Abstract
Purpose
To investigate the efficacy of hyaluronic acid-carboxymethylcellulose (HACM) in facilitating early recovery of erectile function (EF) after radical prostatectomy, we report our initial experience of HACM use on the neurovascular bundle (NVB) after robot-assisted radical prostatectomy (RARP).
Patients and Methods
Between 2008 and 2010, 459 consecutive patients who underwent RARP with bilateral nerve-sparing technique were included in this study. Patients were classified into two groups: HACM (group 1; n=162) and non-HACM (group 2; n=287). HACM was delivered to the anatomic location of the NVB after prostate removal. We retrospectively analyzed the surgical outcomes including EF, continence, and perioperative complications.
Results
At 6 months after surgery, EF recovery rate was 28.5% in group 1 and 17.4% in group 2 (P=0.006). In a subgroup analysis consisting of 225 patients with a preoperative International Index of Erectile Function Short Survey (IIEF)-5 score ≥20, the difference in EF recovery at 6 months was significant with 62.8% in group 1 and 27.0% in group 2 (P=0.002), respectively. HACM use was an independent predictor for EF recovery at 6 months after surgery (odds ratio, 2.735; 95% confidence interval, 1.613–4.638; P<0.001). Age and preoperative IIEF-5 were also independent predictors. No differences in continence at 6 months or perioperative complications were found between the two groups. EF recovery was not different between the two groups after 18 months.
Conclusions
HACM use around the NVBs is safe and facilitates early recovery of EF after nerve-sparing RARP. HACM use is more effective in patients with normal preoperative sexual function.
Introduction
Although radical prostatectomy (RP) remains the gold standard for the surgical treatment of patients with localized prostate cancer, erectile dysfunction (ED) can be a significant postoperative side effect. With regard to erectile function (EF), the landscape of prostate cancer surgery has dramatically changed since the pioneering studies of Walsh and Donker1 of the neurovascular anatomy of the prostate in the 1980s. There has been a rapid acceptance of robot-assisted radical prostatectomy (RARP) since 2000, resulting in technical modifications to preserve EF without compromising cancer control. These include minimization of thermal energy along the apical/posterolateral borders of the prostate,2 intrafascial dissection of the lateral prostatic borders to decrease neurovascular bundle (NVB) transection,3 and high apical release of periprostatic fascia and tissue to minimize traction nerve injury.4
Despite these alterations and the availability of tools to discern the neurovascular anatomy more accurately than ever before (e.g., state-of-the-art cadaveric studies, vision enhancement and magnification, prostate immunohistopathologic analysis, preoperative endorectal MRI), the rates of ED after RARP remain high. While our bilateral athermal intrafascial release (AIR) of the NVBs has increased EF after surgery in preoperatively potent men,3 our goal continues to be earlier return of EF in all men undergoing RARP.
Known elements contributing to postoperative ED include traction, distraction, and thermal nerve injuries.2–4 While intraoperative mechanisms of NVB injury are often considered, inflammation and wound healing after RP may also be critical components. Limiting the local effects of these processes on neurovascular tissue may improve functional outcomes.5,6 Hyaluronic acid-carboxymethylcellulose (HACM) adhesion barrier has been shown to effectively reduce postoperative adhesions in abdominopelvic surgery.7,8 The mechanism of action is the physical separation of traumatized and inflammatory adhesiogenic tissues and organs while normal tissue repair takes place. In terms of safety, the compound begins to break down in 7 days, well after the acute phase of normal tissue repair is finished, and is completely resorbed by the body in 28 days. More interestingly, it has been demonstrated in rabbits that HACM improves peripheral nerve regeneration.9 We hypothesized that placing HACM gel around the anatomic location of the NVBs intraoperatively limits local inflammatory response and reduces fibrosis, thus hastening return of neural activity and potency. We present our initial experience with HACM use in RARP.
Patients and Methods
Patient selection
HACM adhesion barrier use is approved by the Food and Drug Administration for the prevention of adhesions during abdominopelvic surgery. Institutional Review Board approval was obtained for this retrospective study. Between 2008 and 2010, 459 patients underwent standard transperitoneal RARP with bilateral nerve-sparing technique. Patients were divided into three groups based on time: First 137 patients underwent RARP without HACM use, middle 162 patients with HACM use, and the last 150 patients without HACM use. All RARPs were performed by a single surgeon who had performed more than 500 total RARPs before the study initiation. All patients completed the self-administered American Urological Association Symptom Score (AUASS) and International Index of Erectile Function Short Survey (IIEF-5) before and after surgery.
For analysis of the efficacy of HACM use, patients were reclassified into two groups: HACM use (group 1; n=162) and non-HACM use (group 2; n=287). To analyze the influence of the surgeon's learning curve, group 2 included the latter 150 cases after 162 cases with HACM use. Subgroup stratification was performed to analyze HACM use with preoperative IIEF-5 ≥20 (subgroup A; n=74) and non-HACM use with IIEF-5 ≥20 (subgroup B; n=153).
Surgical technique
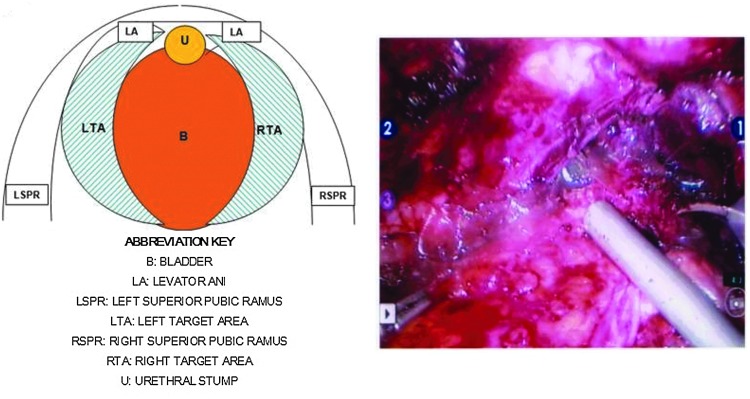
Our intrafascial nerve-sparing surgical technique during RARP has been described previously.3 After completing the vesicourethral anastomosis, 20 mL of dissolved HACM gel was delivered (Fig. 1) using a 35-cm cannulated laparoscopic injector through the assistant trocar in the anatomic area of the left and right NVB. Each target area received 10 mL. This anatomic area was defined laterally by the levator ani muscles, medially by the bladder, cranially by the superior pubic ramus, and caudally by the urethral stump. The injected gel, which congealed on delivery to target area tissues, consisted of two sheets of HACM dissolved in 20 mL of 0.9% physiologic saline for 30 minutes before use. Surgical drains were not used. Patients were routinely discharged home at postoperative day 1 with urethral catheters that were removed on postoperative day 7.
FIG. 1.
Laparoscopic delivery of hyaluronic acid-carboxymethylcellulose in the anatomic area of the neurovascular bundle.
Clinical follow-up
The following perioperative data were retrospectively collected and analyzed: Patient age, operative time, estimated blood loss, preoperative prostate-specific antigen (PSA) level, preoperative AUASS, preoperative IIEF-5, pathologic tumor stage, final Gleason sum, prostate weight, and surgical margin status. All patients underwent routine postoperative follow-up with detailed physical examinations for a minimum of 12 months. An EF assessment with an IIEF-5 survey was completed by the patients at 3, 6, 9, and 12 months. EF recovery was defined as the ability to achieve penetration ≥50% of the time and maintain erections significant for penetration ≥50% of the time as per questions 2 and 3 on the IIEF-5 survey.10,11 All patients were recommended to take a phosphodiesterase 5 inhibitor (PDE5I) (sildenafil 50 mg) every other night for 90 days as part of the penile rehabilitation program.
Statistical analysis
The demographic and clinical characteristics in the two groups (group 1 vs group 2) were analyzed using the chi-square test for categorical variables and an independent t test for continuous variables. For the incidence of perioperative complications, a Fisher exact test was used to determine statistically significant difference between groups. The association between HACM use and the EF recovery on the basis of IIEF-5 was evaluated using an unconditional logistic regression model. The odds ratios (ORs) and 95% confidence intervals (CIs) were estimated. We used a multivariable logistic regression model to indentify independent predictor for EF recovery by including age, IIEF-5 score, prostate weight, PDE5I intake, and HACM use as potential candidates. All statistical analysis were performed using Stata 8.0 (StataCorp LP, College Station, TX). The reported P values are two-sided, and a significance level of <5% was considered statistically significant.
Results
The demographic and clinical characteristics of the two groups as shown in Table 1. Preoperative IIEF-5 score was higher in group 2 compared with those of group 1. No significant differences were found in age, AUASS, prostate weight, PSA levels, and pathologic features (Table 1). With regard to continence at 6 months postsurgery, 136 (85.5%) patients in group 1 and 257 (89.5%) patients in group 2 were pad free. The perioperative complication rate was 8.0% in group 1 and 11.8% in group 2 (P=0.261). The complete list of major and minor complications is presented in Table 2.
Table 1.
Demographic and Clinical Characteristics of Patients
| Characteristic | Group 1 (HACM use; n=162) | Group 2 (non-HACM use; n=287) | P value |
|---|---|---|---|
| Preoperative: | |||
| Age (range) | 59 (41–75) | 59 (36–77) | 0.464 |
| IIEF-5 (range) | 18 (1–25) | 20 (0–25) | 0.023 |
| AUASS (range) | 7 (0–31) | 8 (0–30) | 0.908 |
| PSA, ng/mL (range) | 5.10 (0.46–55.4) | 4.92 (0.49–30.4) | 0.199 |
| Biopsy GS (%) | 0.418 | ||
| 2–6 | 95 (58.6) | 182 (63.6) | |
| 7 | 50 (30.9) | 83 (29.0) | |
| 8–10 | 17 (10.5) | 21 (7.3) | |
| Intraoperative: | |||
| Operative time (range) | 190 (120–335) | 195 (100–400) | 0.617 |
| EBL, mL (range) | 200 (50–1000) | 200 (50–1300) | 0.320 |
| Postoperative: | |||
| Pathologic stage (%) | 0.411 | ||
| pT0 | 0 (0) | 3 (1.0) | |
| pT2a–T2b | 25 (15.5) | 38 (13.2) | |
| pT2c | 96 (59.6) | 184 (64.1) | |
| pT3 | 40 (24.8) | 62 (21.6) | |
| pT4 | 0 (0) | 0 (0) | |
| LN+ | 1 (0.6) | 1 (0.3) | 1.000 |
| Surgical GS (%) | 0.563 | ||
| 2–6 | 72 (44.7) | 134 (47.2) | |
| 7 | 69 (42.9) | 108 (38.0) | |
| 8–10 | 20 (12.4) | 42 (14.8) | |
| Prostate weight, (range) | 48 (24–153) | 45 (18–113) | 0.107 |
| PDE5I intake (%) | 150 (92.6) | 271 (94.4) | 0.543 |
HACM=hyaluronic acid–carboxymethylcellulose; IIEF=International Index of Erectile Function Short Survey; AUASS=American Urological Association Symptom Score; PSA=prostate-specific antigen; GS=Gleason score; EBL=estimated blood loss; LN=lymph node; PDE5I=phosphodiesterase type 5 inhibitor.
Values are median.
Table 2.
Perioperative Complications Including Major and Minor
| Group 1 (HACM use; n=162) | Group 2 (non-HACM use; n=287) | Pvalue | |
|---|---|---|---|
| AUR (%) | 5 (3.1) | 22 (7.7) | |
| BNC (%) | 3 (1.9) | 3 (1.0) | |
| Rectal injury (%) | 1 (0.6) | 1 (0.3) | |
| Ileus/colitis (%) | 3 (1.9) | 4 (1.4) | |
| Lymphocele (%) | 1 (0.6) | 3 (1.0) | |
| Fascial dehiscence (%) | 0 (0) | 1 (0.3) | |
| Total (%) | 13 (8.0) | 34 (11.8) | 0.261 |
AUR=acute urinary retention; BNC=bladder neck contracture.
Analyses of EF recovery after surgery between the two groups are shown in Table 3. No difference was found in the rate of EF recovery at 3 months postsurgery. At 6 months, 45 (28.5%) patients in group 1 showed EF recovery, which was significantly higher than the 50 (17.4%) patients in group 2 (P=0.006). In the multivariable logistic regression model, HACM use was an independent predictor for EF recovery at 6 months postsurgery (OR, 2.432; 95% CI, 1.463–4.044; P=0.001). Age and preoperative IIEF-5 score were also independent predictors (Table 4). At 12 months postsurgery, group 2 was higher in EF recovery than group 1 (Table 3), but HACM use was not an independent predictor based on the results of the multivariable logistic regression model demonstrating age and preoperative IIEF-5 were significant predictors (supplementary Table 1; supplementary data are available online at www.liebertpub.com/end). After 12 months, EF recovery was not different between the two groups.
Table 3.
Recovery of Erectile Function According the Time Following Surgery
| Postoperative time | Group 1 (HACM use; n=162) | Group 2 (non–HACM use; n=287) | Pvalue |
|---|---|---|---|
| 3 months (%) | 12 (7.5) | 24 (8.4) | 0.762 |
| 6 months (%) | 45 (28.5) | 50 (17.4) | 0.006 |
| 9 months (%) | 54 (34.2) | 91 (31.7) | 0.595 |
| 12 months (%) | 66 (41.8) | 153 (53.3) | 0.020 |
| 18 months (%) | 75 (47.5) | 157 (54.7) | 0.144 |
| 24 months (%) | 76 (48.1) | 159 (55.6) | 0.130 |
| 36 months (%) | 76 (48.1) | 159 (55.6) | 0.130 |
Table 4.
Prediction of Erectile Function Recovery at 6 Months After Surgery
| |
Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | 0.923 | 0.895–0.957 | 0.017 | 0.955 | 0.918–0.992 | 0.019 |
| IIEF-5 | 1.142 | 1.091–1.196 | <0.001 | 1.132 | 1.079–1.188 | <0.001 |
| Prostate weight | 0.996 | 0.983–1.010 | 0.582 | 1.005 | 0.991–1.020 | 0.480 |
| HACM use | 1.888 | 1.191–2.993 | 0.007 | 2.432 | 1.463–4.044 | 0.001 |
| PDE5I intake | 1.266 | 0.468–3.424 | 0.642 | 1.084 | 0.361–3.259 | 0.885 |
OR=odds; ratio, CI=confidence interval; IIEF-5=International Index of Erectile Function Short Survey; HACM=hyaluronic acid-carboxymethylcellulose; PDE5I=phosphodiesterase type 5 inhibitor.
Given the difference in EF recovery between the two groups, subgroups with baseline IIEF-5 ≥20 were selected based on their preoperative sexual function. At both 6 months and 9 months, subgroup A (n=74) showed a higher rate of EF recovery compared with that of subgroup B (n=153) (P<0.001 at 6 months; P=0.022 at 9 months; supplementary Table 2). Multivariate logistic regression model demonstrated that HACM use was a strong independent predictor for EF recovery at 6 months after surgery (OR, 3.560; 95% CI, 1.926–6.580; P<0.001; supplementary Table 3). Age was also a significant predictor. At 12 months, age was the only independent predictor for recovery of EF after surgery (supplementary Table 4).
To check the possible influence of the surgeon's learning curve on results, we compared the EF recovery of group 1 with the last 150 patients of group 2 who underwent surgeries subsequent to those of group 1. At 6 months, the rate of EF recovery in group 1 was higher than group 2 (28.5% vs 15.3%; P=0.005, supplementary table 5). In subgroups with baseline IIEF-5 ≥20, similar findings were found at both 6 and 9 months postsurgery (supplementary table 6). HACM use was still an independent predictor for EF recovery at 6 months postsurgery (OR, 3.210; 95% CI, 1.717–6.003; P<0.001) with age and preoperative IIEF-5 score, which were similarly observed in subgroups with baseline IIEF-5 ≥20 (supplementary Table 7).
Discussion
In this study, HACM use had a statistically significant impact on early recovery of EF after surgery, which was more prominent for the subgroup of patients with better preoperative sexual function who underwent nerve-sparing RARP.
Despite recent advances, early return of potency post-RARP remains challenging. Because preoperative factors are generally immutable, discussion on improving both early and overall EF has focused on surgical techniques and concepts. Identifying surgeon-independent factors and therapies contributing to potency may help to standardize functional outcomes. The rationale for use of a HACM adhesion barrier is evidence-based, with our primary focus on the pathophysiology of surgically induced EF and postsurgical wound healing after RARP in the early postoperative period. We attempted to identify potential areas of technical improvement or novel technology that might improve early recovery of EF. Of note, Dubbelman and associates12 identified NVB preservation, patient age, and preoperative sexual function as the most important prognostic indicators for return of potency. The physiology of erections is also significant, because a well-described “neuropraxia” commonly occurs after RP from NVB traction or trauma with subsequent inadequate release of nitric oxide and nitric oxide synthase, resulting in decreased smooth muscle relaxation and inhibition of EF.13
As previously noted, efforts are routinely made to spare injury to the nerves caused by thermal energy, transection, or traction. The possibility that nerve injury (from postoperative inflammation or scarring) may be caused or exacerbated by the body's normal wound healing response, a process that can cause a number of deleterious effects in the body's other systems during the postoperative period, must be considered, however.14 Leungwattanakij and colleagues5 demonstrated increased synthesis of TGF-β1, HIF-1 α, and collagen III in rat cavernosal smooth musculature after cavernous neurotomies were made using electrocautery, indicative of hypoxic stress response. Loss of nocturnal and normal erections and induction of hypoxic pathways might explain the lower rates of recovery and response to therapies for ED in patients who have undergone nerve-sparing RP. Podlasek and coworkers6 have further demonstrated in a rat model that cavernosal nerve injury leads to inhibition of the morphogenic growth protein sonic hedgehog (SHH), causing a 12-fold increase in smooth muscle apoptosis of the penis with associated fibrosis and ED. Dose-dependent SHH treatment in this same model led to a one to threefold decrease in the nerve injury-induced apoptosis.6
Prevention of inflammation and scarring must also be considered. Albersen and associates15 note that no standard prophylactic strategy is currently used to treat potential NVB injury during RARP, despite the development of multiple modalities (hyperbaric oxygen, nitric oxide donor therapy, ligands to block immune system induction and/or downstream targets that stimulate inflammation and scarring, and direct trophic factors to induce nerve regeneration). While intriguing, most agents remain in the early stages of development; few are ready for clinical use. Also, these may trigger systemic immunomodulation, which increases surgical complication rates and may cause inadequate surgical bed and anastomotic healing.16
Our aim in this study was to produce local rather than systemic immunomodulation, an effort that we contend our results support. Both group 1 (HACM use) and group 2 (non-HACM use) demonstrated equivalent complication rates (11.8% vs 8.0%) with a higher rate of EF recovery at 6 months postsurgery when HACM was used. Furthermore, recent studies in the general surgery, colorectal, hepatobiliary, and gynecologic literature have demonstrated the safety and efficacy of HACM adhesion barrier in the reduction of the incidence, severity, and extent of postoperative adhesions in patients having abdominopelvic surgery.7,8,17–19 The role of HACM may be therapeutic as well as protective. The 2003 study by Adanali and colleagues9 concluded that HACM use with concurrent repair of sciatic nerve injuries in rabbits resulted in a statistically significant improvement in the number of viable axons 3 months after surgery and a reduction in perineural fibrosis.9 The introduction of a locally active agent as a physical barrier around the NVBs to separate them from adjacent inflammatory tissue and reduce fibrosis is a novel concept. Unlike an individual's surgical technique, HACM use can be standardized in its dosage, delivery, and target area to improve early potency outcomes after RARP.
Largely because of its retrospective nature, this study does have some limitations. First, with respect to demographic characteristics, there was a difference in preoperative IIEF-5 score between the two groups, which could influence the results of EF recovery after RARP. By multivariative analysis to adjust this difference, we demonstrated HACM use to an independent predictor of EF recovery by adjusting these variables. Notably, group 1 showed lower preoperative IIEF-5 score than group 2. If this baseline characteristic of group 1 is comparable with those of group 2, more prominent results could be observed that favor a larger benefit of HAMC use in outcomes.
Second, the impact of the surgeon's learning curve on the improved the rate of EF recovery postsurgery could be potential that cannot be excluded completely. All RARPs, however, were performed by a single fellowship-trained surgeon with >350 RARP performed using the same surgical technique (AIR) and more than 500 RARPs overall before study commencement. It is generally agreed that the robotic learning curve plateaus by 200 to 300 cases.20,21 Furthermore, group 2 included the latter 150 cases fter 162 cases with HACM use. In subgroup analysis between group 1 and the last 150 cases of group 2, HACM use was found to be a significant factor. Thus, we believe that surgeon-dependent improvement in technique would have a minimal influence on the results.
Third, the HACM adhesion barrier was placed after the vesicourethral anastomosis was performed, limiting the use of the lateral prostatic fascia as a precise landmark to mark the location of the NVB for gel delivery. This was purposefully done to prevent the gel from being placed within the vesicourethral anastomosis because of concerns of a possible anastomotic leak secondary to reduced wound healing. We instead chose to deliver the gel in a carefully defined area (see Methods) encompassing the most likely anatomic location of each NVB after reconstruction was completed. While group 1 demonstrated superiority in early EF recovery, there were no anastomotic leaks in any of the 162 patients.
Finally, one may question the lack of consistent and significant improvement in potency at 3, 9, and 12 months in patients in group 1. While we assert that the use of HACM is protective and may help to facilitate faster return of function, the pathophysiologic cavernosal nerve neuropraxia that most patients experience after surgery is the most likely rate-limiting step in EF recovery until about 3 months after surgery. This argument is supported by the paucity of studies in the urologic literature demonstrating return of potency at 3 months after RARP and their lack of reproducibility.22–25 In 9 and 12 months, the effect of HACM use in EF recovery was not found, but patient characteristics including age and preoperative potency were significant factors in EF recovery at these times.
Conclusions
HACM use around the anatomic location of the NVBs during nerve-sparing RARP has significant impact on early recovery of EF postoperatively and is more pronounced in patients with better preoperative sexual function. The use of a HACM adhesion barrier for separation of the NVBs from adjacent inflammatory tissue during RARP may result in reduced fibrosis and inflammation and promote earlier return of EF. Overall, the results from this study are compelling and warrant further prospective investigation.
Supplementary Material
Abbreviations Used
- AIR
athermal intrafascial release
- AUASS
American Urological Association Symptom Score
- CI
confidence interval
- ED
erectile dysfunction
- EF
erectile function
- HACM
hyaluronic acid-carboxymethylcellulose
- IIEF
International Index of Erectile Function Short Survey
- MRI
magnetic resonance imaging
- NVB
neurovascular bundle
- OR
odds ratio
- PDE-5
phosphodiesterase type-5
- PSA
prostate-specific antigen
- RARP
robot-assisted radical prostatectomy
- RP
radical prostatectomy
- SHH
sonic hedgehog
Acknowledgments
This work has been supported in part by generous grants from the Tanzman Foundation, Jon Runyan's Score for the Cure, and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education Science and Technology (2013-003819) and supported by a grant from the Next-Generation BioGreen 21 Program (No.PJ009621), Rural Development Administration, Republic of Korea.
Disclosure Statement
Isaac Yi Kim is a consultant speaker for Amgen and a consultant for Baxter. For the remaining authors, no competing financial interests exist.
References
- 1.Walsh PC. Donker PJ. Impotence following radical prostatectomy: Insight into etiology and prevention. J Urol. 1982;128:492–497. doi: 10.1016/s0022-5347(17)53012-8. [DOI] [PubMed] [Google Scholar]
- 2.Ahlering TE. Skarecky D. Borin J. Impact of cautery versus cautery-free preservation of neurovascular bundles on early return of potency. J Endourol. 2006;20:586–589. doi: 10.1089/end.2006.20.586. [DOI] [PubMed] [Google Scholar]
- 3.Potdevin L. Ercolani M. Jeong J. Kim IY. Functional and oncologic outcomes comparing interfascial and intrafascial nerve sparing in robot-assisted laparoscopic radical prostatectomies. J Endourol. 2009;23:1479–1484. doi: 10.1089/end.2009.0369. [DOI] [PubMed] [Google Scholar]
- 4.Savera AT. Kaul S. Badani K, et al. Robotic radical prostatectomy with the “Veil of Aphrodite” technique: Histologic evidence of enhanced nerve sparing. Eur Urol. 2006;49:1065–1074. doi: 10.1016/j.eururo.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 5.Leungwattanakij S. Bivalacqua TJ. Usta MF, et al. Cavernous neurotomy causes hypoxia and fibrosis in rat corpus cavernosum. J Androl. 2003;24:239–245. doi: 10.1002/j.1939-4640.2003.tb02668.x. [DOI] [PubMed] [Google Scholar]
- 6.Podlasek CA. Meroz CL. Tang Y, et al. Regulation of cavernous nerve injury-induced apoptosis by sonic hedgehog. Biol Reprod. 2007;76:19–28. doi: 10.1095/biolreprod.106.053926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeuchi H. Kitade M. Kikuchi I, et al. A novel instrument and technique for using Seprafilm hyaluronic acid/carboxymethylcellulose membrane during laparoscopic myomectomy. J Laparoendosc Adv Surg Tech A. 2006;16:497–502. doi: 10.1089/lap.2006.16.497. [DOI] [PubMed] [Google Scholar]
- 8.Vrijland WW. Tseng LN. Eijkman HJ, et al. Fewer intraperitoneal adhesions with use of hyaluronic acid-carboxymethylcellulose membrane: A randomized clinical trial. Ann Surg. 2002;235:193–199. doi: 10.1097/00000658-200202000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adanali G. Verdi M. Tuncel A, et al. Effects of hyaluronic acid-carboxymethylcellulose membrane on extraneural adhesion formation and peripheral nerve regeneration. J Reconstr Microsurg. 2003;19:29–36. doi: 10.1055/s-2003-37188. [DOI] [PubMed] [Google Scholar]
- 10.Hakimi AA. Blitstein J. Feder M, et al. Direct comparison of surgical and functional outcomes of robotic-assisted versus pure laparoscopic radical prostatectomy: Single-surgeon experience. Urology. 2009;73:119–123. doi: 10.1016/j.urology.2008.08.491. [DOI] [PubMed] [Google Scholar]
- 11.Patel VR. Sivaraman A. Coelho RF, et al. Pentafecta: A new concept for reporting outcomes of robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2011;59:702–707. doi: 10.1016/j.eururo.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 12.Dubbelman YD. Dohle GR. Schroder FH. Sexual function before and after radical retropubic prostatectomy: A systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006;50:711–720. doi: 10.1016/j.eururo.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Dean RC. Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32:379–395. doi: 10.1016/j.ucl.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78:606–617. doi: 10.1093/bja/78.5.606. [DOI] [PubMed] [Google Scholar]
- 15.Albersen M. Joniau S. Claes H. Van Poppel H. Preclinical evidence for the benefits of penile rehabilitation therapy following nerve-sparing radical prostatectomy. Adv Urol. 2008:594868. doi: 10.1155/2008/594868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Troppmann C. Pierce JL. Gandhi MM, et al. Higher surgical wound complication rates with sirolimus immunosuppression after kidney transplantation: A matched-pair pilot study. Transplantation. 2003;76:426–429. doi: 10.1097/01.TP.0000072016.13090.4E. [DOI] [PubMed] [Google Scholar]
- 17.Beck DE. Cohen Z. Fleshman JW, et al. A prospective, randomized, multicenter, controlled study of the safety of Seprafilm adhesion barrier in abdominopelvic surgery of the intestine. Dis Colon Rectum. 2003;46:1310–1319. doi: 10.1007/s10350-004-6739-2. [DOI] [PubMed] [Google Scholar]
- 18.Becker JM. Dayton MT. Fazio VW, et al. Prevention of postoperative abdominal adhesions by a sodium hyaluronate-based bioresorbable membrane: A prospective, randomized, double-blind multicenter study. J Am Coll Surg. 1996;183:297–306. [PubMed] [Google Scholar]
- 19.Fazio VW. Cohen Z. Fleshman JW, et al. Reduction in adhesive small-bowel obstruction by Seprafilm adhesion barrier after intestinal resection. Dis Colon Rectum. 2006;49:1–11. doi: 10.1007/s10350-005-0268-5. [DOI] [PubMed] [Google Scholar]
- 20.Ahlering TE. Skarecky D. Lee D. Clayman RV. Successful transfer of open surgical skills to a laparoscopic environment using a robotic interface: Initial experience with laparoscopic radical prostatectomy. J Urol. 2003;170:1738–1741. doi: 10.1097/01.ju.0000092881.24608.5e. [DOI] [PubMed] [Google Scholar]
- 21.Herrell SD. Smith JA., Jr. Robotic-assisted laparoscopic prostatectomy: What is the learning curve? Urology. 2005;66(suppl 5):105–107. doi: 10.1016/j.urology.2005.06.084. [DOI] [PubMed] [Google Scholar]
- 22.Xylinas E. Ploussard G. Salomon L, et al. Intrafascial nerve-sparing radical prostatectomy with a laparoscopic robot-assisted extraperitoneal approach: Early oncological and functional results. J Endourol. 2010;24:577–582. doi: 10.1089/end.2009.0069. [DOI] [PubMed] [Google Scholar]
- 23.Patel VR. Coelho RF. Chauhan S, et al. Continence, potency and oncological outcomes after robotic-assisted radical prostatectomy: Early trifecta results of a high-volume surgeon. BJU Int. 2010;106:696–702. doi: 10.1111/j.1464-410X.2010.09541.x. [DOI] [PubMed] [Google Scholar]
- 24.Coelho RF. Chauhan S. Palmer KJ, et al. Robotic-assisted radical prostatectomy: A review of current outcomes. BJU Int. 2009;104:1428–1435. doi: 10.1111/j.1464-410X.2009.08895.x. [DOI] [PubMed] [Google Scholar]
- 25.Ahlering TE. Eichel L. Skarecky D. Rapid communication: Early potency outcomes with cautery-free neurovascular bundle preservation with robotic laparoscopic radical prostatectomy. J Endourol. 2005;19:715–718. doi: 10.1089/end.2005.19.715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.