Abstract
In this study, we isolated and characterized spontaneously differentiated human embryonic stem cells (SD-hESCs) found in hESC colonies in comparison to the morphologically premature ESCs in the colonies to investigate the potential role of SD-hESCs in embryogenesis. SD-hESCs were distinguished from undifferentiated hESCs by their higher expression of GATA6, a marker for primitive endoderm and transthyretin, a marker visceral endoderm in embryoid bodies (EBs). SD-hESCs expressed OCT4 and NANOG, markers for pluripotent stem cells, at significantly lower levels than undifferentiated hESCs. EBs derived from isolated SD-hESCs were morphologically distinct from cells directly derived from the undifferentiated hESCs; they contained higher number of cysts compared to EBs from undifferentiated hESC-derived EBs (42% vs. 20%). Furthermore, the extracellular signal molecule, BMP2/4, induced a higher GATA4/6 expression and cystic EB formation than control and noggin-treated EBs. Since cystic formation in EBs play a role in primitive endoderm formation during embryogenesis, the SD-hESC may be a relevant cell type equipped to differentiate into primitive endoderm. Our results suggest that SD-ESCs generated during routine hESC culture are not just an artifact of in vitro culture and these cells could serve as a useful model to study the process of embryogenesis.
Introduction
Pluripotent human embryonic stem cell (hESC) lines are derived from the inner cell mass (ICM) of preimplantation embryos [1]. The ICM is a group of cells found in the mammalian blastocyst, which gives rise to the embryo and is potentially capable of forming all embryonic and extraembryonic tissues, except the trophoblast. As the cells of the ICM become rearranged into an epithelial configuration, sometimes they are referred to as the embryonic shield, a thin layer of cells appearing ventral to the main cellular mass. The main upper layer of cells is known as the epiblast, and the lower layer is called the hypoblast or primitive endoderm. The hypoblast is considered as an extraembryonic endoderm, and it ultimately gives rise to the mesodermal lining of the yolk sac. After the hypoblast has become a well-defined layer and the epiblast has taken on an epithelial configuration, the former ICM is transformed into a bilaminar disk, with the epiblast and hypoblast on the dorsal and ventral surface, respectively. The epiblast contains the cells that will make up the embryo itself, but extraembryonic tissues also arise from this layer. The next layer to appear after the hypoblast is the amnion, a layer of extraembryonic ectoderm that ultimately encloses the entire embryo in a fluid-filled chamber called the amniotic cavity [2]. When cultured as aggregated hESCs to form embryoid bodies (EBs), the structures recapitulate the early steps of preimplantation development [3], including the formation of extraembryonic endoderm on the surface of the ICM, and the columnar epithelium with a central cavity [4]. Upon differentiation of hESCs, extraembryonic endoderm markers such as GATA-4, GATA-6, and transthyretin (TTR) are induced, and the stem cell marker OCT-3/4 is diminished. Expression of GATA-4 and GATA-6, which are zinc finger transcriptional activators that bind to the consensus DNA sequence (A/T)GATA(A/G) [5], is restricted to the primitive endoderm and visceral endoderm of the extraembryonic tissues [6–9]. Thus, members of the GATA family are key transcription factors in the formation of extraembryonic endoderm.
When hESC lines are cultured on feeder cells, they form dense clusters of cells (colonies) composed of morphologically and phenotypically heterogeneous cell populations [3,10]. While most colonies of hESCs remain undifferentiated, a portion loses its self-renewal capacity by spontaneously differentiating (denoted here as SD-hESCs). Whereas undifferentiated hESCs are largely confined to the core areas within the colonies, SD-hESCs are positioned surrounding the core of undifferentiated hESCs, with fibroblast-like cell morphology [11]. Formation of the cell complex referred to as an EB structure appears as an intrinsic feature of hESCs and pluripotent stem cell lines. They subsequently convert to heterogeneous cell populations composed of several cell lineages. Induced human pluripotent stem cell lines are also able to form colonies composed of morphologically heterogeneous cell types, including SD-hESCs, which are similar to that seen in conventional hESC cultures [12–14]. It is not known if SD-hESCs are biologically relevant, or if they are distinct cell types that may play a role in embryogenesis. Information obtained from studies of SD-hESCs could be important for improving the efficiency of differentiation as well as for increasing/maintaining pluripotency of hESCs during culture. We have now characterized SD-hESCs and compared them to undifferentiated hESCs for their developmental status at the phenotypic and gene levels using mechanically isolated SD-hESCs from undifferentiated hESC colonies after culture for different time periods.
Our results indicate that the SD-hESCs isolated from undifferentiated hESCs more efficiently develop into primitive endoderm lineage cells than do undifferentiated hESCs. Moreover, EBs derived from isolated SD-hESCs have higher levels of cavities compared to EBs derived directly from undifferentiated hESCs. This suggests that SD-hESCs may be biologically important with the capacity to differentiate into developmentally relevant EB structures, and are likely not simply artifactual cell types artificially generated during in vitro culture.
Materials and Methods
hESC maintenance and formation of EBs
The undifferentiated hESC line H9 was cultured according to protocols from the WiCell Research Institute. As we have previously reported [15,16], hESC line H9 was plated as mechanically isolated colonies and cultured on a feeder layer of mouse embryonic fibroblasts (MEFs), which were first inactivated with 10 μg/mL mitomycin C (seeded at 2×105/35-mm dish) with a daily change of medium that consisted of the Dulbecco's modified Eagle's medium (DMEM)/F12, 20% serum replacement, 1 mM glutamine, 0.1% nonessential amino acids, 0.1% penicillin/streptomycin, 0.1 mM β-mercaptoethanol, and 4 ng/mL recombinant human fibroblast growth factor 2 (basic) (FGF-2) (all supplements were purchased from Invitrogen Corporation) and maintained in 5% CO2 and air. For passaging, hESC colonies were mechanically detached with a glass pipette during transfer to reduce the number of differentiated cells per colony clump. This was done at a 1:2 or 1:3 split ratio. To form EBs with undifferentiated and SD-hESCs, we first separately isolated SD-hESCs from undifferentiated hESCs in the hESC colonies, and then individually seeded each cell type to nonadhesive bacterial dishes and cultured them in hESC media without FGF-2 and in the absence of MEFs.
RNA isolation and RNA expression analysis
Total RNA was extracted from cultured cells using the Trizol reagent (Invitrogen), according to the manufacturer's protocol. One microgram of DNase-I-treated total RNA was reverse transcribed using random priming and Superscript II reverse transcriptase (Invitrogen) according to the manufacturer's instructions. Standard polymerase chain reaction (PCR) conditions were as follows: 10 min at 94°C; followed by cycles of 30 s denatured at 94°C, 30 s annealing at 55°C, and 30 s extension at 72°C. Primer sequences are as follows: GATA6 forward-aaaaagagggaattcaaacc, GATA6 reverse-cctatgtagagcccatcttg; GATA4 forward-ctccttcaggcagtgagagc, GATA4 reverse-gagatgcagtgtgctcgtgc; NEUROD1 forward-attctaagacgcagaagctg, NEUROD1 reverse-actggtaggagtaggggtgt; HAND1 forward-ctccaagatcaagactctgc, HAND1 reverse-gcgtcctttaatcctcttct; AFP forward-agaacctgtcacaagctgtg, AFP reverse-gacagcaagctgaggatgtc; DCN forward-gagctcaggaattgaaaatg, DCN reverse-aagcttgttgttgtccaagt; TTR forward-gtgcatgtgttcagaaaggctgct, TTR reverse-agtcgttggctgtgaataccacct; NANOG forward-caaaggcaaacaacccactt, NANOG reverse-tctgctggaggctgaggtat; OCT4 forward-aactcgagcaatttgccaagctcc, OCT4 reverse-aactcgagcaatttgccaagctcc; SOX2 forward-gccgagtggaaacttttgtcg, SOX2 reverse-gcagcgtgtacttatccttctt; β-ACTIN forward-caggagatggccactgccgca, and β-ACTIN reverse-tccttctgcatcctgtcagca.
Western blotting
hESCs or differentiated hESCs were harvested at the indicated times and lysed with the lysis buffer (20 mM Tris-HCl, pH 7.5, 1 mM EGTA, 1 mM EDTA, 1 mM β-glycerol phosphate, and 1% Triton X-100; Sigma) containing a protease inhibitor cocktail (PIC; Roche, Ltd.). Extracted proteins were denatured using the SDS sample buffer at 100°C for 5 min. The cell lysates were analyzed by SDS-PAGE, transferred onto polyvinylidene difluoride membranes, and immunoblotted with the following primary antibodies: mouse anti-OCT-4 (1:1,000), rabbit anti-GATA6 (1:1,000), rabbit anti-GATA4 (1:500; Santa Cruz Biotechnology), and mouse β-actin (1:2,000; Sigma).
Immunocytochemistry
Cells were fixed with 4% (w/v) paraformaldehyde for 30 min, and permeabilized with 0.1% (v/v) TritonX-100 in phosphate-buffered saline (PBS) for 5 min. After treatment with a blocking solution containing 10% (v/v) goat serum for 30 min, the cells were incubated with primary antibodies at 4°C for overnight. Antibodies used for immunocytochemistry were as follows: mouse anti-OCT-4 (1:500), rabbit anti-GATA6, and rabbit anti-TTR (1:250; BD Biosciences). After washing with PBS, the stained cells were visualized using secondary antibodies conjugated with FITC (Molecular Probe) and rhodamine (Molecular Probe) by confocal microscopy (LSM 510; Zeiss). Two micrograms per milliliter DAPI (Sigma) was added during the last wash.
Statistical analysis
All experiments were independently performed three times, each in triplicate, and data are represented as mean value±SD for statistical comparison. Significance of differences was assessed by an unpaired Student's t-test, where P<0.05 was considered significant.
Results and Discussion
hESCs differentiate spontaneously into heterogeneous cell populations during maintenance culture
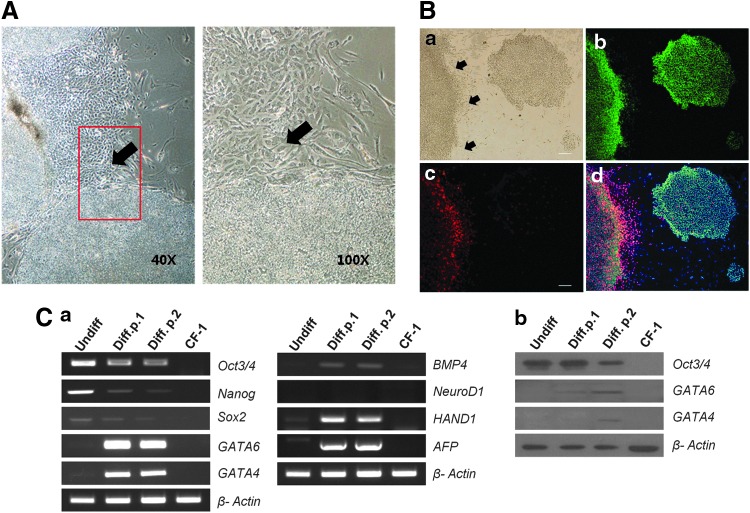
hESCs cultured on feeder layers become heterogeneous. Most remain undifferentiated, but some spontaneously give rise to differentiated cells within the same colony. SD-hESCs resemble fibroblast cells in morphology, and typically reside on the periphery of dense colonies containing morphologically undifferentiated stem cells (Fig. 1A, B; arrows indicate SD-ESCs). Immunocytochemical analysis showed that SD-hESCs found in cultured hESC colonies were positive for GATA6, a marker for primitive endoderm cells, but were negative for OCT4, a pluripotency gene (Fig. 1B-a, b). OCT4-positive (green) cells were localized within the center of the colonies and SD-hESCs surrounding the colonies expressed GATA6 (red), but not OCT4 (Fig. 1B-d). SD-hESCs were manually separated from undifferentiated hESCs using mechanical dissection based on morphological features [17,18] and these cells were subcultured separately for two passages. Real-time PCR (RT-PCR) (Fig. 1C-a) and western blot (Fig. 1C-b) analysis revealed that expression of GATA4/6 in separated SD-hESCs was upregulated in contrast to that of OCT4, SOX2, and NANOG, which was downregulated after subculture, indicating differentiation toward primitive endoderm. In contrast, expression of NEUROD1, a marker for ectoderm lineage, HAND1, a marker for mesoderm lineage and AFP, a marker for endoderm lineage were not noticeably altered, demonstrating that the subculture of SD-hESCs compared to undifferentiated hESCs resulted in changes of gene expression in a specific fashion (Fig. 1C). Subcultured SD-hESCs also expressed BMP4. The segregated specific expression patterns of OCT4 and GATA6 in hESC colonies suggest that organized differentiation was undertaken during the culture possibly into epiblast and hypoblast lineages.
FIG. 1.
hESC colonies contain two different cell types based on Oct4 or GATA6 expression. (A) Phase-contrast image of hESCs forming SD-ESCs (black arrow). The right image was magnified in the red box of the left image. (B) Phase-contrast (a) and fluorescent immune-staining (b–d) analysis was conducted after cell culture for 5 days. OCT4 (green) (b) and GATA6 (red) (c) were detected in the two distinctive colonies consisting of pluripotent and primitive endoderm cells, respectively. Nuclei were stained with DAPI (blue) (d). Scale bar represents 50 μm. (C) Expression of marker genes for pluripotency (OCT3/4, SOX2, and NANOG), primitive endoderm (GATA6 and GATA4), and three germ cell layers (NEUROD1, HAND1, BMP4, and AFP) was examined by RT-PCR (a) and western blot (b) in undifferentiated and SD-hESCs. SD-hESC, spontaneously differentiated human embryonic stem cell; RT-PCR, real-time–polymerase chain reaction. Color images available online at www.liebertpub.com/scd
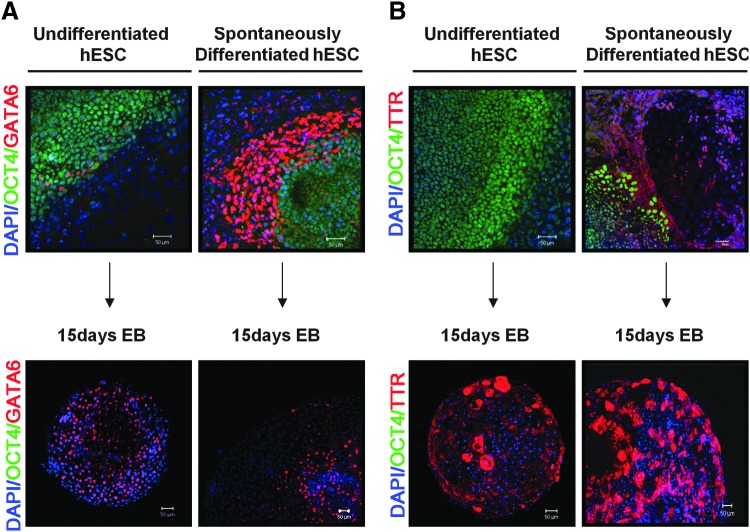
To compare the ability of SD-hESCs and undifferentiated hESCs to differentiate into primitive endoderm, we examined cellular colocalization expression patterns of GATA6 and TTR by immunostaining and confocal microscopy (Fig. 2). SD-hESCs were distinguished from undifferentiated hESCs by expressing higher levels of GATA6 indicating that SD-ESCs have already initiated differentiation into primitive endoderm. Conversely, SD-hESCs rapidly lost GATA6 as they differentiated into day 15 EBs suggesting that GATA6 expression is confined to only early stages of differentiation into primitive endoderm (Fig. 2A). Expression of TTR, a marker for visceral endoderm was not detected in undifferentiated hESCs, but was detected in SD-hESCs (Fig. 2B). TTR was highly expressed in day 15 EBs derived from SD-hESCs as well as from EBs derived from undifferentiated hESCs. The level of TTR was higher in day 15 EBs derived from SD-hESCs than the levels in undifferentiated hESCs (Fig. 2B). Neither EBs from undifferentiated hESCs, nor those from SD-hESCs, expressed OCT-4 (green), indicating that day 15 EBs have completed differentiation beyond the pluripotency stage. Thus, our results demonstrate that SD-hESCs expressed GATA6 at high levels compared to undifferentiated hESCs suggesting that SD-hESCs may initiate differentiation into primitive endoderm. Interestingly, SD-hESCs downregulated GATA6 expression, while simultaneously upregulating TTR during differentiation into day 15 EBs. This switch of GATA6 and TTR expression was more evident in the cells derived from SD-hESCs compared to those from undifferentiated hESCs. This suggests that SD-hESCs may more efficiently differentiate into primitive endoderm than cells from undifferentiated hESCs.
FIG. 2.
SD-hESCs efficiently differentiate into primitive endoderm. Undifferentiated hESCs and SD-hESCs and day-15 EBs derived from each of these respective types of hESCs were analyzed for expression of OCT4 (green; a pluripotent cell marker), GATA6 (red; a primitive endoderm marker (red) (A), and TTR (red; a visceral endoderm marker) (B). Nuclei were counterstained with DAPI (blue). Scale bar represents 50 μm. EBs, embryoid bodies; TTR, transthyretin. Color images available online at www.liebertpub.com/scd
SD-ESCs differentiate into cystic EBs
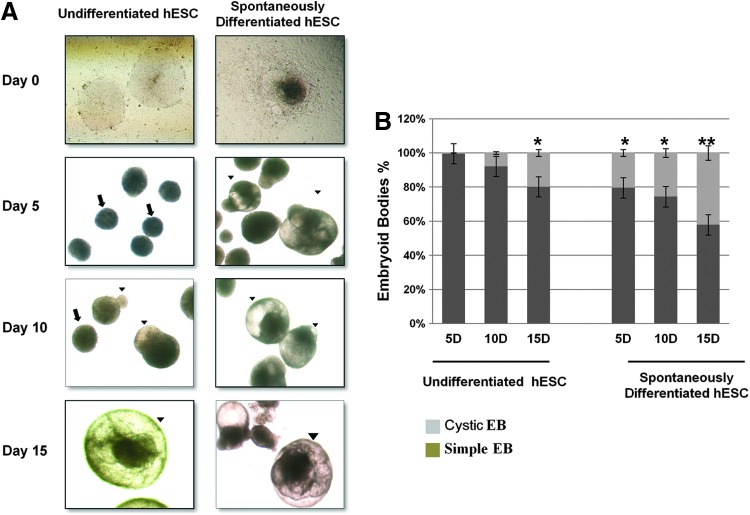
At an early developmental stage, cyst formation in EBs is an essential process for the initiation of gastrulation. To investigate whether GATA6-positive SD-hESCs were different from undifferentiated hESCs in their capacity to develop cavities in EBs, both cell types were separately cultured in suspension and induced to develop EBs. We found that SD-hESCs developed cavities in EBs more efficiently compared to EBs derived from undifferentiated hESCs. EBs from SD-ESCs contained fluid-filled chambers/cavities at times as short as 5 days after initiation of EB formation. In contrast, undifferentiated hESCs failed to form cystic EBs at 5 days of culture. When we cultured EBs derived from SD-hESCs or from undifferentiated hESCs for longer than 15 days, 42% of the SD-hESC-derived EBs exhibited cavities, while only 20% of EBs derived from undifferentiated hESCs developed cysts (Fig. 3A, B). Together, these data suggest that SD-hESCs are a good model to study appropriate differentiation of hESCs into cystic EBs in vitro.
FIG. 3.
Undifferentiated hESCs and SD-hESCs develop morphologically distinctive EBs. (A) Colonies derived from undifferentiated hESCs and SD-hESCs were mechanically separated and cultured to develop EBs and analyzed by phase-contrast imaging at different time points. Colonies derived from undifferentiated hESCs and SD-hESCs were morphologically distinctive with tightly packed cell clusters (indicated by arrows ↑) and distinct layers within EBs with fluid-filled and balloon-like cysts (indicated by arrowhead ▴). (B) Percentages of cystic EBs were counted at serial time points (5, 10, 15 days) after EB formation. Data are presented as mean values±SD from five independent experiments. Student's t-test: *p<0.05 and **p<0.01. Color images available online at www.liebertpub.com/scd
BMP signal induces cystic EB formation
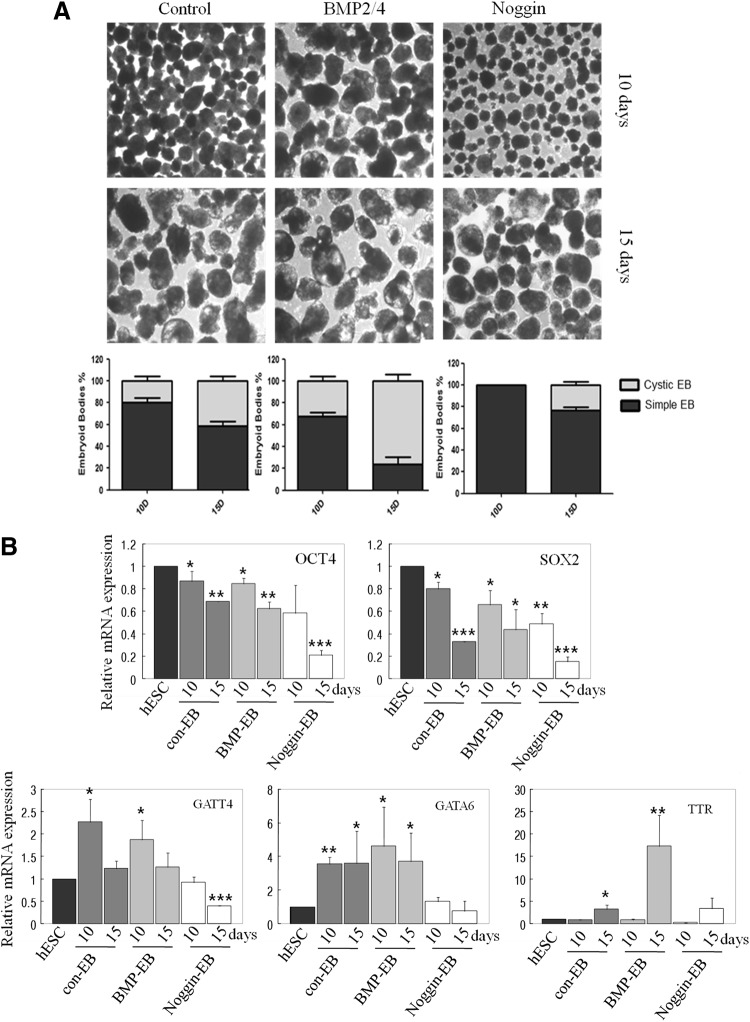
To get more insight into mechanisms of extracellular signal molecular-induced cystic EB formation, we evaluated the BMP pathway. Previous reports have demonstrated that BMP2 and 4 regulate endoderm differentiation [19–21]. To determine whether BMP signaling might be involved in cystic formation, we treated with BMP2/4 or the BMP antagonist, noggin for 3 days after we transferred undifferentiated ESCs to suspension culture. By the next day of culture, >95% of the aggregates differentiated, giving rise to simple EBs with an outer layer. As shown in Figure 4, BMP2/4 treatment resulted in induction of cystic EBs at 10 and 15 days. However, with noggin treatment, the number of cystic EBs was less than control. RT-PCR data showed that GATA4 and 6 expression were increased in 10-day EBs, but reduced in 15-day EBs. However, expression of TTR still increased in late EBs. This suggests that BMP signals are involved in cystic formation and SD-ESCs (Figs. 1C and 3).
FIG. 4.
BMP4 induced cystic EB formation. (A) Colonies derived from undifferentiated hESCs and SD-hESCs were mechanically separated and cultured to develop EBs, and then analyzed by phase-contrast imaging at different time points. EBs derived from undifferentiated hESCs with BMP2/4 treatment were morphologically distinct, with fluid-filled and balloon-like cysts. Percentages of cystic EBs were counted at serial time points (10 and 15 days) after EB formation. Data are presented as mean values±SD from the average of three independent experiments. (B) Expression of undifferentiated specific marker genes (OCT4 and SOX2) and differentiated primitive endoderm marker genes (GATA4, GATA6, and TTR) are displayed in control, BMP2/4, and noggin-treated 10- and 15-day EBs by quantitative RT-PCR analysis. Student's t-test: *p<0.05, **p<0.01, and ***p<0.001.
Cystic EBs differ from noncystic EBs by expressing high levels of TTR when derived from SD-ESCs
To compare the characteristics of noncystic and cystic EBs derived from undifferentiated hESCs or SD-hESCs, we isolated EBs at days 10 and 15 after initiation of EB formation and examined mRNA expression of pluripotent genes (OCT4 and NANOG), primitive endoderm (GATA6), visceral endoderm (TTR), and for other lineage-specific markers (NEURO D1, HAND1, and DCN) by RT-PCR (Fig. 5). Undifferentiated hESCs were used as controls. Results showed that cystic EBs differed from noncystic EBs by expressing significantly lower levels of OCT4 and NANOG, but significantly higher levels of TTR, indicating that cystic EBs likely represent more visceral endoderm differentiated forms of EBs than noncystic EBs. Compared to TTR, GATA6 levels were only marginally different between noncystic and cystic EBs. As expected, hESCs expressed high levels of OCT4 and NANOG, but very little GATA6 and TTR or other lineage markers. In addition, we compared mRNA expression levels of markers for the three germ layers (NEUROD1, HAND1, and DCN) in noncystic and cysytic EBs derived from undifferentiated hESCs and SD-hESCs. There was no significant difference in the expression levels of the lineage-specific genes between noncystic and cystic EBs. Furthermore, cystic EBs derived from undifferentiated hESCs and from SD-hESCs showed comparable expression levels of the lineage-specific genes. Taken together, these results suggest that cystic-EBs represent characteristics of primitive endoderm, and SD-hESCs produce cystic EBs more efficiently than undifferentiated hESCs in vitro.
FIG. 5.
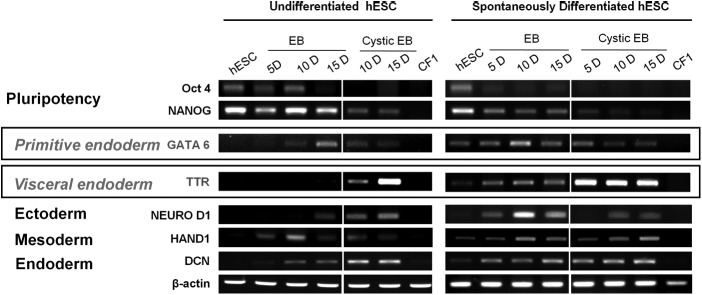
Cystic EBs and noncystic EBs differed in expression levels of genes for pluripotent, primitive, and visceral endoderm cells. Noncystic and cystic EBs were collected from cultured hESCs and SD-hESCs at days 10 and 15 after initiation of EB formation and evaluated for expression of specific genes for pluripotency, primitive endoderm (GATA6), visceral endoderm (TTR), ectoderm (NEURO D1), mesoderm (HAND1), and endoderm (DCN) by RT-PCR analysis. β-ACTIN was used as a loading control.
The ICM is known to consist of heterogeneous cell populations with distinct developmental potentials that become either epiblast or primitive endoderm [22]. It has been reported that the rodent ICM expresses both pluripotency and extraembryonic endoderm-related genes. However, the earliest stages of ICM formation and the role of amniotic cavitation in human development are not completely understood, mainly due to the paucity of human specimens, as well as ethical issues. Fortunately, the process of proamniotic cystic formation can be studied in vitro using hESC lines, which can form advanced derivatives of all three embryonic germ layers, as well as three of the four extraembryonic membranes [23]. Therefore, systematic in vitro differentiation of hESCs represents a powerful tool for analyzing molecular mechanisms controlling preimplantation development. In this study, we characterized SD-hESCs generated in vitro from hESCs. We propose that SD-hESCs may mimic the characteristics of primitive endoderm cells. Since SD-hESCs can be propagated continuously on gelatinized dishes by constitutive activation of GATA-6 and they can differentiate into EBs that are morphologically and genetically similar to visceral endoderm cells, the isolated SD-hESCs can serve as a useful cell model system to study the molecular mechanisms of human ICM and blastocyst cavitation. Our study also suggests that GATA-6 may be important for triggering differentiation of hESCs to extraembryonic cell lineages.
We used a hESC suspension culture system, which produces colonies composed of heterogeneous cell populations, including primitive endoderm cells, to study human early embryonic development. We were able to clearly identify SD-hESCs in colonies. By using our expertise in mechanical dissection to sort out and isolate SD-hESCs from undifferentiated hESCs in hESC colonies under low-magnification microscopy, we characterized SD-hESCs at a cellular and molecular level. SD-hESCs were unique in that they expressed GATA-6, a primitive endoderm marker, and had an ability to develop EBs with high number of cysts. Because cyst formation in the ICM is known to be important for embryogenesis, it is possible that GATA6 may initiate an early stage of development of yolk-like structures and that TTR may take over the next steps for further differentiation of EBs into lineage-specific germinal layers, such as blood islands or endothelial cells, during embryogenesis [24–27]. Visceral endoderm is important for multistep induction leading to complete terminal differentiation at day 7.5 of gestation in mice [28]. Moreover, visceral endoderm-like cells can induce the differentiation of the epiblast to undergo hematopoiesis and vasculogenesis and respecify prospective neuroectodermal cell fates [25]. Mummery et al. previously demonstrated that coculture of hESC lines with visceral endoderm-like cells induce epithelia through formation of large cystic structures that stain positively for α-fetoprotein and are presumably extraembryonic layers [29]. Therefore, we propose that SD-hESCs are not just an artifact cell produced during in vitro hESC culture, but that they may represent an important intermediate cell state related to appropriate germinal layer formation in the ICM during human blastocyst development.
Acknowledgments
This work was supported by the grant for the Bio & Medical Technology Development Program (2012M3A9B4028738) funded by the National Research Foundation of Korea (NRF) of the Ministry of Education, Science and Technology (MEST), Republic of Korea.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Thomson JA. Itskovitz-Eldor J. Shapiro SS. Waknitz MA. Swiergiel JJ. Marshall VS. Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Oron E. Ivanova N. Cell fate regulation in early mammalian development. Phys Biol. 2012;9:045002. doi: 10.1088/1478-3975/9/4/045002. [DOI] [PubMed] [Google Scholar]
- 3.Toyooka Y. Shimosato D. Murakami K. Takahashi K. Niwa H. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development. 2008;135:909–918. doi: 10.1242/dev.017400. [DOI] [PubMed] [Google Scholar]
- 4.Coucouvanis E. Martin GR. Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell. 1995;83:279–287. doi: 10.1016/0092-8674(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 5.Peterkin T. Gibson A. Patient R. Redundancy and evolution of GATA factor requirements in development of the myocardium. Dev Biol. 2007;311:623–635. doi: 10.1016/j.ydbio.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capo-Chichi CD. Rula ME. Smedberg JL. Vanderveer L. Parmacek MS. Morrisey EE. Godwin AK. Xu XX. Perception of differentiation cues by GATA factors in primitive endoderm lineage determination of mouse embryonic stem cells. Dev Biol. 2005;286:574–586. doi: 10.1016/j.ydbio.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 7.Fujikura J. Yamato E. Yonemura S. Hosoda K. Masui S. Nakao K. Miyazaki Ji J. Niwa H. Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev. 2002;16:784–789. doi: 10.1101/gad.968802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrisey EE. Ip HS. Lu MM. Parmacek MS. GATA-6: a zinc finger transcription factor that is expressed in multiple cell lineages derived from lateral mesoderm. Dev Biol. 1996;177:309–322. doi: 10.1006/dbio.1996.0165. [DOI] [PubMed] [Google Scholar]
- 9.Morrisey EE. Tang Z. Sigrist K. Lu MM. Jiang F. Ip HS. Parmacek MS. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 1998;12:3579–3590. doi: 10.1101/gad.12.22.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vieyra DS. Rosen A. Goodell MA. Identification and characterization of side population cells in embryonic stem cell cultures. Stem Cells Dev. 2009;18:1155–1166. doi: 10.1089/scd.2008.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bendall SC. Stewart MH. Menendez P. George D. Vijayaragavan K. Werbowetski-Ogilvie T. Ramos-Mejia V. Rouleau A. Yang J, et al. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448:1015–1021. doi: 10.1038/nature06027. [DOI] [PubMed] [Google Scholar]
- 12.Stewart MH. Bendall SC. Bhatia M. Deconstructing human embryonic stem cell cultures: niche regulation of self-renewal and pluripotency. J Mol Med (Berl) 2008;86:875–886. doi: 10.1007/s00109-008-0356-9. [DOI] [PubMed] [Google Scholar]
- 13.Peerani R. Rao BM. Bauwens C. Yin T. Wood GA. Nagy A. Kumacheva E. Zandstra PW. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 2007;26:4744–4755. doi: 10.1038/sj.emboj.7601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotta A. Cheung AY. Farra N. Vijayaragavan K. Seguin CA. Draper JS. Pasceri P. Maksakova IA. Mager DL, et al. Isolation of human iPS cells using EOS lentiviral vectors to select for pluripotency. Nat Methods. 2009;6:370–376. doi: 10.1038/nmeth.1325. [DOI] [PubMed] [Google Scholar]
- 15.Kim SK. Suh MR. Yoon HS. Lee JB. Oh SK. Moon SY. Moon SH. Lee JY. Hwang JH. Cho WJ. Kim KS. Identification of developmental pluripotency associated 5 expression in human pluripotent stem cells. Stem Cells. 2005;23:458–462. doi: 10.1634/stemcells.2004-0245. [DOI] [PubMed] [Google Scholar]
- 16.Mantel C. Guo Y. Lee MR. Kim MK. Han MK. Shibayama H. Fukuda S. Yoder MC. Pelus LM. Kim KS. Broxmeyer HE. Checkpoint-apoptosis uncoupling in human and mouse embryonic stem cells: a source of karyotpic instability. Blood. 2007;109:4518–4527. doi: 10.1182/blood-2006-10-054247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HS. Oh SK. Park YB. Ahn HJ. Sung KC. Kang MJ. Lee LA. Suh CS. Kim SH. Kim DW. Moon SY. Methods for derivation of human embryonic stem cells. Stem Cells. 2005;23:1228–1233. doi: 10.1634/stemcells.2004-0296. [DOI] [PubMed] [Google Scholar]
- 18.Lee MR. Kim JS. Kim KS. miR-124a is important for migratory cell fate transition during gastrulation of human embryonic stem cells. Stem Cells. 2010;28:1550–1559. doi: 10.1002/stem.490. [DOI] [PubMed] [Google Scholar]
- 19.Coucouvanis E. Martin GR. BMP signaling plays a role in visceral endoderm differentiation and cavitation in the early mouse embryo. Development. 1999;126:535–546. doi: 10.1242/dev.126.3.535. [DOI] [PubMed] [Google Scholar]
- 20.Rong L. Liu J. Qi Y. Graham AM. Parmacek MS. Li S. GATA-6 promotes cell survival by up-regulating BMP-2 expression during embryonic stem cell differentiation. Mol Biol Cell. 2012;23:3754–3763. doi: 10.1091/mbc.E12-04-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conley BJ. Ellis S. Gulluyan L. Mollard R. BMPs regulate differentiation of a putative visceral endoderm layer within human embryonic stem-cell-derived embryoid bodies. Biochem Cell Biol. 2007;85:121–132. doi: 10.1139/o06-145. [DOI] [PubMed] [Google Scholar]
- 22.Yamanaka Y. Ralston A. Stephenson RO. Rossant J. Cell and molecular regulation of the mouse blastocyst. Dev Dyn. 2006;235:2301–2314. doi: 10.1002/dvdy.20844. [DOI] [PubMed] [Google Scholar]
- 23.Stewart MH. Bosse M. Chadwick K. Menendez P. Bendall SC. Bhatia M. Clonal isolation of hESCs reveals heterogeneity within the pluripotent stem cell compartment. Nat Methods. 2006;3:807–815. doi: 10.1038/nmeth939. [DOI] [PubMed] [Google Scholar]
- 24.Shimosato D. Shiki M. Niwa H. Extra-embryonic endoderm cells derived from ES cells induced by GATA factors acquire the character of XEN cells. BMC Dev Biol. 2007;7:80. doi: 10.1186/1471-213X-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dyer MA. Farrington SM. Mohn D. Munday JR. Baron MH. Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neurectodermal cell fate in the mouse embryo. Development. 2001;128:1717–1730. doi: 10.1242/dev.128.10.1717. [DOI] [PubMed] [Google Scholar]
- 26.Byrd N. Becker S. Maye P. Narasimhaiah R. St-Jacques B. Zhang X. McMahon J. McMahon A. Grabel L. Hedgehog is required for murine yolk sac angiogenesis. Development. 2002;129:361–372. doi: 10.1242/dev.129.2.361. [DOI] [PubMed] [Google Scholar]
- 27.Kunath T. Arnaud D. Uy GD. Okamoto I. Chureau C. Yamanaka Y. Heard E. Gardner RL. Avner P. Rossant J. Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development. 2005;132:1649–1661. doi: 10.1242/dev.01715. [DOI] [PubMed] [Google Scholar]
- 28.Arai A. Yamamoto K. Toyama J. Murine cardiac progenitor cells require visceral embryonic endoderm and primitive streak for terminal differentiation. Dev Dyn. 1997;210:344–353. doi: 10.1002/(SICI)1097-0177(199711)210:3<344::AID-AJA13>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 29.Mummery C. Ward-van Oostwaard D. Doevendans P. Spijker R. van den Brink S. Hassink R. van der Heyden M. Opthof T. Pera M, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]