Development of methods for efficient catalytic enantioselective conjugate addition (ECA) of readily accessible carbon-based nucleophiles to α,β-unsaturated carbonyls is a major objective of research in chemical synthesis.[1] Progress has been made in designing effective chiral complexes that promote a variety of catalytic ECA reactions. One especially challenging area corresponds to transformations that furnish all-carbon quaternary stereogenic centers;[2] recent years have witnessed a number of important advances in this regard,[3, 4, 5, 6] including applications to synthesis of complex natural products.[7] Nonetheless, several important limitations remain. One shortcoming is that the majority of processes relate to reactions with cyclic systems.[4-7] The paucity of ECA processes that involve acyclic trisubstituted substrates might be because their transformations, unlike those of cyclic enones, are not facilitated by ring strain; catalysts shown to be effective in differentiating the enantiotopic faces of a Z cyclic olefin might not provide optimal enantioselectivity with commonly used linear E alkenes. The limited number of cases involving acyclic substrates[3] correspond to incorporation of alkyl groups or highly activated Meldrum acid derivatives.[3b-d] There is one report of enantioselective Rh-catalyzed ECA of acyclic enoates with sodium tetraarylborates (one aryl unit transferred);[8] in a recent disclosure, three related examples of Pd-catalyzed ECA with PhB(OH)2 are shown to proceed in up to 80:20 enantiomeric ratio (e.r.).[6c] Another study relates to Cu-catalyzed ECA of methyl units to acyclic α,β-unsaturated aryl- or heteroaryl-substituted ketones; in all but one case (with Et3Al), Me3Al was used.[9]
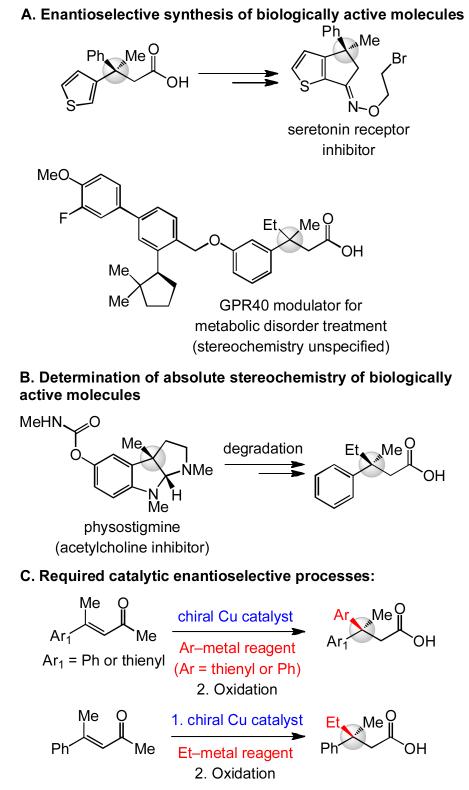
The value of catalytic ECA processes that allow for incorporation of aryl and different alkyl groups is demonstrated in Scheme 1. A carbonyl group with a β-stereogenic center substituted with a phenyl and a thienyl group has been utilized in enantioselective preparation of a serotonin receptor inhibitor;[10] another example is the agent against metabolic disorder.[11] Access to a related enantiomerically enriched carboxylic acid, but one that carries two alkyl and an aryl unit at its quaternary carbon stereogenic site, was required to ascertain the absolute stereochemistry of acetylcholine esterase inhibitor physostigmine.[12]
Scheme 1.
Catalytic ECA of acyclic enones to afford all-carbon quaternary stereogenic centers can be applied to the total synthesis of biologically active molecule and/or facilitate the elucidation of their absolute stereochemical identity.
Herein, we report the first Cu-catalyzed method for efficient ECA of aryl and commonly occurring alkyl groups to a range of trisubstituted acyclic enones. Arylaluminum reagents are easily prepared in situ from aryllithium species and commercially available dialkylaluminum halides; trialkylaluminum reagents are inexpensive. A robust chiral bidentate N-heterocyclic carbene (NHC) of silver and commercially available Cu(OTf)2 are combined to form the chiral catalyst (0.5-3.0 mol %; 0.5-24 h); products are formed in 33-95% yield and 90:10 to >99:1 e.r. It should be noted that, although efficient catalytic enantioselective allylic substitutions (EAS) with the same types of organoaluminum reagents have been reported,[13] ECA processes present a distinct challenge for several reasons. In both cases, nucleophilic addition of an organocopper intermediate is likely followed by reductive elimination; the first key step, however, is reversible only in ECA, requiring C-C bond formation to be sufficiently rapid. Moreover, the relative position of the alkene and the phosphate or carbonyl unit in EAS and ECA processes, respectively, are different; such factors are significant in reactions that likely involve association of the Lewis basic groups with the catalytic complex.[1413]
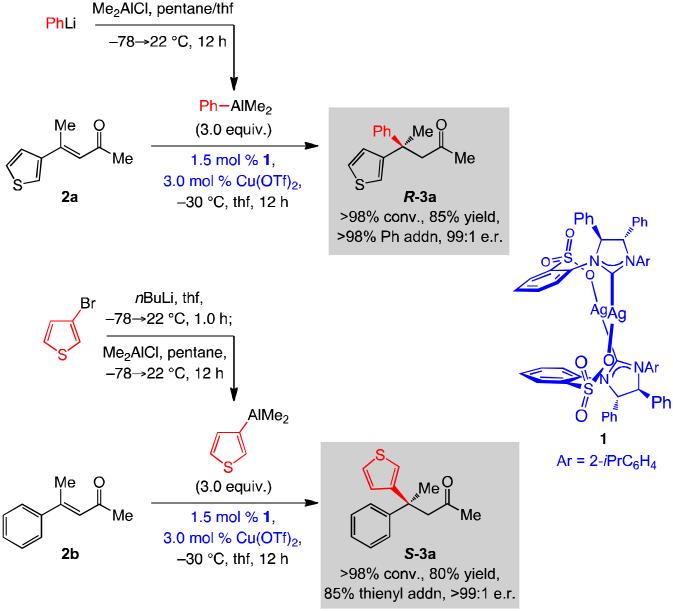
We began by exploring the possibility of accessing the thienyl-containing ketone, used in the synthesis of a serotonin receptor inhibitor (Scheme 1), by an efficient enantioselective ECA. We thus established that treatment of enone 2a with three equivalents of Ph(Me)2Al, generated in situ from reaction of PhLi and Me2AlCl, and 3.0 mol % of an NHC-Cu catalyst derived from Ag complex 1 and Cu(OTf)2[15] leads to the formation of R-3a in 85% yield and 99:1 e.r. (Scheme 2). Reaction is complete in 12 hours at −30 °C without generating any detectable amount of byproducts derived from Me transfer.[13] As further depicted in Scheme 2, we also evaluated the possibility of performing an enantioselective ECA with the corresponding thienyl-aluminum reagent and phenyl-substituted α,β-unsaturated ketone 2b. Under the latter conditions, the transformation proceeds to complete conversion in 12 hours, affording S-3a in 80% yield and >99:1 e.r.; however, there is 15% of the achiral product derived from Me transfer.[16] It should be noted that the NHC-Cu complex derived from 1, which emerged as the superior choice, has not been previously employed.[15]
Scheme 2.
Preparation of aryl(dimethyl)aluminum reagents and their in situ use in NHC-Cu-catalyzed ECA reactions with trisubstituted enones to generate all-carbon quaternary stereogenic centers.
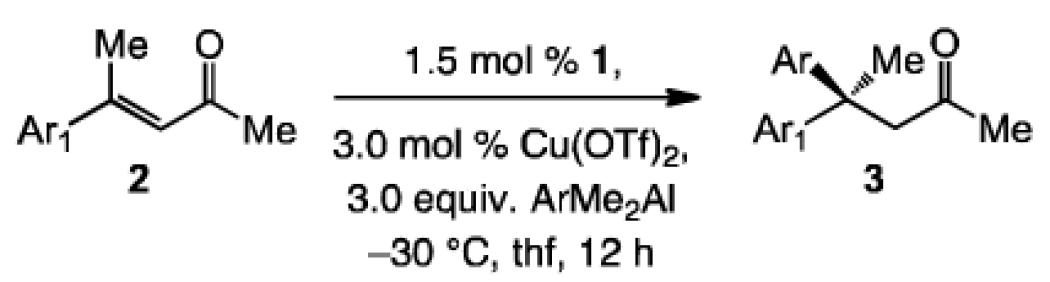
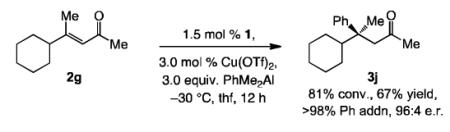
A range of trisubstituted enones and in situ-generated aryl(dialkyl)aluminum reagents can be used (Table 1). Reaction involving a 2-thienyl-substituted (vs. 3-substituted 2a, Scheme 2) with Ph(Me)2Al leads to 73% conversion in 12 hours (entry 1, Table 1), and 3b is isolated in 57% yield with complete transfer of the phenyl unit in 92:8 e.r. Formation of the sterically demanding stereogenic center that contains two aryl groups is relatively sluggish when one bears an ortho unit; the example in entry 2 of Table 1 is illustrative (17% conv. with the derived ortho chloro-aryl substrate). Synthesis of ortho-fluoro-aryl 3c thus proceeds in 33% yield and is accompanied by the product derived from Me transfer (Ph:Me = 55:45); however, the ECA remains exceptionally enantioselective (>99:1 e.r.). Cu-catalyzed ECA with enones that contain electron-deficient or electron-rich aryl units proceed efficiently and with high enantioselectivity: 3d and 3e are obtained in 76% and 82% yield, with 92% and >98% group selectivity and in 94:6 and 98:2 e.r., respectively (entries 3-4, Table 1). Similarly high efficiency and enantioselectivity is observed with aryl(dimethyl)aluminum reagents that carry electron withdrawing or donating groups (entries 5-8, Table 1). The example in Eq. (1), regarding formation of 3j in 67% yield, >98% transfer of Ph group and 96:4 e.r., demonstrates that catalytic ECA can be performed with high selectivity with enones that contain only alkyl substituents.
Table 1.
NHC-Cu-catalyzed ECA with various aryl(dimethyl)aluminum reagents.[a]
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Entry | Ar1 | Ar | Product | Conv. [%];[b]
Yield [%][c] |
Ar vs. Me addn[b] |
e.r.[d] |
| 1 | 2-thienyl; 2c | Ph | 3b | 73; 57 | >98:2 | 92:8 |
| 2 | oFC6H4; 2d | Ph | 3c | 88; 33 | 55:45 | >99:1 |
| 3 | pF3CC6H4; 2e | Ph | 3d | 89; 76 | 92:8 | 94:6 |
| 4 | pMeOC6H4; 2f | Ph | 3e | >98; 82 | >98:2 | 98:2 |
| 5 | Ph; 2b | pF3CC6H4 | 3f | >98; 82 | 92:8 | 98:2 |
| 6 | Ph; 2b | pMeOC6H4 | 3g | >98; 77 | >98:2 | >99:1 |
| 7 | 3-thienyl; 2a | pF3CC6H4 | 3h | >98; 83 | >98:2 | 96:4 |
| 8 | 3-thienyl; 2a | pMeOC6H4 | 3i | >98; 89 | >98:2 | >99:1 |
Reactions were performed under N2 atmosphere.
Determined through analysis of 400 MHz 1H NMR spectra of unpurified mixtures.
Yield of isolated and purified products.
Determined by HPLC analysis (±2%); see the Supporting Information for details.
 |
(1) |
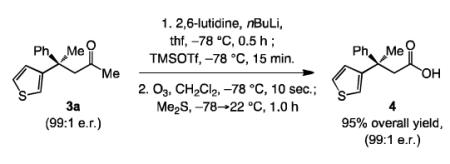
Access to the corresponding enantiomerically enriched carboxylic acid derivatives increases the value of the protocol (cf. Scheme 1); nonetheless, our attempts to identify conditions for efficient ECA with related derivatives (e.g., Weinreb amides, N-acyloxazolidinones, carboxylic esters, thioesters) proved unsuccessful (<10% conv.). To address the above problem, we identified a two-step procedure that can be completed in less than two hours, without the need for purification of the silyl enol ether intermediate, to obtain the derived carboxylic acid; the example leading to 4 in 95% yield is representative [Eq. (2)].
 |
(2) |
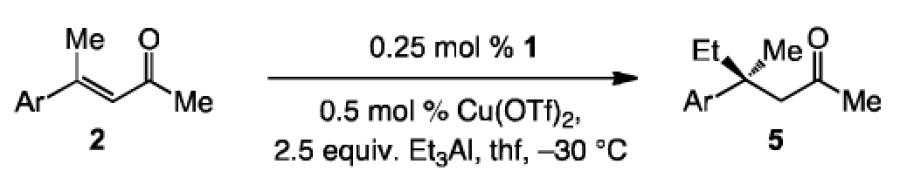
We subsequently turned our attention to catalytic ECA with Et3Al (Table 2), of which a single example exists involving the transformation of a phenyl ketone.[9] We therefore established that aryl- and heteroaryl-substituted enones of different steric and electronic attributes can be used in transformations that require 0.5 mol % of the NHC-Cu complex to proceed to ≥97% conversion, affording the desired products in 96.5:3.5 to >99:1 e.r. It is noteworthy that, in contrast to ECA with the sterically more demanding aryl(dimethyl)aluminum reagents, additions to substrates that possess relatively large substituents, such as a 2-naphthyl or an ortho-bromo unit inentries 5-6 of Table 2, proceed to ≥97% conversion with equally high enantioselectivities as the less hindered acyclic enones.
Table 2.
NHC-Cu-catalyzed ECA of aryl-substituted enones with Et3AI.[a]
| |||||
|---|---|---|---|---|---|
|
| |||||
| Entry | Ar; Substrate | Product | t[h] | Conv. [%];[b]
Yield [%][c] |
e.r.[d] |
| 1 | Ph; 2b | 5a | 0.5 | >98; 93 | 98:2 |
| 2 | 2-thienyl; 2c | 5b | 1.0 | >98; 86 | 98.5:1.5 |
| 3 | pF3CC6H4; 2e | 5c | 1.0 | >98; 89 | 99:1 |
| 4 | pMeOC6H4; 2f | 5d | 1.0 | >98; 92 | 99:1 |
| 5 | 2-naphthyl; 2h | 5e | 2.5 | 97; 94 | 97.5:2.5 |
| 6 | oBrC6H4; 2i | 5f | 12 | 98; 87 | >99:1 |
| 7 | mFC6H4; 2j | 5g | 3.0 | >98; 90 | 96.5:3.5 |
Reactions were performed under N2 atmosphere.
Determined through analysis of 400 MHz 1H NMR spectra of unpurified mixtures.
Yield of isolated and purified products.
Determined by GC analysis (entries 1-2) or HPLC analysis (±2%); see the Supporting Information for details.
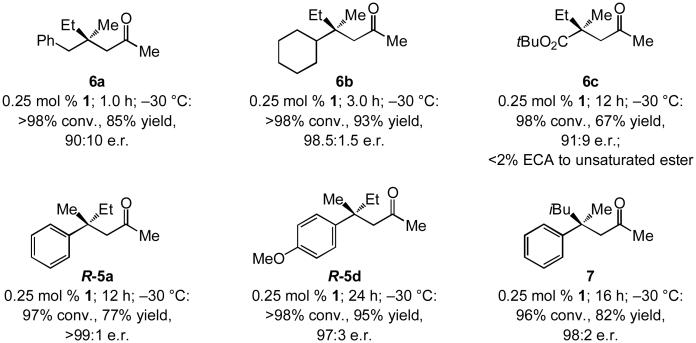
The products shown in Scheme 3 underscore several vital characteristics of the approach. Processes involving Et3Al that lead to the formation of 6a and 6b demonstrate that dialkyl-substituted enones can be used; the lower e.r. in the case of 6a (90:10 vs. 98.5:1.5 for 6b) is likely due to a lower degree of differentiation between a Me and a benzyl group (vs. a cyclohexyl). Enantioselective synthesis of 6c, a product that contains two functionalizable and differentiable carbonyl groups, illustrates that addition to the site β to the ketone unit, versus to the carboxylic ester, is exclusive in spite of formation of a more hindered quaternary carbon stereogenic center. Representative reactions with Me3Al are shown in Scheme 3 as well;[9] R-5a and R-5d are obtained in 77% and 95% yield and >99:1 and 97:3 e.r., respectively. Enantioselective synthesis of i-butyl-substituted ketone 7, generated in 82% yield and 98:2 e.r., shows that the NHC-Cu-catalyzed protocol can be extended to ECA with (iBu)3Al, another commercially available organoaluminum species the ECA of which has not been reported with trisubstituted acyclic enones.
Scheme 3.
Representative cases of efficient and enantioselective NHC-Cu-catalyzed ECA reactions with Et3Al and non-aryl-substituted enones as well as with Me3- and (iBu)3Al reagents.
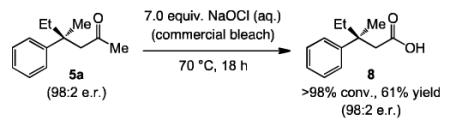
In addition to the efficient two-step protocol depicted in Eq. (2), ECA products without a relatively sensitive heterocyclic substituent prone to adventitious oxidation (such as 3a)[17] can be converted directly to the desired carboxylic acids in a single step with commercial bleach.[18] The transformation in Eq. (3), resulting in the formation of enantiomerically enriched 8 in 61% yield (98:2 e.r.) is illustrative (cf. Scheme 1).
 |
(3) |
Development of additional catalytic ECA protocols and applications to complex molecule synthesis are in progress.
Supplementary Material
Footnotes
Financial support was provided by the NIH (GM-47480); J. A. D. is grateful for a LaMattina Graduate Fellowship. We are grateful to E. M. Vieira for helpful suggestions.
References and Footnotes
- 1.a Harutyunyan SR, den Hartog T, Geurts K, Minnaard AJ, Feringa BL. Chem. Rev. 2008;108:2824. doi: 10.1021/cr068424k. For scholarly reviews on catalytic ECA reactions and their utility in chemical synthesis, see: [DOI] [PubMed] [Google Scholar]; b Jerphagnon T, Pizzuti MG, Minnaard AJ, Feringa BL. Chem. Soc. Rev. 2009;38:1039. doi: 10.1039/b816853a. [DOI] [PubMed] [Google Scholar]; c Ji J-X, Chan ASC. In: Catalytic Asymmetric Synthesis. Ojima I, editor. Wiley; Hoboken: 2010. p. 439. I. [Google Scholar]
- 2.Das JP, Marek I. Chem. Commun. 2011;47:4593. doi: 10.1039/c0cc05222a. For a comprehensive review of enantioselective synthesis of quaternary carbon stereogenic centers within acyclic molecules, see: [DOI] [PubMed] [Google Scholar]
- 3.a Wu J, Mampreian DM, Hoveyda AH. J. Am. Chem. Soc. 2005;127:4584. doi: 10.1021/ja050800f. For catalytic ECA with acyclic substrates that involve alkylmetal reagents, affording quaternary carbon stereogenic centers, see: [DOI] [PubMed] [Google Scholar]; b Fillion E, Wilsily A. J. Am. Chem. Soc. 2006;128:2774. doi: 10.1021/ja056692e. [DOI] [PubMed] [Google Scholar]; c Mauleón P, Carretero JC. Chem. Commun. 2005:4961. doi: 10.1039/b508142d. [DOI] [PubMed] [Google Scholar]; d Wilsily A, Fillion E. Org. Lett. 2008;10:2801. doi: 10.1021/ol800923q. [DOI] [PubMed] [Google Scholar]; e Wilsily A, Fillion E. J. Org. Chem. 2009;74:8583. doi: 10.1021/jo901559d. [DOI] [PubMed] [Google Scholar]; f Kawai H, Yuan Z, Kitayama T, Tokunaga E, Shibata N. Angew. Chem. Int. Ed. 2013 doi: 10.1002/anie.201301123. For related studies involving nitroalkanes as reagents, see: [DOI] [PubMed] [Google Scholar]; g Mazet C, Jacobsen EN. Angew. Chem. Int. Ed. 2008;47:1762. doi: 10.1002/anie.200704461. DOI: 10.1002/anie.201301123. For catalytic ECA leading to quaternary carbon stereogenic centers with cyanide as the nucleophilic agent, see: One case reported: [DOI] [PubMed] [Google Scholar]; h Tanaka Y, Kanai M, Shibasaki M. J. Am. Chem. Soc. 2010;8862;132 doi: 10.1021/ja1035286. [DOI] [PubMed] [Google Scholar]
- 4.a Hird AW, Hoveyda AH. J. Am. Chem. Soc. 2005;127:14988. doi: 10.1021/ja0553811. For catalytic ECA with cyclic substrates that involve alkylmetal reagents, affording quaternary carbon stereogenic centers, see: [DOI] [PubMed] [Google Scholar]; b d′Augustin M, Palais L, Alexakis A. Angew. Chem. Int. Ed. 2005;44:1376. doi: 10.1002/anie.200462137. [DOI] [PubMed] [Google Scholar]; c Lee K-s., Brown MK, Hird AW, Hoveyda AH. J. Am. Chem. Soc. 2006;128:7182. doi: 10.1021/ja062061o. [DOI] [PubMed] [Google Scholar]; d Martin D, Kehrli S, d′Augustin M, Clavier H, Mauduit M, Alexakis A. J. Am. Chem. Soc. 2006;128:8416. doi: 10.1021/ja0629920. [DOI] [PubMed] [Google Scholar]; e Brown MK, May TL, Baxter CA, Hoveyda AH. Angew. Chem. Int. Ed. 2007;46:1097. doi: 10.1002/anie.200604511. [DOI] [PubMed] [Google Scholar]; f May TL, Brown MK, Hoveyda AH. Angew. Chem. Int. Ed. 2008;47:7358. doi: 10.1002/anie.200802910. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Matsumoto Y, Yamada K-i., Tomioka K. J. Org. Chem. 2008;73:4578. doi: 10.1021/jo800613h. [DOI] [PubMed] [Google Scholar]; h Ladjel C, Fuchs N, Zhao J, Bernardinelli G, Alexakis A. Eur. J. Org. Chem. 2009;29:4949. [Google Scholar]; i Kehrli S, Martin D, Rix D, Mauduit M, Alexakis A. Chem. Eur. J. 2010;16:9890. doi: 10.1002/chem.201000471. [DOI] [PubMed] [Google Scholar]; j Kwiatkowski P, Dudziński K, Łyźwa D. Org. Lett. 2011;13:3624. doi: 10.1021/ol201275h. For related studies involving nitroalkanes as reagents, see: [DOI] [PubMed] [Google Scholar]
- 5.May TL, Dabrowski JA, Hoveyda AH. J. Am. Chem. Soc. 2011;133:736. doi: 10.1021/ja110054q. For catalytic ECA involving alkenyl-based nucleophiles and cyclic enones to afford quaternary carbon stereogenic centers, see: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a Shintani R, Duan W-L, Hayashi T. J. Am. Chem. Soc. 2006;128:5628. doi: 10.1021/ja061430d. For catalytic ECA with aryl-based nucleophiles and cyclic substrates to generate quaternary carbon stereogenic centers, see: with Rh-based catalysts and arylboronic acids, [DOI] [PubMed] [Google Scholar]; b with Cu-based catalysts and arylaluminum reagents. Ref. 4f.; c Hawner C, Li K, Cirriez V, Alexakis A. Angew. Chem. Int. Ed. 2008;47:8211. doi: 10.1002/anie.200803436. [DOI] [PubMed] [Google Scholar]; d Shintani R, Tsutsumi Y, Nagaosa M, Nishimura T, Hayashi T. J. Am. Chem. Soc. 2009;131:13588. doi: 10.1021/ja905432x. with Rh-based catalysts and sodium tetraarylborates. [DOI] [PubMed] [Google Scholar]; e Shintani R, Takeda M, Nishimura T, Hayashi T. Angew. Chem. Int. Ed. 2010;49:3969. doi: 10.1002/anie.201000467. with Rh-based catalysts and triarylboroxines. [DOI] [PubMed] [Google Scholar]; f Hawner C, Müller D, Gremaud L, Felouat A, Woodward S, Alexakis A. Angew. Chem. Int. Ed. 2010;49:7769. doi: 10.1002/anie.201003300. with Rh-based catalysts and arylaluminum reagents. [DOI] [PubMed] [Google Scholar]; g Kikushima K, Holder JC, Gatti M, Stoltz BM. J. Am. Chem. Soc. 2011;133:6902. doi: 10.1021/ja200664x. with Pd-based catalysts and arylboronic acids. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Gottumukkala AL, Matcha K, Lutz M, de Vries JG, Minnaard AJ. Chem. Eur. J. 2012;18:6907. doi: 10.1002/chem.201200694. [DOI] [PubMed] [Google Scholar]
- 7.a Brown MK, Hoveyda AH. J. Am. Chem. Soc. 2008;130:12904. doi: 10.1021/ja8058414. For applications of catalytic ECA reactions, which afford quaternary carbon stereogenic centers, to natural product synthesis (all involve cyclic enones), see: [DOI] [PMC free article] [PubMed] [Google Scholar]; b Peese KM, Gin DY. Chem. Eur. J. 2008;14:1654. doi: 10.1002/chem.200701290. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Ref. 5.; d Mendoza A, Ishihara Y, Baran PS. Nature Chem. 2012;4:21. doi: 10.1038/nchem.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Ref. 6h. [Google Scholar]
- 8.Shintani R, Hayashi T. Org. Lett. 2011;13:350. doi: 10.1021/ol102674z. [DOI] [PubMed] [Google Scholar]
- 9.Endo K, Hamada D, Yakeishi S, Shibata T. Angew. Chem. Int. Ed. 2013;52:606. doi: 10.1002/anie.201206297. [DOI] [PubMed] [Google Scholar]
- 10.Badorc A, Courregelongue J, Ducros D, Frehel D, Giudice A, Serradeil-Legal C. 1993 The enantiomerically enriched carboxylic acid was prepared in seven steps including a resolution; see: Patent No. US 005252749 A.
- 11.Brown S, Cao Q, Dransfield PJ, Du X, Houze J, Jiao XY, Kohn TJ, Lai S, Li A-R, Lin D, Luo J, Medina JC, Reagan JD, Pattaropong V, Schwarz M, Shen W, Su Y, Swaminath G, Vimolratana M, Wang X, Xiong Y, Yang L, Yu M, Zhang J, Zhu L. 2009 Patent No. WO 2009/048527 A1.
- 12.Hill RK, Newkome GR. Tetrahedron. 1969;25:1249. doi: 10.1016/s0040-4020(01)82698-3. [DOI] [PubMed] [Google Scholar]
- 13.Gao F, Lee Y, Mandai K, Hoveyda AH. Angew. Chem. Int. Ed. 2010;49:8370. doi: 10.1002/anie.201005124. For application of in situ-generated aryl(dialkyl)aluminum reagents in NHC-Cu-catalyzed enantioselective allylic substitution reactions, see: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao F, McGrath KP, Lee Y, Hoveyda AH. J. Am. Chem. Soc. 2010;132:14315. doi: 10.1021/ja106829k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. For data in connection to chiral ligand and Cu salt screening, see the Supporting Information.
- 16. Determination of the precise origin for the difference in aryl vs. Me group transfer requires detailed investigations, but might be attributed to a stronger thienyl-Cu bond (vs. phenyl-Cu), thus allowing for competitive addition of a Me unit. Such a proposal is made under the assumption that formation of the requisite NHC-Cu-aryl and NHC-Cu-Me complexes from Ar(Me2)Al reagents are reversible under the reaction conditions.
- 17. Attempts to effect oxidation of thienyl-substituted 3a with aqueous NaOCl led to formation of substantial amounts of unidentifiable byproducts.
- 18.Liskin DV, Valente EJ. J. Mol. Structure. 2008;878:149. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.