Abstract
Immunodeficient patients with severe burn injuries are extremely susceptible to infection with Candida albicans (C. albicans). In addition to Th1 cells, IL-17-producing CD4 + T cells (Th17 cells) have recently been described as an important effector cell in host anti-Candida resistance. In this study, therefore, we tried to induce Th17 cells in cultures of severely burned patient PBMC by stimulation with the C. albicans antigen (CAg). In the results, the biomarkers for Th17 cells (IL-17 production and intracellular expression of IL-17 and RORγt) were not displayed by burn patient PBMC stimulated with CAg, while these biomarkers of Th17 cells were detected in cultures of healthy donor PBMC stimulated with CAg. Burn patient sera were shown to be inhibitory on CAg-stimulated Th17 cell generation in healthy donor PBMC cultures; however, Th17 cells were induced by CAg in healthy donor PBMC cultures supplemented with burn patient sera that were previously treated with anti-IL-10 mAb. Also, the biomarkers of Th17 cells were not induced by CAg in healthy donor PBMC cultures supplemented with rIL-10. IL-10 was detected in serum specimens derived from severely burned patients. These results indicate that Th17 cells are not generated in burn patient PBMC cultures supplemented with CAg. IL-10, produced in response to burn injuries, is shown to be inhibitory on Th17 cell generation. The high-susceptibility of severely burned patients to C. albicans infection might be influenced if burn-associated IL-10 production is intervened.
Keywords: T cells, Cytokines, Cellular Activation
Introduction
Despite major advances in the care of severely burned patients, infectious complications remain a leading cause of morbidity and mortality (1, 2). Fungal elements are histopathologically detected in 44% of autopsied patients with an attributable mortality of 33% (3). Adequately managing fungal infections is very important to burned patients with total body surface area (TBSA) burn of more than 30% because high mortality caused by fungal infections was demonstrated in patients with high TBSA burns (4, 5). Candida albicans (C. albicans) infection, especially, causes a high mortality rate in patients with extensive burn injuries (6).
In addition to Th1 cells, IL-17-producing CD4+ T cells (Th17 cells) have been recently described as an anti-Candida effector cell in the host's antifungal resistance (7). In response to stimulation with C. albicans Ag (CAg)3, some of the human peripheral blood CD4+ T cells converted to Th17 cells (8). IL-17, produced by Th17 cells, recruits neutrophils into infected site tissues and increases the antibacterial functions of recruited neutrophils (9, 10). Also, IL-17 enhances the function of epithelial cells, endothelial cells, and macrophages to produce pro-inflammatory cytokines and chemokines, which are factors involved in systemic inflammatory response syndrome (9). These soluble factors contribute to the local eradication of infected cells, because the antimicrobial peptide production by endothelial cells is stimulated with IL-17 (9, 11). IL-17 receptor knockout mice have been described as susceptible mice to C. albicans infection, while normal mice treated with murine IL-17A encoding adenovirus are described as resistant mice against a lethal dose of C. albicans infection (12). Also, hyper-IgE syndrome patients, who are Th17 cell deficient patients, have been reported as susceptible hosts to C. albicans infection (13). Furthermore, inadequate amounts of IL-17 are shown to be produced by chronic mucocutaneous candidiasis patient PBMC (14). All these findings indicate that IL-17, or IL-17-producing CD4+ T cells (Th17 cells), are very important host defense functions against C. albicans infections.
In this study, we tried to generate Th17 cells in cultures of severely burned patient PBMC after stimulation with CAg. However, Th17 cells were not demonstrated in cultures of severely burned patient PBMC. IL-10, a cytokine commonly detected in the sera of burn patients, was shown to be inhibitory on the generation of Th17 cells. The lack of Th17 cell generation in severely burned patients may explain a part of their increased susceptibility to C. albicans infections.
Materials and Methods
Burn patients
A total of 26 patients with 3rd degree thermal injuries (17 males and 9 females), inpatients and outpatients of Shriners Hospital for Children at Galveston and The University of Texas Medical Branch from May 2008 to August 2010, were enrolled in this study (Table I). These patients were adults (40 ± 7.0 years old) and children (6.2 ± 0.9 years old), with the mean TBSA burn of 56.7 ± 3.4% (see Table I). Blood specimen was obtained once from each patient. All patients were subjected to our standard therapeutic protocol, which includes early excision of burn wound, systemic antibiotic therapy, and continuous enteral feeding (15). Within 48 h of admission, each patient underwent total burn wound excision and grafting with autograft skin. Patients returned to the operating room for reharvesting once the autograft donor sites healed (usually 6 to 10 days). Sequential staged surgical procedures for repeat excision and grafting were undertaken until the burn wounds were healed. Patients #1 through #10 were in the acute burn phase (within 1 week of burn injury) and patients #11 through #26 were in the chronic burn phase (over 2 weeks after burn injury). Blood samples were individually withdrawn on 0 (on the admission day, inpatients) to 1488 days (outpatients) after burn injury (Table I). As controls, blood specimens were obtained from 5 healthy donors (3 males and 2 females, the mean age; 30.8 ± 4.1 years old), and subjected to the same assays.
Table I. Burn patients and healthy donors enrolled in the study.
| 1. Healthy donors | |||||
|---|---|---|---|---|---|
|
| |||||
| Donor No. | 1 | 2 | 3 | 4 | 5 |
| Sex (M/F) | M | M | F | M | F |
| Age | 35 | 23 | 45 | 26 | 25 |
| IL-10 (pg/ml) | 10 | 40 | 22 | 8 | 15 |
| 2. Acute phase burn patientsa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Patient No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Sex (M/F) | M | M | M | M | M | F | M | F | M | F |
| Age | 54 | 28 | 28 | 8 | 2 | 4 | 4 | 2 | 2 | 2 |
| TBSA burn (%) | 38 | 80 | 80 | 70 | 35 | 40 | 40 | 40 | 36 | 60 |
| 3rd degree (%) | 38 | 80 | 80 | 70 | 35 | 40 | 40 | 40 | 36 | 60 |
| 2nd degree (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Days after burn injuryb | 0 | 0 | 0 | 1 | 2 | 3 | 3 | 3 | 3 | 4 |
| IL-10 (pg/ml)c | 120 | 76 | 80 | 145 | 120 | 220 | 180 | 250 | 560 | 868 |
| 3. Chronic phase burn patientsa | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Patient No. | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 |
| Sex (M/F) | M | M | F | M | M | F | F | M | F | F | M | M | M | M | F | M |
| Age | 4 | 50 | 3 | 10 | 15 | 4 | 12 | 14 | 3 | 3 | 4 | 8 | 15 | 3 | 9 | 6 |
| TBSA burn (%) | 42 | 80 | 33 | 61 | 55 | 58 | 49 | 85 | 46 | 52 | 84 | 44 | 53 | 83 | 65 | 65 |
| 3rd degree (%) | 42 | 80 | 33 | 37 | 45 | 58 | 34 | 85 | 46 | 52 | 74 | 37 | 53 | 73 | 65 | 60 |
| 2nd degree (%) | 0 | 0 | 0 | 24 | 10 | 0 | 15 | 0 | 0 | 0 | 10 | 7 | 0 | 10 | 0 | 5 |
| Days after burn injuryb | 14 | 17 | 22 | 24 | 29 | 30 | 212 | 305 | 363 | 434 | 449 | 586 | 694 | 704 | 829 | 1488 |
| IL-10 (pg/ml)c | 620 | 880 | 640 | 440 | 380 | 675 | 646 | 310 | 225 | 145 | 220 | 176 | 120 | 180 | 140 | 120 |
All patients were subjected to our standard therapeutic protocol, including early excision of burn wound, systemic antibiotic therapy, and continuous enteral feeding.
Day of blood sample collection relative to day of injury; day 0 = same day as injury, day 1 = 1 day after injury. Blood samples were taken once from each patient.
The amounts of IL-10 in sera of these patients were measured by ELISA.
Reagents and C. albicans
FITC-labeled anti-human IL-17A, PE-labeled anti-human retinoic acid receptor-related orphan receptor γt (RORγt), and isotype control monoclonal antibodies (mAbs) were purchased from eBioscience (San Diego, CA). Recombinant human IL-10 (rIL-10) and Cytofix/Cytoperm plus Fixation/permeabilization kit with GolgiStop were purchased from BD Biosciences (San Diego, CA). Anti-human IL-10, anti-human IL-4, anti-human CCL2 and anti-human CD3 mAbs were purchased from Biolegend (San Diego, CA). ELISA kits for IL-17A and IL-10 were purchased from Biolegend. For the preparation of CAg, C. albicans serotype A (ATCC 36801) was grown for 2 days at 27°C in yeast peptone dextrose broth. The C. albicans cells were washed twice with PBS, and then resuspended in RPMI-1640 medium plus 10% heat-inactivated FBS. These yeast cells were incubated for 2 h at 37°C to promote yeast hyphal transition. Obtained C. albicans hyphae was killed by heating (65°C, 2 h). The heat-killed C. albicans hyphae was designated as CAg. In the experiments, PBMC were stimulated with a constant amount of CAg (corresponded to 105 CFU/ml of viable C. albicans) for 1 to 5 days.
Isolation of PBMC
PBMC were isolated from heparinized whole blood using Ficoll-Hypaque density gradient centrifugation. For the cultivation of PBMC, RPMI-1640 medium supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (complete medium) was utilized.
Induction of Th17 cells
IL-17 (a biomarker of Th17 cells) was produced in cultures of PBMC stimulated with CAg. Also, CD4+ T cells in cultures of PBMC stimulated with CAg were detected by FACSCanto as cells expressing intracellular IL-17 and RORγt. When IL-17+ RORγt+ CD4+ T cells with abilities to produce IL-17 were detected in CAg-stimulated PBMC preparations, we considered these cell preparations to include Th17 cells.
Production and assay of IL-17. To determine the ability of PBMC to produce IL-17, PBMC (2 × 106 cells/ml) from 26 burn patients were individually stimulated with CAg or anti-CD3 mAb (2.5 μg/ml). As controls, PBMC from 5 healthy donors were stimulated with CAg in the same conditions. Culture fluids were harvested 1 to 5 days after cultivation and assayed for IL-17 by ELISA. In our assay system, the detection limit was 8 pg/ml for IL-17.
Intracellular staining for IL-17 and RORγt. To determine the percentage of CD4+ T cells that expressed intracellular IL-17 and RORγt in PBMC, PBMC preparations (2 × 106 cells/ml) were stimulated with CAg for 3 days. Then, CD4+ T cells were isolated from these cells using magnetic beads and cultured for an additional 3 h with GolgiStop (0.7 μg/ml). The cells obtained were washed twice with PBS, incubated with Cytofix/Cytoperm solution at 4°C for 20 min and then washed with Perm/Wash solution. The cells were incubated with FITC-labeled anti-IL-17A mAb and PE-labeled anti-RORγt mAb, or isotype control mAb at 4°C for 30 min. After washing, the cells were analyzed for intracellular IL-17A and RORγt expression by FACSCanto.
Effect of burn patient sera on Th17 cell generation
To determine the inhibitory effect of burn patient sera on Th17 cell generation, PBMC (2 × 106 cells/ml) from 5 healthy donors were individually cultured for 3 days with CAg in media supplemented with 15% healthy donor #3 serum, or sera from burn patients # 10, #11, #12, #15 and #17. Culture fluids harvested from the above cultures were assayed for IL-17 by ELISA, and cells harvested were analyzed for intracellular IL-17 and RORγt expression by FACSCanto. In the same fashion, the inhibitory effect of IL-10, IL-4 and CCL2 on Th17 cell generation were assayed in cultures of healthy donor PBMC supplemented with sera from burn patients # 10, #11, #12, #15 and #17 that were previously treated with 2.5 μg/ml of anti-IL-10 mAb, anti-IL-4 mAb, anti-CCL2 mAb or isotype control Ab (rat IgG2a). To determine the effect of IL-10 on Th17 cell generation, healthy donor PBMC (2 × 106 cells/ml) were stimulated with CAg in the presence of rIL-10 (0.1, 1, or 10 ng/ml). Culture fluids harvested 1 to 5 days after stimulation were assayed for IL-17 by ELISA, and cells harvested 3 days after the stimulation were analyzed for intracellular IL-17 and RORγt expressions by FACSCanto.
Statistical analyses
Statistical analyses were performed using Prism 4.0 software (Graphpad, San Diego, CA). Analysis of variance with post hoc Bonferroni correction and unpaired Student's t test were used. Data are shown as the mean ± SEM. p< 0.05 was considered to statistically significant.
Results
Impaired generation of Th17 cells in cultures of severely burned patient PBMC
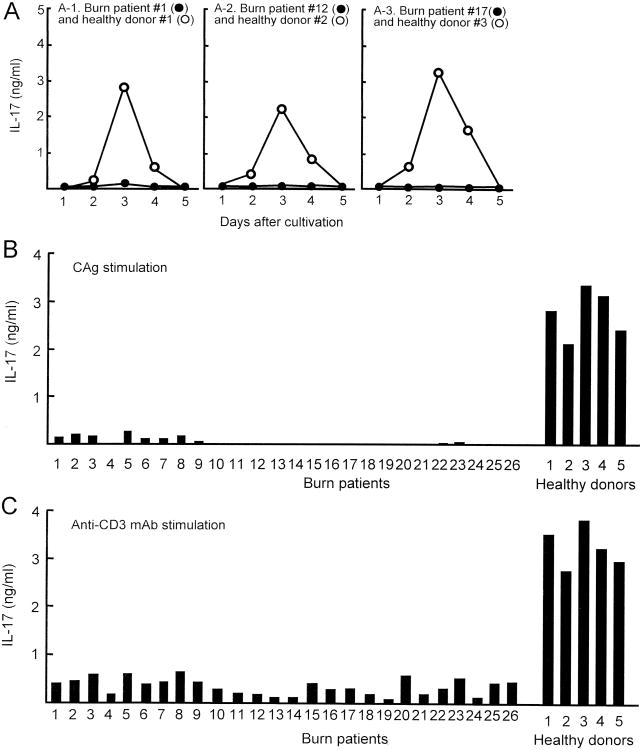
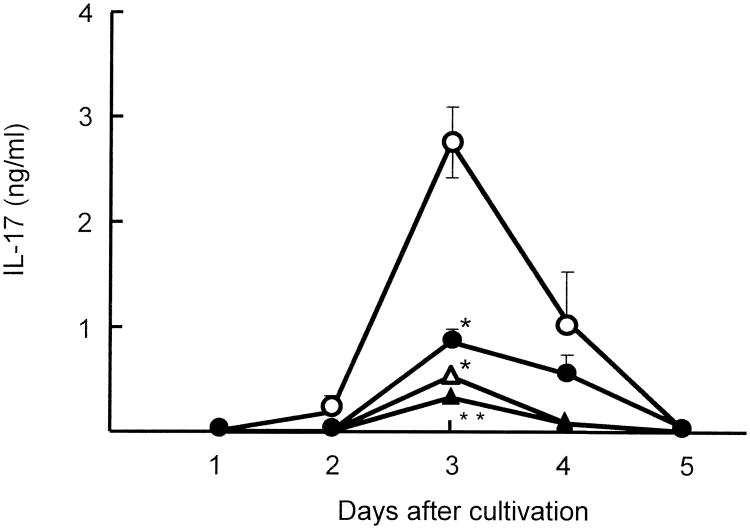
PBMC (2 × 106 cells/ml), isolated from 3 burn patients (#1, acute phase burn; #12 and #17, chronic phase burn) and 3 healthy donors (#1, #2 and #3), were stimulated with CAg in cultures for 1 to 5 days. Culture fluids harvested were assayed for IL-17. The production of IL-17 by healthy donor PBMC peaked 3 days after cultivation, but declined to undetectable levels within 5 days of cultivation. However, IL-17 was not produced in cultures of burn patient PBMC stimulated with CAg (Fig. 1A). In the next experiments, all 26 burn patient PBMC and 5 healthy donor PBMC were stimulated with CAg or anti-CD3 mAb (2.5 μg/ml) for 3 days. Culture fluids harvested were assayed for IL-17. In the results, IL-17 was not produced by any in the 16 chronic phase burn patient PBMC stimulated with CAg (Fig. 1B). After stimulation with CAg, PBMC from acute phase burn patients produced 0 to 0.32 ng/ml of IL-17 (Fig. 1B). During the acute phase burn injuries, production of various cytokines has been demonstrated (16). Healthy donor PBMC stimulated with CAg produced 2.2 to 3.4 ng/ml of IL-17 (Fig. 1B). After stimulation with anti-CD3 mAb, chronic phase burn patient PBMC produced less than 0.56 ng/ml of IL-17 into their culture fluids (Fig. 1C). IL-17 at amounts of 0.1 to 0.75 ng/ml was produced by all patient PBMC when they were stimulated with anti-CD3 mAb, while 2.7 to 3.8 ng/ml of IL-17 were produced by healthy donor PBMC stimulated with anti-CD3 mAb. These results indicate that, in cultures of PBMC isolated from burn patients, IL-17-producing cells are not generated, significantly, after stimulation with CAg.
Figure 1.
IL-17 production by healthy donor and burn patient PBMC stimulated with CAg. Blood samples were individually collected from burn patients (# 1 to # 26) and healthy donors (#1 to #5) (see Table I). A. PBMC (2 × 106 cells/ml), derived from 3 burn patients (#1, #12, and #17) and 3 healthy donors (#1, #2 and #3), were stimulated with CAg in vitro. Culture fluids harvested 1 to 5 days after stimulation were assayed for IL-17. B and C. A total of 31 PBMC samples (26 burn patient PBMC and 5 healthy donor PBMC) were stimulated with CAg (B) or anti-CD3 mAb (C). Culture fluids harvested 3 days after stimulation were assayed for IL-17.
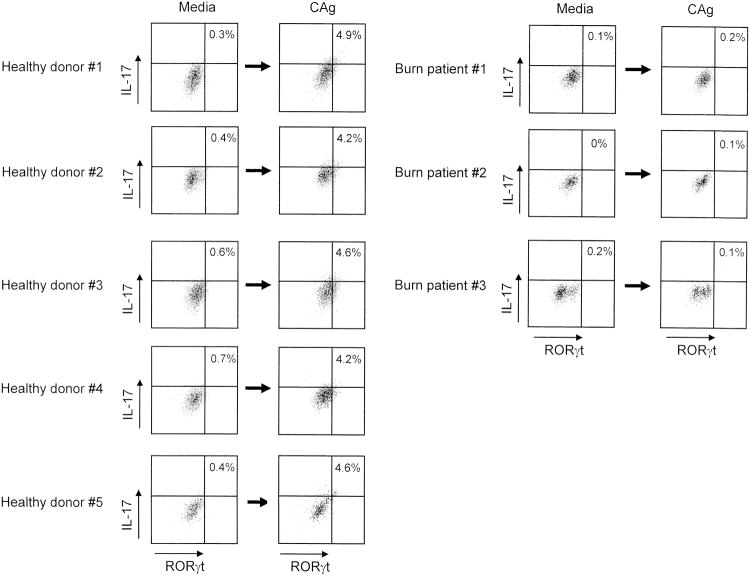
In the next experiments, PBMC (2 × 106 cells/ml) from burn patient #10 and healthy donor #1 were stimulated with CAg for 3 days and analyzed for IL-17+ RORγt+ CD4+ T cells by flow cytometry. The same experiments were performed using healthy donor #2, #3, #4 and #5 and burn patients #11 and #15. Fig. 2 shows the percentage of IL-17+ RORγt+ cells in these two groups of CD4+ T cells. After stimulation with CAg, the increased generation (9.4 fold increase) of IL-17+ RORγt+ cells (%) was demonstrated in cultures of healthy donor CD4+ T cells. However, the generation of IL-17+ RORγt+ CD4+ T cells did not increase in cultures of burn patient PBMC stimulated with CAg. These results indicate that Th17 cells are not easily generated in burn patient PBMC cultures supplemented with CAg.
Figure 2.
Detection of IL-17+ RORγt+ CD4+ T cells in cultures of PBMC stimulated with CAg. PBMC (2 × 106 cells/ml) isolated from 3 burn patients (#10, #11 and #15) and 5 healthy donors (#1 to #5) were stimulated with CAg or without for 3 days. Then, CD4+ T cells isolated from these cells were analyzed for IL-17 and RORγt by flow cytometry.
Burn patient sera are inhibitory on Th17 cell generation
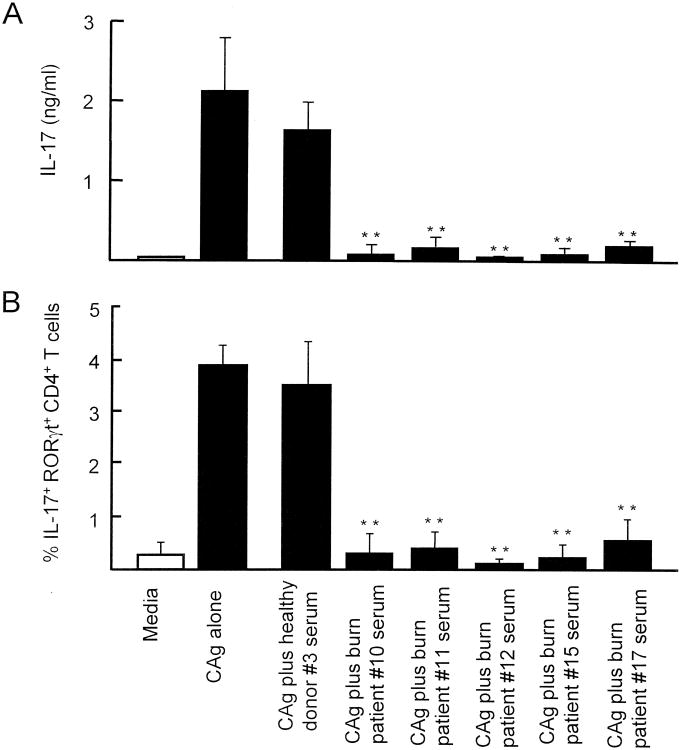
The effect of burn patient sera on Th17 cell generation in CAg-stimulated healthy donor PBMC cultures was examined. PBMC (2 × 106 cells/ml) from healthy donor #1 were cultured with media supplemented together with CAg and sera (15%) from burn patients #10, #11, #12, #15 and #17 for 3 days. The amount of patient serum added to the culture media was decided based on a dose-response curve shown in our preliminary studies. Various concentrations of patient #9 serum were added to the cultures of healthy donor PBMC stimulated with CAg. After cultivation, the ability of these PBMC to produce IL-17 was determined. In the results, 1.8 to 2.5 ng/ml of IL-17 were produced by 2 × 106 cells/ml of PBMC stimulated with CAg, and the similar amounts of IL-17 were produced by the PBMC cultured with 2.5 to 5% (v/v) of patient serum. However, IL-17 production was clearly reduced in PBMC cultures supplemented with 10 to 20% patient serum. Due to the limited amount of patient serum, we have utilized the patient serum at a concentration of 15% throughout the experiments. As a control, the same PBMC were cultured with media supplemented with healthy donor #3 serum in the same fashion. Culture fluids harvested were tested for IL-17 by ELISA, and cells harvested from the cultures were analyzed for intracellular IL-17 and RORγt by flow cytometry. The same experiments were performed using PBMC from healthy donors #2, #3, #4 and #5. Obtained results were combined and displayed in Figures 3A (IL-17 production) and 3B (intracellular expression of IL-17 and RORγt) as a mean ± SEM. In the presence of burn patient sera, IL-17 was not produced by healthy donor PBMC stimulated with CAg (Fig. 3A). Also, the generation of IL-17+ RORγt+ CD4+ T cells were not increased in CAg-stimulated healthy donor PBMC cultures supplemented with burn patient sera (Fig. 3B). These results indicate that soluble factors present in the burn patient serum are inhibitory on CAg-stimulated generation of Th17 cells in PBMC cultures.
Figure 3.
Effect of burn patient sera on CAg-stimulated Th17 cell generation in healthy donor PBMC cultures. PBMC (2 × 106 cells/ml) isolated from healthy donors (#1 to #5) were individually cultured with (1) media alone, (2) media plus CAg, (3) media plus CAg and healthy donor #3 serum (15%), (4-8) media plus CAg and sera (15%) from burn patients #10, #11, #12, #15 and #17. Culture fluids harvested 3 days after cultivation were assayed for IL-17 (A). Cells harvested 3 days after cultivation were analyzed for their intracellular expression of IL-17 and RORγt by flow cytometry (B). The results obtained were combined and displayed in the figure by the mean ± SEM of the 5 experiments. ** p<0.01 vs. PBMC cultured with CAg and healthy donor serum.
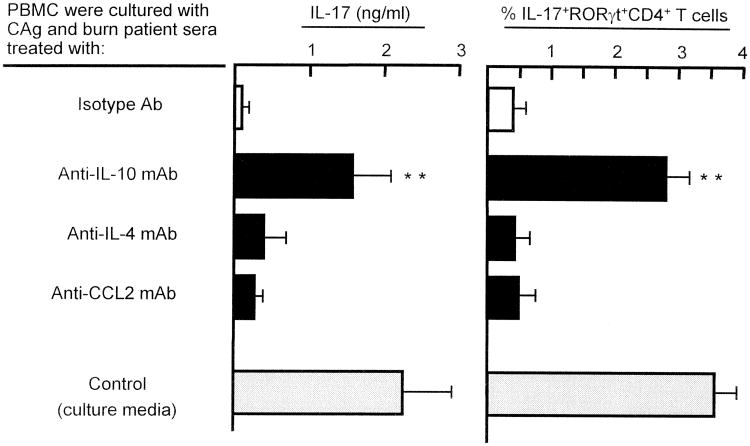
In previous studies, IL-10, IL-4 and CCL2 were detected in the sera of patients with acute and chronic phase burn injuries (17-19). Therefore, the role of IL-10, IL-4 and CCL2 on CAg-stimulated Th17 cell generation in healthy donor PBMC cultures was examined. Thus, in the presence of CAg, healthy donor #1 PBMC (2 × 106 cells/ml) were cultured with media supplemented with sera from burn patients #10, #11, #12, #15 and #17 that were previously treated with 2.5 μg/ml of anti-IL-10 mAb, anti-IL-4 mAb, or anti-CCL2 mAb. Culture fluids harvested 3 days after cultivation were assayed for IL-17 by ELISA, and cells harvested from these cultures were analyzed for intracellular IL-17 and RORγt by flow cytometry. The same experiments were performed using PBMC from healthy donors #2, #3, #4 and #5. Obtained results were combined and displayed in Figure 4 as the mean. By CAg stimulation, IL-17+CD4+RORγt+ T cells were generated in healthy donor PBMC cultures supplemented with burn patient sera previously treated with anti-IL-10 mAb, while these cells were not demonstrated in the same cultures supplemented with burn patient sera that were treated with mAb directed against IL-4 or CCL2. These results suggest that IL-10 present in burn patient sera is inhibitory on CAg-stimulated generation of Th17 cells in PBMC cultures.
Figure 4.
Generation of Th17 cells in healthy donor PBMC cultures supplemented with burn patient sera previously treated with various mAbs. Five PBMC preparations (2 × 106 cells/ml) isolated from healthy donors (#1 to #5) were individually cultured with CAg and sera from burn patients #10, #11, #12 and #15. Before being used to cultures, burn patient sera were treated with 2.5 μg/ml of anti-IL-10, anti-IL-4, anti-CCL2 mAb or isotype control Ab (rat IgG2a) for 30 min at 4°C. Culture fluids harvested 3 days after stimulation were assayed for IL-17, and cells harvested from these cultures were analyzed for intracellular expression of IL-17 and RORγt by flow cytometry. The results obtained were combined and displayed in the figure by the mean ± SEM of the 5 experiments. ** p<0.01 vs. PBMC cultured with CAg and burn patient serum treated with isotype control Ab.
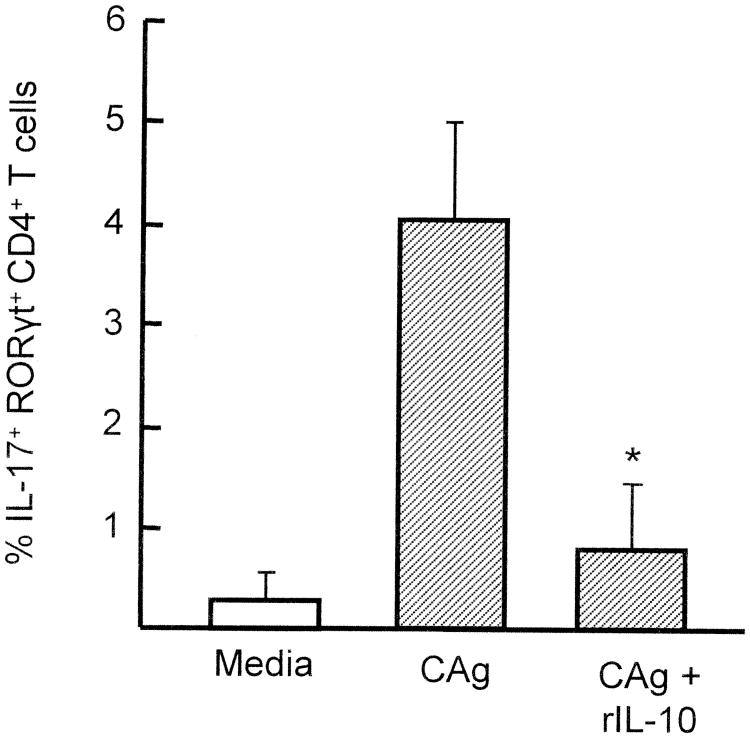
As shown in Table I, IL-10 was detected in sera of all burn patients. Therefore, in the next experiments, the inhibitory effect of rIL-10 on CAg-stimulated generation of Th17 cells in healthy donor PBMC cultures was confirmed. Thus, PBMC (2 × 106 cells/ml) from healthy donor #4 were stimulated with CAg in the presence of various doses of rIL-10 for 1 to 5 days. Culture fluids harvested were assayed for IL-17 by ELISA. In the results, IL-17 was not produced in CAg-stimulated healthy donor #4 PBMC cultures supplemented with rIL-10 (Fig. 5). The inhibitory effect of rIL-10 on IL-17 production by CAg-stimulated healthy donor PBMC was shown as dose-dependent. Also, the generation of IL-17+RORγt+ CD4+ T cells was greatly decreased by 80% when healthy donor PBMC were cultured with 10 ng/ml of rIL-10 (Fig. 6). These results indicate that rIL-10 inhibits CAg-stimulated generation of Th17 cells in healthy donor PBMC cultures.
Figure 5.
Effect of rIL-10 on Th17 cell generation in cultures of healthy donor PBMC stimulated with CAg. Five PBMC preparations (2 × 106 cells/ml) isolated from healthy donors #1,#2, #3, #4 and #5 were individually cultured with CAg and various doses of rIL-10 (none; open circles, 0.1 ng/ml; closed circles, 1 ng/ml; open triangles, 10 ng/ml; closed triangles). Culture fluids harvested 1 to 5 days after stimulation were assayed for IL-17. The results obtained were combined and displayed to the figure by the mean ± SEM of the 5 experiments. * p<0.05, ** p<0.01 vs. cultured with media alone.
Figure 6.
CAg-stimulated generation of IL-17+ RORγt+ CD4+ T cells in healthy donor PBMC cultures supplemented with rIL-10. Five PBMC preparations (2 × 106 cells/ml) isolated from healthy donors #1, #2, #3, #4 and #5 were individually stimulated with CAg in the presence of rIL-10 (10 ng/ml) for 3 days. Harvested cells were analyzed for their intracellular expression of IL-17 and RORγt by flow cytometry. The obtained results were combined and displayed to the figure as mean ± SEM for IL-17+ RORγt+CD4+ T cells. * p<0.05 vs. PBMC cultured with media added without rIL-10.
Discussion
C. albicans is the most significant human fungal pathogen. Although C. albicans is normally commensal, candidiasis may develop in immunosuppressed hosts (20). A major factor in host resistance against systemic C. albicans infection is Th1 cell-mediated immunity (21). In response to the specific antigen, IL-2 and IFN-γ produced by Th1 cells stimulate cytocidal activities of various effector cells against cells infected with C. albicans (21). However, Th1 responses are suppressed by IL-10 (22), which is a regulatory cytokine produced by regulatory T cells, monocytes/macrophages, B cells and some other T cell populations. We have demonstrated that severely burned mice were 50 times or more susceptible to the systemic infection with C. albicans than that of normal mice (23). A predominance of Th2 cells in humans and rodents following severe burn injury has been demonstrated (23-25).
Th17 cells (IL-17+RORγt+ T cells), a new subset of CD4+ T cells, are additionally described as host anti-Candida effector cells (7, 12). In healthy conditions, Th17 cells are generated by the CAg stimulation from CD4+ T cells (8, 12). IL-17 produced by Th17 cells directly and indirectly induces neutrophil recruitment and activation (10, 26). Indeed, IL-17 deficiencies are associated with the defects of neutrophil responses (27). Due to the neutrophil dysfunction, IL-17 receptor knockout mice are highly susceptible to C. albicans infection (12).
In this study, we tried to generate Th17 cells by CAg stimulation in cultures of burn patient PBMC. To detect Th17 cells, three different biomarkers (IL-17 production, intracellular expression of IL-17 and RORγt) were utilized. RORγt is a key transcription factor for the generation of Th17 cells, and transcription of the genes encoding IL-17 is induced by RORγt (8). In the results, CD4+ T cells with abilities to produce IL-17 and express intracellular IL-17 and RORγt, were generated in cultures of healthy donor PBMC stimulated with CAg. However, cells with abilities to produce IL-17 and express RORγt were not induced by the CAg in cultures of chronic phase burn patient PBMC. It has been described in previous papers (3, 28-30) that severely burned patients who survive longer after burn injury have a higher incidence of candidiasis. In contrast, PBMC from acute phase burn patients produced small amounts of IL-17 under CAg stimulation. These results suggest that the generation of Th17 cells is impaired in peripheral blood of severely burned patients exposed to C. albicans infection.
Neutrophils treated with 100 ng/ml or more of IL-17 increased their ability to kill Streptococcus pneumoniae (10). Also, the candidacidal activities of neutrophils were enhanced when they were treated with 1 ng/ml or more of IL-17 (31). These results indicate that 1 ng/ml or more doses of IL-17 are required for the direct activation of neutrophils. Other reports described that 100 ng/ml of IL-17 is needed to induce IL-8 production by epithelial cells. Since IL-8 is known to be a major activator of neutrophils, this suggests that 100 ng/ml of IL-17 is required for indirect activation of neutrophils. Recent paper (16) described that IL-17 is detected in the plasma of patients 0 to 55 days postburn injury. However, the amounts of IL-17 detected in plasma of burn patients were 3 to 9 pg/ml. Therefore, the influence of Th17 cells appeared in response to burn injury on the antifungal activities of neutrophils is minimal.
It is well known that pro- and anti- inflammatory cytokine production is elevated following burn injuries (16, 32, 33). Among these cytokines, IL-10 is associated with frequent occurrence of sepsis and high mortality rate in severely burned patients (34). During infection, IL-10 inhibits the activity of Th1 cells, NK cells and macrophages, all of which play a role on the optimal clearance of the invading pathogen (35). IL-10 allows long-term escape of C. albicans from antifungal host defense and allows persistent infection of the pathogen (36). Recently, the role of IL-10 on Th17 cell generation has been described (37-39). After stimulation with IL-23, IL-6 and TGF-β, IL-17 production and intracellular RORγt expression were greatly increased in cultures of splenic CD4+ T cells isolated from IL-10-knockout mice. When these cells were cultured with IL-23 in the presence of rIL-10, the generation of Th17 cells was clearly reduced (37, 38). Also, IL-17 production was not induced by IL-6, TGF-β and IL-23 in human CD4+ T cell cultures supplemented with rIL-10 (39). These findings strongly suggest that IL-10 is inhibitory on Th17 cell generation.
In this study, IL-17+RORγt+ CD4+ T cells were not generated in healthy donor PBMC cultures supplemented with the burn patient serum. However, these effector cells were generated in healthy donor PBMC cultures supplemented with the same patient serum previously treated with anti-IL-10 mAb. In addition, Th17 cell generation was dose-dependently suppressed by rIL-10 in cultures of healthy donor PBMC stimulated with CAg. These results indicate that IL-10 present in burn patient serum plays a role on the decreased generation of Th17 cells in PBMC cultures. IL-10 has been widely demonstrated in the sera of severely burned patients (40-42). Th2 cells, M2Mφ and PMN-II have been described as sources of IL-10 in these patients (40-42).
The impaired generation and function of Th1 cells in severely burned patients have been well-documented. In severely burned patients lacked with Th1 responses, Th2 responses were shown to develop aggressively (24, 25). Some of Th2 cells were known to remain for a long time in chronic phase-burn patients as IL-4+ cells (43, 44). It has been described in many papers that the generation of Th2 cells occurs under stimulation with small amounts of IL-4 released from IL-4+ cells (45-47). In chronic phase-burn patients who were invaded with pathogens, Th2 responses were easily developed in association with minimal amounts of IL-4 released from IL-4+ cells. In our recent experiments, IL-4+ T cells were detected in the CD3+ cell population derived from patients 434 to 586 days after burn injury. In cultures of PBMC derived from these chronic burn patients, Th2 cells were predominantly induced by CAg stimuli. Since Th2 cells are major IL-10 producer cells, IL-4+ T cells with the ability to produce small amounts of IL-4 may be involved in inhibiting Th17 cell responsiveness in chronic phase-burn patients.
In this study, the inhibition of Th17 cell responsiveness was demonstrated in severely burned patients. This phenomenon may not be particularly attributable to the pathology of burn injury. Physiological threat such as surgery and trauma, and general burn care treatment may be involved in the impaired Th17 cell responsiveness. Since all patients enrolled in this study were diagnosed with SIRS, but not sepsis, the influence of sepsis on Th17 cell responsiveness is unknown. To clarify the correlation between clinical outcome and suppression of Th17 cell responsiveness in severely burned patients, further studies are required.
In conclusion, IL-17+ RORγt+ CD4+T cells were not induced by the CAg stimulation in cultures of burn patient PBMC. IL-10, which was widely present in sera of severely burned patients, was shown to be inhibitory on Th17 cell generation in PBMC cultures. IL-10 was shown to be responsible for the lack of Th17 cells in patients with severe burn injury, this cytokine contributed to the homeostasis of excessive inflammation in such patients. Immunological intervention of IL-10 production should be performed under the careful monitoring of the balance of inflammatory and anti-inflammatory parameters.
Footnotes
This work was supported in part by Shriners of North American Grant 8840 (to F.S.), National Institutes of Health Grant P50 GM060338 (to DNH), and a James W. McLaughlin Postdoctoral Fellowship (to A.A.).
Abbreviation used in this paper: CAg, Candida albicans antigen; RORγt, retinoic acid receptor-related orphan receptor γt; mAb, monoclonal antibody; TBSA, total body surface area.
References
- 1.Mason AJ, McManus A, Pruitt BJ. Association of burn mortality and bacteremia. A 25-year review. Arch Surg. 1986;121:1027–1031. doi: 10.1001/archsurg.1986.01400090057009. [DOI] [PubMed] [Google Scholar]
- 2.Sittig K, Deitch E. Effect of bacteremia on mortality after thermal injury. Arch Surg. 1988;123:1367–1370. doi: 10.1001/archsurg.1988.01400350081012. [DOI] [PubMed] [Google Scholar]
- 3.Murray C, Loo F, Hospenthal D, Cancio L, Jones J, Kim S, Holcomb J, Wade C, Wolf S. Incidence of systemic fungal infection and related mortality following severe burns. Burns. 2008;34:1108–1112. doi: 10.1016/j.burns.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Horvath E, Murray C, Vaughan G, Chung K, Hospenthal D, Wade C, Holcomb J, Wolf S, Mason AJ, Cancio L. Fungal wound infection (not colonization) is independently associated with mortality in burn patients. Ann Surg. 2007;245:978–985. doi: 10.1097/01.sla.0000256914.16754.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballard J, Edelman L, Saffle J, Sheridan R, Kagan R, Bracco D, Cancio L, Cairns B, Baker R, Fillari P, Wibbenmeyer L, Voight D, Palmieri T, Greenhalgh D, Kemalyan N, Caruso D. Positive fungal cultures in burn patients: a multicenter review. J Burn Care Res. 2008;29:213–221. doi: 10.1097/BCR.0b013e31815f6ecb. [DOI] [PubMed] [Google Scholar]
- 6.Vinsonneau C, Benyamina M, Baixench M, Stephanazzi J, Augris C, Grabar S, Paugam A, Wassermann D. Effects of candidaemia on outcome of burns. Burns. 2009;35:561–564. doi: 10.1016/j.burns.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Ye P, Rodriguez F, Kanaly S, Stocking K, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito J, Bagby G, Nelson S, Charrier K, Peschon J, Kolls J. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acosta-Rodriguez E, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 9.Weaver C, Hatton R, Mangan P, Harrington L. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 10.Lu Y, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, Kolls J, Srivastava A, Lundgren A, Forte S, Thompson C, Harney K, Anderson P, Lipsitch M, Malley R. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008;4:e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aujla S, Dubin P, Kolls J. Th17 cells and mucosal host defense. Semin Immunol. 2007;19:377–382. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang W, Na L, Fidel P, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 13.Ma C, Chew G, Simpson N, Priyadarshi A, Wong M, Grimbacher B, Fulcher D, Tangye S, Cook M. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eyerich K, Foerster S, Rombold S, Seidl H, Behrendt H, Hofmann H, Ring J, Traidl-Hoffmann C. Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. J Invest Dermatol. 2008;128:2640–2645. doi: 10.1038/jid.2008.139. [DOI] [PubMed] [Google Scholar]
- 15.Herndon DN. Total Burn Care. 3rd. Saunders Elsevier; 2007. [Google Scholar]
- 16.Finnerty C, Jeschke M, Herndon D, Gamelli R, Gibran N, Klein M, Silver G, Arnoldo B, Remick D, Tompkins R. Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Mol Med. 2008;14:553–560. doi: 10.2119/2007-00132.Finnerty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gauglitz G, Herndon D, Kulp G, Meyer Wr, Jeschke M. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J Clin Endocrinol Metab. 2009;94:1656–1664. doi: 10.1210/jc.2008-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang L, Yao Y, Dong N, Yu Y, He L, Sheng Z. Association between regulatory T cell activity and sepsis and outcome of severely burned patients: a prospective, observational study. Crit Care. 2010;14:R3. doi: 10.1186/cc8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tredget E, Yang L, Delehanty M, Shankowsky H, Scott P. Polarized Th2 cytokine production in patients with hypertrophic scar following thermal injury. J Interferon Cytokine Res. 2006;26:179–189. doi: 10.1089/jir.2006.26.179. [DOI] [PubMed] [Google Scholar]
- 20.Saville S, Lazzell A, Monteagudo C, Lopez-Ribot J. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell. 2003;2:1053–1060. doi: 10.1128/EC.2.5.1053-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romani L. Immunity to Candida albicans: Th1, Th2 cells and beyond. Curr Opin Microbiol. 1999;2:363–367. doi: 10.1016/S1369-5274(99)80064-2. [DOI] [PubMed] [Google Scholar]
- 22.Del Prete GF, De Carli M, Mastromauro C, Biagiotti R, Macchia D, Falagiani P, Ricci M, Romagnani S. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J Clin Invest. 1991;88:346–350. doi: 10.1172/JCI115300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Utsunomiya T, Kobayashi M, Herndon D, Pollard R, Suzuki F. Effects of glycyrrhizin, an active component of licorice roots, on Candida albicans infection in thermally injured mice. Clin Exp Immunol. 1999;116:291–298. doi: 10.1046/j.1365-2249.1999.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mack VE, McCarter MD, Naama HA, Calvano SE, Daly JM. Dominance of T-helper 2-type cytokines after severe injury. Arch Surg. 1996;131:1303–1309. doi: 10.1001/archsurg.1996.01430240057007. [DOI] [PubMed] [Google Scholar]
- 25.O'Sullivan ST, Lederer JA, Horgan AF, Chin DH, Mannick JA, Rodrick ML. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222:482–490. doi: 10.1097/00000658-199522240-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43:402–407. doi: 10.1016/j.cyto.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolls JK. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Desai M, Herndon D, Abston S. Candida infection in massively burned patients. J Trauma. 1987;27:1186–1188. doi: 10.1097/00005373-198710000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Prasad J, Feller I, Thomson P. A ten-year review of Candida sepsis and mortality in burn patients. Surgery. 1987;101:213–216. [PubMed] [Google Scholar]
- 30.Still JJ, Belcher K, Law E. Management of Candida septicaemia in a regional burn unit. Burns. 1995;21:594–596. doi: 10.1016/0305-4179(95)00069-n. [DOI] [PubMed] [Google Scholar]
- 31.Inatsu A, Yoshida S, Asai A, Kobayashi M, Herndon DN, Suzuki F. IL-17 promotes the conversion of normal neutrophils to PMN-I, an immunostimulating subset of neutrophils. J Immunol 2010. 2010;184:34.14. [Google Scholar]
- 32.Yeh F, Lin W, Shen H, Fang R. Changes in serum tumour necrosis factor-α in burned patients. Burns. 1997;23:6–10. doi: 10.1016/s0305-4179(96)00071-x. [DOI] [PubMed] [Google Scholar]
- 33.Yeh F, Lin W, Shen H, Fang R. Changes in circulating levels of interleukin 6 in burned patients. Burns. 1999;25:131–136. doi: 10.1016/s0305-4179(98)00150-8. [DOI] [PubMed] [Google Scholar]
- 34.Csontos C, Foldi V, Pálinkas L, Bogar L, Röth E, Weber G, Lantos J. Time course of pro- and anti-inflammatory cytokine levels in patients with burns-prognostic value of interleukin-10. Burns. 2010;36:483–494. doi: 10.1016/j.burns.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Couper K, Blount D, Riley E. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 36.Netea MG, Sutmuller R, Hermann C, Van der Graaf CA, Van der Meer JW, van Krieken JH, Hartung T, Adema G, Kullberg BJ. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol. 2004;172:3712–3718. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 37.Gu Y, Yang J, Quyang X, Liu W, Li H, Bromberg J, Chen S, Mayer L, Unkeless J, Xiong H. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur J Immunol. 2008;38:1807–1813. doi: 10.1002/eji.200838331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKinstry K, Strutt T, Buck A, Curtis J, Dibble J, Huston G, Tighe M, Hamada H, Sell S, Dutton R, Swain S. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heo Y, Joo Y, Oh H, Park M, Heo Y, Cho M, Kwok S, Ju J, Park K, Cho S, Park S, Kim H, Min J. IL-10 suppresses Th17 cells and promotes regulatory T cells in the CD4+ T cell population of rheumatoid arthritis patients. Immunol Lett. 2010;127:150–156. doi: 10.1016/j.imlet.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Dugan AL, Malarkey WB, Schwemberger S, Jauch EC, Ogle CK, Horseman ND. Serum levels of prolactin, growth hormone, and cortisol in burn patients: correlations with severity of burn, serum cytokine levels, and fatality. J Burn Care Rehabil. 2004;25:306–313. doi: 10.1097/01.bcr.0000124785.32516.cb. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi M, Kobayashi H, Herndon DN, Pollard RB, Suzuki F. Burn-associated Candida albicans infection caused by CD30+ type 2 T cells. J Leukoc Biol. 1998;63:723–731. doi: 10.1002/jlb.63.6.723. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi M, Jeschke MG, Shigematsu K, Asai A, Yoshida S, Herndon DN, Suzuki F. M2b monocytes predominated in peripheral blood of severely burned patients. J Immunol. 2010;185:7174–7179. doi: 10.4049/jimmunol.0903935. [DOI] [PubMed] [Google Scholar]
- 43.Kilani RT, Delehanty M, Shankowsky HA, Ghahary A, Scott P, Tredget EE. Fluorescent-activated cell-sorting analysis of intracellular interferon-γ and interleukin-4 in fresh and frozen human peripheral blood T-helper cells. Wound Rep Reg. 2005;13:441–449. doi: 10.1111/j.1067-1927.2005.130412.x. [DOI] [PubMed] [Google Scholar]
- 44.Tredget EE, Yang L, Delehanty M, Shankowsky H, Scott PG. Polarized Th2 cytokine production in patients with hypertrophic scar following thermal injury. J Interferon Cytokine Res. 2006;26:179–189. doi: 10.1089/jir.2006.26.179. [DOI] [PubMed] [Google Scholar]
- 45.Croft M, Swain SL. Recently activated naive CD4 T cells can help resting B cells, and can produce sufficient autocrine IL-4 to drive differentiation to secretion of T helper 2-type cytokines. J Immunol. 1995;154:4269–4282. [PubMed] [Google Scholar]
- 46.Ikemoto K, Pollard RB, Fukumoto T, Morimatsu M, Suzuki F. Small amounts of exogenous IL-4 increase the severity of encephalitis induced in mice by the intranasal infection of herpes simplex virus type 1. J Immunol. 1995;155:1326–1333. [PubMed] [Google Scholar]
- 47.Schulze-Koops H, Lipsky PE, Davis LS. Human memory T cell differentiation into Th2-like effector cells is dependent on IL-4 and CD28 stimulation and inhibited by TCR ligation. Eur J Immunol. 1998;28:2517–2529. doi: 10.1002/(SICI)1521-4141(199808)28:08<2517::AID-IMMU2517>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]