Abstract
Successful stem cell gene therapy requires high numbers of genetically engineered hematopoietic stem cells collected using optimal mobilization strategies. Here we focus on stem cell mobilization strategies for thalassemia and present the results of a plerixafor-based mobilization trial with emphasis on the remobilization with granulocyte-colony stimulating factor (G-CSF)+plerixafor in those patients who had previously failed mobilization. Plerixafor rapidly mobilized CD34+ cells without inducing hyperleukocytosis; however, 35% of patients failed to reach the target cell dose of ≥6×106 CD34+ cells/kg. Four subjects who failed on either plerixafor or G-CSF were remobilized with G-CSF+plerixafor. The combination proved highly synergistic; the target cell dose was readily reached and the per-apheresis yield was significantly increased over initial mobilization, ultimately resulting in single-apheresis collections, despite a more than 50% reduction of the dose of G-CSF in splenectomized patients to avoid hyperleukocytosis. The total stem and progenitor cells mobilized in G-CSF+plerixafor patients were higher than in patients treated by plerixafor alone. Importantly, the G-CSF+plerixafor-mobilized cells displayed a primitive stem cell phenotype and higher clonogenic capacity over plerixafor-mobilized cells. G-CSF+plerixafor represents the optimal strategy when very high yields of stem cells or a single apheresis is required. The high yields and the favorable transplantation features render the G-CSF+plerixafor-mobilized cells the optimal CD34+ cell source for stem cell gene therapy applications.
Yannaki and colleagues evaluate methods for mobilizing hematopoietic stem cells (HSCs) in patients with thalassemia major. They identify a combination regimen of granulocyte-colony stimulating factor (GCSF) and Plerixafor that circumvents the toxicity of GCSF previously reported in thalassemia patients while also providing high HSC yields in a single apheresis from patients who previously failed single agent regimens. The excellent yield and clonogenic capacity of the mobilized HSCs suggests that this method may be broadly useful for ex vivo HSC gene transfer protocols.
Introduction
Stem cell gene therapy has been successfully applied in several inherited blood diseases; patients with X-linked SCID (Cavazzana-Calvo et al., 2000), ADA deficiency (Aiuti et al., 2009), adrenoleukodystrophy (Cartier et al., 2009), metachromatic leukodystrophy (Biffi et al., 2013), and Wiscott–Aldrich syndrome (Aiuti et al., 2013), and one patient with β-thalassemia (Cavazzana-Calvo et al., 2010), have been either cured or dramatically improved by gene therapy. Application of stem cell gene therapy requires availability of large numbers of pluripotent repopulating stem cells. Stem cell availability is critical for all forms of stem cell gene therapy, but it is especially important in the case of thalassemia. In contrast to immunodeficiencies, the genetically corrected thalassemic hematopoietic stem cells (HSCs) and progenitor cells lack a selective advantage and thus cannot compete effectively for stem cell niche occupancy over the endogenous stem cells, if a nonmyeloablative conditioning of the patient's bone marrow is used. These conditions create a competitive transplantation setting where the infused stem cell dose plays an important role for the outcome.
The preferable source of HSCs for gene therapy is the mobilized peripheral blood because it provides, under a minimally invasive procedure, several-fold higher numbers of HSCs compared with conventional bone marrow harvest (To et al., 1997). Granulocyte-colony stimulating factor (G-CSF)-mobilized blood represents the most widely used source of autologous HSCs containing usually adequate CD34+ cell numbers with robust engrafting capacity (Russel et al., 1993; Bensinger et al., 2001). Plerixafor is a novel mobilizing agent and a reversible inhibitor of CXCR4–SDF-1 interaction resulting in rapid mobilization within hours after administration (Liles et al., 2003; Devine et al., 2004; Broxmeyer et al., 2005). When used as a single agent, plerixafor has been reported to result in modest or even inferior yields as compared with G-CSF (Liles et al., 2003; Broxmeyer et al., 2005; Devine et al., 2008); however, it exhibits marked synergism in combination with G-CSF, increasing CD34+ cell yields by several fold (Liles et al., 2005; DiPersio et al., 2009; Nademanee et al., 2012).
Adult patients with severe thalassemia respond to G-CSF mobilization differently from the normal individuals for various reasons. G-CSF induces splenic enlargement; therefore, G-CSF mobilization in patients with an already enlarged spleen, such as patients with thalassemia, potentially increases the risk of splenic rupture, which is rare (Falzetti et al., 1999). In addition, G-CSF mobilization typically results in leukocytosis, and in splenectomized patients with elevated baseline white blood cells (WBCs), an excessive response to G-CSF expressed as hyperleukocytosis could potentially cause side effects in these patients who are predisposed to thrombosis (Eldor and Rachmilewitz, 2002). In order to define the optimal approach for stem cell mobilization, we have performed clinical trials, G-CSF based or plerixafor based, in adult patients with β-thalassemia.
Initially, we addressed the issues of splenic enlargement and hyperleukocytosis in response to G-CSF by exploring mobilization with G-CSF alone or after hydroxyurea pretreatment (Yannaki et al., 2012). The G-CSF mobilization trial demonstrated that the G-CSF-induced hyperleukocytosis in splenectomized patients is a major dose-limiting factor, ultimately resulting in poor CD34+ cell yields. One-month pretreatment with hydroxyurea and initiation of G-CSF after an optimal 2-week interval decreases the baseline WBCs of splenectomized patients and allows them to tolerate almost the standard G-CSF mobilization dose, thus leading to successful CD34+ cell collections. The limitation, however, of the latter approach is the prolongation of the mobilization procedure by 6 weeks and the cytostatic nature of hydroxyurea. In contrast to G-CSF, plerixafor mobilization in a limited number of patients showed that plerixafor does not induce excessive leukocytosis in the splenectomized patients, resulting, in general, in successful yields, whereas in the nonsplenectomized patients, plerixafor causes significantly less spleen enlargement (Yannaki et al., 2012).
Here, we present the results of the completed plerixafor mobilization trial and we address the issue of remobilization of thalassemic patients who have failed to provide optimal yields of stem cells after single-agent mobilization by G-CSF alone or by plerixafor alone. Our results showing superior yields and qualitative characteristics of stem cells mobilized by the combination of the two mobilizing agents, plerixafor and G-CSF, are of relevance to the overall field of stem cell gene therapy of genetic disorders.
Patients and Methods
Study approval
The study was registered with the European clinical trials database (EudraCT number 2009-014136-37) and at www.clinicaltrials.gov (NCT01206075) and was reviewed and approved by the Greek Independent Ethics Committee at the George Papanicolaou Hospital, the Institutional Review Board of the University of Washington, and the Greek National Organization for Medicines. The study was conducted at the George Papanicolaou Hospital in Thessaloniki, Greece, in compliance with the Declaration of Helsinki. All patients provided written informed consent.
Study design and patients
Plerixafor (provided by Sanofi-Genzyme, gratis) was administered subcutaneously at 0.24 mg/kg/day on two consecutive evenings followed, 10 hours later, by leukapheresis (3X total blood volume) with a Cobe Spectra apheresis system (Terumo BCT Corporate) unless ≥6×106 CD34+ cells/kg were collected in one apheresis. Subjects who failed to provide 6×106 CD34+ cells/kg by plerixafor per two aphereses or those who had failed to mobilize effectively with G-CSF alone in the previous mobilization trial were offered the opportunity to be remobilized with the combination of G-CSF+plerixafor. In this case, G-CSF was administered subcutaneously for 5 or 6 days in the morning, at the standard (10 mcg/kg/day) dose to nonsplenectomized patients or at a dose adjusted to the degree of leucocytosis in splenectomized patients. The evening of the fourth day, the standard dose of plerixafor was administered followed by the fifth dose of G-CSF next morning and leukapheresis 1 hour later. The same procedure was repeated the next day unless ≥6×106 CD34+ cells/kg had already been collected.
Patient eligibility was based on previously published criteria (Yannaki et al., 2012).
The leukapheresis products were enriched for CD34+ cells by a CliniMacs device (Miltenyi Biotech) after the separation of 2×106 unselected CD34+ cells/kg as back up. The final product was sampled for sterility, CD34+ cell content by flow cytometry, and clonogenic capacity (colony-forming unit-granulocyte, macrophage [CFU-GM] and erythroid burst-forming unit [BFU-E]). The bulk product was cryopreserved for a future gene transfer trial, for which these participants would be eligible and willing to participate. Aliquot samples were also stored for transduction with a lenti-viral globin vector and in vitro and in vivo xenograft studies.
Patients were followed by weekly physical and laboratory evaluations for up to 1 month after completion of mobilization.
Efficacy outcomes
Efficacy outcome measures included the number of patients reaching the optimum target number of CD34+ cells/kg (≥6×106 CD34+ cells/kg) within 2 days of aphereses; the number of days of apheresis (1 or 2) to collect the target cell dose or to encounter failure; the total CD34+ cells/kg and colony-forming cells/kg mobilized; the number of CD34+ cells/kg collected per day of apheresis; and the fold increase in blood CD34+ cells/μl.
Safety
Safety was monitored by the incidence of adverse events and severe adverse events in terms of changes from baseline, clinical laboratory measurements, and physical examination findings. In nonsplenectomized patients, the spleen size was evaluated by physical examination daily and measured by ultrasonography (V=0.523×length×thickness×width) (De Odorico et al., 1999) at baseline and after the completion of leukaphereses (plerixafor-mobilized patients) or at baseline and every other day until the completion of leukaphereses (G-CSF+plerixafor-mobilized patients). If the spleen volume increased by more than 80% as compared with baseline, G-CSF should be discontinued and the patient withdrawn. In splenectomized patients, the development of excessive leukocytosis (≥100×103/μl) would trigger drug discontinuation and leukapheresis for the purpose of leukoreduction, regardless of CD34+ counts.
CFU assays and flow cytometry analysis
Fresh, purified CD34+ cells were plated (1×103 cells/ml, in duplicate for CFU-GM and in triplicate for BFU-E colonies) in complete methylcellulose medium (GF H4434; StemCell Technologies) and incubated in a humidified +37°C incubator. CFU-GM and BFU-E colonies were scored after 14 days using an inverted microscope. The absolute mobilized colony-forming cells were calculated per patient weight. The in vitro clonogenic capacity of CD34+ cells mobilized by plerixafor or G-CSF+plerixafor was compared based on the number of colonies generated from equal numbers of CD34+ cells plated per milliliter.
Flow cytometry CD34+ cell subtyping was performed on thawed CD34+ cell samples from plerixafor- and G-CSF+plerixafor-treated individuals. The samples were labeled using the following cell-surface markers: PerCP-Cy5-7AAD, APC-Cy7-CD45, PE-Cy7-CD34, APC-CD38, and PE-HLA-DR (BD Biosciences, Pharmingen). Results were obtained on a FACSCanto flow cytometer (Becton Dickinson) and analyzed with the FACSDiva 6 software.
Statistics
A descriptive analysis of all continuous variables was performed, including mean, median, standard deviation, range, and maximum values. Data are expressed as mean±SD and median (range) values. Means of continuous variables were compared using paired t-test.
Results
Patients and safety
Twenty patients, 12 splenectomized and 8 nonsplenectomized, were enrolled (Table 1). As expected, splenectomized patients presented at baseline with significantly higher WBC and platelet counts (Table 1). From the 20 patients, 18 were evaluable; 1 patient refused to proceed to apheresis because of a need for central venous catheter placement (P15), and in another subject, apheresis was unsuccessful because of technical failure (P10) (Table 2). Seventeen patients received plerixafor alone. One patient received the combination of G-CSF+plerixafor because of previous G-CSF-mobilization failure. Six of the 17 patients who were mobilized with plerixafor alone (35%) failed to reach the target cell dose of ≥6×106 CD34+ cells/kg by two aphereses, and three of them were available for remobilization with the combination of G-CSF+plerixafor (Table 2). In total, four patients were remobilized with the combination of G-CSF+plerixafor (Table 1).
Table 1.
Patient Characteristics
| Patient number | 20 |
| Splenectomized | 12 |
| Nonsplenectomized | 8 |
| Remobilized patients | 4 |
| With prior plerixafor failure | 3: 2 splenectomized, 1 nonsplenectomized |
| With prior G-CSF failure | 1: splenectomized |
| Median age, years (range) | 32 (23–42) |
| Sex, n (M/F) | 15/5 |
| Median weight, kg (range) | 67 (50–84) |
| β-thal genotype | |
| β0/β0 | 5 |
| β+/β+ | 10 |
| β0/β+ | 5 |
| Median ferritin, mg/dl (range) | 678 (65–1318) |
| Chelation | |
| Desferioxamine | 1 |
| Deferiprone | 1 |
| Deferasirox | 3 |
| Deferiprone+desferioxamine | 15 |
| Mean WBCs (×103/μl) baseline | 9.00±3.94 |
| Splenectomized | 11.33±4.00a |
| Nonsplenectomized | 6.31±1.01a |
| Mean PLT counts (×103/μl) baseline | 458±191 |
| Splenectomized | 580±128.95b |
| Nonsplenectomized | 269.25±81.35b |
| Mean CD34+ cells (/μl) baseline | 4.40±2.80 |
| Splenectomized | 4.92±2.99 |
| Nonsplenectomized | 3.00±2.29 |
| Mean spleen volume, baseline (cm3) | 611±290 |
G-CSF, granulocyte-colony stimulating factor; PLT, platelet; WBCs, whole blood counts (total nucleated cells including erythroblasts).
Data are expressed as median (range) or mean±SD.
p=0.002, bp=0.000005.
Table 2.
Individual Characteristics and Mobilization Parameters in Plerixafor-Mobilized Patients
| Patient no. | Age (years) | Weight (kg) | Sex | Splenectomy | WBCs baseline (×103/μl) | Max WBCs (×103/μl) | Max blood CD34+ (cells/μl) | CD34+ cell yield (×106/kg) | No. of aphereses |
|---|---|---|---|---|---|---|---|---|---|
| P01 | 29 | 63 | M | N | 5.30 | 18.30 | 44 | 7.05 | 2 |
| P02 | 28 | 57 | M | Y | 16.47 | 61.40 | 220 | 9.32 | 1 |
| P03 | 23 | 70 | M | Y | 10.60 | 33.00 | 62 | 6.00 | 2 |
| P05 | 42 | 61 | F | Y | 10.30 | 34.00 | 72 | 8.92 | 2 |
| P06 | 34 | 71 | M | Y | 13.80 | 54.50 | 78 | 7.19 | 2 |
| P07 | 39 | 58 | F | N | 5.50 | 18.00 | 57 | 7.28 | 2 |
| P08 | 37 | 69 | M | N | 7.80 | 25.60 | 51 | 7.28 | 2 |
| P09 | 38 | 64 | M | N | 5.80 | 18.00 | 30 | 4.32 | 2 |
| P10 | 42 | 85 | M | Y | 8.50 | 40.00 | 66 | n/a | 2 |
| P11 | 38 | 76 | M | Y | 9.67 | 32.20 | 40 | 4.50 | 2 |
| P12 | 29 | 68 | M | N | 6.74 | 20.80 | 87 | 5.74 | 2 |
| P13 | 25 | 67 | F | N | 7.26 | 22.80 | 18 | 2.53 | 2 |
| P14 | 40 | 73 | M | Y | 5.90 | 15.18 | 61 | 7.81 | 2 |
| P15 | 31 | 51 | F | N | 6.40 | 18.60 | 31 | n/a | 2 |
| P16 | 25 | 50 | F | N | 5.69 | 22.85 | 24 | 5.10 | 2 |
| P17 | 26 | 61 | M | Y | 8.39 | 26.90 | 16 | 1.26 | 2 |
| P18 | 23 | 76 | M | Y | 11.05 | 43.30 | 126 | 6.68 | 1 |
| P19 | 32 | 56 | M | Y | 14.80 | 48.76 | 76 | 6.51 | 2 |
| P20 | 40 | 74 | M | Y | 21.00 | 37.80 | 80 | 9.14 | 2 |
P01–P10 have previously been reported in the study's interim analysis (Yannaki et al., 2012). P10 was initially considered as plerixafor mobilization failure; however, it was later recognized that poor CD34+ collection was because of technical failure during aphereses. P04 is not included because she received only G-CSF+plerixafor in this study, because of previous G-CSF mobilization failure. P11, P13, and P17 were later remobilized with G-CSF+plerixafor. n/a, not applicable (refers to nonevaluable patients described in the Results section). F, female; M, male; N, no; Y, yes.
No serious adverse events occurred. Toxicity was graded according to the Common Terminology Criteria for Adverse Events v4.0. Plerixafor was very well tolerated, and only mild toxicities, most commonly nausea, diarrhea, perioral numbness, headache, and injection-site erythema were encountered. Bone pain and low fever were commonly seen during G-CSF administration. G-CSF-induced leukocytosis was not considered an adverse event because it is a direct and intended effect, and it was not associated with any complaints, discomfort, or clinical findings. Grade 1 thrombocytopenia was a common, apheresis-associated adverse event.
Hematopoietic cell mobilization of thalassemic patients using plerixafor as a single agent
The primary end point of the study was the collection of at least 6×106 CD34+ cells/kg into two or less aphereses; this end point was achieved by 65% of the evaluable subjects who received plerixafor alone (11/17) in 1.83 days required time. Of the remaining 6 patients (35%) who failed to reach the study's target cell dose, only 2/17 (12%) were identified as truly poor mobilizers yielding ≤2.5×106 CD34+ cells/kg by two aphereses (P13, P17) (Table 2).
The mean CD34+ cell yield in all plerixafor-mobilized patients was 6.3±2.2×106 CD34+ cells/kg and it was reached within a mean of 1.88 days (Table 3). The mean peripheral blood CD34+ cells (/μl) during the procedure were 53±37.9, representing an 11-fold increase over baseline (compare Table 3 with Table 1). A higher mean peak both in WBCs and blood CD34+ cells was achieved in the splenectomized than in the nonsplenectomized patient cohort (WBCs×103/μl: 38.99±12.99 vs. 20.6±2.9, p=0.001; CD34+ cells/μl: 65.4±44.0 vs. 37.2±19.2, p=0.03) but it was not translated to significantly higher CD34+ cell yields in this patient group (CD34+ cells/kg: 6.7±2.4 vs. 5.6±1.8, p=not significant [n.s.]) (Table 3). A significant decrease in platelet numbers (×103/μl) was observed after aphereses as compared with baseline (224±109 vs. 458±191, p=0.0001) (Table 3), and thrombocytopenia grade I was a common adverse event at the end of the procedure. In the nonsplenectomized patients, plerixafor induced a modest spleen volume increase over baseline of 10.92%±13.31%, approximately sixfold lower than the mean size increase measured in G-CSF-mobilized thalassemic patients in the G-CSF trial (Table 3) (Yannaki et al., 2012).
Table 3.
Cumulative Data on Mobilization Parameters
| Plerixafor only—all patients | Preceding single-agent mobilization (plerixafor n=3, G-CSF n=1) of remobilized patients | Plerixafor+G-CSF—remobilized patients | |
|---|---|---|---|
| Patient number | 17 (10 SPL, 7 non-SPL) | 4 (3 SPL, 1 non-SPL) | 4 (3 SPL, 1 non-SPL) |
| Blood CD34+ cells during aphereses/μl | 53±37.9a | 20.4±12.4b | 115.5±13.3a,b |
| Splenectomized | 65.4±44c,d | 21.6±14.4e | 118.0±15.1d,e |
| Nonsplenectomized | 37.2±19.2c | 17 | 108 |
| Total CD34+ cell yield,×106/kg | 6.3±2.2 | 2.5±1.4f | 8.9±2.9f |
| Splenectomized | 6.7±2.4 | 2.5±1.5g | 7.6±1.6g |
| Nonsplenectomized | 5.6±1.8 | 2.5 | 12.7 |
| Days of aphereses | 1.88±0.33h | 2±0.0 | 1.00±0.0h |
| CD34+ cell yield per apheresis,×106/kg | 3.6±2.0i | 1.2±0.7j | 8.9±2.9i,j |
| Total CFCs,×106/kg | |||
| CFU-GM | 379±189k | n/a | 737±134k |
| BFU-E | 257±112l | n/a | 496±66l |
| Failure to reach 6×106 CD34+ cells/kg | 6/17 (35%) | 4/4 (100%) | 0/4 (0%) |
| Failure to yield >2.5×106 CD34+ cells/kg | 2/17 (12.5%) | 3/4 (75%) | 0/4 (0%) |
| Max WBCs,×103/μl | 31.3±13.6 | 41.2±28.1 | 76.5±19.7 |
| Splenectomized | 38.9±13m | Plerixafor: 28±17n/G-CSF: 88 | 82.2±19.7m,n |
| Nonsplenectomized | 20.6±2.9 | 27 | 59.5 |
| Platelet counts,×103/μl | |||
| Baseline | 458±191o | 440±139p | 455±151q |
| After leukaphereses | 224±109o | 176±51.2p | 169±23.6q |
| Spleen volume, cm3 | |||
| After mobilization | 703±302.4 | n/a | n/a |
| % Splenic enlargement over baseline(max) | 10.74±13.9 | n/a | n/a |
SPL, splenectomized; non-SPL, non-splenectomized BFU-E, erythroid burst-forming unit; CFU-GM, colony-forming unit-granulocyte, macrophage.
Data are expressed as mean±SD or absolute and relative frequencies.
p=0.003, bp=0.00005, cp=0.03, dp=0.06, ep=0.002, fp=0.0002, g,kp=0.02, hp=0.001, ip=0.0003, jp=0.002, lp=0.007, mp=0.006, np=0.04, op=0.0001, p,qp=0.01.
Remobilization with G-CSF plus plerixafor of thalassemic subjects who failed single-agent mobilization
According to the study design, patients who failed to reach the target cell dose of at least 6×106 CD34+ cells/kg by single-agent mobilization in up to two aphereses were offered the opportunity to be remobilized with the combination of G-CSF+plerixafor. Six of the 17 evaluable patients (35%) met this criterion (P09, P11, P12, P13, P16, P17; Table 2); ultimately, three of them and one patient who had previously experienced G-CSF failure in our first mobilization study were available for remobilization with G-CSF+plerixafor. Three of the remobilized patients were splenectomized and, according to the protocol, they received lower and adjusted to the degree of leukocytosis G-CSF doses (Table 4) to avoid hyperleukocytosis (Yannaki et al., 2012).
Table 4.
Individual Characteristics and Mobilization Parameters in Patients Remobilized with G-CSF+Plerixafor
| Patient no. | Age (years) | Weight (kg) | Sex | Splenectomy | Initial mobilization | Initial mobilization max WBCs (×103/μl) | Remobilization max WBCs (×103/μl) | Remobilization mean G-CSF dose (mcg/kg/d) | Initial mobilization max CD34+(cells/μl) | Remobilization max CD34+(cells/μl) | Initial mobilization mean CD34+ cells/apheresis (×106/kg) | Remobilization mean CD34+ cells/apheresis (×106/kg) | Remobilization aphereses no. to reach target cell dose |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P04 | 38 | 77 | F | Y | G-CSF | 83.0 | 88.0 | 2.2 | 10 | 120 | 0.81 | 6.5 | 1 |
| P11 | 38 | 78 | M | Y | Plerixafor | 32.2 | 60.2 | 5.5 | 40 | 102 | 2.25 | 6.8 | 1 |
| P13 | 25 | 67 | F | N | Plerixafor | 27.0 | 59.5 | 10 | 17 | 108 | 1.25 | 12.7 | 1 |
| P17 | 26 | 62 | M | Y | Plerixafor | 22.8 | 98.3 | 6 | 15 | 132 | 0.63 | 9.4 | 1 |
The combination proved highly synergistic; all remobilized patients readily reached the cell target of ≥6×106 CD34+ cells/kg by a single apheresis (remobilization: mean CD34+ cells/kg: 8.9±2.9×106 in 1 day of apheresis vs. single-agent mobilization: mean CD34+ cells/kg: 2.5±0.7×106 per two aphereses, p=0.0002) (Table 3) and increased the per-apheresis yield over initial, single-agent mobilization, by 9-fold (range 3–14) (p=0.002) (Table 4). This was correlated with a mean value of 115.5±13.3 CD34+ cells/μl of blood (Table 3), representing a 7-fold increase (range 3–12) in the circulating CD34+ cells/μl during remobilization (p=0.00005) (Table 4). The increases both in circulating and harvested CD34+ cells occurred, despite that, in splenectomized subjects, a more than 50% G-CSF dose reduction was applied in order to avoid hyperleukocytosis (Table 4), a major G-CSF dose-limiting factor (Yannaki et al., 2012). Indeed, despite G-CSF dose reductions, in the splenectomized subjects, the mean max WBC (×103/μl) reached 82.1±19.7 during low-dose G-CSF+plerixafor mobilization (Table 3). The synergistic effect of the combination was especially obvious in the splenectomized patient (P04) who had previously failed to yield adequate CD34+ cells by (low dose) G-CSF and was remobilized; although she developed the same degree of leukocytosis in response to similar, adjusted G-CSF doses, the per-apheresis yield and the circulating blood stem cells were increased by 8- and 10-fold, respectively, when a single plerixafor dose was added to 4 days' G-CSF (Table 4).
Superior stem cell mobilization by the combination of G-CSF+plerixafor
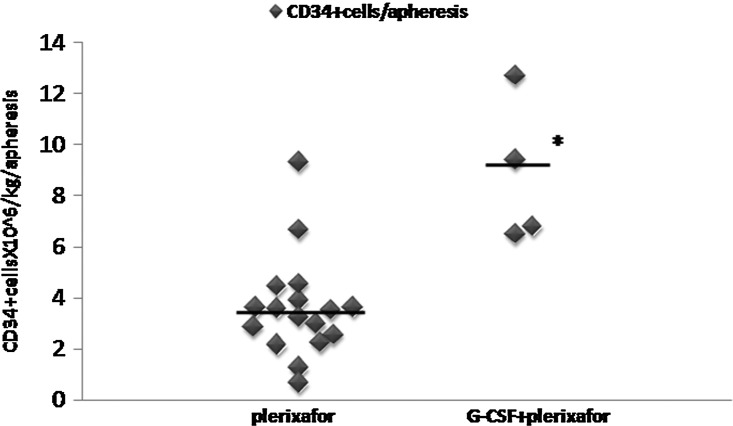
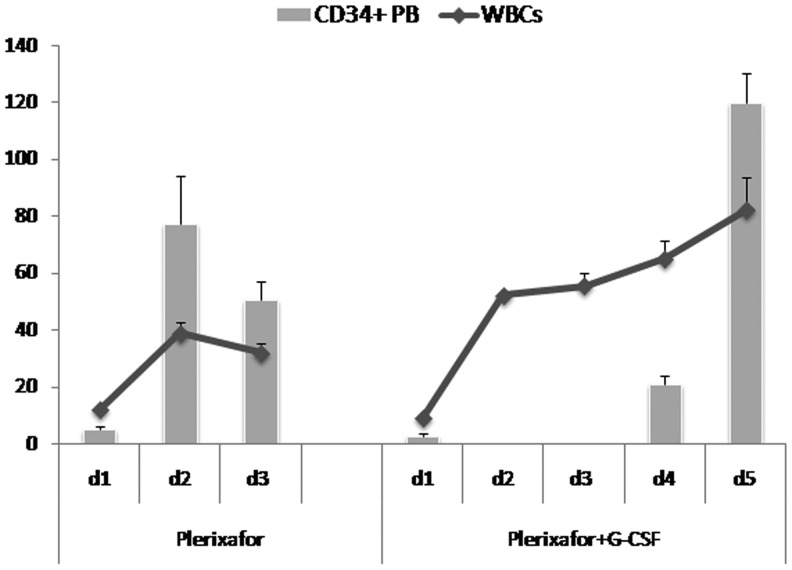
Compared with plerixafor alone, the combination of G-CSF+plerixafor increased the mean peripheral blood CD34+ cells by 2.1-fold (from 53±37.9 to 115.5±13.3 CD34+ cells/μl, p=0.003) and the per-apheresis CD34+ cell yield by 2.5-fold (from 3.61±2.05 to 8.85±2.88×106/kg, p=0.0003), thus significantly reducing the days needed to collect the target cell dose (from 1.83±0.39 to 1.0±0.0, p=0.001) (Fig. 1 and Table 3). In the splenectomized patients, the G-CSF+plerixafor combination, despite the significantly reduced G-CSF doses that were administered, still produced very high WBCs, whereas plerixafor-alone mobilization resulted in only modest leukocytosis (mean max WBC×103/μl: 82.2±19.7 vs. 39.0±13.0, p=0.006) (Fig. 2 and Table 3). Peripheral blood CD34+ cells increased proportionally to WBCs in both plerixafor-alone-mobilized or G-CSF+plerixafor-mobilized splenectomized patients (Fig. 2).
FIG. 1.
Mean CD34+ cell yield per apheresis in splenectomized and nonsplenectomized thalassemic subjects mobilized with plerixafor alone or G-CSF+plerixafor. Each data point represents the mean yield per apheresis of each patient in all evaluable subjects treated with plerixafor and/or G-CSF+plerixafor. The black horizontal line represents the mean CD34+ cell yield per apheresis per mobilization group. *p=0.0003 versus plerixafor. G-CSF, granulocyte-colony stimulating factor.
FIG. 2.
Kinetics of PB CD34+ cells and WBCs in splenectomized patients mobilized by plerixafor and/or G-CSF+plerixafor. The mean values of WBCs (total nucleated cells including erythroblasts,×103/μl) and peripheral blood CD34+ cells (/μl) during mobilization of splenectomized patients are depicted (mean±SEM). PB CD34+ cell counts were not performed on days 2 and 3 in remobilized patients. All evaluable splenectomized patients mobilized with plerixafor alone (n=11) and remobilized with G-CSF+plerixafor (n=3) were included. PB, peripheral blood; WBCs, whole blood cells.
Despite single collections, the G-CSF+plerixafor mobilization was superior to the plerixafor-alone mobilization in terms of the total numbers of CD34+ cells collected (8.85±2.88/kg vs. 6.3±2.2/kg, p=0.06) as well as CFU-GM (737±133.5 vs. 379±188.8×106/kg, p=0.02) and BFU-E (496±66 vs. 257±112×106/kg, p=0.007) mobilized (Table 3). Importantly, the real in vitro clonogenic capacity of G-CSF+plerixafor-mobilized CD34+ cells, based on the same number of CD34+ cells plated/ml, was higher than plerixafor-alone mobilized cells (CFU-GM per 1×103 CD34+ cells/ml: 87±13.6 vs. 56.5±25.6, p=0.05; BFU-E per 1×103 CD34+ cells/ml: 59.3±16.1 vs. 38±13.4, p=0.03; Table 5). Purified CD34+ cells mobilized by either method displayed a primitive CD34+/CD38− and CD34+/CD38−/HLA-DR− phenotype (Table 5).
Table 5.
Clonogenic Capacity and Cell Phenotype
| Plerixafor | G-CSF+plerixafor | p | |
|---|---|---|---|
| CFU-GM per 2×103 cells plated | 113±51.1 | 174±27.2 | 0.05 |
| BFU-E per 3×103 cells plated | 114±40.3 | 178±48.3 | 0.03 |
| CD34+CD38−, % | 23.7±15.0 | 28.0±14.1 | ns |
| CD34+CD38−HLA-DR−, % | 9.93±9.1 | 10.95±9.0 | ns |
Data are expressed as mean±SD. ns, not significant.
Discussion
In stem cell gene therapy protocols, as for thalassemia, in order to ensure stable engraftment of genetically modified stem cells and low peritransplant toxicity, significantly higher CD34+ cell numbers are optimal than the lower CD34+ cell limit of 2×106/kg acceptable for autologous hematopoietic cell transplantation (To et al., 1997; Yannaki et al., 2010). Full myeloablation before the infusion of gene-corrected HSCs is expected to facilitate the establishment of complete vector-carrying cell chimera; however, a nonmyeloablative conditioning should be preferably considered to reduce the risks during the posttransplant phase of bone marrow aplasia or in the case of graft failure. This approach has been successfully applied in the case of inherited immunodeficiencies (Aiuti et al., 2009, 2013), but it presents a challenge when the genetically corrected stem cells lack a selective advantage such as in thalassemia and sickle cell disease. Partial myeloablation creates a competitive setting in the bone marrow, and under such conditions, high numbers of gene-corrected cells are required for infusion in order to prevail over the uncorrected endogenous bone marrow cells. In addition, for safety reasons, a backup of unmodified CD34+ cells is necessarily stored to rescue patients in the case of engraftment failure, further increasing the need for high-yield stem cell collections. Consequently, for stem cell gene therapy the optimal source of HSCs is the source that provides, safely and effectively, high cell doses with increased engrafting capacity.
G-CSF-mobilized hematopoietic cell grafts are currently the preferred source for hematopoietic cell transplantation (Gratwohl et al., 2005; To et al., 2011, and references within) and stem cell gene therapy applications (Ott et al., 2006; Cartier et al., 2009; Boztug et al., 2010; Sadelain et al., 2010). G-CSF mobilization, however, is associated with certain morbidities (Falzetti et al., 1999; Hill et al., 2005); furthermore, a number of patients fail to mobilize effectively or certain normal donors need to undergo extended aphereses (Anderlini et al., 1997; Stiff et al., 2000; Miller et al., 2008). In addition, certain limitations have been raised with the use of G-CSF in autologous transplantation settings, including gene therapy. In sickle cell patients, G-CSF is contraindicated because it can precipitate severe or even fatal sickle cell crisis (Abboud et al., 1998; Adler et al., 2001). In splenectomized thalassemic patients, G-CSF mobilization induces hyperleukocytosis, a significant dose-limiting factor usually resulting in failure to reach optimal cell target doses (Yannaki et al., 2012). Patients with Fanconi anemia mobilize only limited numbers of CD34+ cells with G-CSF (Croop et al., 2001) even after very high (32 mcg/kg/day) G-CSF doses (Kelly et al., 2007).
In view of the limitations of G-CSF mobilization, we investigated plerixafor as a single mobilizing agent and its combination with G-CSF in case of failure to collect sufficient HSC numbers by single-agent mobilization. The target cell dose was defined as equal or more than 6×106 CD34+ cells/kg acquired by two aphereses. This target was set under the assumption that in a gene therapy trial, 2×106 cells/kg will be stored as unmanipulated back up cells to rescue the patient in the case of graft failure and at least 4×106 cells/kg will be purified by positive selection and stored to be later transduced with an optimized beta-globin lentiviral vector and infused into the patients.
Plerixafor reduces the binding and chemotaxis of HSCs to the bone marrow stroma through reversible interruption of the SDF-1a/CXCR4 signaling leading to a rapid mobilization process (Liles et al., 2003; Devine et al., 2004; Broxmeyer et al., 2005). In our study, plerixafor rapidly and effectively mobilized CD34+ cells after injection in thalassemic individuals without the induction of hyperleukocytosis in splenectomized patients or remarkable splenic enlargement in nonsplenectomized patients. This stands in clear contrast with G-CSF mobilization that requires at least 3-day treatment before significant increases in circulating CD34+ cells can be measured (To et al., 1997) and induces excessive leukocytosis in splenectomized thalassemics or significant spleen enlargement in nonsplenectomized subjects (Yannaki et al., 2012). Despite the safe and effective plerixafor mobilization of thalassemic patients, almost one-third of our patients failed to provide by two aphereses the target cell dose of 6×106 CD34+ cells/kg, which we consider as the lower limit to subsequently proceed to stem cell gene therapy. As per the protocol design, patients who failed to mobilize adequately by plerixafor alone in this trial or G-CSF alone in the previous trial were eligible for remobilization by G-CSF+plerixafor.
Our data showed that patients with thalassemia who fail to yield the high target cell doses by single-agent mobilization can greatly benefit from G-CSF+plerixafor remobilization. G-CSF+plerixafor was well tolerated, increased the per-apheresis yield by several fold, and, in all paired cases, resulted in single-apheresis collections of the desired target cell dose. The great synergism of the combination was overt especially in the splenectomized patients, in whom, despite the significant G-CSF dose reductions to control hyperleukocytosis, it still provided high cell yields by single apheresis. This stands in clear contrast to what we had previously seen with splenectomized patients who were mobilized with G-CSF alone and in whom hyperleukocytosis was not associated with a proportional increase of blood CD34+ cells, finally resulting in poor or modest CD34+ cell yields (Yannaki et al., 2012). Importantly, although fewer days of apheresis were needed, G-CSF+plerixafor patients mobilized higher total numbers of CD34+ cells and colony-forming cells as compared with patients mobilized with plerixafor alone. These cells displayed favorable transplantation features such as higher clonogenic capacity and a primitive immunophenotype (Fruehauf et al., 2009), implying a potential engraftment benefit upon transplantation. A faster time to engraftment reduces the peritransplant toxicity, including severe infections and bleeding complications.
The synergistic effect of the combination is so rapid and intense that plerixafor should be considered not only for remobilization but also as immediate salvage for those patients who are predicted to mobilize with G-CSF poorly or inadequately. Indicators to predict a poor or inadequate mobilization may include suboptimal blood CD34+ cell levels (i.e., CD34+ cells <20/μl the fourth day of G-CSF administration) and/or suboptimal yields by the first apheresis, thus implying failure to harvest the target cell dose within an acceptable number of apheresis days. However, for stem cell gene therapy applications where the very high numbers of collected CD34+ cells are critical for the outcome, or failure to single-agent mobilization is highly likely (i.e., patients with Fanconi anemia), or single apheresis collections are desirable, the upfront use of G-CSF+plerixafor combination should be considered. Although the earlier identification of poor or suboptimal CD34+ cell yields and initiation of plerixafor during G-CSF mobilization or the front-line use of G-CSF+plerixafor may increase the per-patient cost of mobilization, the days of aphereses, the failure rates, and the need for remobilizations will be decreased, and these may counterbalance the additional costs linked to the introduction of plerixafor.
Overall, our data, in addition to providing optimal mobilization approaches for thalassemia, have broader implications for stem cell gene therapy of genetic diseases.
Acknowledgments
We thank the patients for their participation and the physicians who referred patients to this program. We thank Dr. Vasilios Perifanis (AHEPA Hospital, Thessaloniki) for performing the medical monitoring locally. We thank Sanofi-Genzyme for providing plerixafor gratis. This work was supported by NIH Grant #P01 HL053750-19 and the “Cooperation-Action I” ESPA Program 09SYN-12-1159.
Author Disclosure Statement
All authors declare that no competing financial interests exist.
References
- Abboud M. Laver J. Blau C.A. Granulocytosis causing sickle-cell crisis. Lancet. 1998;351:959. doi: 10.1016/S0140-6736(05)60614-9. [DOI] [PubMed] [Google Scholar]
- Adler B.K. Salzman D.E. Carabasi M.H., et al. Fatal sickle cell crisis after granulocyte colony-stimulating factor administration. Blood. 2001;97:3313–3314. doi: 10.1182/blood.v97.10.3313. [DOI] [PubMed] [Google Scholar]
- Aiuti A. Cattaneo F. Galimberti S., et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N. Engl. J. Med. 2009;360:447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- Aiuti A. Biasco L. Scaramuzza S., et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341:1233151. doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderlini P. Przepiorka D. Seong C., et al. Factors affecting mobilization of CD34+ cells in normal donors treated with filgrastim. Transfusion. 1997;37:507–512. doi: 10.1046/j.1537-2995.1997.37597293882.x. [DOI] [PubMed] [Google Scholar]
- Bensinger W.I. Martin P.J. Storer B., et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N. Engl. J. Med. 2001;344:175–181. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- Biffi A. Montini E. Lorioli L., et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromaticleukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- Boztug K. Schmidt M. Schwarzer A., et al. Stem-cell gene therapy for the Wiskott-Aldrich syndrome. N. Engl. J. Med. 2010;363:1918–1927. doi: 10.1056/NEJMoa1003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H.E. Orschell C.M. Clapp D.W., et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier N. Hacein-Bey-Abina S. Bartholomae C.C., et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M. Hacein-Bey S. de Saint Basile G., et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–72. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M. Payen E. Negre O., et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croop J.M. Cooper R. Fernandez C., et al. Mobilization and collection of peripheral blood CD34+ cells from patients with Fanconi anemia. Blood. 2001;98:2917–2921. doi: 10.1182/blood.v98.10.2917. [DOI] [PubMed] [Google Scholar]
- De Odorico I. Spaulding K. Pretorius D., et al. Normal splenic volumes estimated using three-dimensional ultrasonography. J. Ultrasound Med. 1999;18:231–236. doi: 10.7863/jum.1999.18.3.231. [DOI] [PubMed] [Google Scholar]
- Devine S.M. Flomenberg N. Vesole D.H., et al. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin's lymphoma. J. Clin. Oncol. 2004;22:1095–1102. doi: 10.1200/JCO.2004.07.131. [DOI] [PubMed] [Google Scholar]
- Devine S.M. Vij R. Rettig M., et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood. 2008;112:990–998. doi: 10.1182/blood-2007-12-130179. [DOI] [PubMed] [Google Scholar]
- DiPersio J.F. Micallef I.N. Stiff P.J., et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J. Clin. Oncol. 2009;27:4767–4773. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- Eldor A. Rachmilewitz E.A. The hypercoagulable state in thalassemia. Blood. 2002;99:36–43. doi: 10.1182/blood.v99.1.36. [DOI] [PubMed] [Google Scholar]
- Falzetti F. Aversa F. Minelli O. Tabilio A. Spontaneous rupture of spleen during peripheral blood stem-cell mobilisation in a healthy donor. Lancet. 1999;353:555. doi: 10.1016/S0140-6736(99)00268-8. [DOI] [PubMed] [Google Scholar]
- Fruehauf S. Veldwijk M.R. Seeger T., et al. A combination of granulocyte-colony-stimulating factor (G-CSF) and plerixafor mobilizes more primitive peripheral blood progenitor cells than G-CSF alone: results of a European phase II study. Cytotherapy. 2009;11:992–1001. doi: 10.3109/14653240903121245. [DOI] [PubMed] [Google Scholar]
- Gratwohl A. Baldomero H. Schmid O., et al. Change in stem cell source for hematopoietic stem cell transplantation (HSCT) in Europe: a report of the EBMT activity survey 2003. Bone Marrow Transplant. 2005;36:575–590. doi: 10.1038/sj.bmt.1705104. [DOI] [PubMed] [Google Scholar]
- Hill J.M. Syed M.A. Arai A.E., et al. Outcomes and risks of granulocyte colony-stimulating factor in patients with coronary artery disease. J. Am. Coll. Cardiol. 2005;46:1643–1648. doi: 10.1016/j.jacc.2005.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P.F. Radtke S. von Kalle C., et al. Stem cell collection and gene transfer in Fanconianemia. Mol. Ther. 2007;15:211–219. doi: 10.1038/sj.mt.6300033. [DOI] [PubMed] [Google Scholar]
- Liles W.C. Broxmeyer H.E. Rodger E., et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102:2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- Liles W.C. Rodger E. Broxmeyer H.E., et al. Augmented mobilization and collection of CD34+ hematopoietic cells from normal human volunteers stimulated with granulocyte-colony-stimulating factor by single-dose administration of AMD3100, a CXCR4 antagonist. Transfusion. 2005;45:295–300. doi: 10.1111/j.1537-2995.2005.04222.x. [DOI] [PubMed] [Google Scholar]
- Miller J.P. Perry E.H. Price T.H., et al. Recovery and safety profiles of marrow and PBSC donors: experience of the National Marrow Donor Program. Biol. Blood Marrow Transplant. 2008;14:29–36. doi: 10.1016/j.bbmt.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Nademanee A.P. DiPersio J.F. Maziarz R.T., et al. Plerixafor plus granulocyte colony-stimulating factor versus placebo plus granulocyte colony-stimulating factor for mobilization of CD34(+) hematopoietic stem cells in patients with multiple myeloma and low peripheral blood CD34(+) cell count: results of a subset analysis of a randomized trial. Biol Blood Marrow Transplant. 2012;18:1564–1572. doi: 10.1016/j.bbmt.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Ott M.G. Schmidt M. Schwarzwaelder K., et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat. Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- Russell N.H. Hunter A. Rogers S., et al. Peripheral blood stem cells as an alternative to marrow for allogeneic transplantation. Lancet. 1993;341:1482. doi: 10.1016/0140-6736(93)90929-b. [DOI] [PubMed] [Google Scholar]
- Sadelain M. Rivière I. Wang X., et al. Strategy for a multicenter phase I clinical trial to evaluate globin gene transfer in beta-thalassemia. Ann. N. Y. Acad. Sci. 2010;1202:52–58. doi: 10.1111/j.1749-6632.2010.05597.x. [DOI] [PubMed] [Google Scholar]
- Stiff P. Gingrich R. Luger S., et al. A randomized phase 2 study of PBPC mobilization by stem cell factor and filgrastim in heavily pretreated patients with Hodgkin's disease or non-Hodgkin's lymphoma. Bone Marrow Transplant. 2000;26:471–481. doi: 10.1038/sj.bmt.1702531. [DOI] [PubMed] [Google Scholar]
- To L.B. Haylock D.N. Simmons P.J. Juttner C.A. The biology and clinical uses of blood stem cells. Blood. 1997;89:2233–2258. [PubMed] [Google Scholar]
- To L.B. Levesque J.P. Herbert K.E. How I treat patients who mobilize hematopoietic stem cells poorly. Blood. 2011;118:4530–4540. doi: 10.1182/blood-2011-06-318220. [DOI] [PubMed] [Google Scholar]
- Yannaki E. Emery D.W. Stamatoyannopoulos G. Gene therapy for β-thalassaemia: the continuing challenge. Expert Rev. Mol. Med. 2010;12:e31. doi: 10.1017/S1462399410001626. [DOI] [PubMed] [Google Scholar]
- Yannaki E. Papayannopoulou T. Jonlin E., et al. Hematopoietic stem cell mobilization for gene therapy of adult patients with severe β-thalassemia: results of clinical trials using G-CSF or plerixafor in splenectomized and nonsplenectomized subjects. Mol. Ther. 2012;20:230–238. doi: 10.1038/mt.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]