Abstract
Derived from mesoderm precursors, hemangioblasts are bipotential common progenitors of hematopoietic cells and endothelial cells. The regulatory events controlling hematopoietic and endothelial lineage specification are largely unknown, especially in humans. In this study, we establish a serum-free and feeder-free system with a high-efficient embryoid body (EB) generation to investigate the signals that direct differentiation of human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs). Consistent with previous studies, the CD34+CD31+VE-cadherin+ (VEC+) cells derived from hPSCs contain hematopoietic and endothelial progenitors. In the presence of hematopoietic and endothelial growth factors, some of CD34+CD31+VEC+ cells give rise to blast colony-forming cells (BL-CFCs), which have been used to characterize bipotential hemangioblasts. We found that the level of the transforming growth factor beta (TGF-β) 1 protein is increased during hPSC differentiation, and that TGF-β signaling has the double-edged effect on hematopoietic and endothelial lineage differentiation in hPSCs. An addition of TGF-β to hPSC differentiation before mesoderm induction promotes the development of mesoderm and the generation of CD34+CD31+VEC+ cells. An addition of TGF-β inhibitor, SB431542, before mesoderm induction downregulates the expression of mesodermal markers and reduces the number of CD34+CD31+VEC+ progenitor cells. However, inhibition of TGF-β signaling after mesoderm induction increases CD34+CD31+VEC+ progenitors and BL-CFCs. These data provide evidence that a balance of positive and negative effects of TGF-β signaling at the appropriate timing is critical, and potential means to improve hematopoiesis and vasculogenesis from hPSCs.
Introduction
In early ontogeny, hematopoiesis is closely associated with vasculogenesis. The earliest hematopoietic and endothelial cells arise at the same time and locations in the yolk sac, and share the expression of molecules, such as Flk-1 (VEGFR2 or KDR). Based on the rationale that the vascular and hematopoietic systems develop together to establish the body's oxygen-delivery system during organogenesis, it has been hypothesized that the hematopoietic and vascular endothelial cells, which line the interior surface of blood vessels, are derived from a common precursor, the hemangioblast [1–3]. The hemangioblasts have been identified in early embryonic life in vivo [4,5], and during mouse and human pluripotent stem cell (hPSC) differentiation in vitro [6–9]. Although the nature of hemangioblast is still debatable, increasing evidences indicate that hemogenic endothelial cells are transient intermediates that contribute to de novo production of hematopoietic cells [10–12]. Whereas hemangioblasts are derived from the extraembryonic mesoderm and couple vasculogenesis and primitive hematopoiesis in the yolk sac, the hemogenic endothelium of intraembryonic mesoderm in the dorsal aorta has been recognized as a source of hematopoietic stem cells [11,13]. It is still unclear whether hemangioblasts give rise to hematopoietic cells directly or through hemogenic endothelial cells by an endothelial to hematopoietic transition in hPSCs.
A bipotential hemangioblast is still poorly characterized and difficult to distinguish from multipotent mesodermal progenitors. Differentiation of hPSCs, including human embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs), reproduces many features of embryonic development and provides an in vitro model to elucidate mechanisms of lineage commitment, practically inaccessible in the human embryo [14]. Although hiPSCs through reprogramming adult somatic cells are similar to hESCs in many aspects, including self-renewal and differentiation into cell types in three germ layers, the full extent of the hiPSC relation to typical pluripotent stem cells, such as hESCs, is still being assessed [15]. Differentiation of hPSCs provides a great model system to characterize signals that direct lineage commitment. Our previous studies demonstrated that hESC-derived CD34+ cell populations contain the progenitor cells for hematopoietic, endothelial, and smooth muscle cells, suggesting that CD34+ cells are heterogeneous and contain common precursors of blood and endothelial cells, hemangioblasts [16,17]. The signals that regulate hematopoietic and endothelial specification are largely unknown.
Transforming growth factor beta (TGF-β) signaling directs different responses in different cell types [18]. Bone morphogenetic protein 4 (BMP4) is one of the TGF-β super-family members that participate in a wide range of processes, including vascular development, angiogenesis, and vascular cell functions [19–21]. We and others have previously found that BMP4, vascular endothelial growth factor (VEGF), and fibroblast growth factor 2 (FGF2) are critical factors to promote hESC differentiation to CD34+ progenitors [16,17]. TGF-β and its closer relatives, such as activin and nodal, activate Smad2 and Smad3 via the type I receptors ALK4, ALK5, and ALK7 (ACVR1B, TGFBR1, and ACVR1C, respectively), are required for hESC pluripotency; whereas most of the BMP subfamily members induce hESC differentiation by activating Smad1, Smad5, and Smad8 (Smad1/5/8) signaling via ALK1, ALK2, ALK3, and ALK6 (ACVRL1, ACVR1, BMPR1A, and BMPR1B, respectively) [17,22–29]. Recent studies demonstrated that lineage-specific factors recruit Smad proteins to many sites in the genome to implement specific differentiation programs. For example, C/EBPα and GATA-1 interact with Smad1 to regulate myeloid and erythroid lineage by BMP signaling, whereas Smad3 mediates TGF-β signaling by interacting with MyoD1 and PU.1 in myotubes and pro-B cells, respectively [30,31].
While BMP and TGF-β pathways are critical for hemangioblast development in the murine system [32–35], the role of TGF-β signaling, including those by endogenous activin and Nodal ligands, in human hemangioblast development is largely unknown. Combinatorial signals of activin/Nodal and BMP4 regulate the early lineage differentiation of hPSCs [36,37]. In the present study, we demonstrated that CD34+CD31+VE-Cadherin+ (VEC+) cells derived from hPSCs have a great potential to give rise to both hematopoietic and endothelial cells. Inhibitory assay with small molecules indicated that the TGF-β signaling pathway played a complicated role in developmental windows of hematopoietic and endothelial progenitors. Here we report that TGF-β signaling is indispensable before mesoderm commitment. We found that TGF-β expression is significantly increased after mesoderm induction, and inhibition of TGF-β enhances the development of CD34+CD31+VEC+ hematopoietic and endothelial progenitors. Our study demonstrated that TGF-β signaling has the double-edged effect on hematopoietic and endothelial lineage specification.
Materials and Methods
Maintenance of hESC and hiPSC cultures
H1 and H9 hESCs were obtained from the WiCell Research Institute. The HDFa-YK26 and TZ1 hiPSCs were kind gifts from Dr. Ren-He Xu at the University of Connecticut Health Center [38]. BC1 hiPSCs were obtained from Dr. Linzhao Cheng at Johns Hopkins University [39]. To assess mesodermal development in hPSCs, a lentiviral transduction on BC1 hiPSCs were performed to generate a hiPSC line, BC1-Brachyury-GFP, in which GFP expression is driven by the Brachyury (T-box) promoter [40]. The hESCs and hiPSCs (passages 30–50) were grown on mitotic-inactivated mouse embryonic fibroblast cells (MEFs) in the ESC culture medium (ESM) containing the DMEM/F-12 (Invitrogen), 20% knockout serum replacement (KSR; Invitrogen), 0.1 mM nonessential amino acids (Invitrogen), 2 mM L-glutamine (Mediatech, Inc.), 0.1 mM beta-mercaptoethanol (Sigma), and 4 ng/mL FGF2 (PeproTech). The growth media were changed every day. MEFs were derived from decapitated E12.5 embryos. MEFs were maintained and treated by mitomycin C as previously described [17,41].
Differentiation of embryoid bodies
To remove feeder cells before differentiation, hESCs or hiPSCs cultured on MEFs were treated by Accutase at room temperature for 2 to 3 min. After washing away MEFs with PBS, hPSC colonies were dissociated to single cells by continuing Accutase treatment at 37°C for 5 min. To generate hanging-drop EBs, 1,000 cells per drop in 25 μL were cultured on Petri dishes to form EBs in the presence of 5 μM Rho-dependent protein kinase (ROCK) inhibitor, Y27632, in the ESM without FGF2. After 2 days of hanging-drop EB formation, EBs were collected and transferred to ultralow attachment dishes in a serum-free differentiation medium (DM-SF; Supplementary Table S1). The differentiation media were changed every 2 days with growth factors and inhibitors as indicated in Fig. 1A.
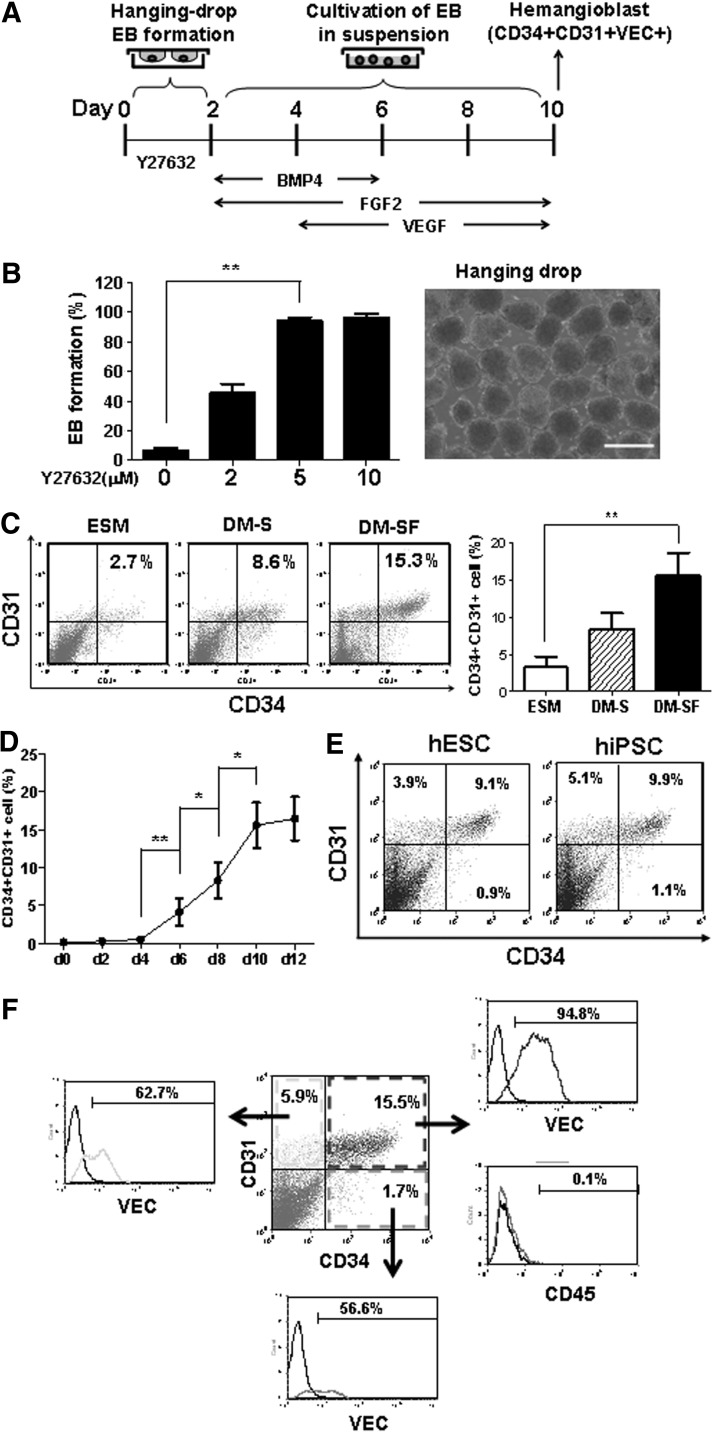
FIG. 1.
Differentiation of human pluripotent stem cells (hPSCs) in embryoid bodies (EBs). (A) Schematic diagram of hPSC differentiation. EBs were initialized in hanging drops. After 2 days, EBs were collected into ultralow attachment dishes with growth factors as indicated. (B) Rho-dependent protein kinase (ROCK) inhibitor, Y27632, enhanced EB formation in hanging drops from day 0 to 2. The percentages of EB formation in hanging drops were counted after 2 days in the presence of different Y27632 concentrations (left panel). A representative morphology of EBs at day 2 was shown (right panel). Scale bar=500 μm. (C) Media for hPSC differentiation from day 2 to 10, including the ESC culture medium (ESM), serum-containing differentiation medium (DM-S), and serum-free differentiation medium (DM-SF). Flow cytometry analyses were performed at day 10. (D) The kinetics of hPSC differentiation to CD34+CD31+ cells. Flow cytometry analyses were performed every 2 days in triplicate experiments. (E) Differentiation of human embryonic stem cells (hESCs) and human induced-pluripotent stem cells (hiPSCs) to CD34+CD31+ cells. Flow cytometry analyses were performed at day 10. (F) CD34+CD31+VEC+ population from hPSC differentiation. Flow cytometry analyses were performed at day 10 of hPSC differentiation. Data are represented as mean±SD from three independent experiments. *P<0.05, **P<0.01.
Hematopoietic and endothelial differentiation
After 10 days of EB differentiation, EBs were treated by TrypLE (Invitrogen) in room temperature for 10 min to obtain a single-cell suspension. The CD34+CD31+VEC+ cells were isolated by fluorescence-activated cell sorting (FACS) with FACSAria II (BD Biosciences). In some experiments, the CD34+CD31+ cells were positively selected by using the MultiSort immunomagnetic separation system (Miltenyi Biotec) following the manufacturer's instruction.
For further differentiation to endothelial cells and hematopoietic cells, CD34+CD31+VEC+ cells were cultured on a collagen-I-coated six-well plate (1×105 cells per well) in a serum-free endothelial cell growth medium (SFEGM; Supplementary Table S1). The adhesive cells were cultured for 6 days before passage. Suspension cells were collected after 4 days of culture for flow cytometry analyses.
Measurement of TGF-β concentration in media
During EB differentiation, supernatants from EB culture were collected every 2 days. The human TGF-β1 concentrations were measured by the Quantikine ELISA kit (R&D Systems) following the manufacturer's instruction. The endpoint absorbencies were determined on iMark Microplate Absorbance Reader (Bio-Rad Laboratories).
Real-time PCR analyses
Total RNAs from undifferentiated hPSCs and EBs at different time points were isolated by using Direct-zol RNA MiniPrep kit (Zymo Research). To eliminate DNA contamination, the RNA samples were treated with DNase I on column following the manufacturer's instruction. Total RNA (1 μg) was used for each reverse transcription reaction with SuperScript III (Invitrogen). Real-time quantitative polymerase chain reaction (qPCR) was performed on iQ5 thermal cycler (Bio-Rad Laboratories). Samples were adjusted to yield equal amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal standard. Oligonucleotide primers are listed in the Supplementary Table S2.
Immunohistochemistry
The cells were fixed with 4% paraformaldehyde in PBS at room temperature for 10 min, permeabilized with 0.1% Triton X-100 in PBS at room temperature for 10 min, and then incubated with 1% BSA in PBS for 30 min to block nonspecific binding. The cells were incubated with the primary antibodies VEC-Alexa Fluor 488 (eBioscience) for 1 h at room temperature. The nuclei were stained by 0.1 μg/mL DAPI for 3 min. The results were examined by a fluorescence microscope (Olympus).
Western blot
To examine the phosphorylation of Smad stimulated by TGF-β, undifferentiated hPSCs or EBs at day 2 were incubated with TGF-β1 (10 ng/mL) and/or SB431542 (10 μM; Sigma) for 30 min. Cells were lysed by the RIPA buffer (1% Triton X-100, 0.1% SDS, 1% sodium deoxycholate, 1 mM EDTA, 1 mM sodium orthovanadate, 1 mM sodium fluoride, 10 μM leupeptin, 150 mM NaCl, 50 mM Tris, pH 7.4) containing 1% protease inhibitor cocktail (Sigma). Western blots were developed with primary antibodies anti-Phospho-Smad1/5/8, anti-Phospho-Smad2/3 (Cell Signaling Technology), and secondary antibody anti-rabbit IgG-HRP (Sigma).
LDL-uptake assay
Endothelial cells derived from hPSCs (hPSC-ECs) were cultured in the SFEGM (Supplementary Table S1) containing 2 ng/mL Dil-acetylated low-density lipoprotein (Dil-Ac-LDL; Invitrogen) for 12 h. After washing twice with PBS, the cells were examined by a fluorescence microscope (Olympus).
Vascular network formation in Matrigel
The assay was performed essentially as previously described [16,42]. Briefly, 24-well plates were coated with 200 μL per well of Matrigel matrix (BD Biosciences) in room temperature for more than 30 min, and hPSC-ECs (5×104 cells) in 500 μL EGM-2 medium (Lonza) were loaded on Matrigel-coated plates. The plates were incubated at 37°C in 5% CO2. The vascular structures in Matrigel were photographed by a phase-contrast microscopy (Zeiss) after 16 h of incubation.
Colony-forming cell assay
Hematopoietic colony-forming cell (CFC) assay was performed as described in our previous studies [16,42] with minor modifications. The 1×104 subpopulation cells isolated from EBs at day 10, including CD34+CD31+ cells, CD34+CD31− cells, or CD34−CD31− cells, were mixed with the methylcellulose medium (StemCell Tech.; H4435) following the manufacturer's instruction. The hematopoietic colonies were photographed and counted after 2 weeks of culture.
To assess hemangioblast potential of CD34+CD31+VEC+ cells, the blast colony-forming cell (BL-CFC) assay was performed according to previous studies [7,9,43]. The methylcellulose medium (StemCell Tech.; H4435) was supplemented with 2 μM Y27632, 10 ng/mL VEGF, 10 ng/mL IGF-1, 5 ng/mL FGF-2, 5 ng/mL epidermal growth factor (EGF), 1 U/mL heparin, 1 μg/mL hydrocortisone, and 20 μg/mL ascorbic acid. The CD34+CD31+VEC+ cells (2×103), isolated from EBs at day 10 were mixed with the methylcellulose medium following the manufacturer's instruction. The blast colony number was counted after 3 days of culture. To analyze endothelial and hematopoietic components, 10 blast colonies were pooled together for analysis of gene expression pattern by reverse transcription polymerase chain reaction (RT-PCR) as previously described [9].
Flow cytometric analysis
The adhesion cells or EBs were dissociated to single-cell suspension by TrypLE (Invitrogen) treatment, and washed with the FACS buffer (0.5% BSA, 1 mM EDTA in PBS). The dissociated cells were suspended in the FACS buffer, and labeled by fluorochrome-conjugated anti-human CD34-APC, CD31-PE (both from Miltenyi Biotec), and VEC-Alexa Fluor 488 (eBioscience). 7-Amino-Actinomycin D (7-AAD; BD Biosciences) was used to detect dead cells. Isotype-matched control antibodies were used to determine the background staining. The flow cytometry was performed on a FACSCalibur (BD Biosciences). Data analysis was performed using CellQuest or FCS Express software.
For cell proliferation analyses, EBs were incubated with 10 μM EdU (5-ethynyl-2′-deoxyuridine) for 3 h, and then dissociated to single cells by TrpyLE treatment. The Click-iT EdU Flow Cytometry Assay Kit (Invitrogen) was used to analyze EdU-positive cells by FACSCalibur.
Statistical analysis
The data were subjected to statistical analysis by the Student's t-test. Results with a value of P<0.05 were considered statistically significant.
Results
Differentiation of hESCs and hiPSCs
To identify molecular signals and the cell population that gives rise to hematopoietic cells and endothelial cells in hPSCs, it is critical to establish a reproducible serum-free and feeder-free system to induce hPSC differentiation. Because of the low survival rate of dissociated single cells, hPSCs do not form embryoid bodies (EBs) efficiently from single-cell suspension. Our previous study demonstrated that attenuation of apoptosis in hESCs by Bcl-xL enhances single-cell survival, and increases the efficiency of EB formation [41]. Recent studies demonstrated that inhibition of ROCK by small molecules improves hESC survival by blocking dissociation-induced cell death [44,45]. The outline of our serum-free and feeder-free differentiation system was shown in a schematic diagram (Fig. 1A). We found that the ROCK inhibitor, Y-27632, significantly enhanced the efficiency of EB formation by a hanging-drop method in serum-free conditions (Fig. 1B). When 1,000 cells were used in each drop, almost 100% of the drops formed uniformed EBs in 2 days in the presence of 5 μM Y-27632 (Fig. 1B). We and others previously showed that BMP4 promotes mesodermal development [17,32,46–48]. However, the efficiency of hanging-drop EB formation from day 0 to 2 was decreased by an addition of BMP4, but not TGF-β (Supplementary Fig. S1C). Several basic media were tested for hESC differentiation into CD34+CD31+ progenitors in the presence of BMP4, VEGF, and FGF2 [16,17]. Compared to the ESM and serum-containing differentiation medium (DM-S), hESCs in the DM-SF differentiated to CD34+CD31+ progenitors effectively (Fig. 1C). The components of the differentiation medium were detailed in Supplementary Table S1. The CD34+CD31+ progenitors emerged after 4 days of differentiation in DM-SF. At day 10, ∼10% to 15% cells were CD34+CD31+ progenitors (Fig. 1D). Similar results were observed in both hESCs and hiPSCs (Fig. 1E). The majority (more than 90%) of CD34+CD31+ cells also expressed the endothelial marker, VEC, but not the hematopoietic marker, CD45 (Fig. 1F).
Hematopoietic and endothelial potential of CD34+CD31+VEC+ cells
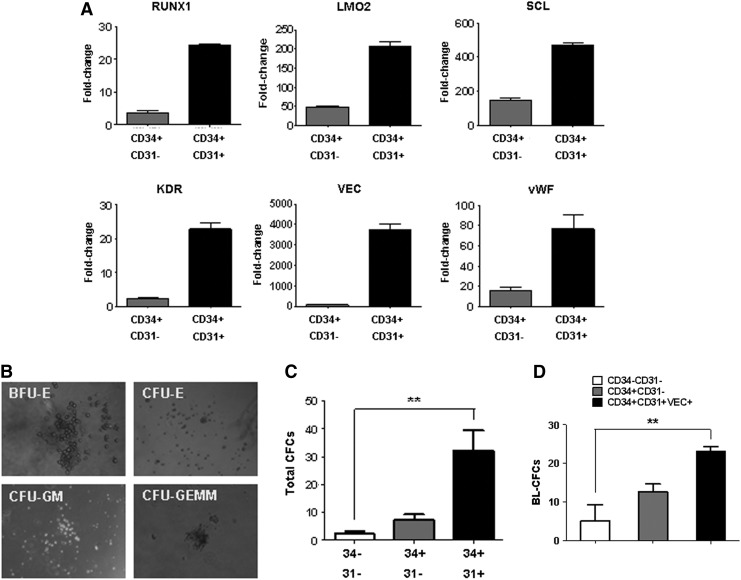
To investigate the hematopoietic and endothelial potential of CD34+CD31+VEC+ cells, CD34+CD31+ and CD34+CD31− cells were isolated by FACS sorting. Gene expression analysis by real-time RT-PCR indicated that CD34+CD31+ progenitors expressed a high level of endothelial markers, including VEC, vWF, and KDR, and hematopoietic stem/progenitor markers, including RUNX1, LMO2, and SCL (Fig. 2A). By hematopoietic CFC assay in methylcellulose (Fig. 2B), isolated CD34+CD31+ cells gave more hematopoietic colonies, compared to CD34+CD31− cells (Fig. 2C). To determine whether CD34+CD31+VEC+ cells contain hemangioblast potential, the BL-CFC assay, which has been used to identify hemangioblast potential by several laboratories [7,9,32,49,50], was performed in the methylcellulose medium containing growth factors to support both endothelial and hematopoietic cell growth. Approximately 2.3% of CD34+CD31+VEC+ cells gave rise to BL-CFCs, compared to ∼1.2% from CD34+CD31− cells and ∼0.5% from CD34−CD31− cells (Fig. 2D). The BL-CFCs contained both endothelial and hematopoietic components, assessed by RT-PCR analysis as previously described [9]. Both endothelial genes (CD31, VE-cad, eNOS, and vWF) and hematopoietic genes (SCL, RUNX1, CD41, GATA-1, HBE, and HBG) were expressed in BL-CFCs and CD34+CD31+VEC+ cells (Supplementary Fig. S2).
FIG. 2.
Characterization of CD34+CD31+ cells. (A) qPCR analyses of hematopoietic stem/progenitor markers (RUNX1, LMO2, and SCL) and endothelial markers (KDR, VEC, and vWF). CD34+CD31+ cells and CD34+CD31− cells were isolated by fluorescence-activated cell sorting (FACS) at day 10 of hPSC differentiation. (B) Hematopoietic colony-forming cell (CFC) assay in the methylcellulose medium. Photos of representative hematopoietic colonies were taken after 2 weeks of culture. (C) CFC assays of different populations that were isolated from day 10 EBs. The cells (1×104) were mixed with the methylcellulose medium (StemCell Tech.; H4435), the colony numbers were counted after 2 weeks of incubation. (D) Blast colony-forming cell (BL-CFC) assay of subpopulation from day 10 EBs. Two thousand or 5,000 cells were cultured in the methylcellulose medium with both hematopoietic and endothelial growth factors. The colony number was counted at day 3 and normalized to per 1,000 cells. Data are represented as mean±SD from three independent experiments. **P<0.01.
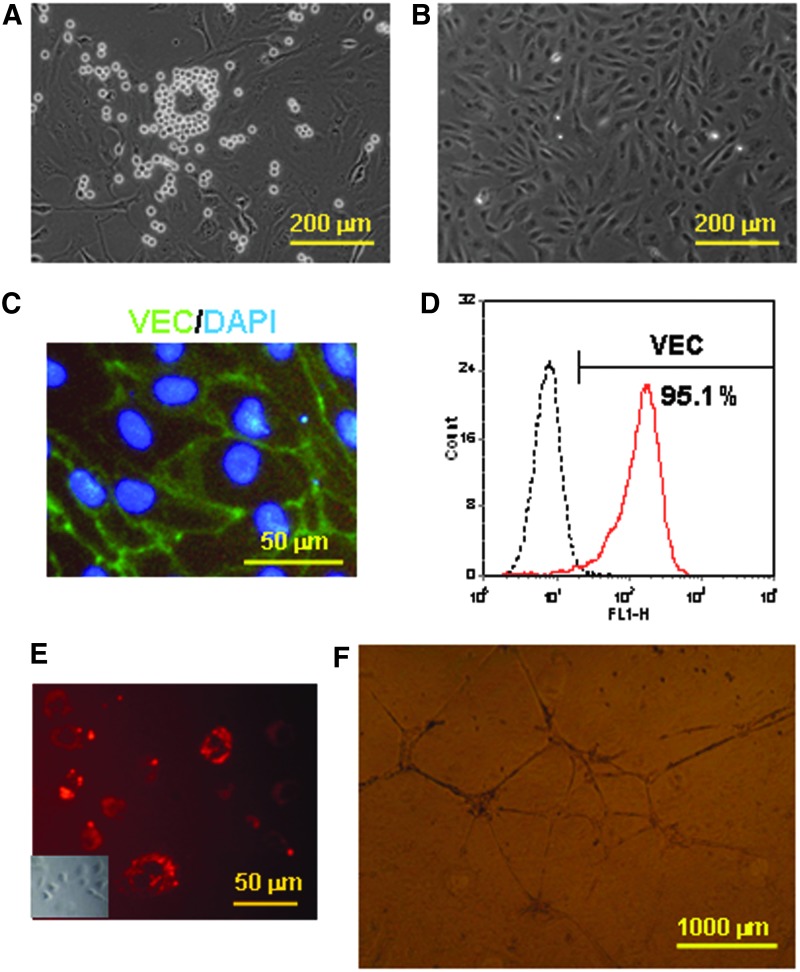
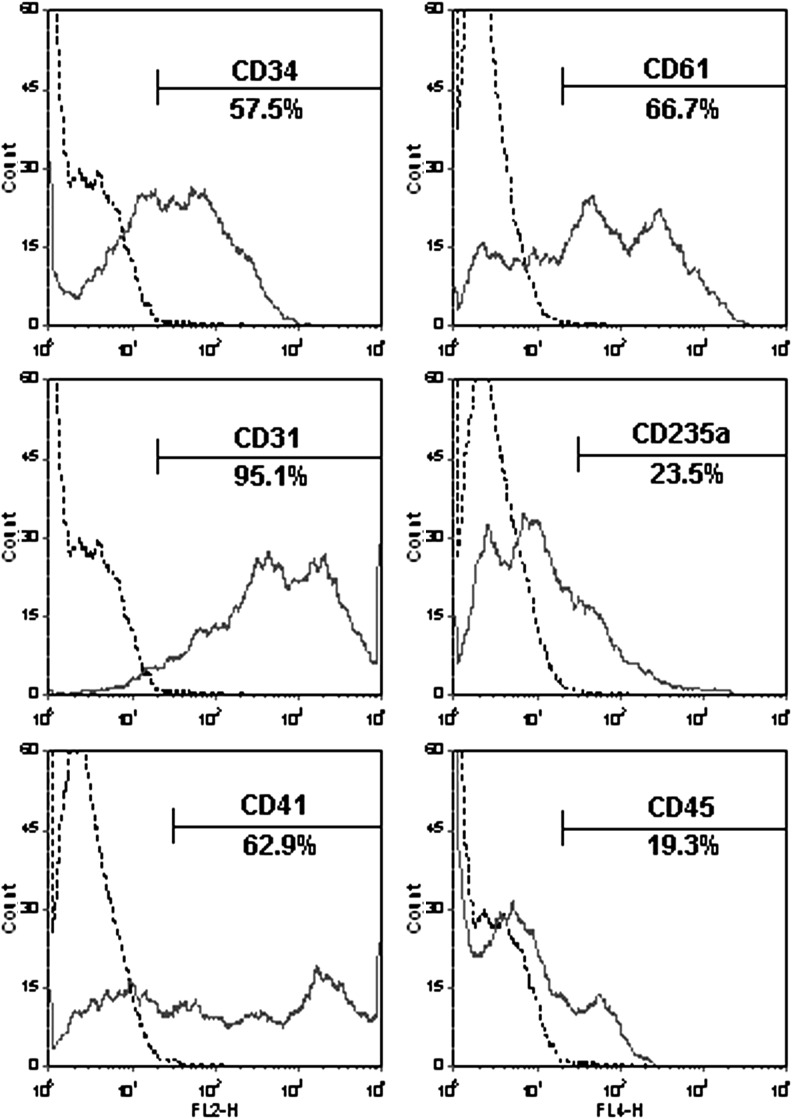
To further characterize hematopoietic and endothelial potential of CD34+CD31+VEC+ cells, the CD34+CD31+VEC+ cells were cultured in a SFEGM without hematopoietic factors. After 4 to 6 days of culture, both adhesion and suspension cells were emerged (Fig. 3A). The adhesion cells were characterized as endothelial cells by (i) their cobblestone morphology (Fig. 3B), (ii) high expression of the endothelial marker VEC by immunohistochemistry (Fig. 3C) and flow cytometry (Fig. 3D), (iii) uptaken Dil-Ac-LDL (Fig. 3E), and (iv) formation of network on Matrigel in vitro (Fig. 3F). The suspension cells after 4 days of culture in the SFEGM were positive for hematopoietic lineage markers, including CD235a, CD61, and CD45 (Fig. 4). Interestingly, the majority (∼90%) of suspension cells expressed CD31, whereas ∼50% of suspension cells were CD34+ cells. Over 60% of cells were positive for CD41, the megakaryocyte marker, and early hematopoietic marker in embryonic development [51,52]. These data demonstrated that CD34+CD31+VEC+ cells contained progenitors to give rise to endothelial cells and multilineage hematopoietic cells without additional hematopoietic growth factors.
FIG. 3.
Endothelial potential of CD34+CD31+VEC+ cells. CD34+CD31+VEC+ cells were isolated from day 10 EBs, and then cultured in the serum-free endothelial cell growth medium (SFEGM). After 4 days of culture, the cells showed hematopoietic-like colonies (A). After 6 days, the adhesive cells displayed endothelial characteristics, including typical morphology of endothelial cells (B), positive for VEC by immunohistochemistry (C) and flow cytometry analysis (D), Dil-acetylated low-density lipoprotein (Dil-Ac-LDL) uptake (E), and vascular network formation on Matrigel (F). A light phase photo was inserted to show the endothelial morphology (E).
FIG. 4.
Hematopoietic potential of CD34+CD31+VEC+ cells. CD34+CD31+VEC+ cells were cultured in the SFEGM. After 2 days, unattached cells were removed by PSB washing, and attached cells were cultured for additional 2 days in the SFEGM. The suspension cells were collected at day 4, and analyzed by flow cytometry. Data were representative of three independent experiments.
Positive effect of TGF-β signaling on mesodermal progenitor development
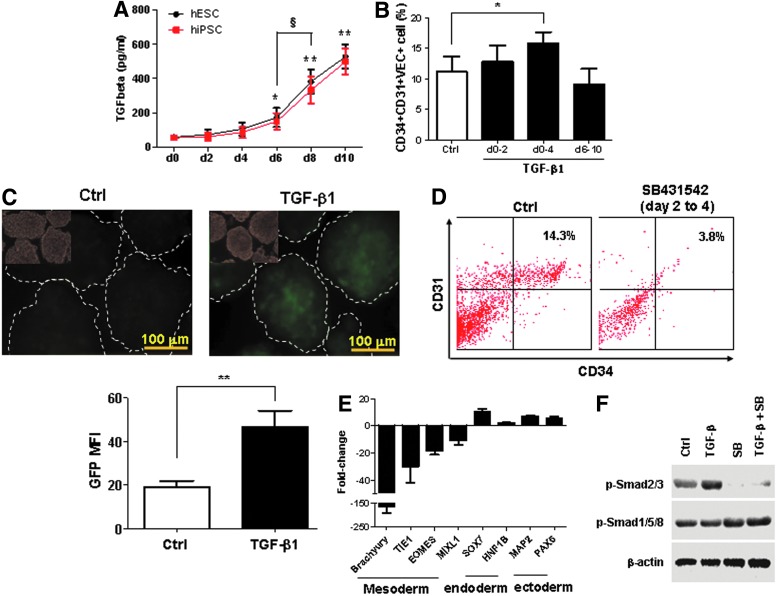
The TGF-β subfamily, including TGF-β, activin, and nodal, regulates both stem cell pluripotency and differentiation [25,27,53]. We measured the level of TGF-β1 proteins during the differentiation of hPSCs by ELISA assay. As shown in Fig. 5A, the expression of TGF-β was increased after 4 days of differentiation of hESCs (H1) or hiPSCs (HDFa-YK26). By day 10, the concentration of TGF-β1 in a culture medium was approximately 500 pg/mL. Interestingly, the pattern of TGF-β expression was similar to kinetics of hemangioblast development (Fig. 1D), showing a robust increase from day 6 to 10. To elucidate the role of TGF-β signaling in the hemangioblast development, we added TGF-β1 (2 ng/mL) in three major steps of hemangioblast generation: (i) the formation of uniformed EBs in hanging-drops (day 0 to 2), (ii) the development of multipotent mesoderm precursors in cultivation of EBs (day 0 to 4), and (iii) the generation of hemangioblasts (day 6 to 10). As shown in Fig. 5B, the addition of TGF-β1 from day 0 to 4 significantly increased the population of CD34+CD31+VEC+ cells. Treatment with TGF-β1 between day 6 and 10 decreased the number of CD34+CD31+VEC+ cells modestly (Fig. 5B). During the early stage of hPSC differentiation (day 0 to 4), the addition of TGF-β1 or other members of the TGF-β family, including TGF-β2, TGF-β3, and activin, induced the expression of mesodermal markers, Brachyury (T), EOMES, and TIE1, and decreased the expression of endoderm marker, SOX7, as well as ectoderm marker, PAX6 (Supplementary Fig. S3). To further assess the role of TGF-β in mesoderm development, we generated a hiPSC line, BC1-Brachyury-GFP, in which the GFP expression is driven by a specific Brachyury promoter [40]. The mesodermal marker, Brachyury, was tracked by GFP expression during hPSC differentiation. In the presence of TGF-β1 for 2 days during EB formation, the Brachyury-GFP expression was significantly increased in the EBs at day 5 when the level of mesodermal progenitors was high (Fig. 5C). These data demonstrated that TGF-β signaling promoted mesoderm development during the early stage of hPSC differentiation.
FIG. 5.
Positive effect of transforming growth factor beta (TGF-β) signaling on early mesodermal development. (A) The kinetics of TGF-β secretion during EB differentiation. The cultured media were collected for ELISA assay every 2 days before changing to fresh media. The TGF-β concentration in cultured media with hESCs (H1) and hiPSCs (HSFa-YK26) were measured by the Quantikine ELISA kit. (B) Promoting effect of TGF-β1 on CD34+CD31+VEC+ cells during early mesoderm development. TGF-β1 (2 ng/mL) was added to EB differentiation at different time points. Flow cytometry analyses were performed at day 10 of EB differentiation. (C) EBs were generated from hiPSC BC1-Brachyury-GFP, in which the GFP expression is driven by the Brachyury promoter, in the medium with or without 2 ng/mL TGF-β1 from day 0 to 2. The morphology and GFP expression were examined at day 5. The GFP mean fluorescence intensity (MFI) of individual EBs was quantified according to histogram of green channel by Photoshop software. (D) Impaired development of CD34+CD31+ cells by inhibition of TGF-β signaling during early mesoderm development. TGF-β inhibitor, SB431542, (10 μM) was added to EB culture from day 2 to 4. Flow cytometry analyses were performed at day 10 of EB differentiation. (E) qPCR analyses of three germ layer gene expressions. EB differentiation was performed with or without SB431542 from day 2 to 4. EBs were collected at day 4 for gene expressions analyses of mesodermal, endodermal, and ectodermal markers. (F) Western blot analysis of phosphorylated Smad. EBs from hiPSCs at day 2 were incubated with 10 ng/mL TGF-β1 and/or 10 μM SB431542 for 30 min. The phosphorylated Smad2/3 (p-Smad2/3) and p-Smad1/5/8 were detected by specific antibodies against p-Smad2/3 or p-Smad1/5/8. β-Actin was used a control. Data are represented as mean±SD from three independent experiments. *P<0.05, **P<0.01, compared with the day 0 group or indicated group. §P<0.05, between two groups.
A small molecule, SB431542, is a specific inhibitor of TGF-β receptors of ALK4, ALK5, and ALK7, and blocks the activation of downstream transducers, Samd2/3 [54,55]. We further investigated whether inhibition of TGF-β1 signaling with SB431542 affects mesodermal development. As shown in Fig. 5D, the addition of SB431542 at day 2 to 4 significantly decreased the development of CD34+CD31+ cells. Real-time RT-PCR analysis showed that the inhibition of TGF-β signaling significantly decreased the expression of mesodermal markers, Brachyury (T), EOMES, TIE1, and MIXL1, and increased the endodermal and ectodermal markers Sox7, HNF1B, MAP2, and PAX6 (Fig. 5E). Western blot analysis of Smad phosphorylation in day 2 EBs of hiPSCs indicated that TGF-β1 stimulated the phosphorylation of Smad2/3 (p-Smad2/3), which was inhibited by SB431542 (Fig. 5F). The phosphorylation of Smad1/5/8 was not affected by TGF-β1. Similar results of p-Smad2/3 were obtained in undifferentiated hESCs (Supplementary Fig. S4). These data indicated that the TGF-β and Smad2/3 signaling pathway is essential for mesodermal progenitor development in hPSCs, whereas inhibition of TGF-β signaling in the early stages of hPSC differentiation promotes ectodermal and endodermal development.
Negative effect of TGF-β on the development of hematopoietic and endothelial progenitors
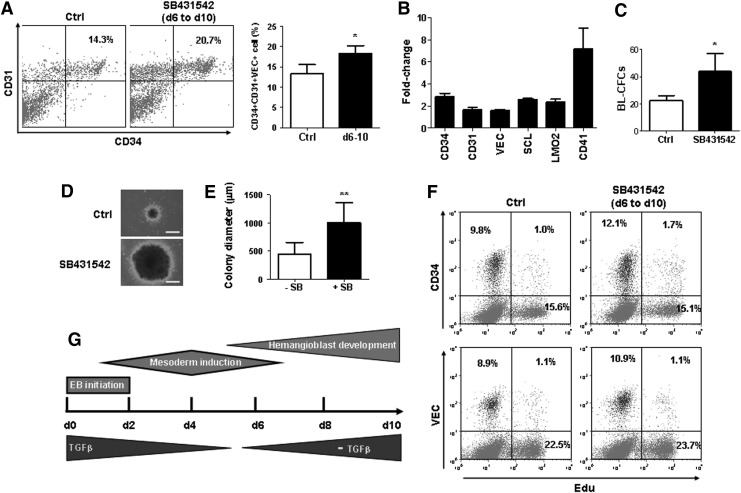
Because of the high expression of TGF-β between day 6 to 10 during hPSC differentiation (Fig. 5A), we tested the hypothesis that endogenous TGF-β expression inhibits the development of hematopoietic and endothelial progenitors from mesodermal precursors. Whereas an addition of TGF-β1 decreased CD34+CD31+ cells modestly (Fig. 5B), inhibition of TGF-β signaling by SB431542 after day 6 of hPSC differentiation promoted the development of CD34+CD31+ cells (Fig. 6A). Gene expression analysis by real-time RT-PCR revealed that inhibition of TGF-β by SB431542 after day 6 of hPSC differentiation increased gene expression of CD34, CD31, and VEC, as well as hematopoietic stem/progenitor markers, SCL, LMO2, and CD41 (Fig. 6B). To examine the effect of TGF-β signaling on BL-CFC formation, we performed BL-CFC assay of CD34+CD31+VEC+ cells with or without TGF-β inhibitor, SB431542. As shown in Fig. 6, the addition of SB431542 not only significantly increased the number of BL colonies (Fig. 6C), but also increased the size of BL-CFCs (Fig. 6D, E), suggesting that TGF-β signaling negatively regulates BL-CFC generation and proliferation. TGF-β signaling has antiproliferative effects in several cell types, including hematopoietic cells, epithelial cells, and primary MEFs [56,57]. To determine whether TGF-β inhibition stimulates CD34+CD31+VEC+ progenitor cell proliferation during EB differentiation, we utilized the EdU cell proliferation assays and flow cytometry to quantify proliferating cells in EBs. As shown in Fig. 6F, the percentages of EdU-positive cells in CD34+ cells and VEC+ cells were not significantly affected by TGF-β inhibition. Our study demonstrated that TGF-β signaling is required for mesodermal development, and inhibition of TGF-β signaling facilitates hematopoietic and endothelial lineage specification after establishment of mesoderm precursors (Fig. 6G).
FIG. 6.
Negative effect of TGF-β on the development of CD34+CD31+VEC+ progenitors. (A) Inhibition of TGF-β enhanced the generation of CD34+CD31+VEC+ cells. EB differentiation was conducted with or without SB431542 from day 6 to 10. Flow cytometry analyses of CD34+CD31+ cells were performed at day 10 of differentiation. (B) qPCR analyses of endothelial and hematopoietic gene expressions. Ten μM SB431542 was added from day 6 to 10. EBs were collected at day 10 for qPCR. The gene expressions levels were normalized by the group of EBs without SB431542 addition. (C) BL-CFC assay of CD34+CD31+VEC+ cells from EB day 10. Two thousand or 5,000 cells were cultured with or without 10 μM SB431542 in the methylcellulose medium containing both hematopoietic and endothelial growth factors. The colony number was counted at day 3 and normalized by per 1,000 cells. The blast colony morphology (D) and statistic size (E) were examined at day 6. (F) Cell proliferation analyses by flow cytometry. Ten mM EdU was added for 3 h before analyses. The CD34-positive and VEC-positive cells with EdU integration were analyzed using the Click-iT EdU Flow Cytometry Assay Kit. Data were representative of three independent experiments. (G) A working model of TGF-β regulating hemangioblast development. TGF-β signaling is required for mesodermal development, and inhibition of TGF-β signaling facilitates hemangioblast development after establishment of mesoderm precursors. Data are represented as mean±SD from three independent experiments. *P<0.05, **P<0.01.
Discussion
Despite decade-long research, the molecular and niche signals, which regulate de novo blood generation in humans, are largely unknown, partially because of the transient presence of the hematopoietic precursors at different developmental stages, and limited accessibility of study materials. Human PSCs differentiate efficiently in vitro and give rise to a differentiated cell mass called EBs. Although an EB is far less organized than an actual embryo, it can partially mimic the spatial organization in the embryo, and provides a great model system to investigate early blood development. We previously used the scratching method to generate EBs of hPSC differentiation [16,42], and found that the random EB formation by the scratching method caused low efficiency of EB formation and various EB sizes, resulting in tremendous deviation of differentiation outcomes (data not shown). Recently, a spin-aggregation method by centrifuge is developed to generate unified EBs from hPSCs [58,59]. However, it is time-consuming and labor intensive if changing the medium is required following a series of sequential steps either in AggreWell plate (StemCell Tech.) or 96-well plate. In this study, we established a serum-free and feeder-free EB differentiation system to investigate the role of TGF-β signaling in the early development of hematopoietic and endothelial lineages from hESCs and hiPSCs. EBs were formed by hanging drop from day 0 to 2, and then transferred to an ultralow attachment dish for up to 10 days of differentiation. By combining the advantages of Accutase and ROCK inhibitor Y27632, as well as optimization of cell numbers and differentiation medium (Supplementary Fig. S1), we achieved extremely high efficiency and uniformity of EB generation by the hanging-drop method. Interestingly, the efficiency of EB formation in the ESM from day 0 to 2 increased compared to in the differentiation medium. An addition of BMP4, but not TGF-β, decreased the efficiency of EB formation in hanging drops. Our study indicated that the promotion of early differentiation reduces the efficiency of EB formation.
Hemangioblast is defined as a common precursor with the potential to differentiate into both hematopoietic and endothelial lineages. In the presence of mesoderm-inducing growth factors (Fig. 1A), we successfully differentiated hPSCs to CD34+CD31+VEC+ progenitor cells that gave rise to endothelial cells and hematopoietic cells (Figs. 3 and 4), as well as BL-CFCs (Fig. 2), which are clonal expansion of hemangioblast [7,9,43]. To explore a role of TGF-β signaling in hematopoietic and endothelial lineage commitment, we applied TGF-β1 and inhibitor (SB431542) during EB differentiation at different stages. During the early stage of hPSC differentiation (day 0 to 4), the addition of TGF-β1 and other members of the TGF-β family, including TGF-β2, TGF-β3, and activin, promoted the mesoderm gene expression and reduced gene expressions of endoderm and ectoderm (Supplementary Fig. S3). Inhibition of TGF-β signaling by SB431542 significantly decreased the expression of mesoderm genes, and increased the expression of endoderm and ectoderm genes (Fig. 5E), resulting in attenuation of the generation of CD34+CD31+VEC+ progenitors (Fig. 5D). At this point, it is unknown whether the effect of TGF-β on mesoderm induction resulted from inhibition of endoderm and ectoderm. Although BMP4 promotes mesoderm induction, addition of BMP4 during EB initiation (day 0 to 2) resulted in poor EB formation (Supplementary Fig. S1C), probably because inducing differentiation by BMP4 before hPSC aggregation leads to apoptosis. These data indicated that TGF-β is indispensable during the early mesoderm developmental stage.
Because of the complexity of TGF-β signaling, the function of the TGF-β family of cytokines depends on their cell context [18]. Although an addition of TGF-β had no significant effect after 6 days of hPSC differentiation (Supplementary Fig. S5), inhibition of TGF-β signaling during the later stage of hPSC differentiation (day 6 to 10) promoted the generation of CD34+CD31+VEC+ progenitors and BL-CFCs (Fig. 6), suggesting that high expression of endogenous TGF-β in EBs negatively regulates hematopoietic and endothelial lineage commitment and hemangioblast development from mesodermal progenitors. Gene expression analysis at different developmental stages indicated that expression of early mesoderm genes, such as Brachyury, was induced by TGF-β signals (Fig. 5E), whereas suppression of TGF-β after mesoderm induction lead to profound enhancement in the levels of hemangioblast-related and key hematopoietic regulators, including CD34, VEC, SCL, CD41, and LMO2 (Fig. 6B). Whether these genes are direct targets of Smad2/3 will be further investigated. A recent study from the Gordon Keller's group [60] demonstrated that TGF-β inhibition promotes the differentiation of hPSC toward hemogenic endothelium and definitive hematopoiesis. In our study, a similar result was obtained when TGF-β inhibitor was added after day 6 EB culture. Interestingly, when TGF-β inhibitor was added before mesoderm induction (before day 4 EB culture), the development of CD34+CD31+VEC+ cells was abolished as a result of mesoderm reduction. Considering the heterogeneity of CD34+CD31+VEC+ cell populations, it would be important to understand whether TGF-β signaling regulates hemangioblast bipotential differentiation of hematopoietic and endothelial cells. The future studies of hemangioblast in clonally derived blast colonies, as well as hematopoietic and endothelial cell lineage commitment from hemangioblast, will shed light on human hemato-vascular development and provide insights into a role of TGF-β signaling in hemangioblast development, proliferation, and differentiation into hematopoietic and/or endothelial cells.
Taken together, we concluded that TGF-β signaling has the double-edged effect on hPSC differentiation to hematopoietic and endothelial progenitors. Whereas TGF-β signaling is required for the early mesoderm induction, inhibition of TGF-β signaling after mesoderm induction enhances the hematopoietic and endothelial lineage commitment.
Supplementary Material
Acknowledgments
This work was partially supported by the MD State Stem Research Cell Fund (2012-MSCRFII-0124) and NIH/NHLBI U01 HL107446, and MOST (2011CB964801 and 2011ZX09102-1004) and NNSF (81090410 and 30825017) to T.C. Additional technical support was provided by the Ross flow cytometry core at Johns Hopkins Medicine.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Sabin FR. Studies on the origin of blood vessels and of red corpuscles as seen in the living blastoderm of the chick during the second day of induction. Contribut Embryol. 1920;9:213–262. [Google Scholar]
- 2.Wagner RC. Endothelial cell embryology and growth. Adv Microcirc. 1980;9:45–75. [Google Scholar]
- 3.Pardanaud L. Yassine F. Dieterlen-Lievre F. Relationship between vasculogenesis, angiogenesis and haemopoiesis during avian ontogeny. Development. 1989;105:473–485. doi: 10.1242/dev.105.3.473. [DOI] [PubMed] [Google Scholar]
- 4.Huber TL. Kouskoff V. Fehling HJ. Palis J. Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimoto M. Yoder MC. Developmental biology: birth of the blood cell. Nature. 2009;457:801–803. doi: 10.1038/457801a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi K. Hemangioblast development and regulation. Biochem Cell Biol. 1998;76:947–956. [PubMed] [Google Scholar]
- 7.Kennedy M. D'Souza SL. Lynch-Kattman M. Schwantz S. Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109:2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy M. Firpo M. Choi K. Wall C. Robertson S. Kabrun N. Keller G. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature. 1997;386:488–493. doi: 10.1038/386488a0. [DOI] [PubMed] [Google Scholar]
- 9.Zambidis ET. Park TS. Yu W. Tam A. Levine M. Yuan X. Pryzhkova M. Peault B. Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood. 2008;112:3601–3614. doi: 10.1182/blood-2008-03-144766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eilken HM. Nishikawa S. Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- 11.Lancrin C. Sroczynska P. Stephenson C. Allen T. Kouskoff V. Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishikawa SI. Nishikawa S. Kawamoto H. Yoshida H. Kizumoto M. Kataoka H. Katsura Y. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity. 1998;8:761–769. doi: 10.1016/s1074-7613(00)80581-6. [DOI] [PubMed] [Google Scholar]
- 13.Dzierzak E. Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 15.Onder TT. Daley GQ. New lessons learned from disease modeling with induced pluripotent stem cells. Curr Opin Genet Dev. 2012;22:500–508. doi: 10.1016/j.gde.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang ZZ. Au P. Chen T. Shao Y. Daheron LM. Bai H. Arzigian M. Fukumura D. Jain RK. Scadden DT. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007;25:317–318. doi: 10.1038/nbt1287. [DOI] [PubMed] [Google Scholar]
- 17.Bai H. Gao Y. Arzigian M. Wojchowski DM. Wu WS. Wang ZZ. BMP4 regulates vascular progenitor development in human embryonic stem cells through a smad-dependent pathway. J Cell Biochem. 2010;109:363–374. doi: 10.1002/jcb.22410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massague J. TGF[beta] signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bobik A. Transforming growth factor-betas and vascular disorders. Arterioscler Thromb Vasc Biol. 2006;26:1712–1720. doi: 10.1161/01.ATV.0000225287.20034.2c. [DOI] [PubMed] [Google Scholar]
- 20.David L. Feige JJ. Bailly S. Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev. 2009;20:203–212. doi: 10.1016/j.cytogfr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Goumans MJ. Liu Z. ten Dijke P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–127. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- 22.Massague J. Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- 23.Valdimarsdottir G. Mummery C. Functions of the TGFbeta superfamily in human embryonic stem cells. APMIS. 2005;113:773–789. doi: 10.1111/j.1600-0463.2005.apm_3181.x. [DOI] [PubMed] [Google Scholar]
- 24.James D. Levine AJ. Besser D. Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- 25.Vallier L. Reynolds D. Pedersen RA. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol. 2004;275:403–421. doi: 10.1016/j.ydbio.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 26.Beattie GM. Lopez AD. Bucay N. Hinton A. Firpo MT. King CC. Hayek A. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2005;23:489–495. doi: 10.1634/stemcells.2004-0279. [DOI] [PubMed] [Google Scholar]
- 27.Xiao L. Yuan X. Sharkis SJ. Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells. Stem Cells. 2006;24:1476–1486. doi: 10.1634/stemcells.2005-0299. [DOI] [PubMed] [Google Scholar]
- 28.Xu RH. Peck RM. Li DS. Feng X. Ludwig T. Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 29.Wang G. Zhang H. Zhao Y. Li J. Cai J. Wang P. Meng S. Feng J. Miao C, et al. Noggin and bFGF cooperate to maintain the pluripotency of human embryonic stem cells in the absence of feeder layers. Biochem Biophys Res Commun. 2005;330:934–942. doi: 10.1016/j.bbrc.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 30.Trompouki E. Bowman TV. Lawton LN. Fan ZP. Wu D-C. DiBiase A. Martin CS. Cech JN. Sessa AK, et al. Lineage regulators direct BMP and Wnt pathways to cell-specific programs during differentiation and regeneration. Cell. 2011;147:577–589. doi: 10.1016/j.cell.2011.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mullen Alan C. Orlando DA. Newman JJ. Lovén J. Kumar RM. Bilodeau S. Reddy J. Guenther MG. DeKoter RP. Young RA. Master transcription factors determine cell-type-specific responses to TGF-β signaling. Cell. 2011;147:565–576. doi: 10.1016/j.cell.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zafonte BT. Liu S. Lynch-Kattman M. Torregroza I. Benvenuto L. Kennedy M. Keller G. Evans T. Smad1 expands the hemangioblast population within a limited developmental window. Blood. 2007;109:516–523. doi: 10.1182/blood-2006-02-004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu B. Sun Y. Jiang F. Zhang S. Wu Y. Lan Y. Yang X. Mao N. Disruption of Smad5 gene leads to enhanced proliferation of high-proliferative potential precursors during embryonic hematopoiesis. Blood. 2003;101:124–133. doi: 10.1182/blood-2002-02-0398. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L. Magli A. Catanese J. Xu Z. Kyba M. Perlingeiro RC. Modulation of TGF-beta signaling by endoglin in murine hemangioblast development and primitive hematopoiesis. Blood. 2011;118:88–97. doi: 10.1182/blood-2010-12-325019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perlingeiro RC. Endoglin is required for hemangioblast and early hematopoietic development. Development. 2007;134:3041–3048. doi: 10.1242/dev.002907. [DOI] [PubMed] [Google Scholar]
- 36.Wu Z. Zhang W. Chen G. Cheng L. Liao J. Jia N. Gao Y. Dai H. Yuan J. Xiao L. Combinatorial signals of activin/nodal and bone morphogenic protein regulate the early lineage segregation of human embryonic stem cells. J Biol Chem. 2008;283:24991–25002. doi: 10.1074/jbc.M803893200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kennedy M. Awong G. Sturgeon CM. Ditadi A. Lamotte-Mohs R. Zuniga-Pflucker JC. Keller G. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2:1722–1735. doi: 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Wang X. Lin G. Martins-Taylor K. Zeng H. Xu RH. Inhibition of caspase-mediated anoikis is critical for basic fibroblast growth factor-sustained culture of human pluripotent stem cells. J Biol Chem. 2009;284:34054–34064. doi: 10.1074/jbc.M109.052290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chou BK. Mali P. Huang X. Ye Z. Dowey SN. Resar LM. Zou C. Zhang YA. Tong J. Cheng L. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011;21:518–529. doi: 10.1038/cr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kita-Matsuo H. Barcova M. Prigozhina N. Salomonis N. Wei K. Jacot JG. Nelson B. Spiering S. Haverslag R, et al. Lentiviral vectors and protocols for creation of stable hESC lines for fluorescent tracking and drug resistance selection of cardiomyocytes. PLoS One. 2009;4:e5046. doi: 10.1371/journal.pone.0005046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bai H. Chen K. Gao Y. Arzigian M. Xie Y. Malcosky C. Yang Y. Wu W. Wang Z. Bcl-xL enhances single-cell survival and expansion of human embryonic stem cells without affecting self-renewal. Stem Cell Res. 2012;8:26–37. doi: 10.1016/j.scr.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen T. Bai H. Shao Y. Arzigian M. Janzen V. Attar E. Xie Y. Scadden DT. Wang ZZ. SDF-1/CXCR4 signaling modifies the capillary-like organization of human embryonic stem cell-derived endothelium in vitro. Stem Cells. 2007;25:392–401. doi: 10.1634/stemcells.2006-0145. [DOI] [PubMed] [Google Scholar]
- 43.D'Souza SL. Elefanty AG. Keller G. SCL/Tal-1 is essential for hematopoietic commitment of the hemangioblast but not for its development. Blood. 2005;105:3862–3870. doi: 10.1182/blood-2004-09-3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watanabe K. Ueno M. Kamiya D. Nishiyama A. Matsumura M. Wataya T. Takahashi JB. Nishikawa S. Nishikawa S. Muguruma K. Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 45.Xu Y. Zhu X. Hahm HS. Wei W. Hao E. Hayek A. Ding S. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc Natl Acad Sci U S A. 2010;107:8129–8134. doi: 10.1073/pnas.1002024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang P. Li J. Tan Z. Wang C. Liu T. Chen L. Yong J. Jiang W. Sun X, et al. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood. 2008;111:1933–1941. doi: 10.1182/blood-2007-02-074120. [DOI] [PubMed] [Google Scholar]
- 47.Yang L. Soonpaa MH. Adler ED. Roepke TK. Kattman SJ. Kennedy M. Henckaerts E. Bonham K. Abbott GW, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 48.Chadwick K. Wang L. Li L. Menendez P. Murdoch B. Rouleau A. Bhatia M. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906–915. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- 49.Laranjeiro R. Alcobia I. Neves H. Gomes AC. Saavedra P. Carvalho CC. Duarte A. Cidadao A. Parreira L. The notch ligand delta-like 4 regulates multiple stages of early hemato-vascular development. PLoS One. 2012;7:e34553. doi: 10.1371/journal.pone.0034553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramirez-Bergeron DL. Runge A. Dahl KD. Fehling HJ. Keller G. Simon MC. Hypoxia affects mesoderm and enhances hemangioblast specification during early development. Development. 2004;131:4623–4634. doi: 10.1242/dev.01310. [DOI] [PubMed] [Google Scholar]
- 51.Mikkola HK. Fujiwara Y. Schlaeger TM. Traver D. Orkin SH. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood. 2003;101:508–516. doi: 10.1182/blood-2002-06-1699. [DOI] [PubMed] [Google Scholar]
- 52.Ferkowicz MJ. Starr M. Xie X. Li W. Johnson SA. Shelley WC. Morrison PR. Yoder MC. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development. 2003;130:4393–4403. doi: 10.1242/dev.00632. [DOI] [PubMed] [Google Scholar]
- 53.Amit M. Shariki C. Margulets V. Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol Reprod. 2004;70:837–845. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- 54.Inman GJ. Nicolas FJ. Callahan JF. Harling JD. Gaster LM. Reith AD. Laping NJ. Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 55.DaCosta Byfield S. Major C. Laping NJ. Roberts AB. SB-505124 is a selective inhibitor of transforming growth factor-beta type I receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2004;65:744–752. doi: 10.1124/mol.65.3.744. [DOI] [PubMed] [Google Scholar]
- 56.Alexandrow MG. Moses HL. Transforming growth factor beta and cell cycle regulation. Cancer Res. 1995;55:1452–1457. [PubMed] [Google Scholar]
- 57.Cheng T. Shen H. Rodrigues N. Stier S. Scadden DT. Transforming growth factor beta 1 mediates cell-cycle arrest of primitive hematopoietic cells independent of p21(Cip1/Waf1) or p27(Kip1) Blood. 2001;98:3643–3649. doi: 10.1182/blood.v98.13.3643. [DOI] [PubMed] [Google Scholar]
- 58.Bratt-Leal AM. Carpenedo RL. Ungrin MD. Zandstra PW. McDevitt TC. Incorporation of biomaterials in multicellular aggregates modulates pluripotent stem cell differentiation. Biomaterials. 2011;32:48–56. doi: 10.1016/j.biomaterials.2010.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ran D. Shia WJ. Lo MC. Fan JB. Knorr DA. Ferrell PI. Ye Z. Yan M. Cheng L. Kaufman DS. Zhang DE. RUNX1a enhances hematopoietic lineage commitment from human embryonic stem cells and inducible pluripotent stem cells. Blood. 2013;121:2882–2890. doi: 10.1182/blood-2012-08-451641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kennedy M. Awong G. Sturgeon CM. Ditadi A. LaMotte-Mohs R. Zuniga-Pflucker JC. Keller G. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2:1722–1735. doi: 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.