Abstract
Background
Both female reproductive hormones and childhood sexual abuse (CSA) are implicated in migraine and in menstrually related mood disorders (MRMD). We examined the association of migraine, including migraine with aura (MA), and history of MRMD or CSA.
Methods
A total of 174 women (mean age 33.9±7.6 years) in this cross-sectional study were evaluated for (1) current MRMD using prospective daily ratings; (2) history of CSA using structured interview; and (3) MA and migraine without aura using the International Classification of Headaches Disorders II criteria.
Results
Ninety-six women met MRMD criteria (21 of whom had history of CSA) and 78 women were non-MRMD controls (16 with CSA histories). Migraine with aura was more prevalent in women with MRMD when compared to non-MRMD controls (11/88 and 0/86, respectively, p=0.001). In MRMD women only, a CSA history was associated with higher MA rates (6/21 and 5/67, respectively, p=0.019). A combination of current MRMD diagnosis and a history CSA was associated with increased risk for MA, even after adjusting for potential confounders (odds ratio=12.08, 95% confidence interval 2.98–48.90, p<0.001).
Conclusions
Women with MRMD may be vulnerable to the development of MA, and a history of CSA in women with a MRMD appears to increase that vulnerability. MRMDs and MA should be included among other poor mental and physical health outcomes of an abuse history. Routine screening for abuse histories would potentially improve identification of women with increased risk of experiencing abuse-related disorders.
Introduction
Migraine is a disabling condition with a cumulative lifetime incidence reaching 43% among women.1 Attacks of migraine with aura (MA) are more prevalent in women, with a lifetime prevalence rate of approximately 5%, and are associated with increased risk of ischemic stroke, especially among women using oral contraceptives.2–5 It is widely accepted that the sudden withdrawal of estrogen can trigger some attacks of migraine without aura (MO).6–9 In contrast to MO, MA is thought to be associated with higher concentrations of estrogen, consistent with the finding that the suppression of endogenous estrogen production reduces the frequency of auras in migraineurs.8,10,11 Estrogens are also implicated in cortical spreading depression, an electrophysiological phenomenon of cortical excitation followed by inhibition that is considered to be an underlying biological mechanism of the aura phase of migraine.12
Psychosocial factors, including traumatic stress during childhood, were shown to be associated with higher rates of adult headache and higher than expected rates of migraines.13,14 For example, a recent multicentered study by Tietjen and colleagues in migraineurs seeking treatment at headache clinics reported that 58% of their patients had a history of childhood maltreatment, and that 44% of patients with childhood sexual abuse (CSA) histories had MA.15 Furthermore, childhood maltreatment was also associated with elevated concentrations of biomarkers of coagulation, inflammation and oxidative stress in migraineurs, suggesting that adverse childhood experiences can play an important role in the association of migraine and stroke.16 However, self-reported questionnaire assessment of abuse histories and lack of a control group of healthy subjects were limitations of previous studies.
There is substantial evidence that adverse childhood experiences increase risk for the development of mood disorders.13,14 Our prior research showed that histories of abuse are significantly more prevalent in women with a menstrually related mood disorder (MRMD),17,18 and that those histories predict a unique experimental pain phenotype in women with a MRMD.19 Consequently, we sought to examine the association of an abuse history and migraine in women with an MRMD. Menstrual mood disorders are characterized by significant emotional and physical symptoms and functional impairment that is isolated to the luteal phase of the menstrual cycle.20,21 Additional rationale that women with a MRMD may be a relevant population in which to examine the link between an abuse history and migraine is based on the following evidence: (1) MRMD women are differentially sensitive to the mood destabilizing effects of gonadal steroid hormones;22 (2) although mood symptoms are a required feature of MRMD, premenstrual headache is among the diagnostic criteria;20 (3) headache and other somatic symptoms contribute to luteal phase functional impairment;20,23 and (4) for both MRMDs24 and MA,25 polymorphisms in the estrogen receptor alpha (ESR1) may have pathophysiological relevance.
Therefore, for the first time, this report systematically examines the association of CSA with migraine, including MA, in women with and without a diagnosis of MRMD, using standardized criteria for migraine, MRMD, and CSA. We hypothesized, based on the pathophysiologic role of estrogen in both MRMD22 and migraine,8 that women with MRMD would have higher rates of migraines than non-MRMD women. Based on the evidence that CSA is linked to increased rates of headaches,15 combined with the evidence that a history of abuse is associated with different biologic, clinical, and pain processing sequelae in women with MRMD,17–19 we hypothesized that in MRMD women, CSA histories would be related to increased rates of migraines headaches.
Methods
Subjects
During the time period from July 2007 until March 2012 we carried out a prospective diagnostic study aimed at identifying women suffering from MRMDs as well as non-MRMD controls in the Chapel Hill, North Carolina area. This diagnostic study was intended to serve as a feeder study for other research studies comparing women with a MRMD diagnosis to non-MRMD controls for differences in neuroendocrine and other physiological functions.19,26 Women were recruited via newspaper, radio or posted advertisements targeting women with severe premenstrual symptoms (MRMD women) or without premenstrual symptoms (non-MRMD women). Advertisements specifically targeting non-MRMD women with abuse histories were necessary to ensure roughly equivalent percentages of CSA in the two diagnostic groups.
During an enrollment visit all women were evaluated for medical history, including MA and MO using the International Classification of Headache Disorders, 2nd edition (ICHD-II) criteria,2 for Diagnostic and Statistical Manual of Mental disorders, 4th edition (DSM-IV) Axis 1 psychiatric disorders using the Mini-international neuropsychiatric interview (MINI) structured psychiatric interview,20,27 and for histories of CSA using a validated structured interview28 (see below for description of instruments).
Based on interviews, all women were in good health, without current chronic medical conditions or current DSM-IV Axis 1 psychiatric disorders. None of the subjects was taking prescription medication, including oral contraceptives or psychotropic medications. The enrollment visit was followed by the prospective evaluation for two to three menstrual cycles of (1) MRMD status using the Daily Record of Severity of Problems (DRSP), which all women completed on a daily basis29; and (2) diagnosis of MO or MA.2 During a subsequent study visit, a history of CSA was assessed by a trained interviewer.28 All interviews were later reviewed and coded by an investigator (J.L.) who was blind to MRMD status.
MRMD diagnosis
MRMD status was established using the well-validated DRSP that allows for the quantification of the severity of physical, emotional and behavioral symptoms using a six-point scale (1, absent; 2, minimal; 3, mild; 4, moderate; 5, severe; 6, extreme).29 In order to discourage retrospective reporting, forms were mailed back weekly. To classify participants with MRMD, each met the following criteria: (1) at least a 30% decrease in emotional symptom severity between the seven luteal phase days preceding menses compared with follicular phase days 4–10; (2) a rating of emotional symptoms as moderate, severe or extreme on at least two of the seven premenstrual days; (3) remission of symptoms shortly after the onset of menses followed by a clear symptom-free period (≥6 consecutive days) during the early-to-mid follicular phase; and (4) criteria 1–3 met in at least two menstrual cycles.29,30 Non-MRMD women met the following criteria: (1) no more than minimal emotional symptoms occurring on fewer than three days during the premenstrual week; (2) less than a 30% decrease in emotional symptom severity from the luteal to the follicular phase; and (3) these criteria met in at least two menstrual cycles.
In all, 316 women presented as MRMD and 86 as non-MRMD. However, based on prospective evaluation with the DRSP, 96 (31%) women that presented as MRMD met the MRMD criteria and 78 (91%) women that presented as non-MRMD met non-MRMD criteria and were studied.
Psychiatric histories
Histories of major depressive disorder, anxiety disorders (panic disorder, generalized anxiety disorder, social phobia, and agoraphobia) and alcohol and/or substance abuse and/or dependence were evaluated using the MINI Psychiatric interview.27 For analytical purposes, histories of all anxiety disorders were considered together.
Migraines
During the enrollment visit, women were interviewed for current and past history of MA and MO. They were also instructed to complete headache diaries that assessed headache duration, location, quality, severity, association with routine physical activity, as well concomitant symptoms, such as nausea, vomiting, sensitivity to light and sounds. Women were also asked about aura symptoms, including visual, sensory and speech symptoms. This allowed for the prospective diagnosis of MA or MO according to ICHD-II criteria.2
Abuse histories
A history of CSA was verified using a validated structured interview.28 Sexual abuse included the following experiences: (a) sexual touching with hands, mouth, or objects; (b) making the subject touch the perpetrator with hands, mouth, or objects; and (c) making the subject have vaginal or anal intercourse. To code for CSA, the subject had to be younger than 13 years of age at the time of the first episode of sexual abuse. Either force or threat of harm was required to meet criteria for CSA, unless it was implied by the age differential between perpetrator and victim. This measure of SA history has been associated with multiple other measures of poor mental and physical health status in previous studies.28,31 In addition, this instrument establishes SA history by using behaviorally specific questions. This type of methodology has been associated with more reliable and valid responses and with greater reported rates of abuse when compared with pencil-and-paper assessments.32 Based on large population-based surveys, most prevalence estimates of lifetime sexual abuse range between 15% and 25% in the general female population,32 with slightly lower rates of CSA. Estimates of sexual abuse are higher when surveying female patients with pain or psychiatric illness.32 Surveys using behaviorally specific questions like the one employed in the current study give the most accurate estimates since relying on documented cases underreports prevalence.
According to MRMD status and CSA histories, women were classified into one of four subgroups: (1) MRMD women with CSA histories (n=21; 12%); (2) MRMD women without CSA histories (n=75; 43%); (3) non-MRMD women with CSA histories (n=16; 9%); and (4) non-MRMD women without CSA histories (n=62; 36%).
The protocol was approved by the University of North Carolina at Chapel Hill Committee on the Protection of the Rights of Human Subjects.
Statistical analyses
All continuous data are presented as means±standard deviations, all categorical data as number and percent. Continuous variables were distributed normally according to the Kolmogorov-Smirnov test.
First, by using one-way analysis of variance (ANOVA) and Pearson Chi-square tests, we evaluated differences in socio-demographic status (age, education, and race), psychiatric histories (depression, anxiety, and alcohol and/or substance abuse and/or dependence) and distributional differences in the percent of women meeting migraine criteria between the four subgroups of women stratified by MRMD status and CSA histories. Significant omnibus differences were followed by post hoc analyses using the Fisher exact test. Next, by performing univariate binary logistic regression analyses we investigated the risk for any migraine, MO, and MA associated with a MRMD diagnosis only, with CSA history only, and with the interaction of a MRMD diagnosis and CSA history. Significant univariate associations were adjusted for socio-demographic factors and psychiatric histories that were different between the four subgroups of women. Data are presented as odds ratios with 95% confidence intervals.
PASW Statistics 18 package was used for data analysis. A criterion for statistical significance was chosen as a two-tailed p value of less than 0.05.
Results
Sociodemographic characteristics and psychiatric histories of all study patients and stratified by MRMD and CSA status are presented in Table 1. The mean age of the women was 33.9±7.6 years. There were no statistically significant differences in age (p=0.42), education (p=0.12), race (p=0.90) or histories of anxiety disorders between the four subgroups of women stratified by MRMD and CSA status (Table 1). There were significant differences in rates of depression histories [Pearson Chi-square (3, 173)=9.14, p=0.03] and alcohol and/or substance abuse and/or dependence [Pearson Chi-square (3, 173)=25.70, p<0.001] between the four subgroups of women stratified by MRMD and CSA status (Table 1). Post hoc analyses revealed that depression histories were associated with CSA histories in non-MRMD women (p=0.005), but not in MRMD women (p=0.27). Histories of alcohol and/or substance abuse and/or dependence were associated with CSA histories in both diagnostic groups (p=0.001 for MRMD and for non-MRMD women).
Table 1.
Sociodemographic and Psychiatric Characteristics of All Study Participants and Stratified by MRMD and Childhood Sexual Abuse Status
| |
All women |
MRMD |
Non-MRMD |
||
|---|---|---|---|---|---|
| N=174 | CSA (n=21) | No CSA (n=75) | CSA (n=16) | No CSA (n=62) | |
| Age, years | 33.9±7.6 | 33.0±7.2 | 33.3±7.8 | 32.9±6.3 | 35.2±7.8 |
| Educationa | 3.1±0.8 | 2.8±0.7 | 3.0±0.8 | 2.9±0.9 | 3.2±0.8 |
| Caucasians, n (%) | 110 (63) | 14 (67) | 49 (65) | 10 (63) | 37 (60) |
| Depression historyb | 61 (35) | 11 (52) | 26 (35) | 9 (56) | 15 (24) |
| Anxiety history, n (%) | 28 (16) | 3 (14) | 13 (17) | 4 (25) | 8 (13) |
| Alcohol and/or substance abuse and/or dependence history, n (%)c | 28 (16) | 9 (43) | 7 (9) | 7 (44) | 5 (8) |
1=less than a high school education; 2=high school degree; 3=college degree; and 4=post-graduate degree.
Non-MRMD women only: CSA>no-CSA, p<0.05.
CSA>no-CSA, p<0.05.
CSA, childhood sexual abuse; MRMD, menstrually related mood disorders.
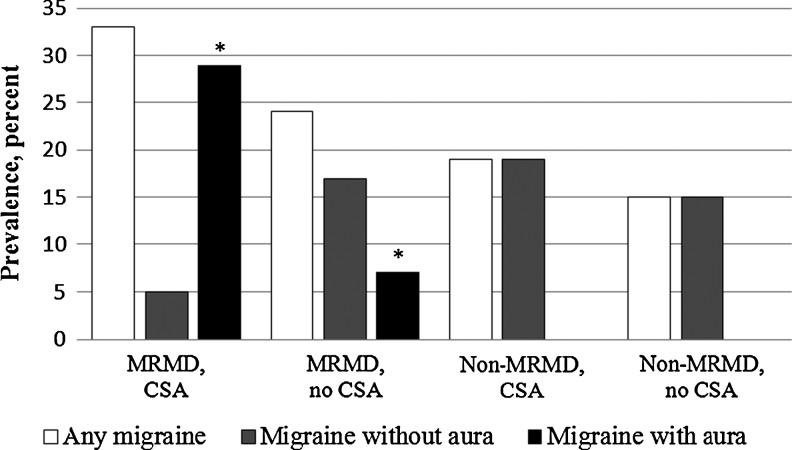
Of the entire sample, 37 (21%) women were migraineurs: 26 (15%) had MO and 11 (6%) had MA. All auras were visual and were not necessarily present during every migraine attack. More women with MRMD compared to non-MRMD women were migraineurs (25/88 and 12/86, respectively; Pearson Chi-square (1, 173)=5.43, p=0.02) and met criteria for MA (11/88 and 0/86; Pearson Chi-square (1, 173)=11.48, p=0.001), but not MO (14/88 and 12/86; Pearson Chi-square (1, 173)=0.13, p=0.72). When examined as a function of CSA, there was a statistically significant difference in percent of the sample with MA between the four subgroups of women [Pearson Chi-square (3, 173)=22.83, p<0.001], because in MRMD women, more women with CSA had MA compared to women without CSA histories (6/21 and 5/67, respectively; Fisher exact test, p=0.019) (see Fig. 1). None of the non-MRMD women had MA, regardless of CSA history. There were no differences in rates of any migraines [Pearson Chi-square (3, 173)=3.91, p=0.27) or MO (Pearson Chi-square (3, 173)=2.24, p=0.52] as a function of CSA histories (Fig. 1).
FIG. 1.
Percent of the sample with any migraine, migraine without aura and migraine with aura in women stratified by MRMD status and CSA histories. CSA, childhood sexual abuse; MRMD, menstrually related mood disorders. *Fisher exact test, p=0.019.
In univarite binary logistic regression analyses, current MRMD was associated with increased risk for any migraine (odds ratio [OR]=2.45, 95% confidence interval [CI] 1.14–5.26, p=0.02), while the interaction of CSA with MRMD status (OR=11.84, 95%CI 3.23–43.45, p<0.001) was associated with increased risk for MA (Table 2). After adjusting for histories of depression and alcohol and/or substance abuse and/or dependence history (see Table 1), current MRMD diagnosis remained an independent predictor of increased risk for any migraine disorder (OR=2.24, 95%CI 1.02–4.85, p=0.04), and the interaction of CSA history with MRMD diagnosis remained a significant predictor of MA (OR=12.08, 95%CI 2.98–48.90, p<0.001).
Table 2.
The Associations of MRMD Diagnosis, Childhood Sexual, Abuse History, and Their Interactions With Any Migraine, Migraine With Aura, and Migraine Without Aura in Univariate and Multivariate Regression Analyses
| Any migraine OR (95% CI) | Migraine with aura OR (95% CI) | Migraine without aura OR (95% CI) | |
|---|---|---|---|
| Univariate association | |||
| MRMD only | 2.45 (1.14–5.26), p=0.02 | N/A | 1.17 (0.51–2.69), p=0.72 |
| CSA history only | 1.51 (0.65–3.49), p=0.33 | N/A | 0.63 (0.20–1.97), p=0.43 |
| MRMD and CSA | 2.1 (0.76–5.52), p=0.16 | 11.84 (3.23–43.45), p<0.001 | 0.19 (0.03–1.99), p=0.19 |
| Multivariate associationa | |||
| MRMD only | 2.24 (1.02–4.85), p=0.04 | - | - |
| CSA history only | - | - | - |
| MRMD and CSA | - | 12.08 (2.98–48.90), p<0.001 | - |
Adjusted for histories of depression and alcohol and/or substance abuse and/or dependence (binary logistic regression: enter).
Discussion
A chief finding of the present study was that in our cohort, only MRMD women suffered from MA, and only in those women a greater prevalence of MA was associated with CSA histories. Specifically, the combination of a current diagnosis of MRMD and a history of CSA was associated with the greatest risk for MA, even after adjusting for histories of depression and substance abuse.
A potential pathophysiological mechanism contributing to the association of MA with MRMD may involve female reproductive hormones since reproductive hormones are implicated in both MRMD22 and MA.8,11,12 For example, during pregnancy, when estrogen concentrations are steadily increasing and the cyclic declines are eliminated, there is an increased risk for first episode MA33 as well as worsening of MA.34 Also, the use of combined oral contraceptives (OCs) is associated with worsening of MA.9 In contrast, continuous treatment with ultra-low dose combined hormonal contraceptives (15μg ethinyl estradiol per 24 hours) decreases the frequency of aura presumably by decreasing endogenous estrogen production, thereby leading to sustained low concentrations of estrogen.11 Estrogens also increase neuronal excitability and are implicated in the development and propagation of cortical spreading depression,12 a putative pathophysiological mechanism underlying MA. In a similar vein, substantial divergent evidence supports a role for reproductive hormones in MRMDs. For example, pharmacologically induced hypogonadism eliminates symptoms in women with a MRMD, while the add-back of estrogen or progesterone results in the return of symptoms in MRMD women but not in controls.22 Additionally, while traditional OCs involving 21 days of active pills and 7 days of placebo have not proven to be effective in MRMD,35 more recent research has shown low dose OCs with extended hormone delivery to be effective in reducing premenstrual symptom severity,36–38 presumably by decreasing endogenous gonadal hormone production and cyclicity.
It is well documented that migraine, especially MA, is highly comorbid with psychiatric disorders, including major depression.39 The bidirectional nature of the association between MA and major depression suggests a common neurobiology between the two disorders. However, to the best of our knowledge, this is the first report of the comorbidity between MA and MRMDs, even after controlling for histories of depression. This comorbidity may be partially accounted for by an overlapping genetic predisposition for both disorders, since the same susceptibility genes are implicated in MRMD and MA. For example, ESR1 polymorphisms are a significant risk factor for MA, especially in women,25 and are also implicated in MRMDs.24 Thus, MRMDs and MA might share a common genetic predisposition with respect to a steroid hormone receptor, which is pathogenically relevant in both disorders and might play a role in cortical spreading depression. However, further studies addressing these assumptions are needed.
Our finding that CSA predicted MA, but only in MRMD women, is consistent with previous work showing that a history of abuse has different biological sequelae in MRMD versus non-MRMD women.17,18 For example, we have previously found that histories of abuse were associated with greater β-adrenergic receptor responsivity in MRMD women but not in non-MRMD women with an abuse history.18 The role of altered β-adrenergic receptor function in migraine is supported by the substantial evidence that β-adrenergic receptor blockers are probably the most commonly used drugs for migraine prophylaxis.40 However, not all migraineurs are responsive to propranolol41, suggesting a heterogeneity in the pathophysiological mechanisms contributing to migraines, and hence, in therapeutic response. An intriguing possibility that remains to be explored is that a history of MRMD or CSA may predict efficacy of beta-adrenergic blockade for migraine prophylaxis.
Another possible mechanism linking CSA histories and MA involves female reproductive hormones, since women with childhood trauma have elevated concentrations of follicle stimulating hormone as well as elevated concentrations of dehydroepiandrosterone, a precursor of female reproductive hormones, when compared to women without such experiences.42,43 Thus, dysregulation in the female reproductive axis associated with CSA could consequently contribute to the development of female reproductive hormone dependent disorders in vulnerable women, including MRMD and migraine. In addition to any effect that childhood adverse experiences may have on the reproductive axis, childhood maltreatment has also been shown to correlate with biomarkers of endothelial dysfunction and inflammation.16 Thus, childhood maltreatment may contribute to deleterious alterations in endothelial function and inflammatory responses, thereby contributing to the migraine-associated increased susceptibility for stroke.4,5 Genotype can also moderate the effect that childhood maltreatment has on long-term mental health outcomes.42,44 Hence, a possible gene–environment interaction in the development of MA and MRMDS, and a possible role of CSA, remains to be examined in future studies. Nonetheless, the results of this study add to growing evidence that MA and MRMDs may be considered additional mental and physical health outcomes associated with childhood adversity. Routine screening for CSA can potentially identify women at increased risk to experience abuse-related disorders, and aid in the management of those disorders.
While the use of validated diagnostic instruments to determine migraine, MRMD and CSA are significant strengths of this study, and enhance the reliability of our findings, this study is not without its limitations. These include a moderate sample size, rendering our results as preliminary, and the potential lack of generalizability to the population of women without MRMD since we over sampled for histories of abuse in that group. Future research should assess female reproductive hormones and vascular biomarkers in relation to migraine characteristics and abuse histories in women with MRMD in order to shed light on potential biological mechanisms mediating the link between CSA and MA.
Conclusions
In summary, these preliminary results suggest that MRMDs are associated with migraine, particularly MA. Moreover, a CSA history predicted MA in MRMD women, even after controlling for potential confounders. These findings indirectly suggest that MA and MRMD might share common pathophysiology and genetic predispositions, and that CSA may moderate the link between MRMD and MA. If confirmed in a larger sample, these results would not only have implications for individualizing prevention and treatment of migraine, particularly MA, in MRMD patients, but would also suggest that administration of carefully selected hormonal therapies or β-adrenergic receptor blockers might be considered in MRMD women with CSA histories.
Acknowledgment
This study was supported by the National Institute of Mental Health (grant numbers: R01 MH051246, RO1 MH081837, and UL 1RR025747) and The Foundation of Hope for the Research and Treatment of Mental Illness.
Author Disclosure Statement
Anne Calhoun, MD, receives research support from GlaxoSmithKline. She is on the advisory board as a consultant for Ferring, GlaxoSmithKline, MAP, Nautilus, and Zogenix. Additionally, AC belongs to the speakers bureau for GlaxoSmithKline, MAP, Nautilus, and Zogenix. For the remaining authors, no financial conflicts exist.
References
- 1.Stewart WF. Wood C. Reed ML. Roy J. Lipton RB. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28:1170–1178. doi: 10.1111/j.1468-2982.2008.01666.x. [DOI] [PubMed] [Google Scholar]
- 2.Headache Classification Subcommittee of the International Headache Society. Cephalalgia. 2nd. Suppl 1. Vol. 24. The International Classification of Headache Disorders; 2004. pp. 9–160. [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen BK. Olesen J. Migraine epidemiology. Cephalalgia. 1993;13:216–217. doi: 10.1046/j.1468-2982.1993.1303214.x. [DOI] [PubMed] [Google Scholar]
- 4.Etminan M. Takkouche B. Isorna FC. Samii A. Risk of ischaemic stroke in people with migraine: Systematic review and meta-analysis of observational studies. BMJ. 2005;330:63. doi: 10.1136/bmj.38302.504063.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurth T. The association of migraine with ischemic stroke. Curr Neurol Neurosci Rep. 2010;10:133–139. doi: 10.1007/s11910-010-0098-2. [DOI] [PubMed] [Google Scholar]
- 6.Serva WA. Serva VM. de Fatima Costa Caminha M, et al. Course of migraine during pregnancy among migraine sufferers before pregnancy. Arq Neuropsiquiatr. 2011;69:613–619. doi: 10.1590/s0004-282x2011000500008. [DOI] [PubMed] [Google Scholar]
- 7.Martin VT. Lipton RB. Epidemiology and biology of menstrual migraine. Headache. 2008;48(Suppl 3):S124–130. doi: 10.1111/j.1526-4610.2008.01310.x. [DOI] [PubMed] [Google Scholar]
- 8.MacGregor EA. Oestrogen and attacks of migraine with and without aura. Lancet Neurol. 2004;3:354–361. doi: 10.1016/S1474-4422(04)00768-9. [DOI] [PubMed] [Google Scholar]
- 9.Granella F. Sances G. Pucci E. Nappi RE. Ghiotto N. Napp G. Migraine with aura and reproductive life events: A case control study. Cephalalgia. 2000;20:701–707. doi: 10.1111/j.1468-2982.2000.00112.x. [DOI] [PubMed] [Google Scholar]
- 10.Nagel-Leiby S. Welch KM. Grunfeld S. D'Andrea G. Ovarian steroid levels in migraine with and without aura. Cephalalgia. 1990;10:147–152. doi: 10.1046/j.1468-2982.1990.1003147.x. [DOI] [PubMed] [Google Scholar]
- 11.Calhoun A. Combined hormonal contraceptives: is it time to reassess their role in migraine? Headache. 2012;52:648–660. doi: 10.1111/j.1526-4610.2011.02051.x. [DOI] [PubMed] [Google Scholar]
- 12.Eikermann-Haerter K. Kudo C. Moskowitz MA. Cortical spreading depression and estrogen. Headache. 2007;47(Suppl 2):S79–85. doi: 10.1111/j.1526-4610.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- 13.Tietjen GE. Peterlin BL. Childhood abuse and migraine: epidemiology, sex differences, and potential mechanisms. Headache. 2011;51:869–879. doi: 10.1111/j.1526-4610.2011.01906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuh JL. Wang SJ. Juang KD. Lu SR. Liao YC. Chen SP. Relationship between childhood physical maltreatment and migraine in adolescents. Headache. 2010;50:761–768. doi: 10.1111/j.1526-4610.2010.01639.x. [DOI] [PubMed] [Google Scholar]
- 15.Tietjen GE. Brandes JL. Peterlin BL, et al. Childhood maltreatment and migraine (part II). Emotional abuse as a risk factor for headache chronification. Headache. 2010;50:32–41. doi: 10.1111/j.1526-4610.2009.01557.x. [DOI] [PubMed] [Google Scholar]
- 16.Tietjen GE. Khubchandani J. Herial NA. Shah K. Adverse childhood experiences are associated with migraine and vascular biomarkers. Headache. 2012;52:920–929. doi: 10.1111/j.1526-4610.2012.02165.x. [DOI] [PubMed] [Google Scholar]
- 17.Girdler SS. Leserman J. Bunevicius R. Klatzkin R. Pedersen CA. Light KC. Persistent alterations in biological profiles in women with abuse histories: influence of premenstrual dysphoric disorder. Health Psychol. 2007;26:201–213. doi: 10.1037/0278-6133.26.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girdler SS. Sherwood A. Hinderliter AL, et al. Biological correlates of abuse in women with premenstrual dysphoric disorder and healthy controls. Psychosom Med. 2003;65:849–856. doi: 10.1097/01.psy.0000088593.38201.cd. [DOI] [PubMed] [Google Scholar]
- 19.Fleischman DS. Bunevicius A. Leserman J. Girdler SS. Menstrually Related mood disorders and a history of abuse: Moderators of pain sensitivity. Health Psychol. 2013 doi: 10.1037/a0031900. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4th. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 21.Cunningham J. Yonkers KA. O'Brien S. Eriksson E. Update on research and treatment of premenstrual dysphoric disorder. Harvard Rev Psychiatry. 2009;17:120–137. doi: 10.1080/10673220902891836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt PJ. Nieman LK. Danaceau MA. Adams LF. Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. New Engl J Med. 1998;338:209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- 23.MacGregor EA. Perimenstrual headaches: Unmet needs. Curr Pain Headache Rep. 2008;12:468–474. doi: 10.1007/s11916-008-0079-1. [DOI] [PubMed] [Google Scholar]
- 24.Huo L. Straub RE. Roca C, et al. Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry. 2007;62:925–933. doi: 10.1016/j.biopsych.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joshi G. Pradhan S. Mittal B. Role of the oestrogen receptor (ESR1 PvuII and ESR1 325 C->G) and progesterone receptor (PROGINS) polymorphisms in genetic susceptibility to migraine in a North Indian population. Cephalalgia. 2010;30:311–320. doi: 10.1111/j.1468-2982.2009.01967.x. [DOI] [PubMed] [Google Scholar]
- 26.Bunevicius A. Leserman J. Girdler SS. Hypothalamic-pituitary-thyroid axis function in women with a menstrually related mood disorder: association with histories of sexual abuse. Psychosom Med. 2012;74:810–816. doi: 10.1097/PSY.0b013e31826c3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheehan DV. Lecrubier Y. Sheehan KH, et al. The mini-international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 28.Leserman J. Drossman DA. Li Z. Toomey TC. Nachman G. Glogau L. Sexual and physical abuse history in gastroenterology practice: How types of abuse impact health status. Psychosom Med. 1996;58:4–15. doi: 10.1097/00006842-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Endicott J. Nee J. Harrison W. Daily record of severity of problems (DRSP): Reliability and validity. Arch Womens Ment Health. 2006;9:41–49. doi: 10.1007/s00737-005-0103-y. [DOI] [PubMed] [Google Scholar]
- 30.Rubinow DR. Roy-Byrne P. Hoban MC. Gold PW. Post RM. Prospective assessment of menstrually related mood disorders. Am J Psychiatry. 1984;141:684–686. doi: 10.1176/ajp.141.5.684. [DOI] [PubMed] [Google Scholar]
- 31.Girdler SS. Pedersen CA. Straneva PA, et al. Dysregulation of cardiovascular and neuroendocrine responses to stress in premenstrual dysphoric disorder. Psychiatry Res. 1998;81:163–178. doi: 10.1016/s0165-1781(98)00074-2. [DOI] [PubMed] [Google Scholar]
- 32.Leserman J. Sexual abuse history: Prevalence, health effects, mediators, and psychological treatment. Psychosom Med. 2005;67:906–915. doi: 10.1097/01.psy.0000188405.54425.20. [DOI] [PubMed] [Google Scholar]
- 33.Cupini LM. Matteis M. Troisi E. Calabresi P. Bernardi G. Silvestrini M. Sex-hormone-related events in migrainous females. A clinical comparative study between migraine with aura and migraine without aura. Cephalalgia. 1995;15:140–144. doi: 10.1046/j.1468-2982.1995.015002140.x. [DOI] [PubMed] [Google Scholar]
- 34.Aube M. Migraine in pregnancy. Neurology. 1999;53:S26–28. [PubMed] [Google Scholar]
- 35.Freeman EW. Kroll R. Rapkin A, et al. Evaluation of a unique oral contraceptive in the treatment of premenstrual dysphoric disorder. J Womens Health Gend Based Med. 2001;10:561–569. doi: 10.1089/15246090152543148. [DOI] [PubMed] [Google Scholar]
- 36.Freeman EW. Halbreich U. Grubb GS, et al. An overview of four studies of a continuous oral contraceptive (levonorgestrel 90 mcg/ethinyl estradiol 20 mcg) on premenstrual dysphoric disorder and premenstrual syndrome. Contraception. 2012;85:437–445. doi: 10.1016/j.contraception.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Lopez LM. Kaptein AA. Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;2:CD006586. doi: 10.1002/14651858.CD006586.pub4. [DOI] [PubMed] [Google Scholar]
- 38.Pearlstein TB. Bachmann GA. Zacur HA. Yonkers KA. Treatment of premenstrual dysphoric disorder with a new drospirenone-containing oral contraceptive formulation. Contraception. 2005;72:414–421. doi: 10.1016/j.contraception.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 39.Frediani F. Villani V. Migraine and depression. Neurol Sci. 2007;28(Suppl 2):S161–165. doi: 10.1007/s10072-007-0771-7. [DOI] [PubMed] [Google Scholar]
- 40.Silberstein SD. Treatment recommendations for migraine. Nat Clin Pract Neurol. 2008;4:482–489. doi: 10.1038/ncpneuro0861. [DOI] [PubMed] [Google Scholar]
- 41.Linde K. Rossnagel K. Propranolol for migraine prophylaxis. Cochrane Database Syst Rev. 2004:CD003225. doi: 10.1002/14651858.CD003225.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Alemany S. Arias B. Aguilera M, et al. Childhood abuse, the BDNF-Val66Met polymorphism and adult psychotic-like experiences. Br J Psychiatry. 2011;199:38–42. doi: 10.1192/bjp.bp.110.083808. [DOI] [PubMed] [Google Scholar]
- 43.Kellner M. Muhtz C. Peter F. Dunker S. Wiedemann K. Yassouridis A. Increased DHEA and DHEA-S plasma levels in patients with post-traumatic stress disorder and a history of childhood abuse. J Psychiatric Res. 2010;44:215–219. doi: 10.1016/j.jpsychires.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Caspi A. McClay J. Moffitt TE, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]