Abstract
Objective
The mechanisms through which ω-3 fatty acids reduce adverse cardiac events remain uncertain. We aimed to investigate the effect of ω-3 fatty acid supplementation on endothelial vasomotor function, endogenous fibrinolysis, and platelet and monocyte activation in patients with coronary heart disease.
Design
Randomised, double-blind, placebo-controlled, cross-over trial.
Setting
Academic cardiac centre.
Participants
20 male patients with a previous myocardial infarction.
Intervention
ω-3 Fatty acid supplementation (2 g/day for 6 weeks) versus olive oil placebo.
Outcome measures
Peripheral blood was taken for analysis of platelet and monocyte activation, and forearm blood flow (FBF) was assessed in a subset of 12 patients during intrabrachial infusions of acetylcholine, substance P and sodium nitroprusside. Stimulated plasma tissue plasminogen activator (t-PA) concentrations were measured during substance P infusion.
Results
All vasodilators caused dose-dependent increases in FBF (p<0.0001). ω-3 Fatty acid supplementation did not affect endothelium-dependent vasodilation with acetylcholine and substance P compared with placebo (p=0.5 and 0.9). Substance P caused a dose-dependent increase in plasma t-PA concentrations (p<0.0001), which was not affected by ω-3 fatty acid supplementation (p=0.9). ω-3 Fatty acids did not affect platelet–monocyte aggregation, platelet P-selectin or CD40L, or monocyte CD40.
Conclusions
We have demonstrated that dietary supplementation with ω-3 fatty acids does not affect endothelial vasomotor function, endothelial t-PA release, or platelet and monocyte activation in patients with coronary heart disease. Cardiac benefits conferred by ω-3 fatty acids in coronary heart disease are unlikely to be mediated through effects on these systems.
Keywords: Nutrition & Dietetics, Vascular Medicine
Article summary.
Strengths and limitations of this study
Randomised, double-blind, placebo-controlled crossover design.
Use of an established and robust model to simultaneously assess endothelial vasomotor tone as well as endogenous fibrinolysis: two important and complementary measures of vascular function.
Limitations: modest sample size.
Introduction
Dietary fish or fish oil supplements containing ω-3 fatty acids may protect against cardiovascular disease.1 Clinical trials have demonstrated beneficial effects on mortality or cardiac events in patients with coronary heart disease.2–4 However, the mechanisms through which they confer cardiac benefits are uncertain. Although an effect on ventricular arrhythmias has been thought to be important due to an observed reduction in sudden death,5 6 subsequent studies have failed to clearly demonstrate an antiarrhythmic effect.7 An alternative mechanism may, therefore, be an effect on the vascular endothelium, as acute myocardial infarction due to plaque rupture and subsequent coronary thrombosis remains the most common cause of sudden cardiac death.8
The endothelium regulates vascular tone and blood flow, and mediates thrombosis through the production of factors that influence fibrinolysis and platelet activation. The endogenous fibrinolytic system is responsible for the dissolution of arterial thrombi and is regulated by the endothelium-derived profibrinolytic factor, tissue plasminogen activator (t-PA) and its inhibitor, plasminogen-activator inhibitor type 1 (PAI-1).9 The rapid release of t-PA from the endothelium is vital, with thrombus dissolution being more effective if t-PA is incorporated early during thrombus formation.10
Endothelial cells regulate thrombosis through the release of paracrine factors that mediate platelet function. Activated platelets can bind to leucocytes through a P-selectin-dependent mechanism,11 and these interactions can also be modulated by the CD40 receptor and its ligand.12 Formation of platelet–leucocyte aggregates or ligation of CD40 can mediate an array of proinflammatory and prothrombotic effects, thereby contributing to endothelial injury and atherothrombosis.13
Patients with coronary heart disease demonstrate impaired endothelial function,14 in addition to increased platelet–monocyte aggregation and upregulation of the CD40/CD40 ligand system.15 16 We have recently demonstrated that ω-3 fatty acid supplements improve endogenous fibrinolysis and endothelial function in healthy cigarette smokers, a group at high risk of adverse cardiac events.17 Previously, we have shown that dietary fish intake reduces platelet–monocyte aggregation in man.18 We, therefore, hypothesised that ω-3 fatty acid supplementation would improve endothelial vasomotor function, endogenous fibrinolysis, and markers of platelet and monocyte activation in patients with coronary heart disease.
Methods
Study participants
Twenty patients with a myocardial infarction at least 3 months previously were recruited to participate in the study. Myocardial infarction was defined as any two of the following: typical clinical history, ECG changes (Q waves in 2 contiguous leads) or elevation of cardiac markers (CKmB or troponin). All participants gave written informed consent.. Exclusion criteria included dietary fish allergy or intolerance, consumption of more than one fish meal per week, renal or hepatic failure, or any intercurrent illness likely to be associated with an inflammatory response. The first patient was randomised in December 2004 and the last study visit took place in June 2006. There were logistical delays in the analysis of frozen plasma samples and the final data became available for analysis in June 2009.
Study design
This was a prospective, double-blind, placebo-controlled, randomised crossover trial. Participants were randomised to receive either ω-3 fatty acid supplements (2 g/day, Omacor capsules, Pronova, Norway) or matching placebo capsules (2 g/day olive oil capsules; Eurocaps Ltd, Gwent, Australia) for a 6-week period. After a 4-week washout phase, participants crossed over to the opposite treatment arm for a further 6-week period. The ω-3 Fatty acid supplements and placebo were packaged and dispensed in identical containers by the Royal Infirmary of Edinburgh Pharmacy. All study participants and investigators were blinded to the study allocation. The randomisation schedule was generated by an investigator not involved in the study, and securely kept in the Royal Infirmary of Edinburgh Pharmacy. The ω-3 fatty acid capsules contained 85–88% eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) as ethyl esters in a ratio of 1.2:1. The ω-3 fatty acid capsules as well as olive oil placebo contained 4 mg α-tocopherol. All 20 participants had peripheral blood taken for fasting lipid profile, plasma fatty acid analysis and flow cytometric analysis of platelet activation at baseline and at the end of each treatment period. Two patients withdrew from the study: one was withdrawn after being admitted with unstable angina and a second was lost to follow-up. A subset of 12 participants also underwent measurement of forearm blood flow (FBF) and endogenous fibrinolysis at the end of each treatment period.
Blood collection protocol
Peripheral venous blood was drawn from a large antecubital vein with a 19-gauge needle and anticoagulated with EDTA (1.6 mg/mL, Sarstedt Monovette) and the direct thrombin inhibitor d-phenylalanine-l-prolyl-l-arginine chloromethyl ketone (75 μM, PPACK, Cambridge Biosciences). Whole blood anticoagulated with PPACK was immunolabelled within 5 min of phlebotomy for subsequent flow cytometric analysis. Plasma was prepared from blood anticoagulated with sodium EDTA by centrifugation (1500×g for 30 min). Plasma samples were stored at −70°C until analysis.
Flow cytometry
The following reagents were used: fluorscein isothiocyanate (FITC)-conjugated CD42a (GRP-P, IgG1), FITC-conjugated CD14 (UCHM1, IgG2a), phycoerythrin (PE)-conjugated CD40 (LOB7/6, IgG1), and their appropriate isotype controls (Serotec Ltd, Oxford, UK) as well as PE-conjugated CD154 (TRAP1, IgG1), PE-conjugated CD14 (Tuk-4, IgG2a), PE-conjugated CD 62P (IE3, IgG2a) and their appropriate isotype controls (Dako Cytomation, Buckinghamshire, UK) and FACS-Lyse (Becton-Dickinson; Cowley, UK). Aliquots of whole blood (60 μL) anticoagulated with PPACK were incubated with appropriate antibodies and their isotype matched controls for 20 min at room temperature. To evaluate platelet–monocyte aggregates and CD40 on monocytes, samples were fixed and red cells lysed by the addition of 500 μL of FACS-Lyse solution. To evaluate platelet surface P-selectin and CD40 ligand, samples were fixed with 1% paraformaldehyde. Samples were analysed using a Coulter EPICS XL flow cytometer equipped with a 488 nm wavelength laser (Beckman Coulter, High Wycombe, UK) within 6 h of labelling. Monocytes and platelets were identified by gating for CD14 and CD42a positive cells, respectively. Platelet–monocyte aggregates were defined as monocytes positive for CD42a. Analyses were performed using EXPO 32 software (Beckman Coulter).
Plasma fatty acid analysis
The fatty acid composition of plasma phospholipids was determined from blood anticoagulated with EDTA. Total lipids were recovered from 500 μL of plasma using dichloromethane–methanol (2:1) containing 0.005% butyrated hydroxytoluene as an antioxidant (Folch extraction). Phospholipids were isolated by solid phase extraction using aminopropyl silica columns (IST International), and fatty acids converted into methyl esters by transmethylation with 0.5 M sodium methoxide. Fatty acid methyl ester analysis was performed with an HP-INNOWAX capillary column (Agilent Technologies). Peaks were identified by comparison of retention times with known fatty acid methyl ester standards and quantified using an internal standard. Plasma total phospholipid fatty acids were expressed as the individual fractions of fatty acids and fatty acid groups as relative values (% of total fatty acids). The mean coefficient of variation for the assay was 2.4%.
Vascular studies
Studies were conducted in a quiet temperature controlled room (22–25°C). Participants fasted for 6 h prior to the study and avoided caffeine and alcohol for the preceding 24 h. Blood pressure and heart rate were recorded throughout the study using a semi-automated non-invasive oscillometric sphygmomanometer (OMRON 705 IT, Kyoto, Japan).
All participants underwent brachial artery cannulation with a 27-standard wire gauge steel needle under controlled conditions. After a 30-min baseline saline infusion, acetylcholine at 5, 10 and 20 µg/min (endothelium-dependent vasodilator that does not release t-PA; Merck Biosciences), substance P at 2, 4 and 8 pmol/min (endothelium-dependent vasodilator that releases t-PA; Clinalfa, Switzerland) and sodium nitroprusside at 2, 4 and 8 µg/min (endothelium-independent vasodilator that does not release t-PA; David Bull Laboratories) were infused for 6 min at each dose. The three vasodilators were separated by 20-min saline infusions and given in a randomised order.
FBF was measured in infused and non-infused arms by venous occlusion plethysmography with mercury-in-silicone elastomer strain gauges as described previously.19 Venous cannulas (17-gauge) were inserted into large subcutaneous veins of the antecubital fossae of both arms. Blood (10 mL) was withdrawn simultaneously from each arm at baseline and during infusion of each dose of substance P and collected into acidified buffered citrate (Stabilyte tubes, Biopool International; for t-PA assays) and into citrate (BD Vacutainer; for PAI-1 assays). Samples were kept on ice before being centrifuged at 2000g for 30 min at 4°C. Platelet-free plasma was decanted and stored at −80°C before assay. Plasma t-PA antigen and activity (t-PA Combi Actibind Elisa Kit; Technoclone, Vienna, Austria) and PAI-1 antigen and activity (Elitest PAI-1 Antigen and Zymutest PAI-1 Activity; Hyphen Biomed, Neuville-Sur-Oise, France) concentrations were determined by ELISAs. Haematocrit was determined by capillary tube centrifugation at baseline.
Data analysis and statistical methods
Continuous variables are reported as mean±SE of the mean. The pre-specified primary endpoint was endothelial vasomotor and fibrinolytic function. The sample size of n=12 was based on power calculations derived from previous studies giving 90% power to detect a 17% difference in the mean t-PA release at a significance level of 5%.19 The pre-specified secondary endpoint was platelet and monocyte activation. The sample size of n=20 was based on power calculations derived from previous studies, giving 90% power to detect a 5% difference in mean platelet–monocyte aggregation at a significance level of 5%. Forearm plethysmographic data were analysed as described previously.17 Estimated net release of plasma t-PA has been defined previously as the product of the infused forearm plasma flow (based on the mean haematocrit and the infused FBF) and the concentration difference between the infused and non-infused arms.19 Statistical analyses were performed using one-way and two-way ANOVA with Bonferroni's post-tests for multiple comparisons where appropriate. The statistical methods for each analysis are detailed in the relevant figure and table legends. All calculations were performed using GraphPad Prism (Graph Pad Software).
Results
Baseline characteristics
Participant flow through the study including a CONSORT diagram is included in the online supplementary file. Patients were relatively young and well treated in terms of blood pressure control and lipid profile (table 1). The mean and median times from myocardial infarction were 12 and 16 months, respectively. Patients were on standard medical therapy including aspirin, β-blockers, statins and ACE-inhibitors, and over half had undergone revascularisation post-MI.
Table 1.
Baseline characteristics.
| Age, years | 53±3 |
| Body mass index, kg/m2 | 28±1 |
| Systolic blood pressure, mm Hg | 137±5 |
| Diastolic blood pressure, mm Hg | 78±3 |
| Heart rate, bpm | 60±2 |
| Total cholesterol, mmol/L | 4.2±0.2 |
| LDL cholesterol, mmol/L | 2.3±0.2 |
| HDL cholesterol, mmol/L | 1.1±0.1 |
| Chol:HDL chol ratio | 3.8±0.2 |
| Triacylglycerol, mmol/L | 1.6±0.2 |
| Fasting glucose, mmol/L | 5.4±0.1 |
| Time from MI, months | 16±4 |
| Revascularisation post-MI, % | 56 |
| Current or ex-smoker, % | 61 |
| Hypertension, % | 11 |
| Diabetes mellitus, % | 0 |
| Hyperlipidemia, % | 78 |
| Family history of premature coronary heart disease, % | 33 |
| Medical therapy | |
| Aspirin, % | 100 |
| Clopidogrel, % | 11 |
| ACE-inhibitor/ angiotensin-receptor blocker, % | 56 |
| β-Blocker, % | 78 |
| Statin, % | 100 |
HDL, high-density lipoprotein; LDL, low-density lipoprotein; MI, myocardial infarction; Mean±SEM.
Effect of ω-3 fatty acid supplementation on plasma phospholipid fatty acid composition
Dietary supplementation with ω-3 fatty acids led to a marked increase in EPA as a percentage of plasma phospholipids compared with both baseline (3.7±0.4% vs 2.0±0.2%, p<0.0001) and placebo (3.7±0.4% vs 1.7±0.1%, p<0.0001; table 2). There was also an increase in DHA compared with baseline (5.6±0.2% vs 4.8±0.3%, p<0.01) and placebo (5.6±0.2% vs 4.4±0.3%, p<0.0001; table 2). We did not detect any carryover of EPA or DHA after 6 weeks of placebo in the group who had ω-3 fatty acids first (data not shown). There was a reduction in the plasma phospholipid percentage of arachidonic acid, but no effect on α-linolenic acid, linoleic acid, palmitic acid, stearic acid or oleic acid with either ω-3 fatty acid supplements or olive oil placebo (table 2).
Table 2.
Effect of ω-3 fatty acid supplementation on plasma phospholipid fatty acid composition.
| Baseline | ω-3 | Placebo | p Value | |
|---|---|---|---|---|
| EPA | 2.0±0.2 | 3.7±0.4 | 1.7±0.1 | p<0.0001 |
| DHA | 4.8±0.3 | 5.6±0.2 | 4.4±0.3 | p<0.0001 |
| α-Linolenic acid | 0.3±0.01 | 0.3±0.02 | 0.3±0.03 | 0.3 |
| Arachidonic acid | 12.5±0.4 | 11.0±0.3 | 11.6±0.5 | 0.0005 |
| Linoleic acid | 18.8±0.6 | 19.0±0.6 | 20.0±0.6 | 0.1 |
| Palmitic acid | 28.2±0.4 | 27.9±0.3 | 28.2±0.3 | 0.6 |
| Stearic acid | 13.8±0.3 | 14.1±0.2 | 13.9±0.2 | 0.4 |
| Oleic acid | 13.3±0.5 | 13.0±0.4 | 13.8±0.5 | 0.1 |
Mean±SEM. Data analysed using one-way ANOVA. p Values in the table are for the difference between the three means. p Values for individual comparisons are below.
EPA: baseline versus ω-3, p<0.0001; baseline versus placebo, p=NS; ω-3 versus placebo, p<0.0001.
DHA: baseline versus ω-3, p<0.01; baseline versus placebo, p=NS; ω-3 versus placebo, p<0.0001.
Arachidonic acid: baseline versus ω-3, p<0.001; baseline versus placebo, p=0.05; ω-3 versus placebo, p=NS.
EPA, Eicosapentaenoic acid; DHA, Docosahexaenoic acid.
Effect of ω-3 fatty acid supplementation on lipid profile
Supplementation for 6 weeks with ω-3 fatty acids did not affect total cholesterol, low-density liproprotein cholesterol, high-density liproprotein cholesterol or triglycerides (data not shown).
Effect of ω-3 fatty acid supplementation on vasomotor function
ω-3 Fatty acid supplementation did not have any effect on systolic blood pressure, diastolic blood pressure or heart rate compared with placebo (data not shown). During forearm vascular studies substance P, acetylcholine and sodium nitroprusside led to a dose-dependent increase in absolute FBF (p<0.0001 for all). Compared with placebo, ω-3 fatty acid supplementation did not affect endothelium-dependent vasodilation in response to acetylcholine or substance P (p=0.5 and 0.9; figure 1), or endothelium-independent vasodilation with sodium nitroprusside (p=0.9; figure 1).
Figure 1.
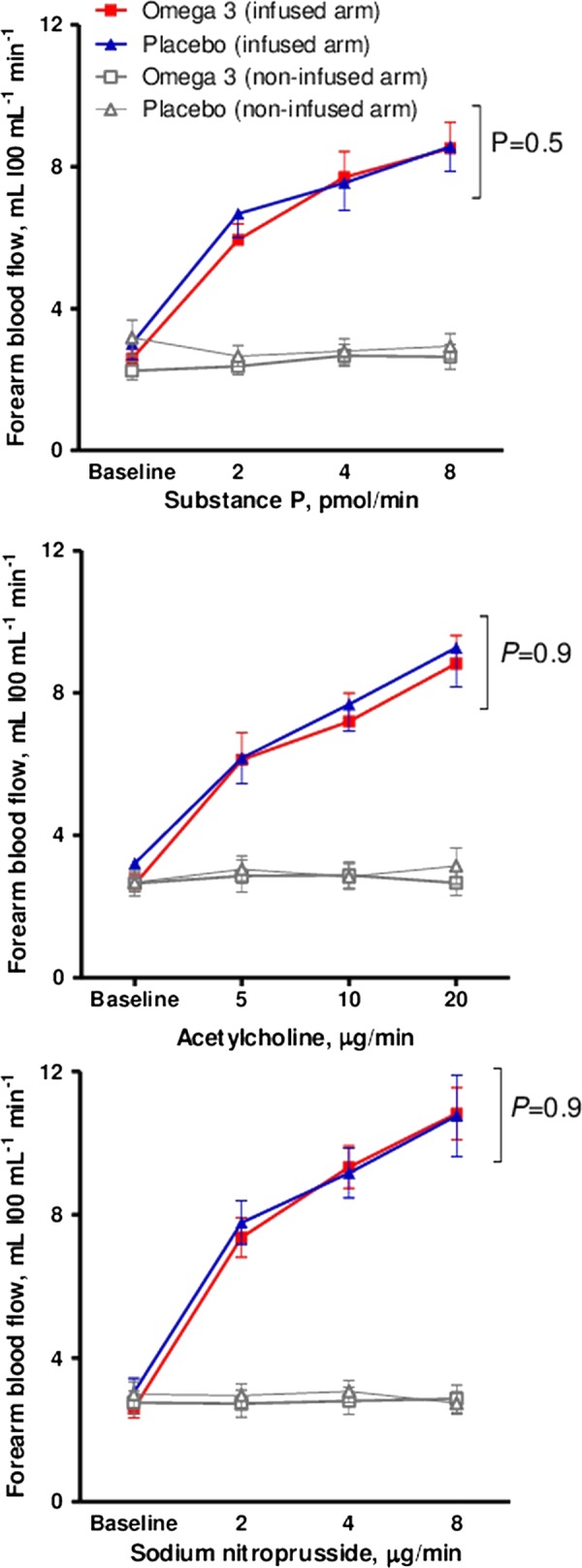
Effect of ω-3 fatty acid supplementation on absolute forearm blood flow in response to endothelium-dependent and endothelium-independent vasodilators. Statistical analyses two-way analysis of variance and Bonferroni's post-tests for multiple comparisons.
Effect of ω-3 fatty acid supplementation on stimulated t-PA activity
Substance P infusion caused a dose-dependent increase in plasma t-PA activity concentrations after both ω-3 fatty acid supplementation and placebo (p<0.0001; table 3). ω-3 Fatty acid supplementation did not affect plasma TPA activity, TPA antigen or PAI-1 concentrations compared to placebo (table 3). There was no difference in net release of t-PA activity after ω-3 fatty acid supplementation compared to placebo (p=0.94; figure 2).
Table 3.
Effect of ω-3 fatty acid supplementation on plasma t-PA activity concentrations.
| Substance P, pmol/min | ω-3 Fatty acids |
Placebo |
||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 8 | 0 | 2 | 4 | 8 | |
| t-PA activity, IU mL−1 | ||||||||
| Non-infused arm | 0.39±0.08 | 0.45±0.09 | 0.54±0.12 | 0.64±0.14 | 0.45±0.07 | 0.52±0.08 | 0.60±0.09 | 0.65±0.11 |
| Infused arm | 0.38±0.08 | 0.83±0.16 | 1.12±0.23 | 1.67±0.38 | 0.43±0.07 | 0.78±0.10 | 1.09±0.11 | 1.26±0.15 |
| t-PA antigen, ng mL−1 | ||||||||
| Non-infused arm | 11.78±1.29 | 12.01±1.0 | 12.69±1.08 | 12.83±1.49 | 13.45±1.40 | 12.93±1.70 | 13.08±1.80 | 12.37±1.27 |
| Infused arm | 11.90±1.45 | 13.98±1.33 | 13.63±1.12 | 14.86±1.40 | 12.55±1.10 | 12.85±1.44 | 13.45±1.35 | 13.97±1.55 |
| PAI-1 activity, ng mL−1 | ||||||||
| Non-infused arm | 1.77±0.53 | 1.84±0.43 | 1.80±0.42 | 1.64±0.45 | 1.44±0.29 | 1.38±0.26 | 1.39±0.47 | 1.34±0.44 |
| Infused arm | 2.33±0.86 | 2.18±0.61 | 2.21±0.69 | 1.92±0.63 | 1.69±0.46 | 1.64±0.41 | 1.54±0.39 | 1.49±0.39 |
| PAI-1 antigen, ng mL−1 | ||||||||
| Non-infused arm | 39.51±9.22 | 40.84±7.08 | 39.99±6.62 | 38.48±5.79 | 45.06±7.09 | 43.33±6.45 | 44.41±6.67 | 44.26±7.03 |
| Infused arm | 37.64±8.36 | 38.83±6.25 | 41.71±5.74 | 40.26±7.32 | 48.89±8.25 | 42.65±6.59 | 43.12±6.60 | 40.61±6.46 |
| Net t-PA antigen release, ng 100 mL−1 of tissue mm−1 | 0.23±0.51 | −0.28±4.7 | 3.92±1.8 | 8.41±2.94 | −0.87±1.1 | −0.84±2.82 | 0.94±4.22 | 8.10±3.67 |
Mean±SEM. Data analysed using two-way analysis of variance.
Tissue plasminogen activator (t-PA) activity: dose response p<0.0001. ω-3 Fatty acids versus placebo; p=0.83 (infused arm).
t-PA antigen: dose response p=0.7. ω-3 Fatty acids versus placebo; p=0.60 (infused arm).
Plasminogen-activator inhibitor type 1 (PAI-1) activity: dose response p=0.94. ω-3 Fatty acids versus placebo; p=0.17 (infused arm).
PAI-1 antigen: dose response p=0.67. ω-3 Fatty acids versus placebo; p=0.40 (infused arm).
Net t-PA antigen: dose response p=0.02. ω-3 Fatty acids versus placebo; p=0.62 (infused arm).
Figure 2.
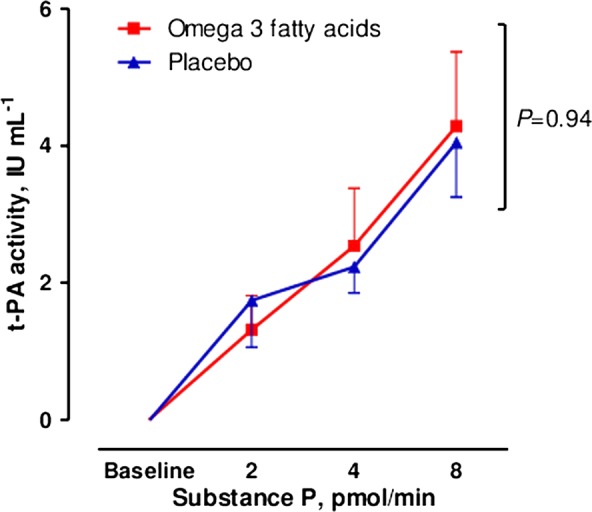
Net release of plasma tissue plasminogen activator activity with ω-3 fatty acid supplementation and placebo. Statistical analyses two-way analysis of variance and Bonferroni's post-tests for multiple comparisons.
Effect of ω-3 fatty acid supplementation on platelet–monocyte aggregation and CD40/CD40 ligand
Supplementation with ω-3 fatty acids did not have any effect on platelet–monocyte aggregation, platelet–neutrophil aggregation, platelet surface expression of P-selectin or CD40L, or monocyte expression of CD40 (data not shown).
Discussion
The current study has demonstrated that dietary supplementation with ω-3 fatty acids does not affect endothelial vasomotor function or endothelial t-PA release in patients with coronary heart disease. There is also no effect on markers of platelet or monocyte activation. These findings suggest that any cardiac benefits conferred by ω-3 fatty acids in coronary heart disease are unlikely to be mediated through effects on endothelial function, endogenous fibrinolysis or platelet activation.
We do not believe the lack of effect on outcome measures in the current study is likely to have been due to poor compliance. The assessment of plasma phospholipid fatty acid composition confirmed substantial increases in the percentage of both EPA and DHA during supplementation with ω-3 fatty acids. The dose and duration of therapy with ω-3 fatty acids are also likely to have been appropriate. We used 2 g/day of ω-3 fatty acids which is similar to the amount shown to reduce mortality in secondary prevention trials.2 3 Although we cannot exclude an effect with a longer duration of therapy, 6 weeks of supplementation caused a large increase in the plasma phospholipid content of ω-3 fatty acids and has previously been long enough to demonstrate clear effects on vascular function and platelet activation.20–22
ω-3 Fatty acids have previously been shown to have inconsistent effects on endothelial function. Although some studies have reported beneficial effects in a variety of populations including healthy volunteers,22 patients with hyperlipidaemia,21 23 diabetes mellitus24 and heart failure,25 others have not found any improvement.26–28 Our findings are in contrast to previous studies in coronary heart disease which demonstrated an improvement in endothelial function with ω-3 fatty acids.20 29 30 These discrepancies could be partly due to differences in study populations or concomitant medication. However, previous studies were all either not randomised or double-blinded, and lacked a control group or placebo. Indeed, our trial is the first double-blinded, placebo-controlled trial investigating the effect of ω-3 fatty acids on endothelial vasomotor function in coronary heart disease; we therefore believe that our study design and findings are likely to be robust.
We also found that ω-3 fatty acids did not augment endogenous fibrinolysis in coronary heart disease. Previous results have varied widely and it has been concluded that ω-3 fatty acids are unlikely to influence the fibrinolytic system.31 While some studies have reported a beneficial impact on fibrinolytic parameters,32 33 others have found an adverse effect34 or no effect.26 35–37 However, previous studies have only measured basal plasma t-PA concentrations that do not reflect the local capacity for acute endothelial t-PA release.9 38 It is the rapid endogenous release of t-PA from the endothelium which regulates the dissolution of thrombus and is of greater pathophysiological relevance. We used an established model of acute endothelial t-PA release that predicts cardiovascular outcome,19 39 but found no effect of ω-3 fatty acid supplementation on acute endogenous fibrinolytic capacity in coronary heart disease.
There are several possible explanations for the lack of effect of ω-3 fatty acids on endothelial function and endogenous fibrinolysis observed in the present coronary heart disease population. The patients were all well treated with modern cardio-active medication known to influence endothelial vasomotor function.40 41 In contrast, patients in previous studies demonstrating improved endothelial function20 29 and cardiac outcomes2 3 with ω-3 fatty acids were much less likely to be taking HMG CoA reductase inhibitors or ACE inhibitors. It is conceivable that endothelial function cannot be further improved by the addition of ω-3 fatty acids in coronary heart disease patients already treated with modern medical therapy. This possibility is supported by the most recent large clinical trials which found a low rate of cardiac events in patients on optimal medical therapy post-myocardial infarction, which could not be improved with ω-3 fatty acid supplementation.42–44
However, concomitant medication may not fully explain the neutral effects on endogenous fibrinolysis. While lipid-lowering therapy improves endothelial vasomotor function, it has not been found to influence acute t-PA release.45 ACE inhibitors only augment bradykinin induced t-PA release; they do not affect t-PA release stimulated by substance P.46 Therefore, there may be other factors that explain why ω-3 fatty acid supplementation can improve endogenous fibrinolytic capacity in healthy cigarette smokers but not in patients with coronary heart disease. Perhaps, the most likely explanation is that the coronary heart disease group was considerably older and may have a dysfunctional endothelium and fibrinolytic system less responsive to dietary interventional measures.
Circulating platelet–monocyte aggregates are increased in stable coronary heart disease and acute coronary syndromes, consistent with an important role in both the development of atherosclerotic lesions and in acute thrombosis.15 We have previously demonstrated that moderate intake of oil-rich fish can significantly reduce platelet–monocyte aggregation.18 However, we did not observe any effect of ω-3 fatty acid supplements on these measures of platelet and monocyte activation in the current study. It is possible that our previous results were due to another active ingredient in oily fish rather than ω-3 fatty acids, and we cannot exclude a dose–effect of ω-3 fatty acids on platelet activation. ω-3 Fatty acids also had no effect on monocyte expression of CD40 or platelet surface CD40 ligand, consistent with previous studies which found no effect of either ω-3 fatty acids or dietary fish on soluble CD40 ligand.18 47
Our study has potential limitations that should be acknowledged. First, the sample size is relatively small which raises the possibility of a type II error due to lack of power. However, the sample size was based on separate power calculations for the vascular function and the platelet monocyte studies, and we have previously detected modest changes in these outcome measures with similar sample sizes.17 18 Although it is possible, we lacked power to detect very small changes; we believe the study had sufficient power to detect any clinically relevant effects of ω-3 fatty acids. Second, as we did not measure fatty acids at the beginning of the second treatment stage we cannot fully exclude the possibility of some carryover of EPA or DHA into the early placebo phase in the group receiving ω-3 fatty acids first. However, we feel any such effect would be modest and unlikely to alter the study outcomes.
Conclusions
We have demonstrated that ω-3 fatty acid supplementation does not affect endothelial function, endogenous fibrinolytic capacity or markers of platelet and monocyte activation in patients with stable coronary heart disease. A major strength of our study is the use of a robust model to simultaneously assess endothelial vasomotor tone as well as endogenous fibrinolysis: two important and complementary measures of vascular function. Our results suggest that any potential cardiac benefits conferred by ω-3 fatty acids in this patient group are unlikely to be mediated by effects on endothelial function or the fibrinolytic system.
Supplementary Material
Acknowledgments
We are grateful to the Clinical Research Facility at the Royal Infirmary of Edinburgh.
Footnotes
Contributors: JD, KL, SH, JS, AF and DN were involved in conception and design or analysis and interpretation of data. JD, JS, AF and DN were responsible for drafting of the article or revising it critically for intellectual content. All authors were involved in the final approval of the article to be submitted.
Funding: JD was supported by a British Heart Foundation Project Grant (PG/2003/009). DEN is supported by the British Heart Foundation. The Wellcome Trust Clinical Research Facility is supported by NHS Research Scotland (NRS) through NHS Lothian.
Competing interests: None.
Ethics approval: Lothian Regional Ethics Committee. The study was undertaken with the approval of the local research ethics committee and in accordance with the Declaration of Helsinki.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Din JN, Newby DE, Flapan AD. Omega 3 fatty acids and cardiovascular disease—fishing for a natural treatment. BMJ 2004;328:30–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burr ML, Fehily AM, Gilbert JF, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 1989;2:757–61 [DOI] [PubMed] [Google Scholar]
- 3.GISSI-Prevenzione Investigators Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet 1999;354:447–55 [PubMed] [Google Scholar]
- 4.Yokoyama M, Origasa H, Matsuzaki M, et al. Japan EPA lipid intervention study (JELIS) Investigators. Lancet 2007;369:1090–817398308 [Google Scholar]
- 5.Marchioli R, Barzi F, Bomba E, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation 2002;105:1897–903 [DOI] [PubMed] [Google Scholar]
- 6.Leaf A, Kang JX, Xiao YF, et al. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation 2003;107:2646–52 [DOI] [PubMed] [Google Scholar]
- 7.Brouwer IA, Raitt MH, Dullemeijer C, et al. Effect of fish oil on ventricular tachyarrhythmia in three studies in patients with implantable cardioverter defibrillators. Eur Heart J 2009;30:820–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowker TJ, Wood DA, Davies MJ, et al. Sudden, unexpected cardiac or unexplained death in England: a national survey. QJM 2003;96:269–79 [DOI] [PubMed] [Google Scholar]
- 9.Oliver JJ, Webb DJ, Newby DE. Stimulated tissue plasminogen activator release as a marker of endothelial function in humans. Arterioscler Thromb Vasc Biol 2005;25:2470–9 [DOI] [PubMed] [Google Scholar]
- 10.Fox KA, Robison AK, Knobb RM, et al. Prevention of coronary thrombosis with subthrombolytic doses of tissue type plasminogen activator. Circulation 1985;72:1346–54 [DOI] [PubMed] [Google Scholar]
- 11.Jungi TW, Spycher MO, Nydegger UE, et al. Platelet-leucocyte interaction: selective binding of thrombin-stimulated platelets to human monocytes, polymorphonuclear leucocytes, and related cell lines. Blood 1986;67:629–36 [PubMed] [Google Scholar]
- 12.Schonbeck U, Lippy P. CD40 signaling and plaque instability. Circ Res 2001;89:1092–103 [DOI] [PubMed] [Google Scholar]
- 13.Huo Y, Schober A, Forlow SB, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med 2003;9:61–7 [DOI] [PubMed] [Google Scholar]
- 14.Zeiher AM, Drexler H, Wollschlager H, et al. Modulation of coronary vasomotor tone in humans: progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation 1991;83:391–401 [DOI] [PubMed] [Google Scholar]
- 15.Sarma J, Laan CA, Alam S, et al. Increased platelet binding to circulating monocytes in acute coronary syndromes. Circulation 2002;105:2166–71 [DOI] [PubMed] [Google Scholar]
- 16.Tousoulis D, Antoniades C, Nikolopoulou A, et al. Interaction between cytokines and sCD40 L in patients with stable and unstable coronary syndromes. Eur J Clin Invest 2007;37:623–8 [DOI] [PubMed] [Google Scholar]
- 17.Din JN, Archer RM, Harding SA, et al. Effect of ω-3 fatty acid supplementation on endothelial function, endogenous fibrinolysis and platelet activation in male cigarette smokers. Heart 2013;99:168–74 [DOI] [PubMed] [Google Scholar]
- 18.Din JN, Harding SA, Valerio CJ, et al. Dietary intervention with oil rich fish reduces platelet-monocyte aggregation in man. Atherosclerosis 2008;197:290–6 [DOI] [PubMed] [Google Scholar]
- 19.Newby DE, Wright RA, Ludlam CA, et al. An in vivo model for the assessment of acute fibrinolytic capacity of the endothelium. Thromb Haemost 1997;78:1242–8 [PubMed] [Google Scholar]
- 20.Tagawa H, Shimokawa H, Tagawa T, et al. Long-term treatment with eicosapentaenoic acid augments both nitric oxide-mediated and non-nitric oxide-mediated endothelium-dependent forearm vasodilation in patients with coronary artery disease. J Cardiovasc Pharmacol 1999;33:633–40 [DOI] [PubMed] [Google Scholar]
- 21.Mori TA, Watts GF, Burke V, et al. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation 2000;102:1264–9 [DOI] [PubMed] [Google Scholar]
- 22.Chin JP, Gust AP, Nestel PJ, et al. Marine oils dose-dependently inhibit vasoconstriction of forearm resistance vessels in humans. Hypertension 1993;21:22–8 [DOI] [PubMed] [Google Scholar]
- 23.Goodfellow J, Bellamy MF, Ramsey MW, et al. Dietary supplementation with marine omega-3 fatty acids improve systemic large artery endothelial function in subjects with hypercholesterolemia. J Am Coll Cardiol 2000;35:265–70 [DOI] [PubMed] [Google Scholar]
- 24.McVeigh GE, Brennan GM, Johnston GD, et al. Dietary fish oil augments nitric oxide production or release in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1993;36:33–8 [DOI] [PubMed] [Google Scholar]
- 25.Morgan DR, Dixon LJ, Hanratty CG, et al. Effects of dietary omega-3 fatty acid supplementation on endothelium-dependent vasodilation in patients with chronic heart failure. Am J Cardiol 2006;97:547–51 [DOI] [PubMed] [Google Scholar]
- 26.Woodman RJ, Mori TA, Burke V, et al. Effects of purified eicosapentaenoic acid and docosahexaenoic acid on platelet, fibrinolytic and vascular function in hypertensive type 2 diabetic patients. Atherosclerosis 2003;166:85–93 [DOI] [PubMed] [Google Scholar]
- 27.Wong CY, Yiu KH, Li SW, et al. Fish-oil supplement has neutral effects on vascular and metabolic function but improves renal function in patients with Type 2 diabetes mellitus. Diabet Med 2010;27:54–60 [DOI] [PubMed] [Google Scholar]
- 28.Skulas-Ray AC, Kris-Etherton PM, Harris WS, et al. Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr 2011;93:243–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tagawa T, Hirooka Y, Shimokawa H, et al. Long-term treatment with eicosapentaenoic acid improves exercise-induced vasodilation in patients with coronary artery disease. Hypertens Res 2002;25:823–9 [DOI] [PubMed] [Google Scholar]
- 30.Haberka M, Mizia-Stec K, Mizia M, et al. N-3 polyunsaturated fatty acids early supplementation improves ultrasound indices of endothelial function, but not through NO inhibitors in patients with acute myocardial infarction: N-3 PUFA supplementation in acute myocardial infarction. Clin Nutr 2011;30:79–85 [DOI] [PubMed] [Google Scholar]
- 31.Kristensen SD, Iversen AM, Schmidt EB. n-3 Polyunsaturated fatty acids and coronary thrombosis. Lipids 2001;36(Suppl):S79–82 [DOI] [PubMed] [Google Scholar]
- 32.Smith P, Arnesen H, Opstad T, et al. Influence of highly concentrated n-3 fatty acids on serum lipids and hemostatic variables in survivors of myocardial infarction receiving either oral anticoagulants or matching placebo. Thromb Res 1989;53:467–74 [DOI] [PubMed] [Google Scholar]
- 33.Mehta J, Lawson D, Saldeen TJ. Reduction in plasminogen activator inhibitor-1 (PAI-1) with omega-3 polyunsaturated fatty acid (PUFA) intake. Am Heart J 1988;116(5 Pt 1):1201–6 [DOI] [PubMed] [Google Scholar]
- 34.Spannagl M, Drummer C, Fröschl H, et al. Plasmatic factors of haemostasis remain essentially unchanged except for PAI activity during n-3 fatty acid intake in type I diabetes mellitus. Blood Coagul Fibrinolysis 1991;2:259–65 [DOI] [PubMed] [Google Scholar]
- 35.Finnegan YE, Howarth D, Minihane AM, et al. Plant and marine derived (n-3) polyunsaturated fatty acids do not affect blood coagulation and fibrinolytic factors in moderately hyperlipidemic humans. J Nutr 2003;133:2210–13 [DOI] [PubMed] [Google Scholar]
- 36.Hellsten G, Boman K, Saarem K, et al. Effects on fibrinolytic activity of corn oil and a fish oil preparation enriched with omega-3-polyunsaturated fatty acids in a long-term study. Curr Med Res Opin 1993;13:133–9 [DOI] [PubMed] [Google Scholar]
- 37.Toft I, Bønaa KH, Ingebretsen OC, et al. Fibrinolytic function after dietary supplementation with omega3 polyunsaturated fatty acids. Arterioscler Thromb Vasc Biol 1997;17:814–19 [DOI] [PubMed] [Google Scholar]
- 38.Hrafnkelsdottir T, Gudnason T, Wall U, et al. Regulation of local availability of active tissue-type plasminogen activator in vivo in man. J Thromb Haemost 2004;2:1960–8 [DOI] [PubMed] [Google Scholar]
- 39.Robinson SD, Ludlam CA, Boon NA, et al. Endothelial fibrinolytic capacity predicts future adverse cardiovascular events in patients with coronary heart disease. Arterioscler Thromb Vasc Biol 2007;27:1651–6 [DOI] [PubMed] [Google Scholar]
- 40.Treasure CB, Klein JL, Weintraub WS, et al. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med 1995;332:481–7 [DOI] [PubMed] [Google Scholar]
- 41.Mancini GB, Henry GC, Macaya C, et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease: the TREND (Trial on Reversing ENdothelial Dysfunction) study. Circulation 1996;94:258–65 [DOI] [PubMed] [Google Scholar]
- 42.Rauch B, Schiele R, Schneider S, et al. OMEGA Study Group. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 2010;122:2152–9 [DOI] [PubMed] [Google Scholar]
- 43.Galan P, Kesse-Guyot E, Czernichow S, et al. SU.FOL.OM3 Collaborative Group. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ 2010;341:c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial Group N-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med 2010;363:2015–26 [DOI] [PubMed] [Google Scholar]
- 45.Newby DE, Witherow FN, Wright RA, et al. Hypercholesterolaemia and lipid lowering treatment do not affect the acute endogenous fibrinolytic capacity in vivo. Heart 2002;87:48–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Witherow FN, Dawson P, Ludlam CA, et al. E. Marked bradykinin-induced tissue plasminogen activator release in patients with heart failure maintained on long-term angiotensin-converting enzyme inhibitor therapy. J Am Coll Cardiol 2002;40:961–6 [DOI] [PubMed] [Google Scholar]
- 47.Aarsetoy H, Brugger-Andersen T, Hetland O, et al. Long-term influence of regular intake of high dose n-3 fatty acids on CD40-ligand, pregnancy-associated plasma protein A and matrix metalloproteinase-9 following acute myocardial infarction. Thromb Haemost 2006;95:329–36 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


