Abstract
Introduction
A noninvasive tool to reposition kidney stones could have significant impact in the management of stone disease. Our research group has developed a noninvasive transcutaneous ultrasound device. A review and update of the current status of this technology is provided.
Discussion of Technology
Stone propulsion is achieved through short bursts of focused, ultrasonic pulses. The initial system consisted of an eight-element annular array transducer, computer, and separate ultrasound imager. In the current generation, imaging and therapy are completed with one ultrasound system and a commercial probe. This generation allows real-time ultrasound imaging, targeting, and propulsion. Safety and effectiveness for the relocation of calyceal stones have been demonstrated in the porcine model.
Role in Endourology
This technology may have applications in repositioning stones as an adjunct to lithotripsy, facilitating clearance of residual fragments after lithotripsy, expelling de novo stones, and potentially repositioning obstructing stones. Human trials are in preparation.
Introduction
All minimally invasive therapies to treat stones have the potential to leave residual stones or fragments, which may grow and require retreatment in up to one-fifth of patients with long-term follow-up.1–6 Small asymptomatic stones that are followed rather than treated may also ultimately require intervention.7 Likewise, patients presenting with an acute obstructing stone and signs of infection, hemodynamic instability, renal insufficiency, or intractable nausea or pain, may require an urgent procedure to provide drainage of the obstructed kidney.8 Currently, the only options to relieve obstruction are surgical.
The management of residual fragments, small untreated stones, and obstructing stones could benefit from a noninvasive device that could reposition stones.9 Our research group has developed a transcutaneous, noninvasive ultrasound prototype that uses acoustic force from focused ultrasound waves to reposition stones.10–15 A review and update of the current status of this technology are provided.
Discussion of the Technology
Device development: first generation model
An experimental ultrasound model was built by combining two separate systems to drive the focused ultrasound therapy probe and perform ultrasound imaging (Fig. 1a). Imaging was performed with a commercial transducer (HDI P4-2; ATL/Philips Healthcare, Andover MA) and HDI 5000 (ATL/Philips Healthcare) ultrasound imager. Therapy was performed with a custom 2 MHz, eight-element annular array curved to a focus of 6 cm. Pulses were generated from synchronized outputs of eight signals from an SC-200 radiofrequency synthesizer (Model H-106; Sonic Concepts, Bothell, WA) amplified by 8, 100W IC-706MKIIG amplifiers (Icom®). A laptop computer controlled the excitation timing of each element.
FIG. 1.
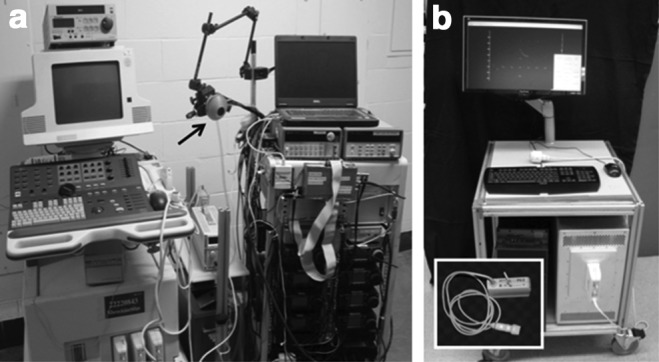
(a) First generation device with HDI5000 commercial diagnostic ultrasound imager (left), cooling system (middle), transducer with water coupling cone (arrow), and acoustic propulsion hardware with laptop (right). (b) Second generation device with Verasonics ultrasound engine, desktop computer, and ATL/Philips commercial transducer (inset).
Feasibility of generating stone motion was first demonstrated in a kidney phantom with 3 to 8 mm human urinary calculi and 2.5 to 4 mm glass beads.14 A subsequent feasibility and safety study in a live porcine model was performed with endoscopically placed beads or human stones placed in the lower or interpolar calyx.13 Stones and beads were moved to the renal pelvis and ureteropelvic junction (UPJ) in all six pigs within 10 minutes of treatment time per stone. Stone composition, which included cystine, calcium oxalate monohydrate, and calcium phosphate stones, did not appear to affect the ability to reposition stones. No thermal injury was observed, with average exposures of 325 W/cm2.
Device development: second generation model
In the current model, imaging and therapy are completed with a single commercial ultrasound imaging probe (HDI C5-2 or P4-1; ATL/Philips), a Verasonics ultrasound engine (Redmond, WA), computer processor, and display monitor (Fig. 1b). The device is portable and can be operated by a single user. Acoustic energy is delivered in 1-second push bursts, consisting of 250 finely focused pulses 0.1 millisecond in duration. Each 0.1 millisecond pulse is separated by 3 milliseconds, thus energy is only being delivered during 3% of the push burst (3% duty cycle). The interval of push bursts is governed by software to prevent transducer probe heating. The pressure and total energy delivered are less than that in current shockwave lithotripsy treatments.10,11
Operation of the device is designed for a single user. The stone is visualized using conventional real-time B-mode ultrasound. The user moves the crosshairs over the stone on the B-mode image and then the push burst is triggered using a computer mouse pad. Acoustic force is delivered to the stone in-line with the ultrasound transducer.
Device therapeutic effectiveness and safety in porcine model: second generation model
Live porcine animal studies have demonstrated effectiveness in repositioning calyceal stones to the renal pelvis, UPJ, or proximal ureter (Fig. 2).10,11 In 12 kidneys, 26 calyceal stones (2–8 mm in size) and metalized beads (2 mm in size, 0.02 g) were endoscopically placed in the lower or interpolar calyx. Overall, 17 stones or beads (65%) were successfully relocated. The stone size did not appear to be associated with success. The average procedure time for successfully repositioned stones was 14.2±7.9 minutes with 23±16 push bursts. Average displacement was estimated to be 5.6±2.7 linear cm on fluoroscopic images. Histologic analyses of the kidneys demonstrated no evidence of injury compared to control animals at maximum clinical exposure settings with intensities of 2400 W/cm2, well below the threshold for injury expected from therapeutic ultrasound (see below).15 A video of the technology used in these experiments is under review with the Journal of Endourology, Part B Videourology.
FIG. 2.
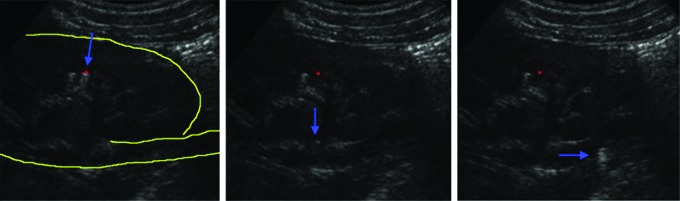
Real-time B-mode imaging of a successfully displaced stone. The red dot indicates the selected target and blue arrow indicates the stone in the collecting system. A burst of ultrasound pulses is applied to move the stone in a single movement from the calyx to the proximal ureter. All motion occurs in about 1 second.
Injury threshold from therapeutic and supratherapeutic focused ultrasound
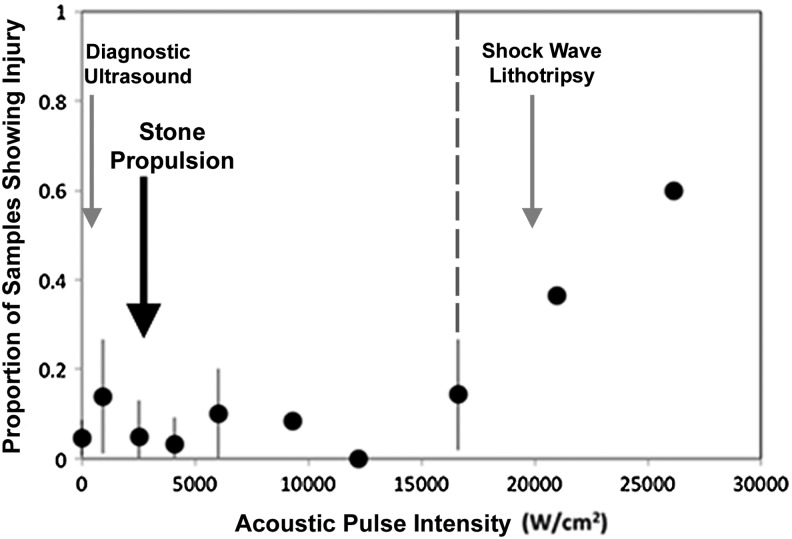
Injury threshold and pattern of injury associated with the pulsing scheme used in the animal studies has been characterized to determine the safe range of intensities with focused ultrasound.15 The first generation system (2 MHz annular array) was required for these experiments to apply spatial peak pulse average intensities up to 28,000 W/cm2 focused on the parenchyma of in vivo porcine kidneys. Three independent experts, blinded to treatment conditions, evaluated the tissue from treated and control animals for injury. Tissue injury consistent with emulsification, necrosis, and hemorrhage appeared to be dose dependent with a threshold of 16,620 W/cm2, well above the intensities generated with the second generation model (Fig. 3).
FIG. 3.
Proportion of samples demonstrating injury with increasing spatial peak pulse averaged intensity at therapeutic and supratherapeutic levels. The large black arrow denotes the intensity at maximum clinical settings for stone propulsion with the current device, well below the dose-dependent injury threshold of >16,620 W/cm2 (dotted line). Gray arrows denote average intensity of shock wave lithotripsy (20,000 W/cm2) and diagnostic ultrasound (200 W/cm2) for reference.
Device imaging in humans
Current commercially available diagnostic ultrasound imagers using B-mode have variable sensitivity (19%–93%) and specificity (84%–100%) for the detection of urinary calculi.16–22 To determine the performance characteristics of the Verasonics imaging system, renal ultrasound images were obtained in nine human stone formers with 17 renal units. Ultrasound images of the upper, middle, lower poles, and the renal pelvis/UPJ were evaluated for stones by blinded reviewers and compared to recent CT imaging (within 60 days), which demonstrated a total of 27 stones with a mean size of 4.4±3.3 mm. The detection of these renal stones with the research ultrasound imager had a sensitivity of 80%, specificity of 90%, positive predictive value of 76%, and negative predictive value of 92% with B-mode, similar to current commercial diagnostic ultrasound imagers.
Role in Endourology
Focused ultrasound technology has several potential applications in the management of upper tract stones. First, ultrasonic propulsion may be used to facilitate the passage of small stones that otherwise would be managed with observation. With long-term follow-up, ∼20% of asymptomatic stones with mean size <6 mm passed spontaneously, while 45% remained within the kidney but enlarged, and 7% ultimately required intervention.7 Most small asymptomatic stones could be managed conservatively, but some patients might desire a noninvasive method to facilitate stone passage due to personal preference or occupational requirements (e.g., pilots). A provoked, but controlled stone event could avoid the unpredictability of stone passage while on observation. Using medical expulsive therapy (e.g., alpha-blockers)23–25 in conjunction with focused ultrasound treatment might reduce stone passage time and colic symptoms.
Second, ultrasonic propulsion may be used to treat residual stone fragments after lithotripsy and improve stone-free rates. Since residual fragments occur after all current surgical techniques,1–5 the technology could have wide applicability. Residual stones lead to stone recurrence and require retreatment in more than 20% of patients.1,6 This technology could reduce the number of retreatment procedures performed.
Third, ultrasonic propulsion may be used to treat an acute stone event due to an obstructing stone. If the stone could be repositioned to a nonobstructing position, the definitive treatment of the stone could be managed in the elective setting rather than as an emergent procedure. This likely could be performed at the bedside without anesthesia or sedation and might reduce utilization of healthcare resources.
Fourth, ultrasonic propulsion may improve stone-free rates with perioperative repositioning of renal stones. This technology could potentially assist with repositioning a difficult to reach stone during ureteroscopy, especially those in the lower pole that can be difficult to access with a laser or basket. In addition, as stone-free rates are lower in stones located in the lower pole,26,27 a lower pole stone could be relocated before shockwave lithotripsy.
Lastly, ultrasonic propulsion may induce movement of a suspected stone to distinguish true stones from renal sinus fat and parenchymal calcifications. The improved diagnostic accuracy could potentially prevent surgeries for false-positive stones reported by ultrasound, and might allow increased use of ultrasound for diagnosis and follow up of stone formers without ionizing radiation exposure from CT imaging.
Despite the promise of this technology, several questions remain unanswered. It is unknown if the ability to reposition stones is the same in human subjects as in pigs or if stones can be moved in the human ureter. Greater skin to stone distances in humans may affect both imaging and therapeutic capabilities of this device although current modifications have allowed successful treatment at target distances of 12 cm. It is unknown if there is an upper limit of stone size for repositioning due to limitations of our animal models, although an 11 mm stone has been moved in vivo. Upper pole stones may be difficult to move due to adjacent organs and overlying ribs. Stones attached to the renal papilla or Randall's plaque may also be difficult to detach, although we have moved attached stones in a separate animal model.12 Many of these questions will be answered with clinical data, and the technology is currently undergoing FDA approval for human trials.
Development of this technology is with the urologist as the end-user in mind. It is anticipated that there will be a learning curve, and efforts are underway to develop a curriculum to train urologists. Early feedback from experts in the field has demonstrated a willingness to adopt this technology and contributed toward future improvements in the prototype.
Abbreviation Used
- UPJ
ureteropelvic junction
Acknowledgments
This work was supported by NIH DK43881, DK092197, and NSBRI through NASA NCC 9-58, the Coulter Foundation, and the University of Washington. This material is the result of work supported by resources from the VA Puget Sound Health Care System, Seattle, Washington. We are very grateful for the help of a large team at the University of Washington and the Consortium for Shock Waves in Medicine, which we cannot list in detail.
Disclosure Statement
No competing financial interests exist.
References
- 1.Osman M. Wendt-Nordahl G. Heger K. Michel MS. Alken P. Knoll T. Percutaneous nephrolithotomy with ultrasonography-guided renal access: Experience from over 300 cases. BJU Int. 2005;96:875–878. doi: 10.1111/j.1464-410X.2005.05749.x. [DOI] [PubMed] [Google Scholar]
- 2.Altunrende F. Tefekli A. Stein RJ, et al. Clinically insignificant residual fragments after percutaneous nephrolithotomy: Medium-term follow-up. J Endourol. 2011;25:941–945. doi: 10.1089/end.2010.0491. [DOI] [PubMed] [Google Scholar]
- 3.Raman JD. Bagrodia A. Gupta A, et al. Natural history of residual fragments following percutaneous nephrostolithotomy. J Urol. 2009;181:1163–1168. doi: 10.1016/j.juro.2008.10.162. [DOI] [PubMed] [Google Scholar]
- 4.Rebuck DA. Macejko A. Bhalani V. Ramos P. Nadler RB. The natural history of renal stone fragments following ureteroscopy. Urology. 2011;77:564–568. doi: 10.1016/j.urology.2010.06.056. [DOI] [PubMed] [Google Scholar]
- 5.Skolarikos A. Papatsoris AG. Diagnosis and management of postpercutaneous nephrolithotomy residual stone fragments. J Endourol. 2009;23:1751–1755. doi: 10.1089/end.2009.1546. [DOI] [PubMed] [Google Scholar]
- 6.Osman MM. Alfano Y. Kamp S, et al. 5-year-follow-up of patients with clinically insignificant residual fragments after extracorporeal shockwave lithotripsy. Eur Urol. 2005;47:860–864. doi: 10.1016/j.eururo.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Koh LT. Ng FC. Ng KK. Outcomes of long-term follow-up of patients with conservative management of asymptomatic renal calculi. BJU Int. 2012;109:622–625. doi: 10.1111/j.1464-410X.2011.10329.x. [DOI] [PubMed] [Google Scholar]
- 8.Preminger GM. Tiselius DG. Assimos D, et al. Guideline for the Management of Ureteral Calculi. 2007. www.auanet.org/content/clinical-practice-guidelines/clinical-guidelines.cfm?sub=uc. [Mar 21;2013 ]. www.auanet.org/content/clinical-practice-guidelines/clinical-guidelines.cfm?sub=uc [DOI] [PubMed]
- 9.Fernandez R. Tan YK. Kaberle W, et al. Determining a performance envelope for capture of kidney stones functionalized with superparamagnetic microparticles. J Endourol. 2012;26:1227–1230. doi: 10.1089/end.2011.0598. [DOI] [PubMed] [Google Scholar]
- 10.Bailey M. Wang YN. Simon JC, et al. Acoustic radiation force to reposition kidney stones. J Acoust Soc Am. 2013;133:3279. doi: 10.1121/1.4799599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harper JD. Sorensen MD. Cunitz BW, et al. Focused ultrasound to expel calculi from the kidney: safety and efficacy of a clinical prototype device. J Urol. 2013;190:1090–1095. doi: 10.1016/j.juro.2013.03.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsi RS. Penniston KL. Crenshaw TD, et al. Focused Ultrasound Repositions De Novo Calculi in an Established Stone-Forming Porcine Model. Engineering in Urology Society Annual Meeting; San Diego, CA. 2013. [Google Scholar]
- 13.Shah A. Harper JD. Cunitz BW, et al. Focused ultrasound to expel calculi from the kidney. J Urol. 2012;187:739–743. doi: 10.1016/j.juro.2011.09.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah A. Owen NR. Lu W, et al. Novel ultrasound method to reposition kidney stones. Urol Res. 2010;38:491–495. doi: 10.1007/s00240-010-0319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang YN. Simon JC. Cunitz BW, et al. Determination of tissue injury thresholds from ultrasound in a porcine kidney model. J Acoust Soc Am. 2013;133:3411. [Google Scholar]
- 16.de Souza LR. Goldman SM. Faintuch S, et al. Comparison between ultrasound and noncontrast helical computed tomography for identification of acute ureterolithiasis in a teaching hospital setting. Sao Paulo Med J. 2007;125:102–107. doi: 10.1590/S1516-31802007000200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowler KA. Locken JA. Duchesne JH. Williamson MR. US for detecting renal calculi with nonenhanced CT as a reference standard. Radiology. 2002;222:109–113. doi: 10.1148/radiol.2221010453. [DOI] [PubMed] [Google Scholar]
- 18.Haroun AA. Hadidy AM. Mithqal AM. Mahafza WS. Al-Riyalat NT. Sheikh-Ali RF. The role of B-mode ultrasonography in the detection of urolithiasis in patients with acute renal colic. Saudi J Kidney Dis Transpl. 2010;21:488–493. [PubMed] [Google Scholar]
- 19.Ray AA. Ghiculete D. Pace KT. Honey RJ. Limitations to ultrasound in the detection and measurement of urinary tract calculi. Urology. 2010;76:295–300. doi: 10.1016/j.urology.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Ripolles T. Agramunt M. Errando J. Martinez MJ. Coronel B. Morales M. Suspected ureteral colic: Plain film and sonography vs unenhanced helical CT. A prospective study in 66 patients. Eur Radiol. 2004;14:129–136. doi: 10.1007/s00330-003-1924-6. [DOI] [PubMed] [Google Scholar]
- 21.Ulusan S. Koc Z. Tokmak N. Accuracy of sonography for detecting renal stone: Comparison with CT. J Clin Ultrasound. 2007;35:256–261. doi: 10.1002/jcu.20347. [DOI] [PubMed] [Google Scholar]
- 22.Viprakasit DP. Sawyer MD. Herrell SD. Miller NL. Limitations of ultrasonography in the evaluation of urolithiasis: A correlation with computed tomography. J Endourol. 2012;26:209–213. doi: 10.1089/end.2011.0177. [DOI] [PubMed] [Google Scholar]
- 23.De Sio M. Autorino R. Di Lorenzo G, et al. Medical expulsive treatment of distal-ureteral stones using tamsulosin: A single-center experience. J Endourol. 2006;20:12–16. doi: 10.1089/end.2006.20.12. [DOI] [PubMed] [Google Scholar]
- 24.Dellabella M. Milanese G. Muzzonigro G. Efficacy of tamsulosin in the medical management of juxtavesical ureteral stones. J Urol. 2003;170(6 Pt 1):2202–2205. doi: 10.1097/01.ju.0000096050.22281.a7. [DOI] [PubMed] [Google Scholar]
- 25.Resim S. Ekerbicer H. Ciftci A. Effect of tamsulosin on the number and intensity of ureteral colic in patients with lower ureteral calculus. Int J Urol. 2005;12:615–620. doi: 10.1111/j.1442-2042.2005.01116.x. [DOI] [PubMed] [Google Scholar]
- 26.El-Nahas AR. Ibrahim HM. Youssef RF. Sheir KZ. Flexible ureterorenoscopy versus extracorporeal shock wave lithotripsy for treatment of lower pole stones of 10–20 mm. BJU Int. 2012;110:898–902. doi: 10.1111/j.1464-410X.2012.10961.x. [DOI] [PubMed] [Google Scholar]
- 27.Pearle MS. Lingeman JE. Leveillee R, et al. Prospective, randomized trial comparing shock wave lithotripsy and ureteroscopy for lower pole caliceal calculi 1 cm or less. J Urol. 2005;173:2005–2009. doi: 10.1097/01.ju.0000158458.51706.56. [DOI] [PubMed] [Google Scholar]