Abstract
Objective
It has been suggested that there is a mechanism by which nonsteroidal anti-inflammatory drugs (NSAIDs) may interfere with antidepressant response, and poorer outcomes among NSAID-treated patients were reported in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. To attempt to confirm this association in an independent population-based treatment cohort and explore potential confounding variables, the authors examined use of NSAIDs and related medications among 1,528 outpatients in a New England health care system.
Method
Treatment outcomes were classified using a validated machine learning tool applied to electronic medical records. Logistic regression was used to examine the association between medication exposure and treatment outcomes, adjusted for potential confounding variables. To further elucidate confounding and treatment specificity of the observed effects, data from the STAR*D study were reanalyzed.
Results
NSAID exposure was associated with a greater likelihood of depression classified as treatment resistant compared with depression classified as responsive to selective serotonin reuptake inhibitors (odds ratio=1.55, 95% CI=1.21–2.00). This association was apparent in the NSAIDs-only group but not in those using other agents with NSAID-like mechanisms (cyclooxygenase-2 inhibitors and salicylates). Inclusion of age, sex, ethnicity, and measures of comorbidity and health care utilization in regression models indicated confounding; association with outcome was no longer significant in fully adjusted models. Reanalysis of STAR*D results likewise identified an association in NSAIDs but not NSAID-like drugs, with more modest effects persisting after adjustment for potential confounding variables.
Conclusions
These results support an association between NSAID use and poorer antidepressant outcomes in major depressive disorder but indicate that some of the observed effect may be a result of confounding.
Two of the most widely used medications in contemporary clinical practice are antidepressants and nonsteroidal anti-inflammatory drugs (NSAIDs) (1), and the co-occurrence of pain and depressive symptoms is common (2). A recent report suggested that NSAIDs interfere with some behavioral and biochemical effects of selective serotonin reuptake inhibitors (SSRIs) in mice (3). The same study also presented an analysis of the multicenter Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study of antidepressant effectiveness, suggesting that NSAID-treated patients with major depression were less likely to achieve remission with citalopram than patients who were not treated with NSAIDs.
Since NSAID use in the STAR*D cohort was not randomly assigned, the risk of confounding was substantial. For example, the indication for NSAID use, rather than NSAID use itself, might have mediated the observed association with outcome, an example of confounding by indication. Chronic pain in particular and greater medical comorbidity in general are known to be associated with greater depression severity and poorer antidepressant treatment outcomes (4, 5).
The strongest means of addressing confounding is a prospective study with randomized treatment assignment. On the other hand, given the prevalence of depression and the costs of treatment resistance (6), the proposed relationship between NSAIDs and SSRIs has potentially large public health implications, making rapid follow-up analysis imperative. Two alternative means of follow-up analysis to address the extent of confounding are 1) confirmation in an independent cohort in which confounding may be addressed and 2) reanalysis using rigorous control of potential sources of confounding. Our aim was to conduct both types of analysis.
To accomplish the first, we used a tool for automated determination of treatment outcome in large electronic medical record systems, the i2b2 (Informatics for Integrating Biology and the Bedside) treatment-resistant depression framework (7), using methods that have previously demonstrated sensitivity to other adverse treatment effects outside of the field of psychiatry (8). We hypothesized that we would observe an association between NSAID use and treatment resistance in more than 1,500 antidepressant-treated patients with major depressive disorder. We then reanalyzed data from this cohort and from the original STAR*D study to better characterize the potential confounding effects of medical comorbidity on the observed association.
Method
i2b2 Treatment-Resistant Depression Cohort
Cohort derivation
The derivation of the i2b2 treatment-resistant depression cohort has been described in detail elsewhere (7). Briefly, the i2b2 system (9, 10) is a scalable computational framework, deployed at more than 45 academic health centers, used to manage clinical data. This tool was applied to the Partners HealthCare electronic medical records system, which includes sociodemographic data, billing codes, laboratory results, clinical problem lists, medications, vital signs, and narrative notes from Massachusetts General Hospital and Brigham and Women’s Hospital, as well as community and specialty hospitals that are part of the Partners HealthCare system in Boston. Patients with at least one diagnosis of major depression determined by the presence of ICD-9 codes 296.2–296.3 in billing data or outpatient medical records were selected for inclusion in a data set (referred to as a data mart), yielding 127,504 individuals from approximately 3.1 million unique patients. Next, we used a validated longitudinal classifier (7), that applies natural language processing to individual narrative notes, followed by a set of rules derived from logistic regression models (7) to identify individuals who either achieved remission with SSRI monotherapy or remained depressed despite two or more antidepressant treatments.
Sociodemographic and clinical data for use as covariates, including age, sex, race, insurance type, and selected comorbidities, were also extracted from the electronic medical records in an automated fashion. A validated tool for estimating burden of comorbidity, the age-adjusted Charlson comorbidity index (11, 12), was applied, along with a measure for overall health care utilization based on the count of total medical facts (the latter method has been used in previous studies [8] and includes billing codes, medications, and procedure codes, i.e., any single data point in the electronic medical records). Concomitant medications, including NSAIDs, cyclooxygenase (COX)-2 inhibitors, and salicylates, were extracted from the prescription list. The Partners HealthCare Institutional Review Board approved all aspects of the study, and the usual safeguards for human subjects data were applied, including data encryption, password protection, and elimination of patient identifiers from derived data sets.
Analysis
Patients were considered to be NSAID-exposed if they received at least one documented prescription during the classified antidepressant treatment period, that is, the interval considered by the longitudinal classifier based on mood status in narrative notes (7). The primary analysis compared antidepressant response between patients with chronic NSAID use and patients unexposed to NSAIDs. For consistency with the previous report by Warner-Schmidt et al. (3), our primary analysis examined all NSAID and NSAID-like treatments (referred to as the broad NSAID analysis), while our secondary analysis distinguished between NSAIDs, COX-2 inhibitors, and salicylates.
Crude (unadjusted) odds ratios for the association between NSAID exposure and treatment outcome were calculated, followed by adjustment of odds ratios for potential confounding variables, including age, sex, ethnicity (White versus non-White), and insurance type (public versus private) (referred to as model 1), and finally the addition of comorbidity and health care utilization measures, including body mass index, history of hypertension, history of hyperlipidemia, history of myocardial infarction, history of stroke, history of type 2 diabetes, age-adjusted Charlson comorbidity index, and total number of medical facts (referred to as model 2).
As a secondary analysis in the i2b2 treatment-resistant depression cohort, the NSAID-exposed group was further divided into patients with chronic use and patients with intermittent use. Patients who received more than two NSAID prescriptions (or refills) at a daily dose (not an as-needed basis) within the study period were included in the chronic use group, and patients with two or fewer NSAID prescriptions or those prescribed NSAIDs for as-needed use only were included in the intermittent use group.
STAR*D Cohort
Cohort derivation
Details of the STAR*D methodology have been reported elsewhere (13), as well as the methodology for examining outcomes across multiple periods or levels of treatment (14). Briefly, STAR*D was a multicenter study of antidepressant effectiveness in outpatients with major depressive disorder. Eligible patients began level-1 treatment with citalopram. Those who did not achieve remission or near-remission status by 12 weeks were randomly assigned to up to four sequential next-step treatments or levels. At level 2, following nonremission with citalopram, patients could receive either augmentation (with bupropion, buspirone, or cognitive-behavioral therapy [CBT]) or switch treatments (to sertraline, venlafaxine, bupropion, or CBT). In our analyses, we focused on level-1 citalopram treatment, with one follow-up analysis examining the subset of individuals who received CBT at level 2. The primary outcome measures in STAR*D were the Hamilton Depression Rating Scale (15), for which data were collected at level entry and exit, and the Quick Inventory of Depressive Symptomatology–Self Report (16), for which data were collected at every visit. Since data from the latter measure are more complete, they were used in our primary analysis, which is consistent with other STAR*D reports.
In STAR*D, concomitant medications were recorded at every study visit. Medication exposure was defined as at least one report of use of a medication in a given category at one or more visits during level 1. Medication categories were confirmed by manual review to correct a modest number of misclassifications in the original data set. Medical comorbidity was characterized using the 14-item Cumulative Illness Rating Scale (17, 18), completed at study entry. In this instrument, each of 14 categories (e.g., cardiac, vascular, upper gastrointestinal, and musculoskeletal) is scored on a five-point scale (0–4=from no problem to severe disability). In our analyses, we included scores for neurologic and musculoskeletal symptoms, hypothesizing that they would be most likely to reflect painful symptoms. Since the distribution of these scores was non-normal, with marked right skew, patients were classified based on a score of 0 or ≥1 in each category in the primary analysis. Other forms of coding of the ordinal values in sensitivity analyses did not meaningfully change results.
Analysis
Consistent with the analysis conducted by Warner-Schmidt et al. (3), we began our analysis by dichotomizing patients based on their exposure or lack of exposure to NSAIDs, COX-2 inhibitors, or salicylates during level 1 of STAR*D. We then performed multiple sets of analyses to address the possibility of confounding effects. First, we repeated the original NSAID analyses performed at level 1, with adjustment for potential clinical confounding variables, including baseline severity, age, sex, ethnicity, and treatment setting (primary versus specialty care), referred to as model 1. A second model examined these covariates, as well as medical comorbidity as measured by scores on the Cumulative Illness Rating Scale. Two follow-up analyses further explored the confounding effects of pain. One examined narcotic use among non-NSAID-treated patients. The second examined level-2 outcomes among patients receiving CBT (alone or in combination with citalopram), comparing those with and without NSAID exposure at this level.
Finally, we performed an additional analysis to examine the effect of NSAID intensity. The STAR*D data set did not include measures of medication dosage or frequency, but date of initiation was recorded. We therefore distinguished acute (initiation within 14 days of study entry) from chronic/subacute NSAID treatment and examined its association with remission at level 1 in the two subgroups, compared with placebo.
We used the R statistical software package, version 2.14.1 (www.r-project.org) and Stata, version 10.0 (StataCorp., College Station, Tex.) in these analyses.
Results
The first cohort was drawn from 1,528 patients with at least one billing code diagnosis of major depressive disorder (ICD-9 codes 296.2–296.3) and who were identified as having treatment-resistant or treatment-responsive depression using the natural language processing single-visit and longitudinal classification algorithms. Of this cohort, 1,245 (81%) patients were exposed to NSAIDs or NSAID-like medications, and 283 (19%) were unexposed.
Table 1 summarizes this cohort’s sociodemographic and clinical characteristics. Patients with NSAID exposure were significantly more likely to be older, female, and non-White, as well as to have public rather than private insurance. As anticipated, NSAID-exposed patients also demonstrated significantly greater medical comorbidity and use of health care services.
TABLE 1.
Sociodemographic and Clinical Characteristics of Patients Exposed and Unexposed to Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in the i2b2 Treatment-Resistant Cohort
| Characteristic | NSAID Exposure | No NSAID Exposure | Comparison | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | t | df | p | |
| Age (years) | 1,245 | 53.0 | 15.3 | 283 | 46.9 | 17.6 | 5.4 | 1526 | <0.001 |
| Body mass index (kg/m2) | 991 | 32.5 | 8.2 | 171 | 28.2 | 7.1 | 7.1 | 1160 | <0.001 |
| N | % | N | % | χ 2 | df | p | |||
| Female | 914 | 73 | 175 | 62 | 14.5 | 1 | <0.001 | ||
| Race/ethnicitya | 46.6 | 4 | <0.001 | ||||||
| White | 841 | 68 | 236 | 83 | |||||
| African American | 92 | 7 | 8 | 3 | |||||
| Hispanic | 272 | 22 | 21 | 7 | |||||
| Asian | 14 | 1 | 6 | 2 | |||||
| Other | 26 | 2 | 12 | 4 | |||||
| Insurance | 57.7 | 2 | <0.001 | ||||||
| Public | 651 | 52 | 85 | 30 | |||||
| Private | 558 | 45 | 176 | 62 | |||||
| Other/unknown | 36 | 3 | 22 | 8 | |||||
| Comorbidity | |||||||||
| History of hypertension | 693 | 56 | 80 | 28 | 68.1 | 1 | <0.001 | ||
| History of hyperlipidemia | 777 | 62 | 101 | 36 | 66.3 | 1 | <0.001 | ||
| History of myocardial infarction |
114 | 9 | 2 | 1 | 22.3 | 1 | <0.001 | ||
| History of stroke | 167 | 13 | 9 | 3 | 22.7 | 1 | <0.001 | ||
| History of type 2 diabetes | 371 | 30 | 26 | 9 | 49.9 | 1 | <0.001 | ||
| NSAID and NSAID-like treatment |
|||||||||
| NSAIDs | 1,037 | 83 | |||||||
| COX-2 inhibitors | 202 | 16 | |||||||
| Salicylates | 564 | 45 | |||||||
| NSAIDs or COX-2 inhibitors or salicylatesb |
1,245 | 100 | |||||||
| NSAIDs and COX-2 inhibitors | 171 | 14 | |||||||
| NSAIDs and salicylates | 444 | 36 | |||||||
| COX-2 inhibitors and salicylates |
126 | 10 | |||||||
| NSAIDs and COX-2 inhibitors and salicylates |
111 | 9 | |||||||
| Antidepressant treatment response |
|||||||||
| Resistant | 586 | 47 | 103 | 36 | 10.2 | 1 | 0.001 | ||
| Responsive | 659 | 53 | 180 | 64 | |||||
| N | Median | Interquartile Range |
N | Median | Interquartile Range |
Kruskal-Wallis Nonparametric Statistic |
df | p | |
| Health care utilization | |||||||||
| Medical facts recorded | 1,245 | 639 | 665 | 283 | 228 | 280 | 286754 | 1 | <0.001 |
| Psychiatry visits | |||||||||
| All psychiatric visits | 1,245 | 12 | 17 | 283 | 9 | 14 | 196625 | 1 | <0.001 |
| Depressed visits | 1,245 | 2 | 6 | 283 | 1 | 4 | 206820 | 1 | <0.001 |
| Well visits | 1,245 | 1 | 5 | 283 | 2 | 4 | 160643 | 1 | <0.001 |
| Antidepressant treatment | |||||||||
| Unique antidepressants | 1,245 | 3 | 3 | 283 | 2 | 2 | 235580 | 1 | <0.001 |
| Refills per antidepressant | 1,245 | 7 | 15 | 283 | 3 | 8 | 229724 | 1 | <0.001 |
| Comorbidity | |||||||||
| Age-adjusted Charlson comorbidity index |
1,245 | 6 | 6 | 283 | 2 | 5 | 245851 | 1 | <0.001 |
Data on race and ethnicity were collected using a single field in the electronic medical records; individuals who identified as Hispanic are not further characterized.
Analysis is consistent with that of Warner-Schmidt et al. (3).
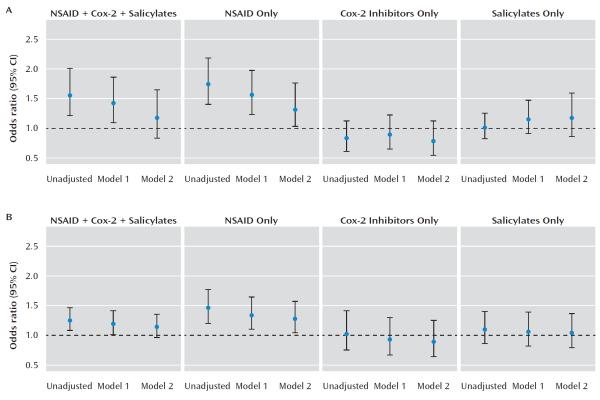
Crude and partially adjusted odds ratios for association between NSAID exposure and outcome category are listed in Table 2 and illustrated in Figure 1. NSAID exposure in the broad analysis was significantly associated with risk for treatment-resistant depression. This effect appeared to be confined to the NSAIDs-only group, since no significant association was observed in the COX-2 inhibitor and salicylate treatment groups (Table 2). In the fully adjusted model (model 2 in Table 2), which incorporated medical comorbidity and health care utilization measures, the beta coefficient for NSAID use changed by more than 10%, a conventional albeit imperfect indicator of confounding (19), and was no longer statistically significant (odds ratio=1.17, 95% confidence interval [CI]=0.83–1.64, p=0.38). When the analysis was limited to NSAIDs only, the effect in the fully adjusted model was likewise reduced but remained significant (odds ratio=1.31, 95% CI=1.03–1.76, p=0.04).
TABLE 2.
Unadjusted and Adjusted Odds Ratios for Treatment Resistance by Medication Class in the i2b2 Treatment-Resistant Cohorta
| Treatment-Resistant Depression |
||||||
|---|---|---|---|---|---|---|
| Medication Class | Unadjusted |
Model 1b |
Model 2c |
|||
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Nonsteroidal anti-inflammatory drugs (NSAIDs) and COX-2 inhibitors and salicylatesd |
1.55 | 1.21–2.00 | 1.42 | 1.09–1.85 | 1.17 | 0.83–1.64 |
| NSAIDs only | 1.74 | 1.40–2.18 | 1.56 | 1.23–1.97 | 1.31 | 1.03–1.76 |
| COX-2 inhibitors only | 0.83 | 0.61–1.12 | 0.89 | 0.65–1.22 | 0.78 | 0.54–1.12 |
| Salicylates only | 1.01 | 0.82–1.25 | 1.15 | 0.91–1.47 | 1.17 | 0.86–1.59 |
The reference group for these analyses is treatment responsive. Likelihood-ratio test compared the fit for model 2 > model 1 > unadjusted (p<0.001 for all comparisons).
The model 1 analysis included age, sex, race, and payer.
The model 2 analysis included model 1 variables as well as log-transformed fact count, age-adjusted Charlson comorbidity index, history of hypertension, history of hyperlipidemia, history of myocardial infarction, history of stroke, and history of type 2 diabetes.
Analysis is consistent with that of Warner-Schmidt et al. (3).
FIGURE 1. Crude and Partially Adjusted Odds Ratios for Association Between Nonsteroidal Anti-Inflammatory Drug (NSAID) Exposure and Outcome Categorya.
a The figure depicts (A) unadjusted and adjusted odds ratios of treatment resistance by medication class in the i2b2 treatment-resistant cohort and (B) unadjusted and adjusted odds ratios of treatment resistance by medication class in the Sequenced Treatment Alternatives to Relieve Depression cohort.
We conducted a secondary analysis in the i2b2 treatment-resistant depression cohort to distinguish between chronic NSAID use and intermittent use. Of the 1,245 patients who were prescribed at least one NSAID, 1,012 (81%) had chronic treatment (>2 prescriptions, excluding as-needed basis only) and 233 (19%) had intermittent treatment (≤2 prescriptions or as-needed basis only prescriptions). Demographic and clinical characteristics were similar between patients with chronic NSAID use and those with intermittent use (see Table S1 in the data supplement accompanying the online edition of this article). In the fully adjusted model (model 2), patients with chronic NSAID use continued to have increased risk for treatment-resistant depression relative to patients without NSAID use (odds ratio=1.47, 95% CI=1.03–1.76, p=0.02), but no increased risk was observed in patients with intermittent use. (For details of the results in the secondary analysis, see Table S2, Table S3, and Figure S1 in the online data supplement.)
We next reanalyzed the STAR*D cohort to better characterize potential confounding variables, particularly those that might mediate confounding by indication (i.e., medical comorbidity). Patients who were exposed or unexposed to NSAIDs or NSAID-like medications differed on most sociodemographic and clinical characteristics (Table 3). As previously reported by Warner-Schmidt et al. (3), without adjusting for confounding variables, NSAID exposure was associated with nonremission at level 1 in STAR*D (crude odds ratio=1.23, 95% CI=1.06–1.44, p<0.01) (Table 4). However, in fully adjusted logistic regression models including terms for Cumulative Illness Rating Scale severity in each category, the effect size was markedly diminished and no longer statistically significant. We observed a pattern similar to that in the i2b2 treatment-resistant depression cohort: the significant effects appeared to be limited to the NSAIDs-only treatment group rather than the NSAID-like treatment groups.
TABLE 3.
Sociodemographic and Clinical Characteristics of Patients Exposed and Unexposed to Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in the Sequenced Treatment Alternatives to Relieve Depression Study
| Characteristic | NSAID Exposure | No NSAID Exposure | Comparison | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | χ 2 | df | p | |||
| Male | 384 | 40 | 1,125 | 37 | 3.69 | 1 | 0.05 | ||
| Race (White) | 732 | 76 | 2,445 | 79 | 4.50 | 1 | 0.03 | ||
| Ethnicity (Hispanic)a | 142 | 15 | 365 | 12 | 5.71 | 1 | 0.02 | ||
| Care setting (primary) | 439 | 46 | 1,136 | 37 | 23.84 | 1 | <0.001 | ||
| Insurance (public) | 248 | 26 | 484 | 16 | 50.29 | 1 | <0.001 | ||
| Cumulative Illness Rating Scale | |||||||||
| Musculoskeletal (≥1 symptom) | 655 | 68 | 1,064 | 35 | 338.56 | 1 | <0.001 | ||
| Neurologic (≥1 symptom) | 352 | 37 | 658 | 21 | 91.04 | 1 | <0.001 | ||
| Treatment | |||||||||
| NSAIDs | 599 | 62 | 0 | 0 | 2253.9 | 1 | <0.001 | ||
| COX-2 inhibitor | 174 | 18 | 0 | 0 | 582.8 | 1 | <0.001 | ||
| Salicylates | 309 | 32 | 0 | 0 | 1072.3 | 1 | <0.001 | ||
| N | Mean | SD | N | Mean | SD | t | df | p | |
| Age (years) | 961 | 46.49 | 13.29 | 3,078 | 39.23 | 12.79 | −15.20 | 1 | <0.001 |
| Baseline Quick Inventory of Depressive Symptomatology–Self Report score |
953 | 15.36 | 4.35 | 3,064 | 15.47 | 4.29 | 0.69 | 1 | 0.49 |
| Cumulative Illness Rating Scale score | |||||||||
| Neurologic symptoms | 961 | 0.49 | 0.74 | 3,080 | 0.28 | 0.60 | −8.71 | 1 | <0.001 |
| Musculoskeletal symptoms | 961 | 1.15 | 0.97 | 3,080 | 0.49 | 0.77 | −21.78 | 1 | <0.001 |
Individuals who identified as Hispanic are not further characterized.
TABLE 4.
Unadjusted and Adjusted Odds Ratios for Nonremission by Medication Class in the Sequenced Treatment Alternatives to Relieve Depression Study Cohorta
| Treatment-Resistant Depression |
||||||
|---|---|---|---|---|---|---|
| Unadjusted |
Model 1b |
Model 2c |
||||
| Medication Class | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI |
| Nonsteroidal anti-inflammatory drugs (NSAIDs) and COX-2 inhibitors and salicylatesd |
1.23 | 1.06–1.44 | 1.17 | 0.99–1.39 | 1.12 | 0.94–1.33 |
| NSAIDs only | 1.44 | 1.18–1.75 | 1.32 | 1.08–1.62 | 1.26 | 1.02–1.55 |
| COX-2 inhibitors only | 1.00 | 0.73–1.39 | 0.91 | 0.65–1.28 | 0.87 | 0.62–1.23 |
| Salicylates only | 1.08 | 0.84–1.38 | 1.04 | 0.80–1.37 | 1.02 | 0.77–1.34 |
| Narcoticse | 1.54 | 1.06–2.24 | 1.44 | 0.99–2.10 | 1.26 | 0.85–1.85 |
| Level 2 (cognitive behavioral therapy) NSAIDs only | 2.42 | 0.85–6.89 | 2.57 | 0.89–7.45 | 2.14 | 0.70–6.57 |
The reference group in the analysis is treatment-responsive.
The model 1 analysis includes age, sex, race, ethnicity, treatment setting, insurance type, and baseline Quick Inventory of Depressive Symptomatology–Self Report scores.
The model 2 analysis includes model 1 variables as well as Cumulative Illness Rating Scale scores for neurologic and musculoskeletal symptoms.
Analyses were consistent with that of Warner-Schmidt et al. (3).
Data are for individuals who did not receive NSAID cotreatment.
To better understand the potential for confounding by pain, we conducted two follow-up analyses. First, we examined narcotic exposure among non-NSAID-treated patients in STAR*D. Although this analysis might carry the risk of the same potential confounding effects observed in the Warner-Schmidt et al. (3) study, it does not consider the same molecular mechanisms. As we expected, narcotic exposure was associated with nonremission (Table 4). We observed similar evidence of confounding; in a fully-adjusted model, the effect was no longer statistically significant.
Second, we examined the effect of NSAID treatment chronicity in STAR*D. We defined a priori a chronic/subacute treatment group (with initiation of NSAID treatment 14 or more days before entering STAR*D, N=337) and an acute treatment group (with initiation of NSAID treatment within 14 days of entering level 1 of STAR*D or during level-1 treatment, N=262). In our comparison of the chronic/subacute treatment group and the no-NSAIDs group, a significant effect on odds of nonremission was observed (fully adjusted model odds ratio=1.44, 95% CI=1.10–1.90, p<0.01). In our comparison of the acute treatment group and the no-NSAIDs group, a substantially more modest and nonsignificant effect was observed (fully adjusted odds ratio=1.07, 95% CI=0.80–1.42, p=0.66).
Finally, we examined NSAID association with response to CBT in level 2 of STAR*D, again to consider an intervention with a presumed different mechanism of action. Among 147 patients receiving CBT (alone or as augmentation of citalopram), 25 (17.0%) also received concomitant NSAID treatment during this level. As observed in the level-1 analyses, NSAID administration was associated with greater likelihood of nonremission in an unadjusted model, with more modest effects in a fully adjusted model, although none of these effects were statistically significant, most likely because of the modest sample size.
Discussion
In this pharmacovigilance study using data from a large health care system, we confirmed a significant association between NSAID exposure and poorer antidepressant treatment outcome in major depressive disorder. In both of the cohorts we analyzed, this effect appeared specific to treatment with NSAIDs rather than with agents that have similar mechanisms of action. Importantly, we also identified evidence of potential confounding effects using measures of medical comorbidity. In a reanalysis of data from the STAR*D study used to support the original report of NSAID risk (3), we likewise identified evidence of confounding using multiple complementary approaches. Notably, the outcomes with CBT at level 2 in STAR*D revealed the same pattern observed for outcomes with citalopram at level 1. This was surprising, since the mechanism of action of CBT is presumably not through direct effects on cytokines. Likewise, citalopram-treated patients who were receiving narcotics at level 1 were also less likely to remit, an effect partially explained by medical comorbidity.
A body of literature suggests that general medical comorbidity is associated with poorer treatment outcomes in major depression (5, 20). In particular, the presence of painful symptoms seems to be associated with greater depression severity and poorer outcomes (4). A portion of the observed effects in both of the cohorts we analyzed appeared to be confounded by this phenomenon. However, a more modest effect remained even after adjustment for these confounding factors.
Two key questions follow from our observations drawn from the two different data sets. The first is whether the observed association, after adjusting for confounding variables, is considered clinically significant and actionable. That is, should clinicians aim to avoid NSAID treatment in depressed patients receiving antidepressant treatment? The finding by Warner-Schmidt et al. (3) is scientifically valuable, even if it does not inform practice, in that it elucidates a putative mechanism of antidepressant effect in vitro. One prediction arising from our data set could be that salicylates and COX-2 inhibitors might be preferable to NSAIDs when indicated for antidepressant-treated patients, absent broader considerations of cost and safety. However, in light of the modest effect size we observed in fully adjusted models, additional investigation in large cohorts or randomized studies is warranted before practice can be altered in this way.
The second question is that of what the appropriate next step in investigation should be. When pilot studies or pharmacovigilance studies identify potential beneficial effects, the gold standard for confirming efficacy is considered to be a randomized controlled trial. On the other hand, when there is preliminary evidence of harm, the next step is less clear. For example, we would probably not proceed with a trial in which some individuals are randomly assigned to smoke cigarettes. At minimum, based on our analysis, there should be sufficient evidence of association to motivate further investigations using large independent data sets, ideally ones with prospectively collected outcomes and detailed exposure data.
A key limitation of our analysis is that, as with any data set that does not include randomization, we cannot fully exclude unmeasured confounding variables. In particular, while we are able to characterize proxy measures of medical comorbidity, electronic medical record data are insufficient to identify specific comorbidities such as pain, which might be more closely associated with NSAID use. In addition, while electronic medical record-based data sets have the advantage of including rich data on medical comorbidity, they often include only limited data on psychiatric comorbidity or on crucial details of pharmacotherapy, such as adherence. Likewise, adequacy of antidepressant treatment can be established by duration (7) but not necessarily by dosage. The precision with which depression treatment outcomes may be assigned in these types of data sets is still less than that of a prospective outcome study that employs systematic use of clinician ratings. The systematic incorporation of scale-based measures in routine clinical practice would greatly improve the utility of future pharmacovigilance studies.
One notable aspect of our analysis could have led to an underestimate of strength of association. In both data sets, there was insufficient detail available to reliably estimate the daily dose, which precludes characterization of dose-response. On the other hand, in both cohorts, we were able to estimate the chronicity of NSAID use. Confidence in our findings is increased by the observation of a stronger effect in the chronic/subacute NSAID-treatment group and little or no effect in the acute NSAID-treatment group.
Despite these limitations, we emphasize the potential utility and efficiency of electronic medical record-based pharmacovigilance systems for rapid identification or confirmation of risk, as well as the consistency of observations between our electronic medical record-based cohort and the more traditionally ascertained STAR*D cohort. Our analysis was initiated within 7 days of the initial report by Warner-Schmidt et al. (3), building on previous investigations using the same methodologies to elucidate association between rosiglitazone and myocardial infarction (8, 21) and between high-serotonin-affinity antidepressants and gastrointestinal bleeding or stroke. By integrating across multiple large data sets, it should be possible to detect early evidence of harm, which might then motivate closer follow-up analysis in more targeted or focused data sets.
Supplementary Material
Clinical Guidance: NSAIDs, Illness, and Antidepressant Resistance.
Coexisting general medical illness accounts for much of the previously reported association between use of nonsteroidal anti-inflammatory drugs (NSAIDs) and nonremission of treated depression. Gallagher et al. found that a modest but smaller relationship for NSAIDs remained when analyses included the comorbidity of patients in a large health care system and in the STAR*D study of antidepressant effectiveness. Resistance to antidepressant treatment is related to long-term NSAID use but not to intermittent use or to use of cyclo-oxygenase-2 (COX-2) inhibitors and salicylates. Shelton in an editorial (p. 1012) suggests that the remaining association between NSAIDs and antidepressant nonresponse is likely due to residual confounding from imperfect measurement of medical conditions or incomplete adjustment for them in analyses.
Acknowledgments
Dr. Fava has received research support from Abbott Laboratories, Alkermes, Aspect Medical Systems, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Forest Pharmaceuticals, GlaxoSmithKline, J & J Pharmaceuticals, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, Novartis, Organon, PamLab, Pfizer, Pharmavite, Roche, Sanofi/Synthelabo, Solvay Pharmaceuticals, and Wyeth-Ayerst Laboratories; he has received advisory/consulting fees from Aspect Medical Systems, AstraZeneca, Bayer AG, Biovail Pharmaceuticals, BrainCells, Bristol-Myers Squibb, Cephalon, Compellis, Cypress Pharmaceuticals, Dov Pharmaceuticals, Eli Lilly, EPIX Pharmaceuticals, Fabre-Kramer Pharmaceuticals, Forest Pharmaceuticals, GlaxoSmithKline, Grunenthal GmBH, Janssen Pharmaceutica, Jazz Pharmaceuticals, J & J Pharmaceuticals, Knoll Pharmaceutical Company, Lundbeck, MedAvante, Neuronetics, Novartis, Nutrition 21, Organon, PamLab, Pfizer, PharmaStar, Pharmavite, Roche, Sanofi/Synthelabo, Sepracor, Solvay Pharmaceuticals, Somaxon, Somerset Pharmaceuticals, and Wyeth-Ayerst Laboratories; and he has received speaking fees from AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Cephalon, Eli Lilly, Forest Pharmaceuticals, GlaxoSmithKline, Novartis, Organon, Pfizer, PharmaStar, and Wyeth-Ayerst Laboratories. Dr. Iosifescu has served as a consultant to CNS Response; he has received grant support from Aspect Medical Systems, Forest Laboratories, Janssen Pharmaceuticals, NARSAD, and NIH; he has received speaker’s honoraria from Eli Lilly, Pfizer, Forest Laboratories, and Reed Medical Education. Dr. Perlis has received research support from Proteus Biomedical; he has received advisory/consulting fees from Eli Lilly, Genomind, Proteus Biomedical, and RidVentures; and he has equity holdings and patents with Concordant Rater Systems.
Supported by the National Library of Medicine award U54LM008748 (to Dr. Kohane) and NIMH grant R01MH-086026 (to Dr. Perlis).
Footnotes
The other authors report no financial relationships with commercial interests.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official view of the National Library of Medicine or the National Institutes of Health.
References
- 1.IMS U.S. Sales and Prescription Information IMS Health. 2009 http://www.imshealth.com/portal/site/ims/menuitem.d248e29c86589c9c30e81c033208c22a/?vgnextoid=9c61ba440c900310VgnVCM10000071812ca2RCRD&cpsextcurrchannel=1.
- 2.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 3.Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc Natl Acad Sci USA. 2011;108:9262–9267. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B, Peterson D, Rutter CM, McGregor M, McCulloch D. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363:2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, Ritz L, Nierenberg AA, Lebowitz BD, Biggs MM, Luther JF, Shores-Wilson K, Rush AJ. STAR*D Study Team: Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354:1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 6.Gibson TB, Jing Y, Smith Carls G, Kim E, Bagalman JE, Burton WN, Tran QV, Pikalov A, Goetzel RZ. Cost burden of treatment resistance in patients with depression. Am J Manag Care. 2010;16:370–377. [PubMed] [Google Scholar]
- 7.Perlis RH, Iosifescu DV, Castro VM, Murphy SN, Gainer VS, Minnier J, Cai T, Goryachev S, Zeng Q, Gallagher PJ, Fava M, Weilburg JB, Churchill SE, Kohane IS, Smoller JW. Using electronic medical records to enable large-scale studies in psychiatry: treatment resistant depression as a model. Psychol Med. 2012;42:41–50. doi: 10.1017/S0033291711000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brownstein JS, Murphy SN, Goldfine AB, Grant RW, Sordo M, Gainer V, Colecchi JA, Dubey A, Nathan DM, Glaser JP, Kohane IS. Rapid identification of myocardial infarction risk associated with diabetes medications using electronic medical records. Diabetes Care. 2010;33:526–531. doi: 10.2337/dc09-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy S, Churchill S, Bry L, Chueh H, Weiss S, Lazarus R, Zeng Q, Dubey A, Gainer V, Mendis M, Glaser J, Kohane I. Instrumenting the health care enterprise for discovery research in the genomic era. Genome Res. 2009;19:1675–1681. doi: 10.1101/gr.094615.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy SN, Weber G, Mendis M, Gainer V, Chueh HC, Churchill S, Kohane I. Serving the enterprise and beyond with informatics for integrating biology and the bedside (i2b2) J Am Med Inform Assoc. 2010;17:124–130. doi: 10.1136/jamia.2009.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 13.Rush AJ, Fava M, Wisniewski SR, Lavori PW, Trivedi MH, Sackeim HA, Thase ME, Nierenberg AA, Quitkin FM, Kashner TM, Kupfer DJ, Rosenbaum JF, Alpert J, Stewart JW, McGrath PJ, Biggs MM, Shores-Wilson K, Lebowitz BD, Ritz L, Niederehe G, STAR*D Investigators Group Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials. 2004;25:119–142. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 14.Perlis RH, Uher R, Ostacher M, Goldberg JF, Trivedi MH, Rush AJ, Fava M. Association between bipolar spectrum features and treatment outcomes in outpatients with major depressive disorder. Arch Gen Psychiatry. 2011;68:351–360. doi: 10.1001/archgenpsychiatry.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 16.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 17.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 18.Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF., 3rd Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 19.Bliss R, Weinberg J, Vieira V, Webster T. Detecting Confounding and Evaluating the 10% Rule. the Proceedings of the Joint Statistical Meetings; Miami Beach, Fla. 2011. [Google Scholar]
- 20.Iosifescu DV, Nierenberg AA, Alpert JE, Smith M, Bitran S, Dording C, Fava M. The impact of medical comorbidity on acute treatment in major depressive disorder. Am J Psychiatry. 2003;160:2122–2127. doi: 10.1176/appi.ajp.160.12.2122. [DOI] [PubMed] [Google Scholar]
- 21.Castro VM, Gallagher PJ, Clements CC, Murphy SN, Gainer VS, Fava M, Weilburg JB, Churchill SE, Kohane IS, Iosifescu DV, Smoller JW, Perlis RH. Incident user cohort study of risk for gastrointestinal bleed and stroke in individuals with major depressive disorder treated with antidepressants. BMJ Open. 2012;2:e000544. doi: 10.1136/bmjopen-2011-000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.