.255.5.H1259. [DOI] [PubMed] [Google Scholar]
- 34.Stone J.R. Walker S.A. Povlishock J.T. The visualization of a new class of traumatically injured axons through the use of a modified method of microwave antigen retrieval. Acta Neuropathol. 1999;97:335–345. doi: 10.1007/s004010050996. [DOI] [PubMed] [Google Scholar]
- 35.Marmarou C.R. Povlishock J.T. Administration of the immunophilin ligand FK506 differentially attenuates neurofilament compaction and impaired axonal transport in injured axons following diffuse traumatic brain injury. Exp. Neurol. 2006;197:353–362. doi: 10.1016/j.expneurol.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Shibasaki F. Kondo E. Akagi T. McKeon F. Suppression of signaling through transcription factor NF-AT by interactions between calcineurin and Bcl-2. Nature. 1997;386:728–731. doi: 10.1038/386728a0. [DOI] [PubMed] [Google Scholar]
- 37.Mills J.D. Stone J.R. Rubin D.G. Melon D.E. Okonkwo D.O. Periasamy A.P. Helm G.A. Illuminating protein interactions in tissue using confocal and two-photon excitation fluorescent resonance energy transfer microscopy. J. Biomed. Opt. 2003;8:347–356. doi: 10.1117/1.1584443. [DOI] [PubMed] [Google Scholar]
- 38.Koizumi H. Povlishock J.T. Posttraumatic hypothermia in the treatment of axonal damage in an animal model of traumatic axonal injury. J. Neurotrauma. 1998;89:303–309. doi: 10.3171/jns.1998.89.2.0303. [DOI] [PubMed] [Google Scholar]
- 39.Polderman K.H. Mechanisms of action, physiological effects, and complications of hypothermia. Crit. Care Med. 2009;37:S186–S202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- 40.Clifton G.L. Valadka A. Zygun D. Coffey C.S. Drever P. Fourwinds S. Janis L.S. Wilde E. Taylor P. Harshman K. Conley A. Puccio A. Levin H.S. McCauley S.R. Bucholz R.D. Smith K.R. Schmidt J.H. Scott J.N. Yonas H. Okonkwo D.O. Very early hypothermia induction in patients with severe brain injury (the national acute brain injury study: hypothermia ii): A randomised trial. Lancet Neurol. 2011;10:131–139. doi: 10.1016/S1474-4422(10)70300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faridar A. Bershad E.M. Emiru T. Laizzo P.A. Suarez J.I. Divani A.A. Therapeutic hypothermia in stroke and traumatic brain injury. Front. Neurol. 2011;2:80. doi: 10.3389/fneur.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sasakawa T. Sasakawa Y. Matsunaga T. Fujitsu T. Hirayama Y. Ohkubo Y. Mutoh S. FK506 suppress E-selectin, ICAM-1 and VCAM-1 expression on vascular endothelial cells by inhibiting tumor necrosis factor alpha secretion from peripheral blood mononuclear cells. Cytokine. 2005;29:67–71. doi: 10.1016/j.cyto.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Thomale U.W. Bender M. Casalis P. Rupprecht S. Griebenow M. Neumann K. Woiceichowsky C. Unterburg A.W. Stover J.F. Tacrolimus depress local immune cell infiltration but fails to reduce cortical contusion volume in brain-injured rats. Immunology. 2007;212:567–576. doi: 10.1016/j.imbio.2007.01.007. [DOI] [PubMed] [Google Scholar]