Abstract
Background
White matter hyperintensity (WMH), or leukoaraiosis, is a radiological finding generally assumed to reflect diseased small cerebral vasculature. WMH has significant functional impact through its relationship to cognitive decline and risk of ischemic and hemorrhagic stroke. Accumulating evidence suggests that some manifestations of small vessel disease such as intracerebral hemorrhage (ICH) are associated with low levels of cholesterol. We sought to determine the relationship between hyperlipidemia (HL) and WMH severity in patients with acute ischemic stroke (AIS).
Methods
We analyzed two independent hospital-based AIS cohorts. Demographic and clinical data were collected prospectively. WMH was measured using semi-automated volumetric image analysis and a semi-quantitative visual grading scale. Univariate and multivariable regression analyses were used to assess the relationship between WMH severity and study variables.
Results
A total of 631 and 504 subjects in the first and second cohorts, respectively, were included. In univariate analyses, advancing age and hypertension were associated with severity of WMH (p<0.001) in both cohorts. In the multivariable analysis, after controlling for age, gender, and those significant risk factors in the univariate and age-adjusted analyses, patients with a history of HL had less severe WMH in both cohorts (p<0.01).
Conclusions
Results from two independent cohorts demonstrate that AIS patients with a history of HL have less severe WMH at the time of stroke. These data support the hypothesis that HL may play a relatively protective role on cerebral small vessel disease.
Keywords: white matter disease, leukoaraiosis, hyperlipidemia, risk factors
INTRODUCTION
White matter hyperintensity (WMH), also known as leukoaraiosis, is frequently detected by MRI in the aging brain 1. Its presence and severity have substantial clinical impact through associations with cognitive decline 2, dementia 3, deterioration in gait 4 and increased risk of stroke (both ischemic and hemorrhagic) 5,6. Furthermore, WMH appears to play an important role in the brain’s response to acute ischemia, as increasing severity of WMH predicts infarct progression and poor clinical outcome after acute ischemic stroke (AIS)7,8.
Initial studies have linked a variety of traditional cardiovascular risk factors to WMH, raising the hypothesis that it is primarily a result of processes similar to those that give rise to coronary atherosclerosis. These risk factors have included, in addition to the presence of coronary disease, presence of hypertension, cigarette smoking 9,10, elevated homocysteine levels 11 and chronic kidney disease 12.
However, it has long been noted that diseases of the small caliber vessels of the cerebrovasculature may differ markedly in pathogenesis from those of the larger cerebral vessels commonly affected by atherosclerosis 13. Indeed, hyperlipidemia (HL), although related to increased risk of ischemic stroke generally 14, appears to have a limited role in intracranial hemorrhage (ICH) due to small vessel disease. Accumulating evidence suggests, in fact, that it is low, rather than high levels of circulating cholesterol, that may contribute to risk of primary ICH 15 and to a higher mortality rate in these patients 16.
Given the presumed overlap in biology between ICH and WMH 13, we sought to investigate whether HL is associated with severity of WMH. Reasoning that WMH in patients with AIS was more likely to be related to cerebral small vessel disease, and that they represent a subset of individuals where WMH has substantial clinical relevance, we used two independent cohorts of AIS to investigate this relationship.
SUBJECTS AND METHODS
Study Population
The study included two independent cohorts of AIS patients from different institutions, independently collected and analyzed retrospectively.
Common inclusion criteria were: 1) neuroimaging-confirmed AIS, 2) availability of an MRI in the first 7 days, 3) complete data on vascular risk factors (VRF) available, and 4) absence of ICH or non-vascular diagnosed diseases that could interfere with the WMH interpretation, including neoplasms, demyelinating and autoimmune diseases, and vasculitides. All patients were assessed and classified by a neurologist.
Massachusetts General Hospital (MGH) cohort
All patients with AIS consecutively assessed in the Emergency Department (ED) of MGH (Boston, USA) who provided informed consent were considered for inclusion. The subjects were recruited from 2003–2008 as part of an ongoing prospective hospital-based study. A cohort-specific inclusion criterion was a T2 fluid-attenuated inversion recovery (FLAIR) axial MRI sequence available within the same study and in usable format for volumetric analysis of WMH.
Hospital del Mar (HM) cohort
Consecutive consenting patients from 2005–2008 with a diagnosis of AIS fulfilling the World Health Organization criteria 17 were considered for inclusion. The subjects were enrolled in BasicMar (Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III; FIS Number PI051737) 18 an ongoing prospective registry of AIS at the IMIM-Hospital Universitari del Mar (Barcelona, Spain).
Baseline characteristics
In both cohorts age, gender and VRF were recorded for every subject. Data were abstracted directly via patient, and/or proxy interview, and medical chart review. VRF were coded following the definitions of international guidelines 19 as follows: arterial hypertension (HTN; evidence of at least 2 raised blood pressure measurements, systolic >140 mm Hg or diastolic >90 mm Hg recorded on different days before stroke onset, a physician diagnosis, or use of an anti-hypertensive medication), diabetes (DM; a physician diagnosis or use of diabetes medication), hyperlipidemia (HL; physician diagnosis, a previous to stroke recorded serum cholesterol concentration >220 mg/dL, or serum triglyceride concentration >150 mg/dL, and use of medication prescribed to control HL. Statin medication that was prescribed for an indication other than HL did not qualify patients for the diagnosis of HL), coronary artery disease (CAD; documented history of angina pectoris or myocardial infarction), atrial fibrillation (AF; documented history or diagnosis during hospitalization), prior stroke (history of prior ischemic stroke). Alcohol and smoking habits were also recorded. In the MGH cohort the threshold for alcohol abuse was “current alcohol intake >3 oz/day” and for smoking was “current smoker or quit smoking < 7 years ago”. In the HM cohort alcohol abuse was considered with a “current or <1 year ago alcohol intake higher than 60gr /day” 20 and smoking habit was considered in “current smokers or quit < 5 years ago”. For the purpose of the study, we focused exclusively on those risk factors collected in a comparable manner in both cohorts.
Image Acquisition and Analysis
Brain MRI studies were performed using 1.5-T whole body scanner (GE Signa Excite II; GE Medical Systems) in both institutions on all subjects. Hyperintense foci in DWI and chronic-infarction lesions (reported or detected in T2) were excluded from the quantification.
MGH cohort
WMH volumetric analysis was performed on FLAIR sequences using a previously published semi-automated method 21,22. All raters displayed high inter-rater reliability for determination of WMH volume compared to “gold standard” WMH volumes derived from scans analyzed in previous publications (intra-class correlation coefficient >0.92) 21. To account for differences in head size, WMH volumes were standardized by comparing the subject’s midline sagittal intracranial cross-sectional area 22, to the study average midline sagittal intracranial cross-sectional area, as in previous studies.
HM cohort
WMH was evaluated in FLAIR sequences using a semi-quantitative visual grading method and classified by a neurologist according to the Fazekas scale23. Leukoaraiosis (LA) scores were distributed in 4 groups (LA0: absence of WMH or minimal PV thin-lining, LA1: caps and thin-lining of PV region and punctuate deep hyperintense foci, LA2: early confluence foci or smooth halo of PV region, LA3: confluent foci or irregular hyperintensity in PV region extending into the deep white matter).
Ethical considerations
All study aspects were approved by the local Institutional Review Board/Institutional Ethic Committee for each cohort. All participants or their approved proxy provided informed consent for participation.
Statistical analysis
In the MGH cohort, the standardized WMH volume (WMHV) was natural log transformed for all linear regression analyses (log_WMHV) (Figure). Univariate analyses evaluating the relationship of log_WMHV to dichotomous baseline variables were tested by Student’s t, and to age by Pearson’s correlation. In the HM cohort, chi-square tests for the dichotomous variables and ANOVA for age were used to assess relationship with LA, an ordinal variable.Given the potent and well-established relationship between advancing age and WMH progression, all univariate analyses were also completed after adjusting for age, using linear regression and ordinal logistic regression, depending on the cohort.
Multivariable models included those variables associated with WMH (log_WMHV or LA) with a p-value <0.1 in at least one analysis (univariate or age-adjusted) in either of the two cohorts. The variables were cross tabulated to assess for multicollinearity, concluding that the model fit was adequate. The model was tested in the MGH cohort with a multiple linear regression, and with an ordinal regression in the HM cohort.
Statistical significance level set at a p-value <0.05. Tests were performed using SPSS package 13.0 for (2009 SPSS Inc., Il, USA).
RESULTS
Of all patients with confirmed AIS who consented to participate in the study (MGH n=965, HM n=835), those with other diseases that could interfere in WMH interpretation (MGH n=59;HM n=43), absence of brain MRI (MGH n=19;HM n=249), MRI formats that could not be used for volumetric analysis (MGH n=178), or lacking complete VRF information (MGH n=78;HM n=39) were excluded. A total of 631 (65.39%) patients from MGH, and 504 (60.36%) from HM cohort met all the inclusion criteria. Characteristics and demographic data are shown in Table 1. The MGH cohort was younger (p<0.05). There were non-significant differences in non-modifiable vascular risk factors except for DM (19.2% vs 33.3%). The MGH cohort also showed a higher rate of smoking habit and alcohol abuse that was not explained by the different age of the cohorts (probably due to different coding definitions between centers).
Table 1.
Characteristics and demographic data. Comparison between cohorts:
| MGH n= 631 |
HM n= 504 |
||
|---|---|---|---|
| Age, years (mean, SD) | 64.8 (15.63)* | 69.1 (12.82)* | |
| Gender (%male) | 58.3 | 61.4 | |
| Hypertension (%) | 62.3 | 67.3 | |
| Diabetes (%) | 19.2* | 33.3* | |
| Hyperlipidemia (%) | 38.7 | 39.8 | |
| Coronary artery disease (%) | 17.7 | 14.3 | |
| Atrial Fibrillation (%) | 14.1 | 17.2 | |
| Prior Stroke (%) | 15.3 | 19.2 | |
| Smoking habit (%) | 54.3* | 34.9* | |
| Alcohol abuse (%) | 55.0* | 14.9* | |
| WMHV, cc (median, IQR) | 7.99 (3.89 – 16.24) | - | |
| LA %(LA0-LA1-LA2-LA3) | - | (33.3 – 37.7 – 20.2 – 8.7) | |
WMHV: White matter hyperintensity volume; LA: Leukoaraiosis
Variables significantly different (p<0.05) between cohorts.
In our univariate analyses (Table 2), advancing age (p<0.001), HTN and smoking (p<0.05) correlated with WMH in both cohorts. There were associations that were not shared between the two samples. AF (p<0.001) and CAD (p=0.02) in the MGH cohort, and prior stroke (p=0.003) and smoking (p<0.001) in the HM cohort were also significantly associated with WMH. Hyperlipidemia (HL) was not associated in any cohort. After the age-adjustement, however, HL emerged as protective in the MGH cohort (p=0.02), and with a similar trend in HM cohort (p=0.06) (Table 3).
Table 2.
Univariate analyses for “natural logarithm of standardized white matter hyperintensity volume” (Log_WMHV) in MGH cohort and “leukoaraiosis” (LA) in HM cohort.
| MGH |
HM |
||||
|---|---|---|---|---|---|
| Median WMHv (cc)* No / Yes |
CC | p | LA Grade (LA0 - LA1 - LA2 - LA3)† |
p | |
| Age (years) | - | 0.53 | <0.001 | (60.5 – 71.1 – 75.8 – 78.7) | <0.001 |
| Gender (F) | 7.7 / 7.8 | - | 0.457 | (36.3 – 38.9 – 42.2 – 36.4) | 0.797 |
| Hypertension | 5.2 / 9.1 | - | <0.001 | (50.6 – 74.2 – 79.4 – 75.0) | <0.001 |
| Diabetes | 7.7 / 8.3 | - | 0.253 | (28.0 – 34.7 – 40.2 – 29.5) | 0.188 |
| Hyperlipidemia | 7.3 / 8.3 | - | 0.455 | (45.8 – 38.4 – 34.3 – 36.4) | 0.241 |
| Coronary Artery Disease | 7.5 / 8.8 | - | 0.027 | (11.3 – 16.8 – 15.7 – 11.4) | 0.440 |
| Atrial Fibrillation | 7.0 / 11.9 | - | <0.001 | (14.9 – 18.4 – 19.6 – 15.9) | 0.730 |
| Prior Stroke | 7.8 / 8.7 | - | 0.085 | (11.9 – 19.5 – 24.5 – 34.1) | 0.003 |
| Smoking habit | 6.9 / 8.7 | - | 0.033 | (49.4 – 31.6 – 26.5 – 13.6) | <0.001 |
| Alcohol abuse | 7.5 / 8.1 | - | 0.383 | (17.9 – 16.3 – 12.7 – 2.3) | 0.061 |
Abreviations: CC: Pearson’s correlation coefficient; LA: Leukoaraiosis F: Female
No / Yes: Median of WMHv in each group of subjects that qualify (Yes) or do not qualify (No) for the referred variable [f.e: Median WMHv in those subjects without HTN (No)= 5.2cc; in subjects with HTN (Yes)= 9.1cc] .
LA Grade: Groups of Leukoaraiosis semiquantitative scale. Age is represented with the mean of years in each subgroup. The other variables are represented with the % of individuals that qualify for this variable in each subgroup of LA.
Table 3.
Age-adjusted analyses for “natural logarithm of standardized white matter hyperintensity volume” (Log_WMHV) in MGH cohort and “leukoaraiosis” (LA) in HM cohort.
| MGH |
HM |
|||
|---|---|---|---|---|
| Beta | p | Coef. | p | |
| Age (years) | - | - | - | - |
| Gender (F) | − 0.03 | 0.344 | −0.30 | 0.090 |
| Hypertension | 0.04 | 0.264 | 0.58 | 0.002 |
| Diabetes | 0.01 | 0.725 | 0.25 | 0.151 |
| Hyperlipidemia | − 0.08 | 0.026 | −0.33 | 0.062 |
| Coronary Artery Disease | − 0.01 | 0.871 | −0.01 | 0.980 |
| Atrial Fibrillation | 0.01 | 0.752 | −0.46 | 0.044 |
| Prior Stroke | 0.05 | 0.210 | 0.65 | 0.002 |
| Smoking habit | 0.10 | 0.004 | −0.09 | 0.624 |
| Alcohol abuse | 0.06 | 0.128 | −0.17 | 0.486 |
In the multivariable analyses (Table 4) only advancing age and absence of HL were independently associated with increasing severity of WMH in both cohorts (p<0.01). HTN appeared associated with WMH severity with robust significance among HM patients (p=0.001) and with borderline significance (p = 0.06) among MGH patients. There were associations that did not replicate at all. Prior stroke and AF were independently associated only in the HM cohort but not in the MGH, whereas smoking habit showed only a risk effect in the MGH cohort.
Table 4.
Multivariate analyses for “natural logarithm of standardized white matter hyperintensity volume” (Log_WMHV) in MGH cohort and “leukoaraiosis” (LA) in HM cohort.
| MGH | HM | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Multiple Linear Regression |
Ordinal Logistic Regression |
||||||||
| B | 95% CI for B | Beta | p | OR | 95% CI | p | |||
| Low | High | Low | High | ||||||
| Age | 0.038 | 0.032 | 0.043 | 0.535 | < 0.001 | 1.095 | 1.076 | 1.115 | < 0.001 |
| Gender (M) | − 0.051 | − 0.209 | 0.106 | − 0.024 | 0.523 | 0.655 | 0.446 | 0.964 | 0.032 |
| Hypertension | 0.162 | − 0.006 | 0.331 | 0.075 | 0.059 | 1.916 | 1.302 | 2.821 | 0.001 |
| Hyperlipidemia | − 0.215 | − 0.375 | − 0.056 | − 0.101 | 0.008 | 0.619 | 0.433 | 0.885 | 0.009 |
| Coronary Artery Disease | − 0.055 | − 0.256 | 0.146 | − 0.020 | 0.591 | 0.920 | 0.561 | 1.508 | 0.741 |
| Atrial Fibrillation | 0.056 | − 0.163 | 0.276 | 0.019 | 0.614 | 0.577 | 0.366 | 0.912 | 0.018 |
| Prior Stroke | 0.148 | − 0.057 | 0.352 | 0.051 | 0.157 | 1.935 | 1.264 | 2.963 | 0.002 |
| Smoking habit | 0.204 | 0.048 | 0.360 | 0.097 | 0.011 | 0.849 | 0.556 | 1.297 | 0.449 |
| Alcohol abuse | 0.054 | − 0.100 | 0.208 | 0.026 | 0.492 | 0.758 | 0.452 | 1.271 | 0.294 |
We performed an additional analysis for previous statin treatment (ST). It was not associated with WMH in the univariate or age-adjusted analyses in either cohort. Forcing its inclusion in the multivariate model again gave a non-significant result with opposite directions of effect between cohorts (OR=1.142, p=0.183 in MGH; OR=0.678, p=0.123 in HM). Though ST did not change the HL effect direction on WMH severity, it rendered the association non-significant. ST exposure and HL also demonstrated a high degree of colinearity in both cohorts(p<0.01).
DISCUSSION
These data, from two independent cohorts, demonstrate that HL may, in fact, be associated with reduced WMH severity in individuals with AIS. This observation is consistent with prior studies demonstrating an inverse relationship between hyperlipidemia and ICH risk 15. Together they suggest that patients with a history of hyperlipidemia appear to be at reduced risk of cerebral small vessel disease.
In this study, the approach to WMH measurement differed between the cohorts. Whereas the Fazekas scale for leukoaraiosis used in HM cohort is wildly validated and well established 23, the semi-automated volumetric measurement carried out in MGH cohort may add accuracy and reliability 21,22.
HTN has been reported as a main risk factor for small vessel diseases 24 and also related to WMH 9. We found that HTN increased the risk of severe WMH in the univariate analyses, but after the age-adjustment and the multivariable analysis, it has not achieved statistical significance in the MGH cohort. This might be explained through the cohort-specific age differences, meaning that the older the individual, the longer the time of exposure to HTN and the stronger is its effect. Given that MGH individuals are younger, the effect size of HTN may be still not sufficiently strong to withstand the adjustment. That might suggest that depending on the age-distribution of the cohort the strength of HTN effect could vary.
The associations of prior stroke, AF, smoking habit or alcohol abuse with WMH burden found in one cohort were not replicated in the other. Smoking and alcohol abuse had different rates between cohorts (probably because different coding definitions between centers), and this might explain possible differences. However, this is not applicable to prior stroke and AF. Whether these discrepancies are the result of between-study variability or due to unpredictable relationships between these variables and WMH severity is unclear.
Apart from advancing-age, the only risk factor clearly related to WMH in our study is HL. This inverse association is strong, consistently significant in both cohorts and independent following multivariable adjustment. HL is widely recognized as a risk factor for stroke13,14 and lipid-lowering therapies have demonstrated benefits in stroke prevention and prognosis 25,26. However, there is also evidence of higher cholesterol levels being related to better outcomes and decreased mortality after AIS or ICH 16,27–29, and lower risk of ICH and microbleeds 15,30. While this issue was not addressed in prior studies directly, data suggests an association between higher cholesterol levels and less severe WMH31–33. Whereas hypercholesterolemia appears to impair endothelial reparative processes 34, it is also known that cholesterol play a fundamental role in the development of the central nervous system (CNS), and in the creation and maintenance of new synapses 35–37. This could explain the role of elevated cholesterol levels in better response to an acute injury such as stroke, but also in better response to a chronic cerebral injury (such as the processes involved in the WMH development).
Other possible explanations might be based in some shared genetic burden, given that both, HL and severity of WMH, have a significant heritability component 38,39. Future studies may unveil novel clues in this field.
In our study, we used the HL variable instead of the serum lipid measurement, because the time of blood draw was not equivalent between the cohorts and the lipid values might be altered by lipid-lowering treatment (and different intensities of such treatment). The history of HL may represent a more uniform way to measure an altered lipid profile between different cohorts and might have been more informative with regard to long term conditions such as WMH. However, HL is still subject to measurement heterogeneity, and should be further investigated to confirm the specific lipid levels implications, and to reveal the underlying mechanisms that might be involved in this phenomenon.
Similarly, the wide use of ST makes its effect difficult to analyze. In case of WMH burden, statins have not demonstrated any consistent role; moreover, adding that the study does not have enough power to address the interaction between HL, ST and WMH burden, especially because of its high collinearity, the inclusion of ST in the model is unwarranted. However, whether ST has any true effect on WMH severity or might partially contribute to the beneficial effect of HL is uncertain. A specifically-designed randomized controlled trial would best be suited to address this issue.
The limitations of the study relate first to its retrospective design. Second, the characteristics of the study do not permit to clearly separate the contribution of ST in HL effect. Third, though the AIS population characteristics allow to powerfully test our hypothesis, the results may warrant future study in population-based cohorts prior to reaching full generalizability. Fourth, the observed differences in baseline characteristics between the two cohorts, especially in age and rates of alcohol abuse and smoking habit, may have reduced the power to find the same results in both. And fifth, the non-harmonized WMH measurement between cohorts, with the consequent use of different statistical tests in each one may also reduce the capability of finding reproducible associations. However, these limitations would have led, if anything, to null results. This is reassuring that our observations are unlikely to be the result of biases introduced through limitations of the study design.
SUMMARY
This study describes for the first time that a history of HL independently relates to lower severity of WMH in patients with acute ischemic stroke. Together with previous studies relating HL to a lower ICH risk, our data suggest a protective role of HL in cerebral small vessel disease. Data also confirmed that advancing-age strongly correlates to WMH severity in these patients. Consistency in the results from independent cohorts reinforces these findings.
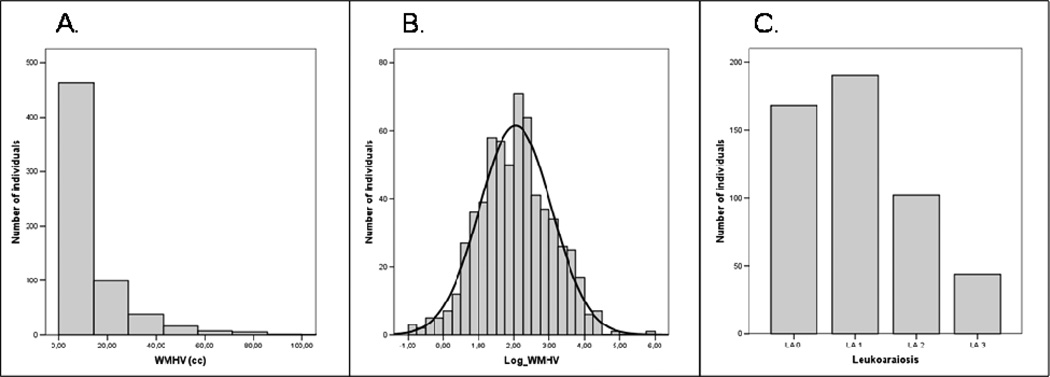
Figure 1.
Distribution of WMH: A: white matter hyperintensity volume (WMHV) in MGH cohort; B: natural logarithm of white matter hyperintensity volume (Log_WMHV) in the MGH cohort ; C: Semi-quantitative scale-based measurement of leukoaraiosis in the HM cohort.
Acknowledgements and funding
This study was funded in part by the Bugher Foundation – American Stroke Association; National Institutes of Neurological Disorders and Stroke (5P50NS051343, R01 NS04217), the Deane Institute for Integrative Study of Atrial Fibrillation and Stroke, and the Ministerio de Sanidad y Consumo de España, Instituto de Salud Carlos III with the grants: “Registro BASICMAR” Funding for Research in Health (PI051737); Contract for Research Training for Professionals with Specialty (CM06100067) and Grant from Spanish Research Networks “Red HERACLES” (RD06/ 0009).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts on Interest Disclosures:
The authors have reported no conflicts of interest.
REFERENCES
- 1.de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, Hofman A, Jolles J, van Gijn J, Breteler MM. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study The Rotterdam Scan Study. J.Neurol.Neurosurg.Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and subjective cognitive dysfunction: the Rotterdam Scan Study. Neurology. 2001;56:1539–1545. doi: 10.1212/wnl.56.11.1539. [DOI] [PubMed] [Google Scholar]
- 3.Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Cerebral white matter lesions and the risk of dementia. Arch.Neurol. 2004;61:1531–1534. doi: 10.1001/archneur.61.10.1531. [DOI] [PubMed] [Google Scholar]
- 4.Wolfson L, Wei X, Hall CB, Panzer V, Wakefield D, Benson RR, Schmidt JA, Warfield SK, Guttmann CR. Accrual of MRI white matter abnormalities in elderly with normal and impaired mobility. J.Neurol.Sci. 2005;232:23–27. doi: 10.1016/j.jns.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Kuller LH, Longstreth WT, Jr, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ., Jr Cardiovascular Health Study Collaborative Research Group White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke. 2004;35:1821–1825. doi: 10.1161/01.STR.0000132193.35955.69. [DOI] [PubMed] [Google Scholar]
- 6.Neumann-Haefelin T, Hoelig S, Berkefeld J, Fiehler J, Gass A, Humpich M, Kastrup A, Kucinski T, Lecei O, Liebeskind DS, Rother J, Rosso C, Samson Y, Saver JL, Yan B. MR Stroke Group. Leukoaraiosis is a risk factor for symptomatic intracerebral hemorrhage after thrombolysis for acute stroke. Stroke. 2006;37:2463–2466. doi: 10.1161/01.STR.0000239321.53203.ea. [DOI] [PubMed] [Google Scholar]
- 7.Ay H, Arsava EM, Rosand J, Furie KL, Singhal AB, Schaefer PW, Wu O, Gonzalez RG, Koroshetz WJ, Sorensen AG. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke. 2008;39:1409–1413. doi: 10.1161/STROKEAHA.107.501932. [DOI] [PubMed] [Google Scholar]
- 8.Arsava EM, Rosand J, Lu S, Rost NS, Smith EE, Singhal AB, Lev MH, Furie KL, Koroshetz WJ, Sorensen AG, Ay H. Severity of leukoaraiosis predicts clinical outcome after ischemic stroke. Stroke. doi: 10.1212/WNL.0b013e3181a18823. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D'Agostino RB, DeCarli C. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 10.Liao D, Cooper L, Cai J, Toole JF, Bryan NR, Hutchinson RG, Tyroler HA. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control. The ARIC Study Atherosclerosis Risk in Communities Study. Stroke. 1996;27:2262–2270. doi: 10.1161/01.str.27.12.2262. [DOI] [PubMed] [Google Scholar]
- 11.Wright CB, Paik MC, Brown TR, Stabler SP, Allen RH, Sacco RL, DeCarli C. Total homocysteine is associated with white matter hyperintensity volume: the Northern Manhattan Study. Stroke. 2005;36:1207–1211. doi: 10.1161/01.STR.0000165923.02318.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khatri M, Wright CB, Nickolas TL, Yoshita M, Paik MC, Kranwinkel G, Sacco RL, DeCarli C. Chronic kidney disease is associated with white matter hyperintensity volume: the Northern Manhattan Study (NOMAS) Stroke. 2007;38:3121–3126. doi: 10.1161/STROKEAHA.107.493593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tirschwell DL, Smith NL, Heckbert SR, Lemaitre RN, Longstreth WT, Jr, Psaty BM. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology. 2004;63:1868–1875. doi: 10.1212/01.wnl.0000144282.42222.da. [DOI] [PubMed] [Google Scholar]
- 14.Gorelick PB, Schneck M, Berglund LF, Feinberg W, Goldstone J. Status of lipids as a risk factor for stroke. Neuroepidemiology. 1997;16:107–115. doi: 10.1159/000109679. [DOI] [PubMed] [Google Scholar]
- 15.Woo D, Kissela BM, Khoury JC, Sauerbeck LR, Haverbusch MA, Szaflarski JP, Gebel JM, Pancioli AM, Jauch EC, Schneider A, Kleindorfer D, Broderick JP. Hypercholesterolemia, HMG-CoA reductase inhibitors, and risk of intracerebral hemorrhage: a case-control study. Stroke. 2004;35:1360–1364. doi: 10.1161/01.STR.0000127786.16612.A4. [DOI] [PubMed] [Google Scholar]
- 16.Roquer J, Rodriguez Campello A, Gomis M, Ois A, Munteis E, Bohm P. Serum lipid levels and in-hospital mortality in patients with intracerebral hemorrhage. Neurology. 2005;65:1198–1202. doi: 10.1212/01.wnl.0000180968.26242.4a. [DOI] [PubMed] [Google Scholar]
- 17.Aho K, Harmsen P, Hatano S, Marquardsen J, Smirnov VE, Strasser T. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull.World Health Organ. 1980;58:113–130. [PMC free article] [PubMed] [Google Scholar]
- 18.Ois A, Cuadrado-Godia E, Jimenez-Conde J, Gomis M, Rodriguez-Campello A, Martinez-Rodriguez JE, Munteis E, Roquer J. Early arterial study in the prediction of mortality after acute ischemic stroke. Stroke. 2007;38:2085–2089. doi: 10.1161/STROKEAHA.107.482950. [DOI] [PubMed] [Google Scholar]
- 19.Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Greenland P, Grundy SM, Hong Y, Miller NH, Lauer RM, Ockene IS, Sacco RL, Sallis JF, Jr, Smith SC, Jr, Stone NJ, Taubert KA. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106:388–391. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 20.Beghi E, Boglium G, Cosso P, Fiorelli G, Lorini C, Mandelli M, Bellini A. Stroke alcohol intake in a hospital population A case-control study. Stroke. 1995;26:1691–1696. doi: 10.1161/01.str.26.9.1691. [DOI] [PubMed] [Google Scholar]
- 21.Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Bottiglieri T, Rosand J, Growdon JH, Greenberg SM. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66:23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- 22.Nandigam RN, Chen YW, Gurol ME, Rosand J, Greenberg SM, Smith EE. Validation of intracranial area as a surrogate measure of intracranial volume when using clinical MRI. J.Neuroimaging. 2007;17:74–77. doi: 10.1111/j.1552-6569.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- 23.Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 24.Khan U, Porteous L, Hassan A, Markus HS. Risk factor profile of cerebral small vessel disease and its subtypes. J.Neurol.Neurosurg.Psychiatry. 2007;78:702–706. doi: 10.1136/jnnp.2006.103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA. Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators High-dose atorvastatin after stroke or transient ischemic attack. N.Engl.J.Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez-Sabin J, Huertas R, Quintana M, Rubiera M, Delgado P, Ribo M, Molina CA, Montaner J. Prior statin use may be associated with improved stroke outcome after tissue plasminogen activator. Stroke. 2007;38:1076–1078. doi: 10.1161/01.STR.0000258075.58283.8f. [DOI] [PubMed] [Google Scholar]
- 27.Vauthey C, de Freitas GR, van Melle G, Devuyst G, Bogousslavsky J. Better outcome after stroke with higher serum cholesterol levels. Neurology. 2000;54:1944–1949. doi: 10.1212/wnl.54.10.1944. [DOI] [PubMed] [Google Scholar]
- 28.Dyker AG, Weir CJ, Lees KR. Influence of cholesterol on survival after stroke: retrospective study. BMJ. 1997;314:1584–1588. doi: 10.1136/bmj.314.7094.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuadrado-Godia E, Jimenez-Conde J, Ois A, Rodriguez Campello A, Garcia-Ramallo E, Roquer J. Gender differences in the prognostic value of lipid profile after the first ischemic stroke. J Neurol. 2009;256:989–95. doi: 10.1007/s00415-009-5059-9. [DOI] [PubMed] [Google Scholar]
- 30.Lee SH, Bae HJ, Yoon BW, Kim H, Kim DE, Roh JK. Low concentration of serum total cholesterol is associated with multifocal signal loss lesions on gradient-echo magnetic resonance imaging: analysis of risk factors for multifocal signal loss lesions. Stroke. 2002;33:2845–2849. doi: 10.1161/01.str.0000036092.23649.2e. [DOI] [PubMed] [Google Scholar]
- 31.Longstreth WT, Jr, Arnold AM, Beauchamp NJ, Jr, Manolio TA, Lefkowitz D, Jungreis C, Hirsch CH, O'Leary DH, Furberg CD. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 32.Soljanlahti S, Autti T, Lauerma K, Raininko R, Keto P, Turtola H, Vuorio AF. Familial hypercholesterolemia patients treated with statins at no increased risk for intracranial vascular lesions despite increased cholesterol burden and extracranial atherosclerosis. Stroke. 2005;36:1572–1574. doi: 10.1161/01.STR.0000169920.64180.fa. [DOI] [PubMed] [Google Scholar]
- 33.Smith JA, Turner ST, Sun YV, Fornage M, Kelly RJ, Mosley TH, Jack CR, Kullo IJ, Kardia SL. Complexity in the Genetic Architecture of Leukoaraiosis in Hypertensive Sibships from the GENOA Study. BMC Med.Genomics. 2009;2:16. doi: 10.1186/1755-8794-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez C, Slevin M, Rodriguez-Calvo R, Kumar S, Krupinski J, Tejerina T, Martinez-Gonzalez J. Modulation of endothelium and endothelial progenitor cell function by low-density lipoproteins: implication for vascular repair, angiogenesis and vasculogenesis. Pathobiology. 2009;76:11–22. doi: 10.1159/000178151. [DOI] [PubMed] [Google Scholar]
- 35.Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr.Opin.Lipidol. 2001;12:105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 37.Goritz C, Mauch DH, Pfrieger FW. Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol.Cell.Neurosci. 2005;29:190–201. doi: 10.1016/j.mcn.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Austin MA, Edwards KL, McNeely MJ, Chandler WL, Leonetti DL, Talmud PJ, Humphries SE, Fujimoto WY. Heritability of multivariate factors of the metabolic syndrome in nondiabetic Japanese americans. Diabetes. 2004;53:1166–1169. doi: 10.2337/diabetes.53.4.1166. [DOI] [PubMed] [Google Scholar]
- 39.Atwood LD, Wolf PA, Heard-Costa NL, Massaro JM, Beiser A, D'Agostino RB, DeCarli C. Genetic variation in white matter hyperintensity volume in the Framingham Study. Stroke. 2004;35:1609–1613. doi: 10.1161/01.STR.0000129643.77045.10. [DOI] [PubMed] [Google Scholar]