Abstract
This work describes the step-by-step development of a novel, serum-free, in vitro cell culture system resulting in the formation of robust, contracting, multinucleate myotubes from dissociated skeletal muscle cells obtained from the hind limbs of fetal rats. This defined system consisted of a serum-free medium formulation developed by the systematic addition of different growth factors as well as a non-biological, cell growth promoting substrate, N-1[3-(trimethoxysilyl) propyl] diethylenetriamine (DETA). Each growth factor in the medium was experimentally evaluated for its effect on myotube formation. The resulting myotubes were evaluated immunocytochemically using embryonic skeletal muscle, specifically the myosin heavy chain antibody. Based upon this analysis, we propose a new skeletal muscle differentiation protocol that reflects the roles of the various growth factors which promote robust myotube formation. Further observation noted that the proposed skeletal muscle differentiation technique also supported muscle-nerve co-culture. Immunocytochemical evidence of nerve-muscle co-culture has also been documented. Applications for this novel culture system include: biocompatibility and skeletal muscle differentiation studies, understanding myopathies, neuromuscular disorders and skeletal muscle tissue engineering.
Keywords: Muscle Differentiation, Muscle-nerve coculture, Myotube, Serum-free Medium, Synthetic Substrate
Introduction
The goal of this study was to develop a serum-free cell culture model to study and understand skeletal muscle differentiation and to better understand the process by which individual skeletal muscle cells migrate and align in order to form functional, contractile, multinucleated myotubes (Arnold and Winter 1998; Brand-Saberi 2005; Brand-Saberi and Christ 1999; Brand et al. 2000; Christ and Brand-Saberi 2002; Li and Olson 1992; Olson 1992a; Olson 1992b; Olson and Perry 1992; Scaal et al. 1999; Schwarz et al. 1992). The serum-free cell culture model required the development of a medium, which was supplemented with different growth factors and utilized a synthetic, biocompatible silane substrate for cell growth. We formulated the serum-free medium by systematically studying the effects of individual growth factors on myotube formation in vitro.
In vivo and in vitro studies carried out in the previous two decades have indicated that skeletal muscle differentiation involves the specific interaction of multiple growth factors with both the myocytes and, subsequently, the developing myotubes. The different growth factors implicated in skeletal muscle differentiation include vitronectin, bFGF, CT1, GDNF, BDNF, NT3, and NT4 (Arnold and Winter 1998; Brand-Saberi and Christ 1999; Brand et al. 2000; Christ and Brand-Saberi 2002; Hornik et al. 2004; Li and Olson 1992; Olson 1992a; Olson 1992b; Olson and Perry 1992; Scaal et al. 1999; Schwarz et al. 1992) However, no systematic in vitro study had been carried out that combined these different growth factors in order to develop a defined medium. In this study, the effects of bFGF, CT1, GDNF, BDNF, NT3, NT4, and vitronectin on myocyte fusion and skeletal muscle differentiation were analyzed. Through this analysis, a simple chemical and growth factor based medium formulation was developed and a novel technique, which promoted the formation of robust, functional, contractile, multinucleated myotubes in culture was proposed. Immunocytochemical characterization was performed on the different myotube morphologies using embryonic myosin heavy chain (MHC) antibody (F1.652). Preliminary reports documented the enhanced myotube formation as well as their integration on silicon microstructures but not the mechanism of myotube formation(Das et al. 2006; Das et al. 2007b). This technique, which promoted robust myotube formation, was also observed to support nerve-muscle coculture(Das et al. 2007a). Immunocytochemical evidences of the muscle-nerve coculture was also established. We believe that this chemically defined formulation and the proposed mechanistic development model will be useful tools in studying myocyte biocompatibility, muscle differentiation, myopathies, muscle tissue engineering and neuromuscular junction formation.
Materials and Methods
Surface modification
Glass coverslips (Thomas Scientific 6661F52, 22 × 22mm No.1) were cleaned using an O2 plasma cleaner (Harrick PDC-32G) for 20 minutes at 100 mTorr. The DETA (United Chemical Technologies Inc. T2910KG) films were formed by the reaction of the cleaned surface with a 0.1% (v/v) mixture of the organosilane in freshly distilled toluene (Fisher T2904). The DETA coated coverslips were heated to just below the boiling point of the toluene, rinsed with toluene, reheated to just below the boiling temperature, and then oven dried (Das et al. 2007b; Hickman et al. 1994)
Surface characterization
Surfaces were characterized by contact angle measurements using an optical contact angle goiniometer (KSV Instruments, Cam 200) and X-ray photoelectron spectroscopy (XPS) (Kratos Axis 165). XPS survey scans, as well as high-resolution N1s and C1s scans, utilizing monochromatic Al Kα excitation, were obtained(Das et al. 2006; Ravenscroft et al. 1998).
Skeletal muscle culture and serum free medium
The skeletal muscle was dissected from the thighs of the hind limbs of fetal rats (17–18 days old). The tissue was collected in a sterile 15 ml centrifuge tube containing 1 ml of phosphate-buffered saline (calcium- and magnesium-free) (Gibco 14200075). The tissue was enzymatically dissociated using 1 ml of 0.05% of trypsin-EDTA (Gibco 25300054) solution for 30 minutes in a 37°C water bath (100 rpm). After 30 minutes the trypsin solution was removed and 2 ml of L15 + 10% fetal calf serum (Gibco 16000044) was added to terminate the trypsin action. The tissue was then mechanically triturated. The supernatant was then transferred to a 15 ml centrifuge tube. The same process was repeated two more times by adding 2 ml of L15 + 10% FBS each time. The 6 ml cell suspension obtained after mechanical trituration was suspended on a 2 ml, 4% BSA (Sigma A3059) (prepared in L15 medium) cushion and centrifuged at 300g for 10 minutes at 4°C. The pellet obtained was washed 5 times with L15 medium, then resuspended in 10 ml of L15 and plated on 100 mm uncoated dishes for 30 min. The non-attached cells were removed, centrifuged on a 4% BSA cushion, and plated on the coverslips. The cells were plated at a density of 700–1000 cells/mm2. The cells attached to the substrate in 1 h. The serum-free medium (containing different formulations of growth factors) was added to the culture dish after 1 h and the cells were maintained in a 5% CO2 incubator (relative humidity 85%). Half of the medium was changed every 4 days (Das et al. 2007b).
Three cell types coculturing in the defined medium
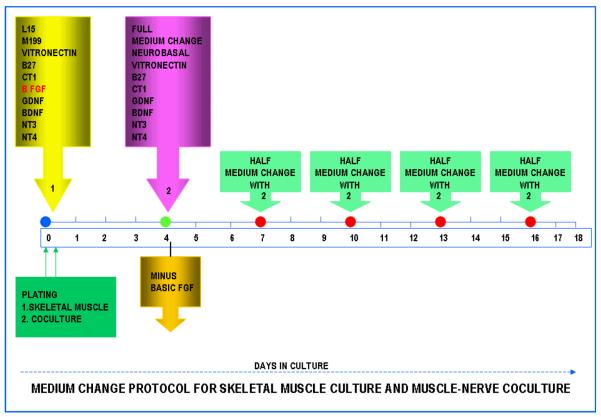
For coculturing of the skeletal muscle, DRG (Sensory Neurons) and spinal cord neurons, a simple coculture technique was developed. The dissociated muscle cells were mixed with dissociated sensory neurons (DRG) and the spinal cord cells. The DRG isolation protocol was described in an earlier paper (Liu et al. 2008). In brief, DRG's were isolated from embryonic, day 14 (E14) rat embryos and dissociated using trypsin. The resulting single cell suspension was then mixed with the dissociated muscle cells in the serum-free medium. Similarly, the whole spinal cord was removed from E14 embryos and dissociated in a trypsin solution. Subsequently, the single cell suspension of the spinal cord cells were combined with the dissociated muscle/DRG cell suspension mixture. The combined three cell suspension was then plated on the coverslips at a density of 800 cells/mm2. After 30 minutes, the well containing coverslips were filled with serum-free medium (Figure 1). The first medium change was done at day 4 as described in Figure 1 and the cultures were maintained for 3 weeks.
Figure 1.
Flow chart showing the technique utilized to grow robust myotubes and muscle-nerve cocultures. The Formulation IX and the medium change protocol, which is the most optimal for myotube and coculture growth.
Immunocytochemistry of skeletal muscle
Coverslips were prepared for immunocytochemical analysis as previously described (Das et al. 2007b). Briefly, coverslips were rinsed with PBS, fixed in −20°C methanol for 5–7 min, washed in PBS, incubated in PBS supplemented with 1% BSA and 0.05% saponin (permeabilization solution) for 10 minutes, and blocked for 2h with 10% goat serum and 1% BSA. Cells were incubated overnight with primary antibodies against embryonic myosin heavy chain (F1.652) (Developmental Studies Hybridoma Bank) diluted (1:5) in the blocking solution. Cells were washed with PBS and incubated with the secondary antibody (Cy3 conjugated anti-mouse, Jackson Labs., 1:200 dilution in PBS) for 2 hours. After 2 hours the coverslips were rinsed with PBS and mounted on glass slides and observed under a confocal microscope.
Immunocytochemistry of cocultures double stained with neurofilament 150 and embryonic myosin heavy chain antibodies
Co-cultures were processed for immunocytochemistry as described above. Next, cells were incubated overnight at 4°C with rabbit anti-neurofilament M polyclonal antibody, 150 kD (AB1981, diluted 1:2000; Millipore/Chemicon, Temecula, CA, USA) and fetal MHC (F1.652, IgG, Developmental Studies Hybridoma Bank, diluted 1:5). After incubating overnight, the coverslips were rinsed three times with PBS and incubated again with the appropriate secondary antibodies for 2 h. After rinsing three times in PBS, the coverslips were mounted with Vectashield_DAPI mounting medium onto the glass slides. The coverslips were visualized and images collected using a confocal microscope (UltraVIEW™ LCI, PerkinElmer). Controls without primary antibody were negative.
AChR labeling of the myotubes
AChRs were labeled as described previously (Dutton et al. 1995) by incubating cultures with 5×10−8 M of α-bungarotoxin, Alexa Fluor® 488 conjugate (B-13422; Invitrogen/Molecular Probes, Carlsbad, CA, USA) for 1.5 h at 37°C before observation. Following incubation in α-bungarotoxin, the cultures were fixed as mentioned above for further staining with embryonic myosin heavy chain (F1.652) antibodies.
Results
DETA surface modification and characterization
Static contact angle and XPS analysis was used for validation of the surface modifications and for monitoring the quality of the surfaces. Stable contact angles (40.64° ± 2.9 /mean ± SD) throughout the study indicated a high reproducibility and quality of the DETA coatings and were similar to previously published results(Hickman et al. 1994)(Das et al. 2006; Das et al. 2003; Ravenscroft et al. 1998; Schaffner et al. 1995; Spargo et al. 1994; Stenger et al. 1992). Based on the ratio of the N (401 and 399 eV) and the Si 2p3/2 peaks, XPS measurements indicated that a complete monolayer of DETA was formed on the coverslips.
Development of serum-free medium formulation
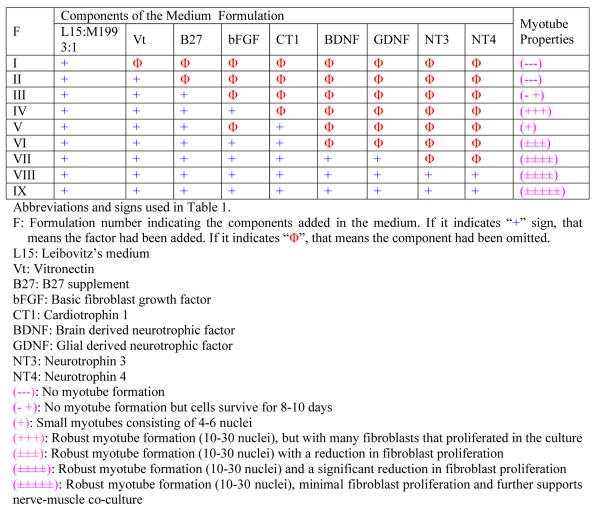
The results have been summarized in Table 1. Below the effects of the different growth factors present in the nine different formulations are discussed. Unless otherwise stated, half of the medium was changed every three to four days.
Table 1.
Development of the chemically defined serum-free medium by systematically adding individual growth factors in the culture.
|
Formulation I (F I) was the basal medium. It consisted of Leibovitz's medium and M199 in a 3:1 ratio (v/v). The basal medium did not promote myotube formation and the cells died after 4 days in culture.
In Formulation II (F II) vitronectin was added to the basal medium. Myotube formation was not observed and cells did not survive in the culture for more than 4 days.
In Formulation III (F III) B27 supplement was added to Formulation II (F II). The cells survive for 8–10 days, but there was no myotube formation.
In Formulation IV (F IV) bFGF was added to Formulation III (F III). The addition of bFGF led to the formation of robust myotubes. Myotubes started appearing by day 2 in culture. Contracting myotubes were observed by day 4. Myotubes covered 50% of the total area of the coverslip and they survived for 10–12 days in culture. Many of the myotubes popped out of the coverslip due to extensive contractions. Extensive proliferation of fibroblasts in culture was also observed.
In Formulation V (F V) CT1 was added to Formulation III (F III). The addition of CT1 led to the formation of small myotubes with 4–6 nuclei. Myotubes started appearing by day 2 in culture and by day 4 most of these small myotubes showed mild contractions. Contractile myotubes covered 10% of the total area of the coverslips and the myotubes survived for 6–7 days in culture. The Formulation V had no bFGF in it. Small myotube formation was observed even without the presence of bFGF.
In Formulation VI (F VI) both bFGF and CT1 were added to the Formulation III (F III). Robust myotube formation with a reduction in fibroblast proliferation was observed. Fibroblast proliferation was less compared to Formulation IV (F IV) where only bFGF was added. Contractile myotubes covered 60% of the total surface area of the coverslips. Most of the myotubes began contracting by day 4 and exhibited extensive contractions as well as surviving for 10–12 days in culture.
In Formulation VII (F VII) GDNF and BDNF were added to Formulation VI (F VI). Robust myotube formation as well as a significant reduction in fibroblast proliferation was observed. The presence of GDNF and BDNF significantly reduced fibroblast proliferation and increased the total surface area covered with myotubes. Almost 65% of the total surface area of the coverslip was covered with contractile myotubes. Most of the myotubes began to show contractions by day 4.
In Formulation VIII (F VIII) NT3 and NT4 were added to formulation VII (F VII). No significant qualitative difference from Formulation VII (F VII) was observed. Functionally, the myotubes started contracting by day 2. Additionally, a significantly reduced fibroblast proliferation was observed compared to Formulation III (F III).
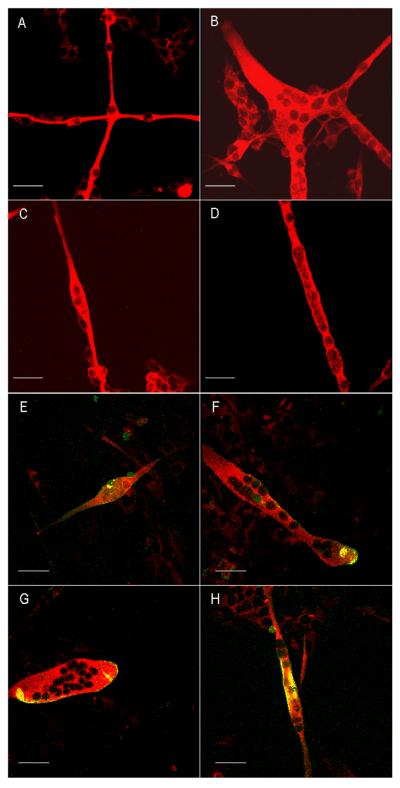
In Formulation IX (F IX) no additional growth factors were added; instead two changes were made in the growth factor application. First, instead of replacing half of the medium during the first change, the entire medium was replaced. Second, in the process of changing the whole medium, bFGF was withdrawn, which resulted in the following formulation: Neurobasal/ vitronectin/ B27/ CT1/ GDNF/ BDNF/ NT3/ NT4. These two changes brought about a significant increase in the total number of myotubes formed. The final medium formulation has been enumerated in Table 2 and the medium change protocol is represented in Figure 1. By day 6, 90% of the total surface area of the coverslip was covered with robust, contractile myotubes. Additionally, there was minimal fibroblast contamination. Contractions began by day 2. The myotubes survived in culture for 16–20 days. All the different morphologies of myotubes observed in the culture were stained with embryonic myosin heavy chain antibodies (Figure 2 A–D). Chain like (Fig 2 A), branched (Fig 2 B), spindle shaped (Fig 2 C) and cylinder like (Fig 2 D) morphologies of myotubes were observed in the culture. The clustering of acetylcholine receptors on the membrane surface of the different morphologies of myotubes are shown in Figure 1 E–H. All of the myotubes showed clustering of acetylcholine receptors on their membrane surface.
Table 2.
Composition of novel serum-free medium for a 500 ml sample.
| S. No. | Component(s) | Catalogue # | Company | Amount |
|---|---|---|---|---|
| 1. | L15 | Invitrogen | 11415064 | 375 ml |
| 2. | M199 | Invitrogen | 11150059 | 125 ml |
| 3. | Vitronectin | Sigma | V0132 | 50 μg |
| 4. | B27 | Invitrogen | 17504044 | 10 ml |
| 5. | Basic FGF | Invitrogen | 13256029 | 10ng/ml |
| 6. | CT1 | Cell Sciences | CRC700B | 10 μg |
| 7. | GDNF | Invitrogen | 10907012 | 10 μg |
| 8. | BDNF | Invitrogen | 10908019 | 10 μg |
| 9. | NT3 | Cell Science | CRN 500B | 10 μg |
| 10. | NT4 | Cell Science | CRN501B | 10 μg |
| 11. | Sodium Bicarbonate | Fisher | 5233500 | 0.7 g |
| 12. | Osmolarity | 320–325 mOsm | ||
| 13. | pH | 7.3 |
Figure 2.
Different morphologies of myotubes stained with embryonic, myosin heavy chain antibodies (Red) and clustering of acetylcholine receptors (Green) on the membrane surface of myotubes. Scale bar is 50 μm. A. Chain like morphology of the myotubes. B. Branched morphology of the myotubes. C. Spindle shaped morphology of a single myotube. B. Cylinder shaped morphology of a single myotube. E–H. Different morphologies of myotubes indicating the clustering of acetylcholine receptors (Green) on the membrane surface of different myotubes.
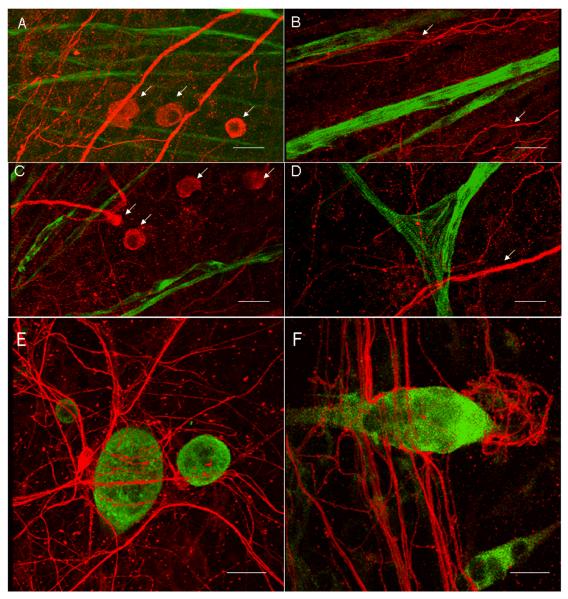
Medium Formulation IX (F IX) and the subsequent change at day 4, further supported the nerve-muscle co-culture (Figure 3 A–F). In Fig 3 A, the sensory neurons along with the myotubes in the cultures were observed. The sensory neurons were seen as large cell bodies. In Fig 3 B, the neuron processes run parallel to the mytotubes. In Fig 3 C, multi-polar cell morphologies of motoneurons, along with the large sensory neurons were detected. In Fig 3 D, branched, striated myotubes, and neuronal process crossing over a myotube was noted. In Fig 3 E–F, the wrapping of a neuronal process on a myotube is represented in three dimensions.
Figure 3.
Coculture of skeletal muscle* sensory neurons (DRG)* spinal cord neurons. All neurons were stained with the antibody against neurofilament-M (150 KD) (Red) and the myotubes were stained with the antibody against embryonic myosin heavy chain (F 1.652) (Green). Scale bar is 75 μm. A. The large sensory neurons (DRG) were stained with NF 150 (Red). The neurons are shown with white arrows. The myotubes stained with F1.652 (Green) were seen in the same field. B. The neuron process in red are noted running parallel to the myotubes (Green). C. The multi-polar motoneuron was noted in close proximity with the myotubes. In the frame multiple large sensory neurons were observed. All the neurons are pointed out by white arrows. D. A bundle of multiple processes of neurons crossed the striated, branched myotube. E. A single multi-polar motoneuron was observed to wrap around the mytotube. F. Neuron processes wrapping on the myotubes.
Discussion
In this study, a step-by-step process was described to develop of a novel, serum-free, in vitro cell culture system which results in the formation of robust, contracting, multinucleate myotubes from dissociated skeletal muscle cells that were obtained from the hind limbs of fetal rats. The step-by step development consisted of experimentally evaluating the effect of individual growth factors on myocyte survival and subsequent myotube formation. At the conclusion of the study, the most optimal formulation i.e. Formulation IX (F IX), was shown to support muscle as well as nerve-muscle co-culture growth. This serum-free medium supporting the survival, proliferation and fusion of fetal rat myoblasts into contractile myotubes. The rational for selecting these growth factors (vitronectin, B27, bFGF, CT1, GDNF, BDNF, NT3 and NT4) was solely based upon the distribution of their receptors within the developing myotubes in the rat fetus.
Role of Vitronectin
Vitronectin was added to the culture medium because its receptors promote cell adhesion and provide an anchoring function during the skeletal muscle differentiation process in vitro (Biesecker 1990; Gullberg et al. 1995). The addition of vitronectin by itself did not promote myotube formation.
Role of B27
As previously documented, B27 supplement had supported cardiomyocyte growth (Das et al. 2004). In this culture medium B27 supplement was added to the medium as a serum-replacement. Although the addition of B27 supplement promoted cell survival, it did not promote myotube formation.
Role of bFGF and the controversy concerning the role of bFGF in differentiation
Basic fibroblast growth factor (FGF-2) is a 17-kDa member of the heparin binding growth factors. Basic FGF (bFGF) plays a complex, yet poorly understood, role in skeletal muscle differentiation. Several studies have indicated that bFGF promoted limb development, and some in vitro studies indicate that bFGF promotes the division of skeletal muscle cells, but inhibits the differentiation process. It had been documented that the terminal differentiation of the skeletal muscle occurs in the G1 phase but is repressed by fibroblast growth factor (Alterio et al. 1990; Anderson et al. 1991; Burgess and Maciag 1989; Clegg et al. 1987; Gonzalez et al. 1990; Hannon et al. 1996; Moore et al. 1991; Morrow et al. 1990; Nugent and Iozzo 2000; Ohuchi and Noji 1999). Interestingly, in this study, even in the presence of bFGF, most myoblasts fused and differentiated to form functional myotubes. These in vitro results support the hypothesis that there are at least two different pools of myoblasts present in the developing limb bud. In one population, bFGF promotes differentiation and in the other it inhibits muscle differentiation.
Role of CT1
Cardiotrophin-1 (CT1) is a cytokine belonging to the IL-6 family. It is expressed at high levels in the embryonic limb bud development and secreted by differentiated myotubes. CT1 promotes cardiac myocyte survival, regeneration of extraocular muscle, exhibits increased immunoreactivity in regenerating muscle and promotes motoneuron survival (Bordet et al. 2001; Chen and von Bartheld 2004; Dolcet et al. 2001; Lesbordes et al. 2002; Mitsumoto et al. 2001; Nishikawa et al. 2005; Oppenheim et al. 2001; Peroulakis and Forger 2000; Sheng et al. 1996). In this study, the addition of CT1 to basal medium/ vitronectin/ B27 was observed to promote the formation of small myotubes. This indicated that CT1 by itself has the potential to initiate myotube formation, but it needs support from other growth factors.
Role of GDNF and BDNF
The glial cell line derived neurotrophic factor (GDNF) is a glycosylated, disulfide-bonded homodimer that is a distantly related member of the transforming growth factor-beta superfamily. GDNF plays a role in the differentiation and survival of central and peripheral neurons as well as in kidney organogenesis. GDNF is widely expressed in the development of skeletal muscle and is involved in regulating the distribution of acetylcholine receptors in mouse, primary skeletal muscle cells (Choi-Lundberg and Bohn 1995; Golden et al. 1999; Henderson et al. 1994; Lin et al. 1993; Yang and Nelson 2004).
Brain-derived neurotrophic factor (BDNF) is a ligand for the low-affinity NGF receptor, p75, and for the high affinity neurotrophin receptor, trkB. It is expressed in the developing skeletal muscle, promotes motoneuron survival and also plays a vital role in the formation of the neuromuscular junction. BDNF rescues myosin, heavy chain, IIB muscle fibers after neonatal nerve injury (Heinrich 2003; Mousavi et al. 2004; Simon et al. 2003) and along with NT4 promotes phenotypic recovery of both fast and slow twitch fibers. We believe that the above mentioned effects of GDNF and BDNF promote enhanced myotube formation in culture.
Role of NT3 and NT4
Neurotrophin-3, or NT3, is a neurotrophic factor in the NGF (Nerve Growth Factor)-family of neurotrophins. It is one of five neurotrophin growth factors which shape the development of the nervous system by regulating neuronal survival and differentiation (Oakley et al. 1997). Recent studies have indicated that NT3 has an essential non-neuronal function. It plays a key role in cardiac development (Donovan et al. 1996). NT4 (Carrasco and English 2003; Heinrich 2003; Simon et al. 2003) promotes the normal development of slow muscle fiber phenotypes and phenotypic recovery of fast and slow twitch fibers. Previously, studies in this lab indicated that the addition of NT3 and NT4 results in early contractions in the myotubes(Das et al. 2006). Speculation is that the NT3 and NT4 have a synergistic effect in muscle differentiation.
Co-culture studies
One interesting feature of the differential application of growth factors in Formulation IX (F IX) was that it supported the survival and growth of skeletal muscle, sensory neurons and spinal cord motoneurons in a three cell co-culture model. While this seems intuitive, it was a crucial finding for easily building functional muscle-motoneuron, muscle-sensory neuron and muscle-motoneuron-sensory neuron constructs and for ultimately reconstructing the stretch reflex arc in vitro.
Conclusions
This work documents the development of a medium formulation that resulted in robust myotube formation and provided an analysis of the role of the individual factors and their mechanism of action in that process. Furthermore, the myotubes developed a MHC profile, which resulted in functional contractile properties. The final medium formulation was determined to support the growth of motoneurons and sensory neurons as well as their co-culture with myotubes. Consequently, this medium will be a powerful tool in nerve-muscle tissue engineering, discovering the molecules which triggers the switching between different myosin heavy chain proteins during muscle development, dissecting the molecules involved in synapse formation at the neuromuscular junction and for applications in diseases such as ALS, muscular dystrophy, other diseases and injuries of spinal cord.
Acknowledgements
The F1.652 monoclonal antibody developed by Helan Blau was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA. This work was supported by DARPA grant DARPA-ITO N65236-01-1-7400 and NIH grant number 5R01 NS 050452. The initial experiments for this work were performed in the Bioengineering Department at Clemson University, Clemson, SC.
References
- Alterio J, Courtois Y, Robelin J, Bechet D, Martelly I. Acidic and basic fibroblast growth factor mRNAs are expressed by skeletal muscle satellite cells. Biochem Biophys Res Commun. 1990;166(3):1205–12. doi: 10.1016/0006-291x(90)90994-x. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Liu L, Kardami E. Distinctive patterns of basic fibroblast growth factor (bFGF) distribution in degenerating and regenerating areas of dystrophic (mdx) striated muscles. Dev Biol. 1991;147(1):96–109. doi: 10.1016/s0012-1606(05)80010-7. [DOI] [PubMed] [Google Scholar]
- Arnold HH, Winter B. Muscle differentiation: more complexity to the network of myogenic regulators. Curr Opin Genet Dev. 1998;8(5):539–44. doi: 10.1016/s0959-437x(98)80008-7. [DOI] [PubMed] [Google Scholar]
- Biesecker G. The complement SC5b-9 complex mediates cell adhesion through a vitronectin receptor. J Immunol. 1990;145(1):209–14. [PubMed] [Google Scholar]
- Bordet T, Lesbordes JC, Rouhani S, Castelnau-Ptakhine L, Schmalbruch H, Haase G, Kahn A. Protective effects of cardiotrophin-1 adenoviral gene transfer on neuromuscular degeneration in transgenic ALS mice. Hum Mol Genet. 2001;10(18):1925–33. doi: 10.1093/hmg/10.18.1925. [DOI] [PubMed] [Google Scholar]
- Brand-Saberi B. Genetic and epigenetic control of skeletal muscle development. Ann Anat. 2005;187(3):199–207. doi: 10.1016/j.aanat.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Brand-Saberi B, Christ B. Genetic and epigenetic control of muscle development in vertebrates. Cell Tissue Res. 1999;296(1):199–212. doi: 10.1007/s004410051281. [DOI] [PubMed] [Google Scholar]
- Brand T, Butler-Browne G, Fuchtbauer EM, Renkawitz-Pohl R, Brand-Saberi B. EMBO Workshop Report: Molecular genetics of muscle development and neuromuscular diseases Kloster Irsee, Germany, September 26-October 1, 1999. Embo J. 2000;19(9):1935–41. doi: 10.1093/emboj/19.9.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess WH, Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Carrasco DI, English AW. Neurotrophin 4/5 is required for the normal development of the slow muscle fiber phenotype in the rat soleus. J Exp Biol. 2003;206(Pt 13):2191–200. doi: 10.1242/jeb.00412. [DOI] [PubMed] [Google Scholar]
- Chen J, von Bartheld CS. Role of exogenous and endogenous trophic factors in the regulation of extraocular muscle strength during development. Invest Ophthalmol Vis Sci. 2004;45(10):3538–45. doi: 10.1167/iovs.04-0393. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Bohn MC. Ontogeny and distribution of glial cell line-derived neurotrophic factor (GDNF) mRNA in rat. Brain Res Dev Brain Res. 1995;85(1):80–8. doi: 10.1016/0165-3806(94)00197-8. [DOI] [PubMed] [Google Scholar]
- Christ B, Brand-Saberi B. Limb muscle development. Int J Dev Biol. 2002;46(7):905–14. [PubMed] [Google Scholar]
- Clegg CH, Linkhart TA, Olwin BB, Hauschka SD. Growth factor control of skeletal muscle differentiation: commitment to terminal differentiation occurs in G1 phase and is repressed by fibroblast growth factor. J Cell Biol. 1987;105(2):949–56. doi: 10.1083/jcb.105.2.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Gregory CA, Molnar P, Riedel LM, Wilson K, Hickman JJ. A defined system to allow skeletal muscle differentiation and subsequent integration with silicon microstructures. Biomaterials. 2006;27(24):4374–80. doi: 10.1016/j.biomaterials.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Das M, Molnar P, Devaraj H, Poeta M, Hickman JJ. Electrophysiological and morphological characterization of rat embryonic motoneurons in a defined system. Biotechnol Prog. 2003;19(6):1756–61. doi: 10.1021/bp034076l. [DOI] [PubMed] [Google Scholar]
- Das M, Molnar P, Gregory C, Riedel L, Jamshidi A, Hickman JJ. Long-term culture of embryonic rat cardiomyocytes on an organosilane surface in a serum-free medium. Biomaterials. 2004;25(25):5643–7. doi: 10.1016/j.biomaterials.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Das M, Rumsey JW, Gregory CA, Bhargava N, Kang JF, Molnar P, Riedel L, Guo X, Hickman JJ. Embryonic motoneuron-skeletal muscle co-culture in a defined system. Neuroscience. 2007a;146(2):481–8. doi: 10.1016/j.neuroscience.2007.01.068. [DOI] [PubMed] [Google Scholar]
- Das M, Wilson K, Molnar P, Hickman JJ. Differentiation of skeletal muscle and integration of myotubes with silicon microstructures using serum-free medium and a synthetic silane substrate. Nat Protoc. 2007b;2(7):1795–801. doi: 10.1038/nprot.2007.229. [DOI] [PubMed] [Google Scholar]
- Dolcet X, Soler RM, Gould TW, Egea J, Oppenheim RW, Comella JX. Cytokines promote motoneuron survival through the Janus kinase-dependent activation of the phosphatidylinositol 3-kinase pathway. Mol Cell Neurosci. 2001;18(6):619–31. doi: 10.1006/mcne.2001.1058. [DOI] [PubMed] [Google Scholar]
- Donovan MJ, Hahn R, Tessarollo L, Hempstead BL. Identification of an essential nonneuronal function of neurotrophin 3 in mammalian cardiac development. Nat Genet. 1996;14(2):210–3. doi: 10.1038/ng1096-210. [DOI] [PubMed] [Google Scholar]
- Dutton EK, Uhm CS, Samuelsson SJ, Schaffner AE, Fitzgerald SC, Daniels MP. Acetylcholine receptor aggregation at nerve-muscle contacts in mammalian cultures: induction by ventral spinal cord neurons is specific to axons. J Neurosci. 1995;15(11):7401–16. doi: 10.1523/JNEUROSCI.15-11-07401.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JP, DeMaro JA, Osborne PA, Milbrandt J, Johnson EM., Jr. Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp Neurol. 1999;158(2):504–28. doi: 10.1006/exnr.1999.7127. [DOI] [PubMed] [Google Scholar]
- Gonzalez AM, Buscaglia M, Ong M, Baird A. Distribution of basic fibroblast growth factor in the 18-day rat fetus: localization in the basement membranes of diverse tissues. J Cell Biol. 1990;110(3):753–65. doi: 10.1083/jcb.110.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullberg D, Sjoberg G, Velling T, Sejersen T. Analysis of fibronectin and vitronectin receptors on human fetal skeletal muscle cells upon differentiation. Exp Cell Res. 1995;220(1):112–23. doi: 10.1006/excr.1995.1297. [DOI] [PubMed] [Google Scholar]
- Hannon K, Kudla AJ, McAvoy MJ, Clase KL, Olwin BB. Differentially expressed fibroblast growth factors regulate skeletal muscle development through autocrine and paracrine mechanisms. J Cell Biol. 1996;132(6):1151–9. doi: 10.1083/jcb.132.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich G. A novel BDNF gene promoter directs expression to skeletal muscle. BMC Neurosci. 2003;4:11. doi: 10.1186/1471-2202-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Simpson LC, et al. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266(5187):1062–4. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Hickman JJ, Bhatia SK, Quong JN, Schoen P, Stenger DA, Pike CJ, Cotman CW. Rational Pattern Design for in-Vitro Cellular Networks Using Surface Photochemistry. Journal of Vacuum Science & Technology A. 1994;12(3):607–616. [Google Scholar]
- Hornik C, Brand-Saberi B, Rudloff S, Christ B, Fuchtbauer EM. Twist is an integrator of SHH, FGF, and BMP signaling. Anat Embryol (Berl) 2004;209(1):31–9. doi: 10.1007/s00429-004-0412-3. [DOI] [PubMed] [Google Scholar]
- Lesbordes JC, Bordet T, Haase G, Castelnau-Ptakhine L, Rouhani S, Gilgenkrantz H, Kahn A. In vivo electrotransfer of the cardiotrophin-1 gene into skeletal muscle slows down progression of motor neuron degeneration in pmn mice. Hum Mol Genet. 2002;11(14):1615–25. doi: 10.1093/hmg/11.14.1615. [DOI] [PubMed] [Google Scholar]
- Li L, Olson EN. Regulation of muscle cell growth and differentiation by the MyoD family of helix-loop-helix proteins. Adv Cancer Res. 1992;58:95–119. doi: 10.1016/s0065-230x(08)60292-4. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–2. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Liu J, Rumsey JW, Das M, Molnar P, Gregory C, Riedel L, Hickman JJ. Electrophysiological and immunocytochemical characterization of DRG neurons on an organosilane surface in serum-free medium. In Vitro Cell Dev Biol Anim. 2008;44(5–6):162–8. doi: 10.1007/s11626-008-9097-x. [DOI] [PubMed] [Google Scholar]
- Mitsumoto H, Klinkosz B, Pioro EP, Tsuzaka K, Ishiyama T, O'Leary RM, Pennica D. Effects of cardiotrophin-1 (CT-1) in a mouse motor neuron disease. Muscle Nerve. 2001;24(6):769–77. doi: 10.1002/mus.1068. [DOI] [PubMed] [Google Scholar]
- Moore JW, Dionne C, Jaye M, Swain JL. The mRNAs encoding acidic FGF, basic FGF and FGF receptor are coordinately downregulated during myogenic differentiation. Development. 1991;111(3):741–8. doi: 10.1242/dev.111.3.741. [DOI] [PubMed] [Google Scholar]
- Morrow NG, Kraus WE, Moore JW, Williams RS, Swain JL. Increased expression of fibroblast growth factors in a rabbit skeletal muscle model of exercise conditioning. J Clin Invest. 1990;85(6):1816–20. doi: 10.1172/JCI114640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi K, Parry DJ, Jasmin BJ. BDNF rescues myosin heavy chain IIB muscle fibers after neonatal nerve injury. Am J Physiol Cell Physiol. 2004;287(1):C22–9. doi: 10.1152/ajpcell.00583.2003. [DOI] [PubMed] [Google Scholar]
- Nishikawa J, Sakuma K, Sorimachi Y, Yoshimoto K, Yasuhara M. Increase of Cardiotrophin-1 immunoreactivity in regenerating and overloaded but not denervated muscles of rats. Neuropathology. 2005;25(1):54–65. doi: 10.1111/j.1440-1789.2004.00587.x. [DOI] [PubMed] [Google Scholar]
- Nugent MA, Iozzo RV. Fibroblast growth factor-2. Int J Biochem Cell Biol. 2000;32(2):115–20. doi: 10.1016/s1357-2725(99)00123-5. [DOI] [PubMed] [Google Scholar]
- Oakley RA, Lefcort FB, Clary DO, Reichardt LF, Prevette D, Oppenheim RW, Frank E. Neurotrophin-3 promotes the differentiation of muscle spindle afferents in the absence of peripheral targets. J Neurosci. 1997;17(11):4262–74. doi: 10.1523/JNEUROSCI.17-11-04262.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi H, Noji S. Fibroblast-growth-factor-induced additional limbs in the study of initiation of limb formation, limb identity, myogenesis, and innervation. Cell Tissue Res. 1999;296(1):45–56. doi: 10.1007/s004410051265. [DOI] [PubMed] [Google Scholar]
- Olson E. Activation of muscle-specific transcription by myogenic helix-loop-helix proteins. Symp Soc Exp Biol. 1992a;46:331–41. [PubMed] [Google Scholar]
- Olson EN. Interplay between proliferation and differentiation within the myogenic lineage. Dev Biol. 1992b;154(2):261–72. doi: 10.1016/0012-1606(92)90066-p. [DOI] [PubMed] [Google Scholar]
- Olson EN, Perry WM. MyoD and the paradoxes of myogenesis. Curr Biol. 1992;2(1):35–7. doi: 10.1016/0960-9822(92)90429-e. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, Wiese S, Prevette D, Armanini M, Wang S, Houenou LJ, Holtmann B, Gotz R, Pennica D, Sendtner M. Cardiotrophin-1, a muscle-derived cytokine, is required for the survival of subpopulations of developing motoneurons. J Neurosci. 2001;21(4):1283–91. doi: 10.1523/JNEUROSCI.21-04-01283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peroulakis ME, Forger NG. Ciliary neurotrophic factor increases muscle fiber number in the developing levator ani muscle of female rats. Neurosci Lett. 2000;296(2–3):73–6. doi: 10.1016/s0304-3940(00)01649-9. [DOI] [PubMed] [Google Scholar]
- Ravenscroft MS, Bateman KE, Shaffer KM, Schessler HM, Jung DR, Schneider TW, Montgomery CB, Custer TL, Schaffner AE, Liu QY, Li YX, Barker JL, Hickman JJ. Developmental neurobiology implications from fabrication and analysis of hippocampal neuronal networks on patterned silane-modified surfaces. Journal of the American Chemical Society. 1998;120(47):12169–12177. [Google Scholar]
- Scaal M, Bonafede A, Dathe V, Sachs M, Cann G, Christ B, Brand-Saberi B. SF/HGF is a mediator between limb patterning and muscle development. Development. 1999;126(21):4885–93. doi: 10.1242/dev.126.21.4885. [DOI] [PubMed] [Google Scholar]
- Schaffner AE, Barker JL, Stenger DA, Hickman JJ. Investigation of the factors necessary for growth of hippocampal neurons in a defined system. Journal of Neuroscience Methods. 1995;62(1–2):111–119. doi: 10.1016/0165-0270(95)00063-1. [DOI] [PubMed] [Google Scholar]
- Schwarz JJ, Chakraborty T, Martin J, Zhou JM, Olson EN. The basic region of myogenin cooperates with two transcription activation domains to induce muscle-specific transcription. Mol Cell Biol. 1992;12(1):266–75. doi: 10.1128/mcb.12.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z, Pennica D, Wood WI, Chien KR. Cardiotrophin-1 displays early expression in the murine heart tube and promotes cardiac myocyte survival. Development. 1996;122(2):419–28. doi: 10.1242/dev.122.2.419. [DOI] [PubMed] [Google Scholar]
- Simon M, Porter R, Brown R, Coulton GR, Terenghi G. Effect of NT-4 and BDNF delivery to damaged sciatic nerves on phenotypic recovery of fast and slow muscles fibres. Eur J Neurosci. 2003;18(9):2460–6. doi: 10.1046/j.1460-9568.2003.02978.x. [DOI] [PubMed] [Google Scholar]
- Spargo BJ, Testoff MA, Nielsen TB, Stenger DA, Hickman JJ, Rudolph AS. Spatially Controlled Adhesion, Spreading, and Differentiation of Endothelial-Cells on Self-Assembled Molecular Monolayers. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(23):11070–11074. doi: 10.1073/pnas.91.23.11070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenger DA, Georger JH, Dulcey CS, Hickman JJ, Rudolph AS, Nielsen TB, McCort SM, Calvert JM. Coplanar Molecular Assemblies of Aminoalkylsilane and Perfluorinated Alkylsilane - Characterization and Geometric Definition of Mammalian-Cell Adhesion and Growth. Journal of the American Chemical Society. 1992;114(22):8435–8442. [Google Scholar]
- Yang LX, Nelson PG. Glia cell line-derived neurotrophic factor regulates the distribution of acetylcholine receptors in mouse primary skeletal muscle cells. Neuroscience. 2004;128(3):497–509. doi: 10.1016/j.neuroscience.2004.06.067. [DOI] [PubMed] [Google Scholar]