Abstract
A serological survey for West Nile virus (WNV) infection involved 395 horses from 43 administrative districts of the Czech Republic (163 animals) and 29 districts of Slovakia (232 animals), sampled between 2008 and 2011. Using a plaque-reduction neutralization microtest, antibodies to WNV were not detected in any horse from the Czech Republic, whereas 19 nonvaccinated horses from Slovakia had specific antibodies to WNV (no cross-reactions were observed with tick-borne encephalitis and Usutu flaviviruses in those animals). The seropositivity rate of nonvaccinated horses in Slovakia was 8.3% (95% confidence interval [CI] 4.7–11.9%), and autochthonous local infection with WNV occurred at least in 11, i.e., 4.8% (95% CI 2.0–7.6%) of the animals. All seropositive horses lived in six lowland districts of southern Slovakia; overall, 15.1% (95% CI 8.8–21.4%) of 126 nonvaccinated horses were seropositive in those districts, situated relatively closely to the border with Hungary, i.e., the country where WNV disease cases have been reported in birds, horses and humans since 2003.
Key Words: Flavivirus, Mosquito-borne virus, West Nile virus, Neutralizing antibodies, Horses
Introduction
West Nile virus (WNV, a Flavivirus of the Japanese encephalitis antigenic group, family Flaviviridae) circulates in natural foci between birds and bird-feeding mosquitoes largely of the genus Culex (e.g., Cx. pipiens and Cx. modestus in Europe). Humans and horses are regarded as “dead-end” hosts of WNV because of the low and short viremia produced. However, equids are very susceptible to WNV infection, which can be responsible for encephalomyelitis in a fraction of infected animals, and lethality in horses can occur (Cantile et al. 2000, Salazar et al. 2004, Venter et al. 2009). Horses also seroconvert rapidly upon WNV infection, and WNV antibodies can be easily detected in serological tests, facilitating the assessment of the epidemiological situation (surveillance) of WNV activity in particular areas.
WNV has recently re-emerged and spread in Europe, including central Europe (Hubálek and Halouzka 1999, Autorino et al. 2002, Durand et al. 2002, Zeller and Schuffenecker 2004, Angelini et al. 2010, Monaco et al. 2010, Papa et al. 2010, Sirbu et al. 2011). For instance, in the Czech Republic (Czechland, for short), West Nile fever was diagnosed in five persons in south Moravia in 1997, and the virus was also isolated from mosquitoes in both Czechland (Hubálek et al. 1999) and Slovakia (Labuda et al. 1974). However, serological surveys in humans and other vertebrates (Hubálek et al. 1999) have not yet detected a remarkable WNV activity in these countries. On the other hand, significant WNV activity involving cases in birds and horses has been demonstrated in adjacent southern countries—Hungary and Austria—in the last years (Bakonyi et al. 2006, Kutasi et al. 2011, Wodak et al. 2011). The aim of our study was to investigate indirectly for the first time whether WNV circulates among horses in Czechland or Slovakia, using a serosurvey. Signs of WNV circulation in horses (cases, seroconversion) might be an early indicator before the identification of human cases (Chevalier et al. 2011).
Materials and Methods
Serum samples
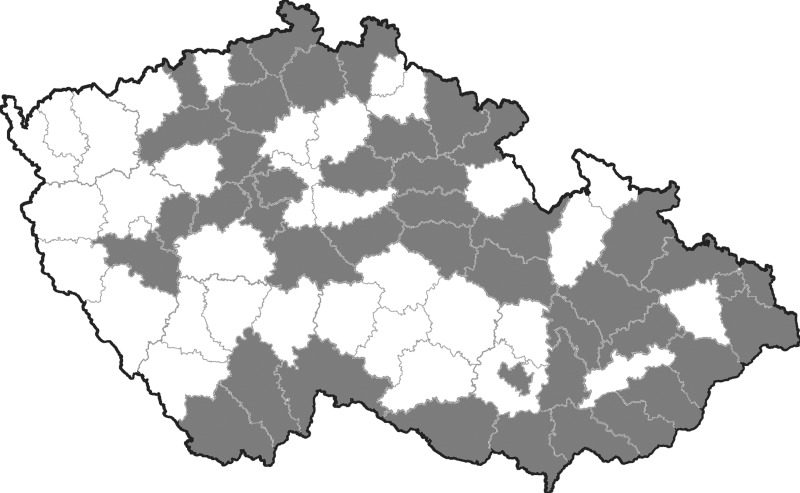
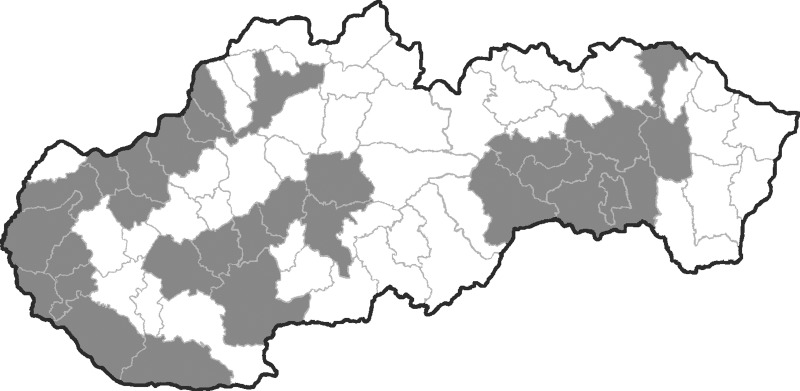
Equine blood samples were randomly collected from 43 out of 77 administrative districts in Czechland (163 horses) and from 29 out of 72 administrative districts in Slovakia (232 horses) between the years 2008 and 2011 (Figs. 1 and 2). A census of horse populations, conducted by the Ministries of Agriculture of the Czech and Slovak Republics, estimated approximately 80,000 and 15,000 individuals, respectively. In general, criteria for selection of animals were lowland regions with presence of abundant mosquito populations (and a potential risk of mosquito-borne infections). None of the sampled horses had moved from the stable locality during the last summer/autumn season at least. The age of examined animals was between 1 and 30 years. The median age of Czech animals was 7 (range, 1–23) years, and that of Slovak horses 9 (range, 1–30) years. Among the Czech animals, there were 75 males (stallions, geldings) and 84 females (mares); the figures for Slovak horses were 96 and 110, respectively. The blood sera were stored at −20°C.
FIG. 1.
Map of Czechland (Czech Republic), with administrative districts; the districts where horses were examined are given in gray.
FIG. 2.
Map of Slovakia, with administrative districts; the districts where horses were examined are given in gray.
Viruses
Three flaviviruses were used for the neutralization test: (1) WNV strain Eg-101 - Egyptian topotype of WNV, lineage 1, passaged 15 times in suckling mouse brain (SMB), homogenized in phosphate-buffered saline (PBS; pH 7.2) with 0.4% bovine serum albumin fraction V (BSA) and antibiotics, and cleared by centrifugation at 1500×g for 15 min (+4°C). (2) Tick-borne encephalitis virus (TBEV) strain Hypr, passaged 10 times in mouse brain, then 55 times in HeLa cells, and finally once in SMB; infectious SMB was homogenized in PBS with 0.4% BSA and antibiotics, and centrifuged. (3) Usutu virus (USUV) strain Vienna 939 passaged three times in Vero cells and once in SMB, homogenized in PBS with 0.4% of BSA and antibiotics, and cleared by centrifugation.
Plaque-reduction neutralization microtest
The method described by Madrid and Porterfield (1974) was adapted for use in 96-well (flat-bottomed) microplates for cell cultures (Hubálek et al. 1979, Hubálek et al. 2008). Briefly, 30 μL of thermally inactivated (at 56°C for 30 min) sera diluted 1:10 (screening) in Leibowitz L-15 medium with antibiotics were mixed with 30 μL of WNV in L-15 medium with 3% fetal calf serum (FCS) for cell culture (Sigma), containing about 30 plaque-forming units (PFU). The serum–virus mixture was incubated at 37°C for 60 min; then 60 μL of a Vero E6 cells (grown at 37°C for 3–4 days) suspension in L-15 with 3% FCS and antibiotics were added to each test well (about 20,000 cells per well). After an incubation at 37°C for 4 h, 120 μL of overlay (1.5% carboxymethylcellulose sodium salt in L-15 supplemented with 3% FCS and antibiotics) was added to each well. The microplates were covered with lids, sealed in small plastic bags, and incubated at 37°C. The cells were checked for plaques and cytopathic effect under an inverted microscope after 3 and 4 days, and then stained with 0.1% Naphthalene Black on the fifth day. Control sera (positive and negative) were included in each run of the test. The micro-plaque-reduction neutralization microtest (PRNT) was validated earlier using positive and negative equine (Weissenböck et al. 2003), other mammalian (including human), and avian sera; this test is used routinely in our laboratory for detection of neutralizing antibodies to WNV, TBEV, and USUV.
Serum samples that neutralized WNV with a 90% or greater reduction of PFU numbers at the 1:10 dilution during screening were titrated in duplicate by two-fold dilutions in L-15 medium, and the dilutions corresponding to 90% reduction of PFU were regarded as the antibody titers (PRNT90). Sera were considered positive if they had a neutralizing activity at dilutions superior to 1:20.
The sera reacting with WNV were also tested against other flaviviruses occurring in central Europe—TBEV and USUV. The PRNT90 assay for these viruses was carried out in the same way as for WNV.
Results
Antibodies neutralizing WNV were not detected in any of the 163 examined horses from Czechland, whereas 22 of 232 examined horses from Slovakia revealed specific antibodies to WNV, with the antibody titers ranging from 1:40 to 1:640 (Table 1); they were all seronegative with TBEV (the PRNT90 titer against TBEV was less than 1:10 in all cases), whereas three of them gave a very low-titer (1:10) reaction with USUV (nos. 20, 27, and 108). WNV-seropositive animals were between 2 and 12 years old, and consisted of 10 males (stallions or geldings) and 12 mares.
Table 1.
PRNT90 Reciprocal Titers of Antibodies against West Nile Virus in Equine-Specific Seroreactors, and Their History
| Horse no. | District | Sex | Age (years) | Date collected | WNV titer | Origin (country) | Past stay in WNV-endemic countries | WNV vaccine |
|---|---|---|---|---|---|---|---|---|
| 20 | Dun.Streda | M | 5 | Sep. 2010 | 160 | US | Russia | Yes |
| 23 | Dun.Streda | F | 3 | Sep. 2010 | 320 | US | Russia | Yes |
| 26 | Komárno | F | 4 | Sep. 2010 | 640 | Slovakia | — | No |
| 27 | Komárno | M | 4 | Sep. 2010 | 320 | Slovakia | — | No |
| 30 | Komárno | M | 8 | Sep. 2010 | 40 | Hungary | Hungary, Italy | No |
| 46 | Bratislava | M | 7 | Oct. 2010 | 160 | Italy | Hungary, Austria | No |
| 51 | Bratislava | M | 12 | Oct. 2010 | 320 | Germany | Austria, Hungary | Yes |
| 63 | Bratislava | M | 11 | Oct. 2010 | 40 | Slovakia | — | No |
| 67 | Bratislava | M | 2 | Oct. 2010 | 80 | Slovakia | — | No |
| 107 | Levice | F | 10 | Mar. 2011 | 80 | Slovakia | — | No |
| 108 | Levice | F | 6 | Mar. 2011 | 320 | Slovakia | — | No |
| 121 | Senec | M | 7 | Apr. 2011 | 320 | Czechland | — | No |
| KP3 | Komárno | F | 6 | Aug. 2011 | 40 | Slovakia | Hungary (2011) | No |
| KP4 | Komárno | F | 12 | Aug. 2011 | 40 | Slovakia | — | No |
| KP7 | Komárno | F | 11 | Aug. 2011 | 80 | Slovakia | Hungary | No |
| KP9 | Komárno | F | 12 | Aug. 2011 | 40 | Slovakia | Hungary | No |
| KP22 | Komárno | M | 8 | Aug. 2011 | 40 | Slovakia | — | No |
| KP24 | Komárno | F | 8 | Aug. 2011 | 640 | Slovakia | — | No |
| KP41 | Pezinok | F | 8 | Aug. 2011 | 80 | Slovakia | — | No |
| SVU20 | Senica | F | 18 | Mar. 2011 | 80 | Italy | Hungary | No |
| SVU100 | Holíč | F | 10 | Aug. 2011 | 320 | US | — | No |
| SVU118 | Pezinok | M | 7 | Jul. 2011 | 40 | Czechland | Hungary, Austria | No |
All tested animals were asymptomatic, and seronegative for tick-borne encephalitis virus (the PRNT90 titer with TBEV was <10) and Usutu virus.
WNV, West Nile virus; M, male; F, female; PRNT, plaque-reduction neutralization test; TBEV, tick-borne encephalitis virus.
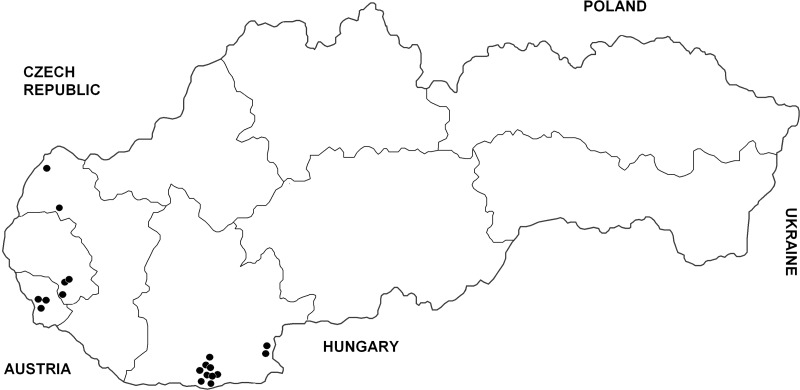
The history of each WNV-seropositive horse was checked. No marked clinical signs compatible with WNV disease (high fever and/or neurological abnormalities) were recorded in these seroreactors in the past. They were born in Slovakia (13), Czechland (2), Italy (2), and Hungary (1), and four originated from the United States and Germany. However, three seropositive horses had been immunized with WNV vaccine (no other seroreactor was vaccinated in the past). The latter three seroreactors therefore were excluded from the Slovak prevalence study, giving a seroprevalence rate in nonvaccinated animals of 19/229, i.e., 8.3% (95% CI 4.7–11.9%). All WNV-seropositive horses only lived in six districts of southern Slovakia (Komárno, Levice, Senec, Bratislava, Pezinok, and Senica), situated exclusively in a lowland part of the country below 200 meters above sea level (Fig. 3). The overall prevalence of antibodies neutralizing WNV was 15.1% (95% confidence interval [CI] 8.8–21.4%) in 126 nonvaccinated horses examined from those six affected districts, and the difference in seroprevalence rate based on local infection with WNV (11 animals) between the six positive districts in southern Slovakia and all other Slovakian districts was statistically significant (χ2=7.59; p=0.006).
FIG. 3.
Distribution of horses with antibodies neutralizing WNV in Slovakia, 2010–2011. (Three animals immunized with a WNV vaccine have not been included here.)
When the 11 autochthonous horse infections with WNV were analyzed for age factor, it was found that their average age was 7.4 (median 8) years versus 10.5 (median 10) years in all seronegative Slovak horses, but the difference was statistically insignificant (Mann–Whitney test, p=0.143). The seropositivity rate in the age group 1–4 years was 8.6% (n=35), in the group 5–8 years 8.2% (n=61), 9–12 years 6.5% (n=46), and in the horses older than 12 years 0.0% (n=64).
Discussion
Out of 22 WNV-seropositive horses in Slovakia, at least 11 (i.e., 4.8% of 229 nonvaccinated animals; 95% CI 2.0–7.6%; five males, six females) revealed autochthonous (local) infection with WNV (they were born in Slovakia or Czechland and did not travel to WNV-endemic countries), confirming circulation of WNV in southern Slovakia, whereas in eight other animals it cannot be excluded with certainty that they could have been infected in the country where they were born or had lived for a certain period (i.e., Italy, Hungary, United States). The remaining three seroreactors developed immunity after a previous WNV vaccination.
Detection of specific antibodies neutralizing WNV in local horses in Slovakia (for the first time in the country) has indicated enzootic transmission of the virus. Although no equine serosurvey for WNV was carried out previously in Slovakia, it is probable that WNV activity in southern Slovakia started only a few years ago. For instance, one 2-year-old animal (no. 67 in Table 1) was found to be positive (and stayed in Slovakia), indicating that WNV had circulated in the last 2 years preceding the sampling (cf. also other young horses nos. 26 and 27). The decreasing trend of seropositivity along the age gradient also indicates a recent WNV activity in southern Slovakia, possibly reflecting an expansion from the WNV endemic area in northwestern Hungary. There is no marked geomorphological or climatological barrier between these two regions.
The WNV lineages 1 and 2 were detected in Hungary recently (Bakonyi et al. 2006, Kutasi et al. 2011). However, it is impossible to differentiate infections caused by individual genomic lineages of WNV using a neutralization test. Thus we do not know which WNV lineage occurs in southern Slovakia at present.
PRNT is regarded a “gold standard” in flavivirus serology and also used for confirmation of other serological tests [enzyme-linked immunosorbent assay (ELISA), hemagglutination-inhibition test] because it is well known that flaviviruses present a high degree of serological cross-reactivity, sometimes even in the neutralization test (Madrid and Porterfield 1974, Calisher et al. 1989, Niedrig et al. 2007). Often several antigenically similar flaviviruses of the same or related flavivirus group might co-occur in one area. Therefore, we examined WNV seroreactors also against TBEV and USUV (i.e., the flaviviruses occurring in central Europe).
In a similar Central European study, sera of 350 horses from eastern Austria were examined for WNV antibodies in 2002 and all were found negative, except for four seropositives out of 35 horses (11.4%) that were transported from Hungary (the country of their origin) via Austria to Germany; these animals had no obvious clinical signs when examined at the border (Weissenböck et al. 2003). A recent study demonstrated WNV-neutralizing antibodies in 3.4% of 2098 horses in western Croatia (Barbic et al. 2012). In Spain, WNV antibodies were detected in 8.3% of 157 feral horses from the Guadalquivir marshes (NP Doñana) in 2005 (Jiménez-Clavero et al. 2007). In southern France (Camargue, a WNV endemic zone), overall 8.5% seropositive horses were detected in 2000 (Durand et al. 2002) and 5.3% in 2001 (Leblond et al. 2005). The seropositivity rate (in terms of neutralizing antibodies to WNV) found in Slovak horses in this study (8.3%) is very similar to that observed in Spain and southern France. However, equine seroprevalence rates for WNV in hyperendemic areas can sometimes be as high as 34%—Danube delta in Romania (Savuta et al. 2007), 22%—Volga delta in southern Russia (Lvov et al. 2005), or even 78%—Ferlo area in Senegal (Chevalier et al. 2006). Selective serosurveys for WNV in nonvaccinated, local horses obviously present a very useful indicator of the virus activity in an area, and a predictor for potential risk of occurrence of human cases or epidemics of West Nile fever (Mattar et al. 2005, Corrigan et al. 2006, Jiménez-Clavero et al. 2007, Epp et al. 2008, Angelini et al. 2010).
It would be interesting to continue monitoring horses to obtain information on the timing of WNV circulation in Slovakia, and in particular to detect or isolate the virus following determination of its origin.
Acknowledgments
Our thanks are due to additional veterinarians Stefan Ambrus, Eva Cikrytová, Libor Hlacik, and Vladimir Januschke, who collected the equine blood samples. The Usutu virus strain was supplied by Prof. Norbert Nowotny. This study was funded by EU grant FP7-261504 EDENext and is catalogued by the EDENext Steering Committee as EDENext005 (http://www.edenext.eu). The contents of this publication are the sole responsibility of the authors and do not necessarily reflect the views of the European Commission.
Author Disclosure statement
No competing financial interests exist.
References
- Angelini P. Tamba M. Finarelli AC. Bellini R, et al. West Nile virus circulation in Emilia-Romagna, Italy: The integrated surveillance system 2009. Euro Surveill. 2010;15 [PubMed] [Google Scholar]
- Autorino GL. Battisti A. Deubel V. Ferrari G, et al. West Nile virus epidemic in horses, Tuscany region, Italy. Emerg Infect Dis. 2002;8:1372–1378. doi: 10.3201/eid0812.020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakonyi T. Ivanics E. Erdélyi K. Ursu K, et al. Lineage 1 and 2 strains of encephalitic West Nile virus, Central Europe. Emerg Infect Dis. 2006;12:618–623. doi: 10.3201/eid1204.051379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbic L. Listes E. Katic S. Stevanovic V, et al. Spreading of West Nile virus infection in Croatia. Vet Microbiol. 2012;159:504–508. doi: 10.1016/j.vetmic.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Calisher CH. Karabatsos N. Dalrymple JM. Shope RE, et al. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol. 1989;70:37–43. doi: 10.1099/0022-1317-70-1-37. [DOI] [PubMed] [Google Scholar]
- Cantile C. Di Guardo G. Eleni C. Arispici M. Clinical and neuropathological features of West Nile virus equine encephalomyelitis. Equine Vet J. 2000;32:31–35. doi: 10.2746/042516400777612080. [DOI] [PubMed] [Google Scholar]
- Chevalier V. Lancelot R. Diaité A. Mondet B, et al. Serological assessment of West Nile fever virus activity in the pastoral system of Ferlo, Senegal. Ann NY Acad Sci. 2006;1081:216–225. doi: 10.1196/annals.1373.026. [DOI] [PubMed] [Google Scholar]
- Chevalier V. Lecollinet S. Durand B. West Nile virus in Europe: A comparison of surveillance system designs in a changing epidemiological context. Vector Borne Zoonot Dis. 2011;11:1085–1091. doi: 10.1089/vbz.2010.0234. [DOI] [PubMed] [Google Scholar]
- Corrigan R. Waldner C. Epp T. Wright J, et al. Prediction of human cases of West Nile virus by equine cases, Saskatchewan, Canada, 2003. Prev Vet Med. 2006;76:263–272. doi: 10.1016/j.prevetmed.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Durand B. Chevallier V. Pouillot R. Labie J, et al. West Nile virus outbreak in horses, southern France, 2000: Results of a serosurvey. Emerg Infect Dis. 2002;8:777–782. doi: 10.3201/eid0808.010486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp T. Waldner C. Corrigan R. Curry P. Public health use of surveillance for West Nile virus in horses: Saskatchewan, 2003–2005. Transbound Emerg Dis. 2008;55:411–416. doi: 10.1111/j.1865-1682.2008.01051.x. [DOI] [PubMed] [Google Scholar]
- Hubálek Z. Chanas AC. Johnson BK. Simpson DIH. Cross-neutralization study of seven California group (Bunyaviridae) strains in homoiothermous (PS) and poikilothermous (XTC-2) vertebrate cells. J Gen Virol. 1979;42:357–362. doi: 10.1099/0022-1317-42-2-357. [DOI] [PubMed] [Google Scholar]
- Hubálek Z. Halouzka J. West Nile fever—a reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis. 1999;5:643–650. doi: 10.3201/eid0505.990505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubálek Z. Halouzka J. Juřicová Z. West Nile fever in Czechland. Emerg Infect Dis. 1999;5:594–595. doi: 10.3201/eid0504.990430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubálek Z. Halouzka J. Juřicová Z. Šikutová S, et al. Serologic survey of birds for West Nile Flavivirus in southern Moravia (Czech Republic) Vector Borne Zoonot Dis. 2008;8:659–666. doi: 10.1089/vbz.2007.0283. [DOI] [PubMed] [Google Scholar]
- Jiménez-Clavero MA. Tejedor CG. Rojo G. Soriguer R, et al. Serosurvey of West Nile virus in equids and bovids in Spain. Vet Rec. 2007;161:212. doi: 10.1136/vr.161.6.212. [DOI] [PubMed] [Google Scholar]
- Kutasi O. Bakonyi T. Lecollinet S. Biksi I, et al. Equine encephalomyelitis outbreak caused by a genetic lineage 2 West Nile virus in Hungary. J Vet Intern Med. 2011;25:586–591. doi: 10.1111/j.1939-1676.2011.0715.x. [DOI] [PubMed] [Google Scholar]
- Labuda M. Kožuch O. Grešíková M. Isolation of West Nile virus from Aedes cantans mosquitoes in West Slovakia. Acta Virol. 1974;18:429–433. [Google Scholar]
- Leblond A. Zientara S. Chadouef J. Comby N, et al. Prévalence de l'infection par le virus West Nile chez le cheval en Camargue en 2001. Revue Méd Vét. 2005;156:77–84. [Google Scholar]
- Lvov DK. Butenko AM. Gromashevsky VL. Shchelkanov MY, et al. West Nile and other emerging-reemerging viruses in Russia. In: Berencsi G, editor; Khan AS, editor; Halouzka J, editor. Emerging Biological Threat. Amsterdam: IOS Press; 2005. pp. 33–42. [Google Scholar]
- Madrid AT. Porterfield JS. The flaviviruses (group B arboviruses): A cross-neutralization study. J Gen Virol. 1974;23:91–96. doi: 10.1099/0022-1317-23-1-91. [DOI] [PubMed] [Google Scholar]
- Mattar S. Edwards E. Laguado J. Gonzales M, et al. West Nile virus antibodies in Colombian horses. Emerg Infect Dis. 2005;11:1497–1498. doi: 10.3201/eid1109.050426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco F. Lelli R. Teodori L. Pinoni C, et al. Re-emergence of West Nile virus in Italy. Zoon Publ Hlth. 2010;57:476–486. doi: 10.1111/j.1863-2378.2009.01245.x. [DOI] [PubMed] [Google Scholar]
- Niedrig M. Sonnenberg K. Steinhagen K. Paweska JT. Comparison of ELISA and immunoassays for measurement of IgG and IgM antibody to West Nile virus in human sera against virus neutralisation. J Virol Meth. 2007;139:103–105. doi: 10.1016/j.jviromet.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Papa A. Danis K. Baka A. Bakas A, et al. Ongoing outbreak of West Nile virus infections in humans in Greece, July–August 2010. Euro Surveill. 2010;15 doi: 10.2807/ese.15.34.19644-en. [DOI] [PubMed] [Google Scholar]
- Salazar P. Traub-Dargatz JL. Morley PS. Wilmot DD, et al. Outcome of equids with clinical signs of West Nile virus infection and factors associated with death. J Am Vet Med Assoc. 2004;225:267–274. doi: 10.2460/javma.2004.225.267. [DOI] [PubMed] [Google Scholar]
- Savuta G. Ionescu A. Dragomir G. Anita A, et al. Serological investigations of WNV infection in horses from the south-east of Romania. Bull Univ Agricult Sci Vet Med (Cluj-Napoca) 2007;64:527–530. [Google Scholar]
- Sirbu A. Ceianu CS. Panculescu-Gatej RI. Vázquez A, et al. Outbreak of West Nile virus infection in humans, Romania, July to October 2010. Euro Surveill. 2011;16 [PubMed] [Google Scholar]
- Venter M. Human S. Zaayman D. Gerdes GH, et al. Lineage 2 West Nile virus as cause of fatal neurologic disease in horses, South Africa. Emerg Infect Dis. 2009;15:877–884. doi: 10.3201/eid1506.081515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenböck H. Hubálek Z. Halouzka J. Pichlmair A, et al. Screening for West Nile virus infections in susceptible animal species in Austria. Epidem Infect. 2003;131:1023–1027. doi: 10.1017/s0950268803001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodak E. Richter S. Bagó Z. Revilla-Fernández E, et al. Detection and molecular analysis of West Nile virus infections in birds of prey in the eastern part of Austria in 2008 and 2009. Vet Microbiol. 2011;149:358–366. doi: 10.1016/j.vetmic.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Zeller HG. Schuffenecker I. West Nile virus: An overview of its spread in Europe and the Mediterranean Basin in contrast to its spread in Americas. Eur J Clin Microbiol Infect Dis. 2004;23:147–156. doi: 10.1007/s10096-003-1085-1. [DOI] [PubMed] [Google Scholar]