Abstract
Purpose
To evaluate the efficacy of Rhodiola rosea extract in terms of alleviating the renal damage induced by unilateral ureter obstruction (UUO) in rats.
Material and Methods
Thirty Wistar albino male rats were divided into five groups: (I) Control, (II) UUO 7 days, (III) UUO 7 days+extract,(IV) UUO 14 days, and (V) UUO 14 days+extract. Seven or 14 days after the initiation of the experimental procedure, the left kidneys of rats in all five groups were removed for histological examination, and their blood was drawn for biochemical measurements.
Result
Median malondialdehyde (MDA) and glutathione peroxidase (GPx) levels were, respectively, 39.4 (5.04) nmol/mL and 25.8 (8.01) nmol/minute/mL in group I, 77.9 (12.38) nmol/mL and 5.8 (1.95) nmol/minute/mL in group II, 48.7 (12.1) nmol/mL and 9.1 (2.3) nmol/minute/mL in group III, 58.5 (23.83) nmol/mL and 8.4 (2.1) nmol/minute/mL in group IV, and 44.8 (4.97) nmol/mL and 13.8 (3.73) nmol/minute/mL in group V. There was a statistically significant difference among the groups in terms of MDA and GPx levels (p<0.05 for both). The median numbers of apoptotic cells were 1 (1), 8 (2.25), 3 (1.25), 23.5 (9), and 7 (I) in groups I, II, III, IV, and V, respectively. There was a statistically siginificant difference among the groups in terms of apoptotic cell number (p<0.05).
Conclusion
R. rosea extract was shown to alleviate the renal damage induced by UUO through its antioxidant effects. The mechanism by which R. rosea extract causes these effects merits further investigation.
Introduction
Obstructive uropathy is one of the most common causes of renal insufficiency, with known etiology in all age groups.1,2 Decreases in renal blood flow and glomerular filtration occur after obstruction. Increased hydrostatic pressure causes damage to the tubulointerstitial compartment of the kidney.3 Apoptosis in tubular cells, capillary rarefaction, and interstitial cell inflammatory infiltration can be observed. The ensuing progressive fibrosis results in loss of parenchyma.4
Rhodiola rosea, also known as golden root or Arctic root, is a plant with a wide geographical range, from Europe to Asia, especially at high altitudes. It has been used in traditional medicine globally, particularly in regions in which it is cultivated.5 R. rosea extract has cardioprotective, neuroprotective, anti-anxiety, anti-fatigue, antidepressant, and nootropic effects, and exerts positive effects on memory, learning, and mental capacity.6
R. rosea exerts antioxidant effects and thereby reduced tissue injury, resulting from oxidative stress.7,8 However, the efficacy of R. rosea in renal tissue damage after ureter obstruction has not been investigated. In the present study, we assessed the efficacy of R. rosea at alleviating the renal damage induced by unilateral ureter obstruction (UUO) in rats.
Material and Methods
This study was carried out at Abant Izzet Baysal University Experimental Animals, Application and Research Center, following approval from the Ethics Board for Animal Studies of Abant Izzet Baysal University. Six-month-old male Wistar albino rats weighing 250–300 g were used. Animals were housed in standard cages in a controlled environment (22°C±2°C, 40%–70% humidity, and a 12 hour/12 hour light-dark cycle). Rats were fed standard rat pellets and had ad libitum access to water.
For the R. rosea extract, we used arctic root SHR-5 (containing salidroside, tyrosol, and rosavin), produced by the Swedish Herbal Institute (Gothenburg, Sweden). Extract (360 mg) was diluted in 5-mL saline. Each rat received an extract dose of 20 mg/kg/day. The extract was given via the intragastric (i.g.) route after 12 h of fasting. The animals were allowed free access to food 2.5 h after the procedure.
Before the procedures, 90 mg/kg ketamine HCl and 10 mg/kg xylazine were administered via the intramuscular route for general anesthesia.
To induce UUO, the left ureter, accessed through an abdominal incision, was ligated using 5/0 silk suture thread and bisected 2 cm distal to the renal hilus. After the procedure, the incision was closed in the usual manner using 3/0 polyglactin (Vicryl).
Thirty rats were randomly divided into five groups (six rats per group). The rats in group I (the control group) were sacrificed on day 14 without undergoing any procedure, while the rats in group II (UUO 7 days) were sacrificed on day 7 of UUO. In addition to the procedures carried out in group II, the rats in group III (UUO+extract 7 days) were given R. rosea extract for 7 days starting from the day of UOO induction. The rats in group IV (UUO 14 days) were sacrificed on day 14 of UUO. In addition to the procedures carried out in group IV, the animals in Group V (UUO+extract 14 days) were administered R. rosea extract for 14 days starting from the day of UOO induction.
Seven or 14 days after the initiation of the experimental procedure, the left kidneys of rats in all five groups were removed for histological examination, and their blood was drawn by cardiac puncture for biochemical measurements.
Histological examination
Tissue samples were fixed in 10% neutral formaldehyde. After routine histological processing, 4-μm-thick sections were obtained and subjected to hematoxylin–eosin and Masson's trichrome staining to assess fibrosis, inflammation, congestion, and hemorrhage. Sections were photographed using a Nikon 50i photomicroscope and NIS elementary software. Apoptosis was evaluated by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL). The renal sections were examined under a light microscope at 40×magnification. Apoptotic cells were enumerated by counting TUNEL-positive cells in six different regions. All histological evaluations were performed by an experienced histologist.
Biochemical evaluation
Serum separator tubes were used to obtain blood. The blood samples were allowed to clot for 2 h and centrifuged for 15 minutes at 1000 g. Malondialdehyde (MDA), which demonstrates the basic by-products of peroxidation of polyunsaturated fatty acids, was measured by a specific enzyme-linked immunosorbent assay (ELISA) method using reagents from Cusabio Biotech (Hubei, P.R. China). Glutathione peroxidase (GPx), which helps organisms protect themselves against oxidative stress, was measured by a specific ELISA method using a reagent from Cayman (Ann Arbor, MI).
Statistical evaluation
Analysis of the data was carried out using the statistical package SPSS for Windows 11.5. Normality of the distribution of continuous variables was tested by Shapiro–Wilk test, while the homogeneity of the variances was analyzed by Levene's test. Descriptive statistics for MDA, GPx, and total number of apoptotic cells are expressed as medians (interquartile ranges). The significance of differences in medians was analyzed by Kruskal–Wallis test. When the p-values from the Kruskal–Wallis tests were found to be statistically significant, post-hoc Tukey HSD or Conover's non-parametric multiple-comparison test was used to identify which group differed from the other. A p-value<0.05 was considered to indicate statistical significance.
Results
Median MDA levels in the control group, group II, group III, group IV, and group V were 39.4 (5.04), 77.9 (12.38), 48.7 (12.1), 58.5 (23.83), and 44.8 (4.97) nmol/mL, respectively. MDA levels were significantly higher in groups II, III, IV, and V than in the control group (p<0.05). The MDA level in group III was significantly lower than that in group II, while the MDA level in group V was lower than that in group IV (p<0.001 and p=0.002, respectively). Compared with group II, the MDA level in group IV was slightly, but significantly, lower (p=0.049). There was no statistically significant difference in MDA level between groups III and V (p=0.136).
Median GPx levels in the control group, group II, group III, group IV, and group V were 25.8 (8.01), 5.8 (1.95), 9.1 (2.3), 8.4 (2.1), and 13.8 (3.73), nmol/minute/mL, respectively. Compared with the control group, GPx levels in groups II, III, IV, and V were significantly lower (p<0.05). The GPx level was significantly lower in group II versus group III and in group IV versus group V (p=0.002 and p=0.002). Group IV had a higher GPx level than group II, and group V had a higher GPx level than group III (p=0.013 and p=0.010).
The median numbers of apoptotic cells were 1 (1), 8 (2.25), 3 (1.25), 23.5 (9), and 7 (1) in groups I, II, III, IV, and V, respectively. There were significant differences in the median numbers of apoptotic cells among the groups, except for groups I and III (p<0.05). Compared with groups II and IV, groups III and V, respectively, had significantly fewer apoptotic cells (p<0.001). Groups IV and V had higher median numbers of apoptotic cells than groups II and III, respectively (p=0.043 and p=0.038, respectively). (Table 1) shows multiple comparisons of the MDA, GPx levels, and apoptotic cell counts among the groups.
Table 1.
Multiple Comparisons of the Malondialdehyde, Glutathione Peroxidase Levels, and Apoptotic Cell Counts Among the Groups, and Associated p-Values
| Groups | MDA | GPx | ACCs |
|---|---|---|---|
| Group I vs. Group II | p<0.001 | p<0.001 | p<0.001 |
| Group I vs. Group III | p<0.001 | p<0.001 | p=0.055 |
| Group I vs. Group IV | p<0.001 | p<0.001 | p<0.001 |
| Group I vs. Group V | p=0.015 | p=0.038 | p<0.001 |
| Group II vs. Group III | p<0.001 | p=0.002 | p<0.001 |
| Group II vs. Group IV | p=0.049 | p=0.013 | p=0.043 |
| Group II vs. Group V | p<0.001 | p<0.001 | p=0.043 |
| Group III vs. Group IV | p=0.062 | p=0.449 | p<0.001 |
| Group III vs. Group V | p=0.136 | p=0.010 | p=0.038 |
| Group IV vs. Group V | p=0.002 | p=0.002 | p<0.001 |
MDA=malondialdehyde; GPx=glutathione peroxidase;
ACCs=apoptotic cell counts.
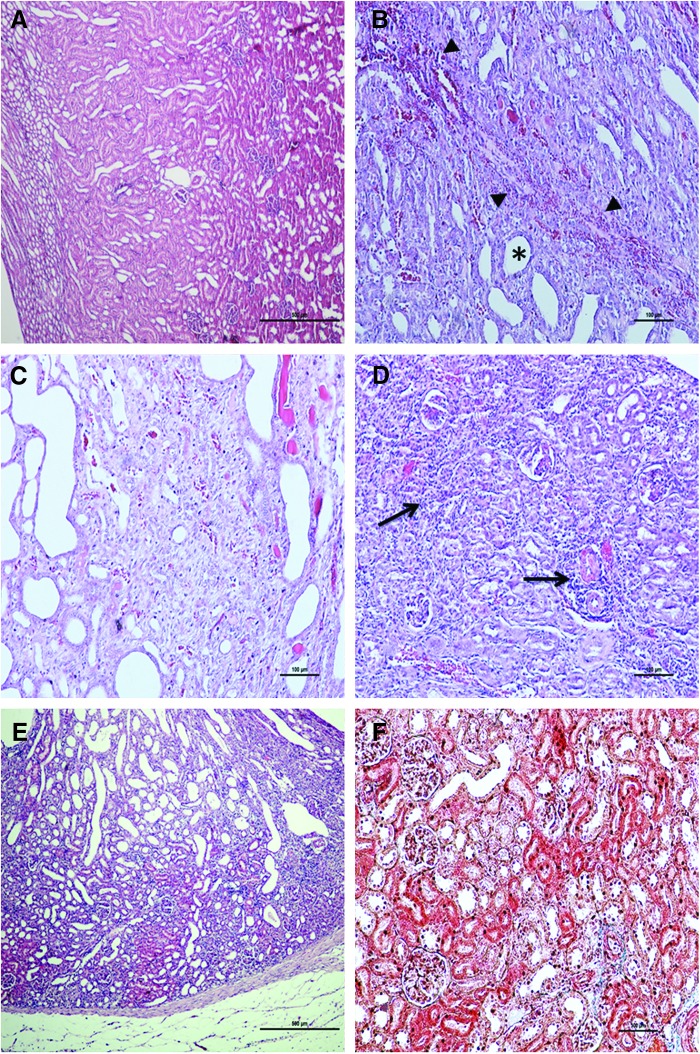
A histological examination revealed dilation of tubular structures in the cortex and medulla, and hemorrhage, congestion, and accumulation of inflammatory cells between the tubules in group II. Moreover, debris was noted within some of the dilated tubules. In some regions in which hemorrhage and inflammation were especially prominent, the tubules had disappeared and fibrotic changes characterized by increased amounts of connective tissue were present in these areas. In group III, tubules were present and dilated, but hemorrhage, congestion, and inflammation were less pronounced and fibrotic areas smaller, compared with group II. The renal cortex had more widespread and denser inflammation in group IV compared with group II. Notably, in areas in which inflammatory cells were abundant, tubules and glomeruli were absent and connective tissue levels were increased around the dilated tubules. Inflammation, hemorrhage, and fibrotic connective tissue increases groups III and V were more prominent than in group I, but less prominent than in groups II and IV. (Figure 1) shows histological sections.
FIG. 1.
(A) Appearance of normal renal structures in the control group. (B) In group II, the tubules in the cortex and medulla were dilated (*), and there was hemorrhage (◀) between the tubules. (C) In group III, the tubules were dilated, but hemorrhage, congestion, and inflammation were less pronounced (HE, scale bar 500 μm), and fibrotic areas were smaller compared with group II (HE, scale bar 100 μm). (D) Dense inflammation was observed in group IV (→). (HE, scale bar 500 μm). (E, F). In group V, decreased inflammation and hemorrhage were detected compared with group IV (HE, scale bar 500 μm). Compared with group IV, decreased fibrotic connective tissue was observed in group V (Masson's trichrome staining, scale bar 100 μm).
Discussion
Obstructive uropathy, caused by prevention of urine flow, results in permanent renal damage and loss of renal function. The obstruction can occur at any level of the urinary tract. The most common cause of obstruction in adults is urolithiasis, while obstructive nephropathy in children is mostly congenital.9,10 Acute obstruction of the ureter rapidly triggers a cascade of events in the kidneys. First, renal blood flow and the glomerular filtration rate drop. Within a few days, hydronephrosis starts to develop, followed by interstitial inflammatory infiltration, apoptosis, and necrosis11
The damage in the kidneys can increase gradually if the obstruction persists. Damage considered obscure or insignificant on day 7 of the obstruction can become significant by day 14 if the obstruction persists. Therefore, in UUO models, it is important to interpret the results of studies according to the time points at which the data were obtained.12 In this study, we aimed at examining the efficacy of R. rosea in preventing UUO-induced renal damage and assessed biochemical and histological parameters on days 7 and 14. Tissue damage was more severe on day 14 than on day 7. R. rosea alleviated damage in all groups, especially at day 7.
The class of biomolecules that is mostly affected during tissue damage due to oxidative stress is lipids. MDA is one of the basic by-products of peroxidation of polyunsaturated fatty acids. Hence, MDA is frequently used to assess tissue damage.13 GPx, a selenoenzyme, reduces harmful hydroperoxides, thereby helping organisms protect themselves against oxidative stress.14 Oxidative stress plays a pivotal role in the pathogenesis of renal damage due to obstruction. After UUO, levels of lipid peroxidation species, such as 8-iso prostaglandin F2α (8-iPGF2α) and MDA, increase, while levels of protective antioxidant enzymes such as GPx, catalase, and dismutase decrease.4 In the present study, MDA levels were elevated in all groups in which obstruction was induced. However, in the R. rosea-treated groups, MDA levels were significantly lower. GPx levels were also reduced in all groups compared with the control group, and were significantly higher in the R. rosea-treated groups. These findings may suggest the oxidative stress-alleviating properties of R. rosea.
A study of the effects of taxol and taurine on renal damage on day 28 of UUO showed that both molecules reduced the amounts of fibrotic and necrotic tissues. In that study, Taxol was administered intraperitoneally, while taurine, as in the present study, was given via i.g route.15 The efficacy of febuxostat was explored by Omori et al. The anti-inflammatory effect of febuxostat on the kidneys was evident as early as 1 day after ureter obstruction—the authors argued that the molecule reduced renal tissue fibrosis.16 In another study, low-dose paclitaxel was found to reduce tubulointerstitial fibrosis on days 7 and 14.17 Karabuga et al. examined the efficacy of lisinopril at alleviating renal damage on days 4 and 14 in rats with induced UUO. The researchers found that the number of apoptotic cells in rats which received treatment was lower than in untreated rats, and concluded that lisinopril was more effective at reducing damage during the early stages.18 In contrast, a further study showed that rapamycin could delay tubulointerstitial renal fibrosis and that the results on day 7 were better than those on day 14. The authors concluded that treatment might be modified according to the stage of the renal damage.19 In the present study, we chose to assess the efficacy of R. rosea extract on days 7 and 14 after obstruction. Hemorrhage, inflammation, fibrosis, and the number of apoptotic cells were more pronounced/higher in the 14-day groups than in the 7-day groups, irrespective of whether they were given R. rosea. However, the increases were lower than in the groups for the same time point that did not receive the extract. Compared with the control group, the increase in the number of apoptotic cells in rats that received R. rosea for 7 days was not significantly different (p=0.055). We interpret these results to indicate that R. rosea alleviated renal damage at all stages after obstruction, but most notably during the early stages.
R rosea is thought to be a safe extract with a lethal dose at which 50% of animals die (LD50) of 3.360 mg/kg.20 The anti-inflammatory effects of R. rosea extract on tissue damage were investigated in a study in which various anti-inflammatory agents were administered to rats. The results showed that R. rosea had anti-inflammatory effects and reduced tissue injury.21 In another experimental study, R. rosea given via the i.g. route exerted antidepressant, adaptogenic, anti-anxiety, and stimulatory effects.22 Moreover, R. rosea exhibited antioxidant activity and increased the resistance of human erythrocytes during oxidative stress caused by exposure to hypochlorous acid.23 Keratinocytes are frequently exposed to potent oxidants. A study carried out on keratinocytes revealed that R. rosea increased antioxidant enzyme levels, demonstrating reliable antioxidant activity.7 Palumbo et al. reported that R. rosea had antioxidant properties and acted as a neuroprotector of cortical neurons.8 Our results also indicate that R. rosea possesses antioxidant activity. R. rosea may alleviate the renal damage induced by UUO through its antioxidant effects.
Conclusions
In the present study, we demonstrated that R. rosea alleviated the renal damage induced by UUO in short and middle terms. R. rosea may be an effective alternative in the treatment of renal damage through ureter obstruction, possibly due to its antioxidant properties. However, long-term and comparative animal and human studies are needed to confirm our findings.
Abbreviations Used
- ELISA
enzyme-linked immunosorbent assay
- GPx
glutathione peroxidase
- i.g.
intragastric
- MDA
malondialdehyde
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling
- UUO
unilateral ureter obstruction
Disclosure Statement
The authors declare that they have no conflict of interest.
References
- 1.Chevalier RL. Pathogenesis of renal injury in obstructive uropathy. Curr Opin Pediatr. 2006;18:153–160. doi: 10.1097/01.mop.0000193287.56528.a4. [DOI] [PubMed] [Google Scholar]
- 2.Sountoulides P. Pardalidis N. Sofikitis N. Endourologic management of malignant ureteral obstruction: Indications, results, and quality-of-life issues. J Endourol. 2010;24:129–142. doi: 10.1089/end.2009.0157. [DOI] [PubMed] [Google Scholar]
- 3.Yeh CH. Chiang HS. Lai TY. Chien CT. Unilateral ureteral obstruction evokes renal tubular apoptosis via the enhanced oxidative stress and endoplasmic reticulum stress in the rat. Neurourol Urodyn. 2011;30:472–479. doi: 10.1002/nau.20855. [DOI] [PubMed] [Google Scholar]
- 4.Dendooven A. Ishola DA., Jr. Nguyen TQ, et al. Oxidative stress in obstructive nephropathy. Int J Exp Pathol. 2011;92:202–210. doi: 10.1111/j.1365-2613.2010.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly GS. Rhodiola rosea: A possible plant adaptogen. Altern Med Rev. 2001;6:293–302. [PubMed] [Google Scholar]
- 6.Panossian A. Wikman G. Sarris J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine. 2010;17:481–493. doi: 10.1016/j.phymed.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Calcabrini C. De Bellis R. Mancini U, et al. Rhodiola rosea ability to enrich cellular antioxidant defences of cultured human keratinocytes. Arch Dermatol Res. 2010;302:191–200. doi: 10.1007/s00403-009-0985-z. [DOI] [PubMed] [Google Scholar]
- 8.Palumbo DR. Occhiuto F. Spadaro F. Circosta C. Rhodiola rosea extract protects human cortical neurons against glutamate and hydrogen peroxide-induced cell death through reduction in the accumulation of intracellular calcium. Phytother Res. 2012;26:878–883. doi: 10.1002/ptr.3662. [DOI] [PubMed] [Google Scholar]
- 9.Manucha W. Biochemical-molecular markers in unilateral ureteral obstruction. Biocell. 2007;31:1–12. [PubMed] [Google Scholar]
- 10.Zecher M. Guichard C. Velasquez MJ. Figueroa G. Rodrigo R. Implications of oxidative stress in the pathophysiology of obstructive uropathy. Urol Res. 2009;37:19–26. doi: 10.1007/s00240-008-0163-3. [DOI] [PubMed] [Google Scholar]
- 11.Chevalier RL. Forbes MS. Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009;75:1145–1152. doi: 10.1038/ki.2009.86. [DOI] [PubMed] [Google Scholar]
- 12.Okamura DM. Pasichnyk K. Lopez-Guisa JM, et al. Galectin-3 preserves renal tubules and modulates extracellular matrix remodeling in progressive fibrosis. Am J Physiol Renal Physiol. 2011;300:F245–F253. doi: 10.1152/ajprenal.00326.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Rio D. Stewart AJ. Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Bhabak KP. Mugesh G. Functional mimics of glutathione peroxidase: Bioinspired synthetic antioxidants. Acc Chem Res. 2010;43:1408–1419. doi: 10.1021/ar100059g. [DOI] [PubMed] [Google Scholar]
- 15.Karbalay-Doust S. Noorafshan A. Pourshahid SM. Taxol and taurine protect the renal tissue of rats after unilateral ureteral obstruction: A stereological survey. Korean J Urol. 2012;53:360–367. doi: 10.4111/kju.2012.53.5.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omori H. Kawada N. Inoue K, et al. Use of xanthine oxidase inhibitor febuxostat inhibits renal interstitial inflammation and fibrosis in unilateral ureteral obstructive nephropathy. Clin Exp Nephrol. 2012;16:549–556. doi: 10.1007/s10157-012-0609-3. [DOI] [PubMed] [Google Scholar]
- 17.Zhang D. Sun L. Xian W, et al. Low-dose paclitaxel ameliorates renal fibrosis in rat UUO model by inhibition of TGF-beta/Smad activity. Lab Invest. 2010;90:436–447. doi: 10.1038/labinvest.2009.149. [DOI] [PubMed] [Google Scholar]
- 18.Karabuga I. Akbay K. Turna B, et al. Effect of lisinopril on renal tissue damage in unilateral ureteral obstruction in rats. Urol Res. 2012;40:27–34. doi: 10.1007/s00240-011-0393-7. [DOI] [PubMed] [Google Scholar]
- 19.Wu MJ. Wen MC. Chiu YT. Chiou YY. Shu KH. Tang MJ. Rapamycin attenuates unilateral ureteral obstruction-induced renal fibrosis. Kidney Int. 2006;69:2029–2036. doi: 10.1038/sj.ki.5000161. [DOI] [PubMed] [Google Scholar]
- 20.Kurkin V. Zapesochnaya G. Chemical composition and pharmacological characteristics of Rhodiola rosea. J Med Plants. 1985:1231–1445. [Google Scholar]
- 21.Bawa AS. Khanum F. Anti-inflammatory activity of Rhodiola rosea—“a second-generation adaptogen”. Phytother Res. 2009;23:1099–1102. doi: 10.1002/ptr.2749. [DOI] [PubMed] [Google Scholar]
- 22.Perfumi M. Mattioli L. Adaptogenic and central nervous system effects of single doses of 3% rosavin and 1% salidroside Rhodiola rosea L. extract in mice. Phytother Res. 2007;21:37–43. doi: 10.1002/ptr.2013. [DOI] [PubMed] [Google Scholar]
- 23.Battistelli M. De Sanctis R. De Bellis R. Cucchiarini L. Dacha M. Gobbi P. Rhodiola rosea as antioxidant in red blood cells: Ultrastructural and hemolytic behaviour. Eur J Histochem. 2005;49:243–254. [PubMed] [Google Scholar]