Abstract
Axonal and dendritic degeneration is a common, early pathological feature of many neurodegenerative disorders and thought to be regulated by mechanisms distinct from that of the soma death. The unique structures of axons and dendrites (collectively neurites) may cause them to be particularly vulnerable to the accumulation of protein aggregates and damaged organelles. Autophagy is a known catabolic mechanism whereby cells clear protein aggregates and damaged organelles. Basal autophagy occurs continuously as a housekeeping function, and can be acutely expanded in response to various stress or injuries. Emerging evidence shows that insufficient or excessive autophagy contributes to neuritic degeneration. Here, we review the recent progress that has begun to reveal the role of autophagy in neuritic functions and degeneration.
Keywords: autophagy, axon, dendrite, neurodegenerative diseases
Introduction
Neurons comprise the soma, axon and dendrites. The latter two are collectively known as neurites, in which molecules including proteins are synthesized, delivered, and degraded for their dynamic functions in synaptic growth and activity. Upon certain stimuli, the axons and dendrites of cultured neurons exhibit features of degeneration, including terminal degradation, neuritic retraction, synaptic pathology, and marked focal beading or swelling. The term ‘dystrophic neurites’ is used to describe these processes, which is analogous to neurite dystrophy in vivo. In neurodegenerative disorders, axons, dendrites and synapses often degenerate prior to the loss of the cell bodies, which may contribute to pre-clinical episodes of disease process and eventual clinical symptoms [1, 2].
Autophagy is a cell self-digesting system that is responsible for the clearance of long-lived proteins and damaged organelles by the lysosome. A number of evolutionarily conserved gene products, known as the Atg proteins, are required for autophagosome (autophagic vacuoles) synthesis, maturation, trafficking and clearance [3]. Several functionally distinct groups are responsible for autophagy execution, including the Ulk1-Atg13-FIP200 kinase complex, the class III PI3K complex containing the core proteins Vps34, p150 and Beclin 1, the lipid-binding Atg18 homologs, the multi-spanning transmembrane protein Atg9, the Atg12 conjugation system and the ubiquitin-like LC3 conjugation system [3]. Examples of upstream signaling molecules that regulate autophagic activity include the mammalian target of rapamycin (mTOR), a Ser/Thr kinase which prevents autophagy induction through Ulk1 inactivation, and AMP-activated protein kinase (AMPK), an energy-sensing kinase that promotes autophagy by inducing Ulk1 activation [4]. In fact, pharmacological induction of autophagy can be achieved using rapamycin, which inhibits mTOR. In addition, class III PI3K-Beclin 1 complex controls the nucleation process of the phagophore, a precursor of autophagosome. 3-methyladenine (3MA) or wortmannin, which inhibits class III PI3K activity, is often used to block autophagy.
Emerging evidence demonstrates a critical role of macroautophagy (hereafter referred to as “autophagy”) in the maintenance of protein and organelle homeostasis in the axons and protection of axons. Dysfunctional autophagy has been observed in experimental neuritic degeneration in both primary neuronal cultures [5–7] and in vivo animal models [8–13]. Insufficient autophagic clearance and perhaps excessive autophagy induction, characterized by the accumulations of autophagy-related vesicular compartments, are also observed in degenerating neurites of brain specimens from patients with several neurodegenerative diseases, including Alzheimer’s disease (AD) [14–17], Parkinson’s disease (PD) [18, 19] and Huntington’s disease (HD) [20]. A growing body of evidence shows the indispensible role of constitutive autophagy in preventing neuritic degeneration, whereas imbalanced induction of autophagy can contribute to neuritic degeneration. This review focuses on current progress that has begun to reveal autophagic processes in the axons and dendrites. The roles for autophagy in neuronal death and neurological disorders, and the potential molecular mechanism for the pathogenic function of autophagy, have been recently reviewed [21–23].
Biogenesis and fate of autophagosomes in neurites
Biogenesis of autophagosomes
Although there has been limited investigation of autophagosome (see Glossary) synthesis in neurons, accumulation of autophagosome-like vacuoles has frequently been observed in dystrophic axon terminals [24, 25] and axonal swellings [8, 14, 17, 26, 27] of affected neurons in diverse injury models. Time-lapse confocal imaging of cultured cerebellar granule neurons shows that vesicles labeled with green fluorescent protein-tagged microtubule-associated protein light chain 3 (GFP-LC3) travel along neurites in a retrograde direction [28], raising the possibility that autophagosomes may arise in the distal tips of axons and transport their engulfed cargo toward the soma through retrograde transport [29] (Figure 1a). The cargo may include soluble or aggregated proteins, mitochondria and possibly even nerve growth factor (NGF) [29, 30]. Indeed, a recent live-imaging study demonstrates that autophagosome initiation is a constitutive and spatially restricted process in the distal ends of in vitro cultured dorsal root ganglion (DRG) neurons [30]. In addition, de novo formation of autophagosomes is also detected in cell bodies of primary cortical neurons [31]. However, it remains unclear whether autophagosome biogenesis occurs along the axons. The origin of the membrane also remains to be established but limited evidence may suggest that autophagosomes in the axons are originated by budding from existing vesicles [31].
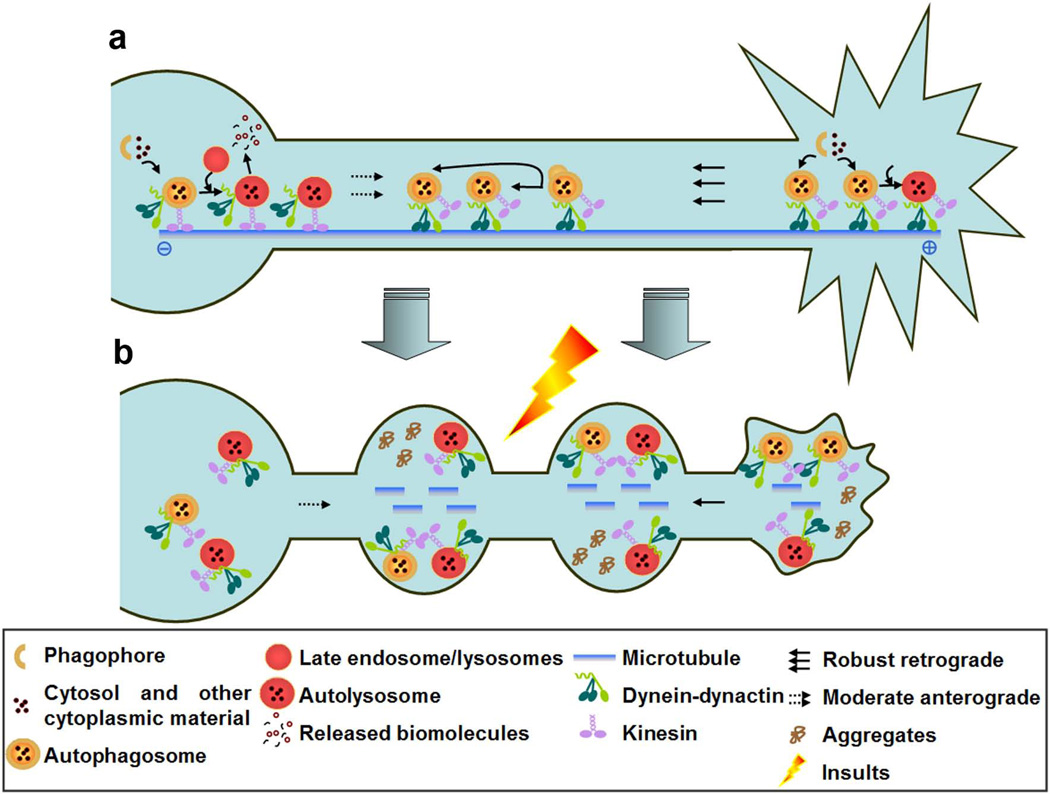
Figure 1.
Biogenesis, maturation and dynamic transport of autophagosomes and autolysosomes in healthy and degenerating axons. (a) Under normal conditions, autophagosomes may arise in the distal ends of healthy axons, become acidified (matured), and initiate retrograde transport along axons depending on microtubules and dynein/dynactin complex. Mature autophagosomes (autolysosomes) switch to bidirectional motility and initiate the proteolytic clearance of the autophagy substrates. (b) Upon certain insults, autophagosomes are induced in a large number, exceeding the rate of clearance. As result, autophagosomes/autolysosomes accumulate in axonal swellings or beadings associated with protein aggregates and collapsed cytoskeleton.
Maturation of autophagosomes
Autophagosome maturation requires fusion of the autophagosomes with late endosomes and/or lysosome, which provide an acidic environment and digestive function to the interior of the autophagosomes [32]. In the past, two major approaches have been applied to monitor the maturation of autophagosomes, both of which are effective for analyzing autophagosome maturation in living neurites. First neurons have been labeled with an autophagosome marker (e.g. LC3 [33]) and a lysosome/endosome marker, such as the late endosome/lysosome marker lysosomalassociated membrane protein 1 (LAMP1) [30, 31], Rab7 [31], LysoTracker [30, 34] and lysosome-associated membrane glycoprotein 1 (Lgp120) [34], followed by analysis of co-localization or comigration. Alternatively, dual-color fluorescent LC3 reporters have been used; these fluoresce in different colors depending on the pH. Examples of these are: mRFP-GFP tandem fluorescent-tagged LC3 (tfLC3) [35], mCherry-EGFP-LC3 [36] and a recently reported mTagRFP-mWasabi-LC3 [37]. GFP does not fluoresce under acidic and degradative conditions whereas mRFP or mCherry do. mCherry-EGFP-LC3-labeled DRG neurons show a reducing gradient of autophagosomes positive for both mCherry and EGFP between distal and proximal axon regions, suggesting that autophagosomes are transported to proximal axons and become fully acidified [30]. Thus, following synthesis, autophagosomes exit from the distal pool, become acidified, and initiate retrograde transport along the axons [28, 30, 31] (Figure 1a).
Autophagosomes mature to autolysosomes after fusion with late endosomes and lysosomes during their axonal transport [31], and an impairment of autophagosome maturation could alter their transport properties and favor autophagosome accumulation (Figure 1b). Once fully acidified in the proximal part of the axons, autophagosomes may switch to bidirectional motility resembling that of lysosomes [30, 34] and initiate the proteolytic clearance of the autophagy substrates [31] (Figure 1a). In contrast to a number of studies of the biogenesis and maturation of autophagosomes in axons, no report has described the similar process in dendrites.
Dynamics of autophagosomes and autolysosomes in neurites
Microtubule-dependent transport
Microtubules are involved in autophagosome formation and appear to facilitate targeting and fusion of autophagosomes to lysosomes [38], although some of these conclusions have been questioned and the precise mechanism remains unclear [39]. MAP1B plays an essential role in regulating microtubule stability in the axons. Thus LC3-MAP1B interaction may regulate autophagosomes in an axon-specific, microtubule-dependent manner [8, 28] (Figure 1a). The administration of nocodazole, a microtubule disruption agent, clearly reduced the activity of autolysosomes in PC12 neurites under starvation conditions [34]. In accordance with the results, a recent report shows that microtubule cytoskeleton and molecular motor defects are the early pathogenic events in the PS1/APP mouse amyloid model, which may lead to transport abnormalities, local accumulation of autophagosomes and promote neuritic dystrophy [40]. It is well known that axons of vertebrate neurons display uniformly plus-enddistal microtubules, whereas their dendrites display non-uniformly oriented microtubules [41]. Although axons and dendrites differ in the arrangement of microtubules, it remains unclear whether there is any difference in the autophagosome dynamics between the two different compartments.
Dynein-dependent retrograde transport
Approximately all moving autophagosomes transport retrogradely along the axons [28, 31]. Increased retrograde transport of autophagic vacuoles has been previously observed when axonal elongation is blocked [42]. In cultured neurons, autophagosomes transport along the axons with a retrograde velocity ranged from 0.25 to 0.45 µm/s during the observation period [30, 31, 43]. The retrograde transport kinetics of autophagosomes in the axons differs from that of endocytic vesicles, which show faster retrograde velocity (0.2 to 1.2 µm/s) than autophagosomes [44]. Dynein is the major minus-end motor that transports various cargos on microtubules (Figure 1a, b). Expression of a fluorescently tagged subunit of dynein in GFP-LC3 labeled neurons reveals the co-migration of dynein with GFP-LC3 puncta in the axons [30]. Such retrograde movement can be largely arrested by dynein motor inhibitor EHNA [43] or by expression of the dominant-negative dynein inhibitor CC1, which blocks the dynein-dynactin interaction [30]. Likewise, EHNA dramatically reduced the percentage of retrogradely transporting autolysosomes in PC12 neurites [34]. The study of dynein mutants links motor neuron degeneration to the defects in retrograde transport [45]. Disruption of the dynein-dynactin interaction inhibits axonal transport in motor neurons and causes late onset degeneration [46], implicating dysfunctional dynein/dynactin complex in autophagy dysregulation in neurodegenerative disease, such as amyotrophic lateral sclerosis (ALS) [47].
Kinesin-dependent anterograde transport
Kinesin-1, a plus-end directed motor on microtubules, is essential for anterograde neuritic transport (Figure 1a, b). Reduction of kinesin-1 promotes Aβ generation and its intraneuronal accumulation as well as the formation of axonal swelling in a mouse amyloid model [48]. Treatment of kinesin inhibitor AMP-PNP or knock-down of kinesin light chains KLC1 or KLC2 suppresses anterograde transport of autolysosomes in PC12 neurites [34], suggesting that the kinesin motors regulate the anterograde movement of autolysosomes in neurites. Furthermore, kinesin-1 and kinesin-2 motors are copurified with isolated autophagosomes [30]. However, these results cannot exclude the possibility that the copurification of autophagosomes and kinesin motors is due to the engulfed fraction of motor proteins in autophagosomes.
Autophagy dysfunction is a common pathological event in diverse neuritic degeneration
Terminal degradation
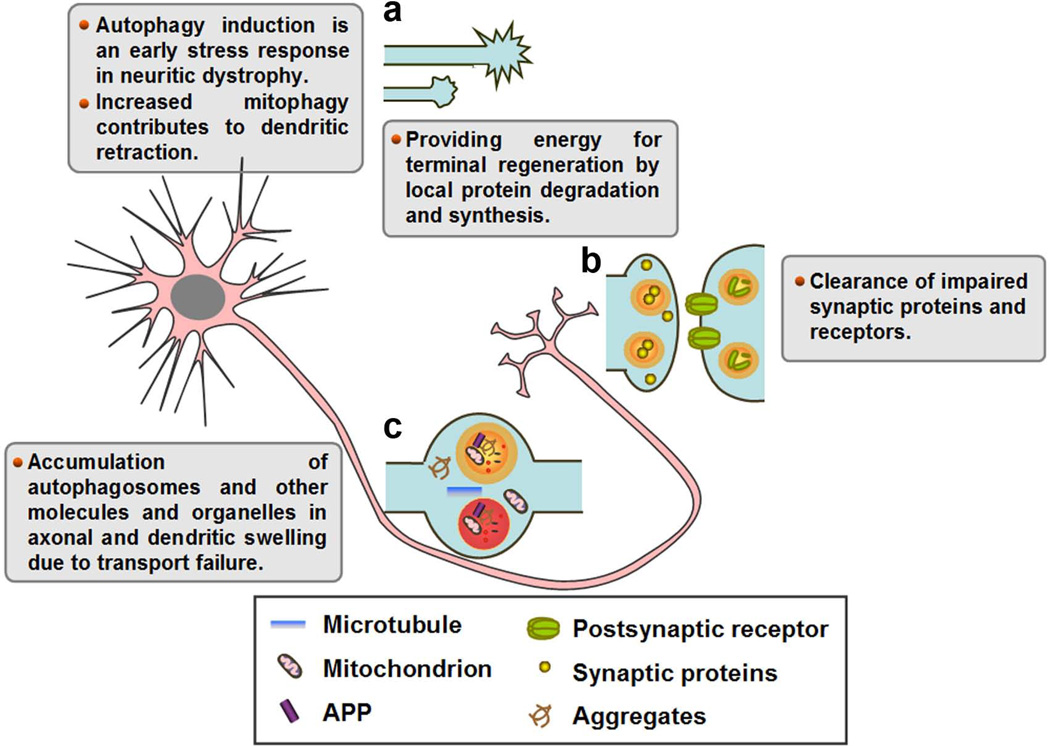
Growth cone collapse and degeneration of the terminal regions of long axons are recognized as the initial events in “dying back” degeneration, which is the most common pathology seen in peripheral nerve diseases caused by a wide variety of toxic, metabolic, and infectious insults [2, 49]. Our previous study shows that 3-MA prevents terminal neurites of sympathetic neurons from degeneration induced by zinc depletion [50]. Moreover, local protein degradation and synthesis are required for growth cone initiation and axonal regeneration following injury [51]. Increased number of autophagic vacuoles has been found in the axon initial segment of axotomised neurons [52], implicating autophagy in terminal regeneration through local protein degradation (Figure 2a). Nevertheless, the role of autophagy in terminal regeneration following injury remains speculative. The direct evidence that the autophagy-mediated degradation contributes to the onset of the terminal regeneration has yet to be shown. It is worth mentioning that axon terminal dystrophy of Purkinje cells deficient in atg7 proceeds with little sign of dendritic atrophy, suggesting that axon terminals are much more vulnerable to autophagy impairment than the dendrites in this particular neuron type [25].
Figure 2.
Link of autophagy and diverse neuritic degenerative features. Autophagic process may be involved in a variety of neuritic degenerative features, including terminal degeneration (a), neuritic retraction (a), synaptic dysfunction (b) and neuritic swelling (c). APP, amyloid precursor protein.
Neuritic retraction
Neuritic retraction, or shortening, is a progressive reduction in axonal and dendritic length. It represents a common hallmark of neurodegenerative diseases. Although little is known about the mechanism of neuritic retraction, several extrinsic and intrinsic cues are suggested to actively signal axonal and dendritic retraction [49, 53]. Dendritic retraction and shrinkage in Purkinje cell degeneration (pcd) mice [9] or axonal dystrophy in Lurcher mice [8] each precedes Purkinje cell loss. The dendritic and axonal degeneration of Purkinje cells in both models is associated with the increased activity of autophagy pathway [8, 9] (Figure 2a). In Lurcher mice, the induction of autophagy is an early stress response in axonal dystrophy and may participate in the remodeling of axon structures. In pcd mice, aberrant autophagymediated mitochondria clearance (mitophagy) contributes to the Purkinje cell degeneration and dendritic retraction. Cultured cortical neurons overexpressing G2019S mutant of leucine-rich repeat kinase 2 (LRRK2) displayed dendritic retraction due to calcium dysregulation and induction of dendritic mitophagy [54, 55]. Furthermore, autophagy inhibition prevents the neuritic retraction, whereas autophagy stimulation potentiates LRRK2-induced neuritic shortening in SH-SY5Y cells [56]. The data suggest that excessive mitochondrial removal could be one of the contributing factors for dendritic retraction (Figure 2a). Nonetheless, the involvement of mitophagy in axonal dystrophy remains to be further clarified.
Synaptic pathology
Synaptic pathology, accompanied by abnormal accumulation of autophagosomes, is found in the hippocampus of young Alzheimer’s model mice [40]. In G2019S LRRK2 transgenic mice, an animal model for PD, autophagosomes appear in synaptic terminal in cerebral cortex at advanced age (17–18 months) [57]. Synaptic loss is an early and major feature of the brain pathology induced by prion diseases [58]. Prion infection induced accumulation of autophagosomes within synaptic terminals in various brain regions of infected hamsters and mice [59]. In brain biopsies from the patients with prion diseases, autophagosomes are also found within damaged synapses [27]. The evidence links dysfunctional autophagy and synaptic pathology.
A recent report reveals that induction of autophagy by rapamycin elevated the number of autophagosomes in prejunctional dopaminergic axons, concomitant with decreased axonal profile volumes, synaptic vesicle numbers, and evoked dopamine release, suggesting the critical role of autophagy in regulation of presynaptic structure and neurotransmission [60]. In addition, autophagosomes are located in the postsynaptic cells that selectively degrade cell surface receptors, such as GABAA receptor in C. elegans [61]. In Drosophila, autophagy and ubiquitin-proteasome systems converge to regulate synaptic development [62]. Moreover, decreased autophagic activity is associated with reduced number of neuromuscular junction synapses in Drosophila dynein light chain 1 (ddlc1) mutant [63]. The neuron-specific synaptic v-soluble NSF attachment protein receptor (v-SNARE) n-syb (neuronal Synaptobrevin) plays an important role during synaptic vesicle exocytosis. Increased autophagy might represent a secondary response to a primary vesicle trafficking defect downstream of endocytosis in Drosophila loss of n-syb [64]. Hence, autophagy may contribute to the clearance of the synaptic proteins and receptors and thus involved in the pathology of synapses (Figure 2b).
Beading and swelling formation
Beadings and swellings are thought to be the early features of axonal or dendritic degeneration, often associated with jamming of intracellular organelles [65–67] and accumulation of autophagosomes [8, 68] (Figure 2c). Methamphetamine (METH) selectively elicits pathogenic effect in the neurites of dopamine neurons without inducing cell death. METH was reported to promote the formation of autophagosomes, particularly in neuritic swellings and, ultimately, within cell bodies of dopaminergic neurons [5]. The disruption of organelle trafficking induced by lysosomal proteolysis inhibition caused autophagosomes to accumulate in axons, especially within focal swellings [31]. Lee et al. detected the cathepsin-containing vesicular organelles were sequestered in axonal swellings during lysosomal proteolysis disruption [31]. In addition, nonvesicular axonal constituents, such as mitochondria, ubiquitin and phosphorylated neurofilaments, were also enriched in swellings with LC3 accumulation [31, 48]. Despite the above observation, the pathological significance of beading and swelling formation is poorly understood. It may result from neuritic transport failure of organelles, or from a failure of autophagy in the axons because ablation of atg7 specifically in Purkinje cells initially induces the axonal swellings [25].
Autophagy in neurite protection and degeneration
Autophagy induction in diverse experimental models of neurite injury
A variety of experimental injury models have been applied to study the mechanism of axonal and dendritic degeneration in vitro (Table 1). Induction of autophagy, characterized by an increase in autophagosome synthesis, is detected in dopaminergic axons in anterior axotomy and posterior axotomy models [69]. Conditional deletion of the essential autophagy gene atg7 in adult mice achieves striking axon protection in this acute model of retrograde degeneration [69], suggesting that autophagy may in fact contribute to axonal degeneration. The exact mechanism whereby autophagy is involved in axonopathy is unclear. In rats with root avulsion or distal axotomy, autophagy is initiated in motor neurons independently of the distance and severity of the lesion [13]. Administration of 3-MA or downregulation of atg7 prevents neuritic degeneration of cultured SCG neurons following the transection [68]. Enhanced autophagic activity was also described in various types of neurons during neuritic degeneration induced by pharmacological toxins, including METH [5], zinc depletion [50], NMDA [6], serum deprivation [34, 70] or growth factor withdrawal [68]. A similar pattern of neuritic dystrophy accompanied by autophagy activation is observed when lysosomal degradation is inhibited by deleting one or more cathepsins [71] or by using cysteine protease inhibitors or general lysosomal enzyme inhibitors [72, 73].
Table 1.
Neuritic accumulation of autophagosomes and autolysosomes in diverse experimental models of neurite injuries.
| Insult | In vivo/ in vitro |
Tissues/cell type/cell line |
Effects | Phenotype | Refs |
|---|---|---|---|---|---|
|
Mechanical injury | |||||
| Root avulsion/distal axotomy |
In vivo | Motor neuron | Undetermined. | Autophagy is initiated independently of the distance and severity of the lesion. |
[13] |
| Anterior/posterior axotomy |
In vivo | DA | Harmful. | Conditional deletion of Atg7 in adult mice offers axon protection. |
[69] |
| Axotomy | In vivo | RGC | Harmful. | Autophagy inhibition prevents axonal degeneration. |
[12] |
| Posterior transection | In vitro | SCG | Harmful. | Autophagy inhibition prevents neuritic degeneration. |
[68] |
|
Pharmacological toxin exposure | |||||
| METH | In vitro | DA | Undetermined. | Degradative autophagic vacuoles accumulate in neuritic varicosities. |
[5] |
| Zinc depletion | In vitro | SCG | Harmful. | Autophagy inhibition prevents terminal neurites from degeneration. |
[50] |
| NMDA | In vitro | CGN | Harmful. | Autophagy inhibition prevents neuritic degeneration. |
[6] |
|
Nutrition withdrawal | |||||
| NGF deprivation | In vitro | SCG, PC12 | Harmful. | Autophagy inhibition prevents neuritic degeneration. |
[68] |
| Serum deprivation | In vitro | PC12 | Undetermined. | Autophagosomes and autolysosomes accumulate in degenerating neurites. |
[34, 70] |
|
Lysosome inhibitors or inactivation of degradation | |||||
| Vinblastine | In vitro | Cortical neurons, SCG |
Harmful. | Autophagosomes and autolysosomes accumulate in degenerating neurites. Autophagy inhibition prevents neuritic degeneration. |
[7, 68] |
| Cathepsins deletion | In vivo | Brain tissues | Undetermined. | Autophagosomes accumulate in degenerating neurites. |
[71] |
| Cysteine protease inhibitors |
In vivo | Hippocampus | Beneficial. | Activated caspase-3 and autophagic vacuoles colocalize in degenerating neurites, which accelerates apoptosis. |
[72] |
| Cathepsins inhibitors | In vitro | Hippocampal neurons |
Undetermined. | Lysosomes and fused dense bodies (FDBs) composed of secondary lysosomes, postlysosomal compartments, and autophagic vacuoles accumulate selectively in the axon hillock and initial segment of the axon. |
[73] |
3-MA, 3-methyladenine; CGN, rat cerebellar granule neuron; DA, Dopamine neurons; METH, methamphetamine; NGF, nerve growth factor; NMDA, N-methyl-D-aspartate; RGC, retinal ganglion cell; SCG, superior cervical ganglion.
Given that protein synthetic and degradative machineries may vary between different neuron types in response to insults [51], it is plausible that autophagy may play differential roles in axonal and dendritic degeneration under different experimental paradigms.
Compartmentalized regulation of autophagy - an example of RGC neuron
Axons and dendrites may also differ from the soma in their autophagy response to various insults. Autophagy inhibition by 3-MA potentiates the harmful effect of axotomy in cultured RGCs, and thus, autophagy induction may serve as a survival mechanism in this model [74]. In addition, a recent report reveals that autophagy promotes the survival of RGCs after optic nerve axotomy in mice [75]. Genetic inhibition of autophagy, for example, knockout of atg4B in mice or specific deletion of atg5 in RGCs, reduces cell survival following optic nerve transection, while pharmacological induction of autophagy by rapamycin in vivo increases the number of surviving cells [75]. Therefore, autophagy may supply energy by self-digestion when the neurons re-program their mode to initiate regeneration. Conversely, an in vivo study shows that blocking autophagic activity with 3-MA results in slower disintegration of RGC axons compared to the control within 360 min postcrush [12]. It appears that, in some scenarios, axon degeneration is a self-destructive program, which has a distinct mechanism from the cell body death [2, 67]. Emerging evidence suggests that molecularly distinct degenerative pathways underlie the destruction of RGC soma and axons [76]. It remains possible that autophagy may participate in a compartmentalized program that regulates axonal or dendritic degeneration distinct from that of the soma.
Distinct role of autophagy in neuritic degeneration associated with neurological disorders
The available evidence that autophagy acts in both pro-survival and pro-death pathways suggest that autophagy is a double-edged sword in health and disease [77]. The appearance of autophagic structures in dying neurites has led to the initial hypothesis that autophagy play a causative role in neurite damage or degeneration induced by acute or chronic injuries, while recent evidence shows protective autophagy as a homeostatic response of neurites required to preserve their integrity. Despite the increase in autophagosome and autolysosome number widely observed in various disease brains, the precise role of autophagy in regulating neuritic degeneration remains unclear (Table 2). Disease-relevant cell and animal models are important for the dissection of the molecular mechanism underlying the exact function of autophagy in neuritic degeneration in the neurological disorders.
Table 2.
Examples of autophagy alteration in degenerating neurites in different neurodegenerative disorders.
| Disease (Organism/animal or cell model) |
Tissues/cells | Effects | Phenotype | Refs |
|---|---|---|---|---|
|
AD | ||||
| Human patients | Neocortex | Undermined. | Autophagosomes and other prelysosomal autophagic vacuoles accumulate in degenerating neurites. |
[14] |
| Hippocampus and inferior temporal cortex |
Undermined. | Enhanced immunoreactivity of Atg12 in tau-immunopositive dystrophic neurites and some neurofibrillary tangles. |
[15] | |
| Hippocampus | Undermined. | APP accumulates in the lysosomal system of the degenerating neurites present in senile plaques. |
[16] | |
| Biopsied brain material | Undermined. | PS1 immunoreactivity is identified in autophagic vacuoles in degenerating neurites. |
[17] | |
| APP, PS/APP transgenetic mice |
Cerebral cortex, hippocampus |
Beneficial. | Autophagosomes and autolysosomes accumulate in degenerating neurites. Recovery of lysosomal proteolysis reverses axonal dystrophy and enhances maturation of accumulated autophagosomes. |
[17, 31, 40, 72] |
| Lysosomal proteolysis inhibition |
Mouse cortical neurons | Beneficial. | Lysosomal dysfunction disrupts axonal transport of degradative organelles and causes Alzheimer-like neurite dystrophy. |
[31] |
|
ALS | ||||
| SOD1 mutant mice | Spinal cord motor neurons |
Harmful. | Autophagic vacuoles accumulated in degenerating axons. Autophagy stimulation accelerates the motor neuron degeneration. |
[11] |
| Human patients | Motor neurons | Undermined. | The autophagy features were also found in close association with the characteristic inclusions of ALS. |
[95] |
|
Epilepsy | ||||
|
Atg7 conditional knockout mice |
Forebrain neurons | Beneficial. | Atg7-deficiency promotes the development of spontaneous seizures. |
[81] |
| Human TSC patients | Brain tissues | Undermined. | Autophagy is suppressed in brains. | [81] |
| HD | ||||
| Human patients | Striatum | Beneficial. | Beclin1-positive inclusion-like deposits accumulate in axon or neuropil of HD samples. However, striatal neurons in normal control brains show diffuse immunoreactivity for Beclin1. |
[20] |
| Hdh140Q/ΔQ mice | Striatum | Beneficial. | LC3 colocalizes with neuropil htt aggregates in the Hdh140Q/ΔQ striatum at 1 and 2 years of age, and such phenomenon is absent in the Hdh140Q/+ striatum. |
[78] |
|
Inherited ataxias | ||||
| Human patients | Cerebral and brainstem | Undermined. | Widespread axonal aggregates, immunopositive for autophagy associated shuttle protein p62, appear in fiber tracts known to undergo neurodegeneration in SCA3 brains. |
[96] |
|
Lurcher mice, atg5 and atg7 conditional knockout mice |
DCN | Tentatively Beneficial. |
Autophagy serves as an early stress response in axonal dystrophy. Atg7 and Atg5 are both required for the maintenance of axonal homeostasis and the prevention of axonal degeneration. |
[8, 25, 97] |
| pcd mutant mice | DCN | Harmful. | Autophagosomes accumulate in Purkinje cell axons. Increased or aberrant mitophagy may contribute to the Purkinje cell degeneration in pcd mice. |
[9] |
|
PD | ||||
| Human patients | SNpc | Beneficial. | LC3 immunoreactivity is detected in a-synuclein-positive Lewy neurites, a neuropathological feature of PD indicative of axonal pathology. |
[18, 19] |
| G2019S LRRK2 mutant mice |
Cerebral cortex, striatum |
Undermined. | Early and late autophagosomes accumulate in axons and synapses in animals with advanced age. |
[57] |
|
Atg7 conditional knockout mice |
Midbrain DA neurons | Beneficial. | Disrupted autophagy leads to dopaminergic axon and dendrite degeneration and promotes presynaptic accumulation of a-synuclein and LRRK2 in the brain. |
[79, 80] |
| G2019S LRRK2 overexpression |
Mouse cortical neurons | Harmful. | LRRK2 mutants selectively cause dendrite retraction due to calcium dysregulation and induction of dendritic mitophagy. |
[54,55] |
| G2019S LRRK2 overexpression |
SH-SY5Y cells | Harmful. | Autophagosomes accumulate in neurites. Autophagy inhibition prevents neuritic retraction, whereas autophagy stimulation potentiates LRRK2-induced neuritic shortening. |
[56] |
| MPP+ intoxication | Mouse DA neurons | Undermined. | Neuritic degeneration and autophagy occurs before cell body loss. |
[98] |
|
Prion disease | ||||
| Human patients | Brain tissues obtained from CJD patients |
Undermined. | Autophagosomes appear in the affected synapses. | [27] |
| CJD-infected hamsters or GSS- infected mice |
Parietal cortex, corpus callosum, hippocampus, thalamus, cerebellum, brainstem |
Undermined. | The major target of autophagy is dystrophic neurites, mostly dendrites but also axonal terminals and preterminals. |
[59] |
| Scrapie prion strain infection |
Mouse brain aggregate | Beneficial. | The LC3-II immunoreactivity is seen as deposits scattered throughout the neuropil. |
[99] |
AD, Alzheimer’s disease; ALS, amyotrophic lateral sclerosis; APP, amyloid precursor protein; CJD, Creutzfeldt-Jakob disease; DA, dopamine; DCN, deep cerebellar nuclei; GSS, Gerstmann-Sträussler-Scheinker; HD, Huntington’s disease; Hdh140Q/ΔQ, full-length htt lacking its polyglutamine stretch in a knockin mouse model for HD; htt, huntingtin; LRRK2, leucine rich repeat kinase 2; MPP+, 1-methyl-4-phenylpyridinium ion; pcd, Purkinje cell degeneration; PD, Parkinson’s disease; PS1, presenilin 1; PS/APP, presenilin/amyloid precursor protein; SCA3, spinocerebellar ataxia type 3; SNpc, substantia nigra pars compacta; TSC, tuberous sclerosis complex.
In LRRK2 G2019S-transefected SH-SY5Y cells, it was suggested that autophagy contributed to the progression of neuritic degeneration [56]. Knockdown of lc3 or atg7 reverses the toxic effects of LRRK2 G2019S expression in neuritic length, whereas rapamycin potentiates these effects [56]. Similarly, in deep cerebellar nuclei (DCN) of pcd mutant mice, an animal model of inherited ataxias, Purkinje cell axons frequently contain autophagosomes [9]. In addition, treatment of rapamycin accelerates the motor neuron degeneration and shortens the life span of the ALS mice [11]. The above evidence therefore suggests that aberrantly hyperactive autophagy may lead to accelerated neuritic degeneration under specific conditions.
On the other hand, autophagy may act as a quality control pathway that prevents neuritic degeneration in neurodegenerative disorders. The autophagy-lysosome system clears disease-related proteins particularly in the form of aggregates or inclusions, such as APP in AD [31], α-synuclein in PD [18, 19], and N-terminal fragments of huntingtin (htt) in HD [78]. Genetic disruption of autophagy specifically in midbrain dopaminergic neurons results in the axonal and dendritic degeneration, the presynaptic accumulation of α-synuclein and LRRK2, and the formation of ubiquitinated protein aggregates, recapitulating some of the pathologic features of PD [79, 80]. Moreover, conditional knockout of atg7 in mouse forebrain neurons is sufficient to promote the development of spontaneous seizures [81]. Therefore, basal autophagy is neuroprotective by constant removal of ubiquitinated or aggregated proteins.
Dysfunctional neuritic mitophagy in neurological disorders
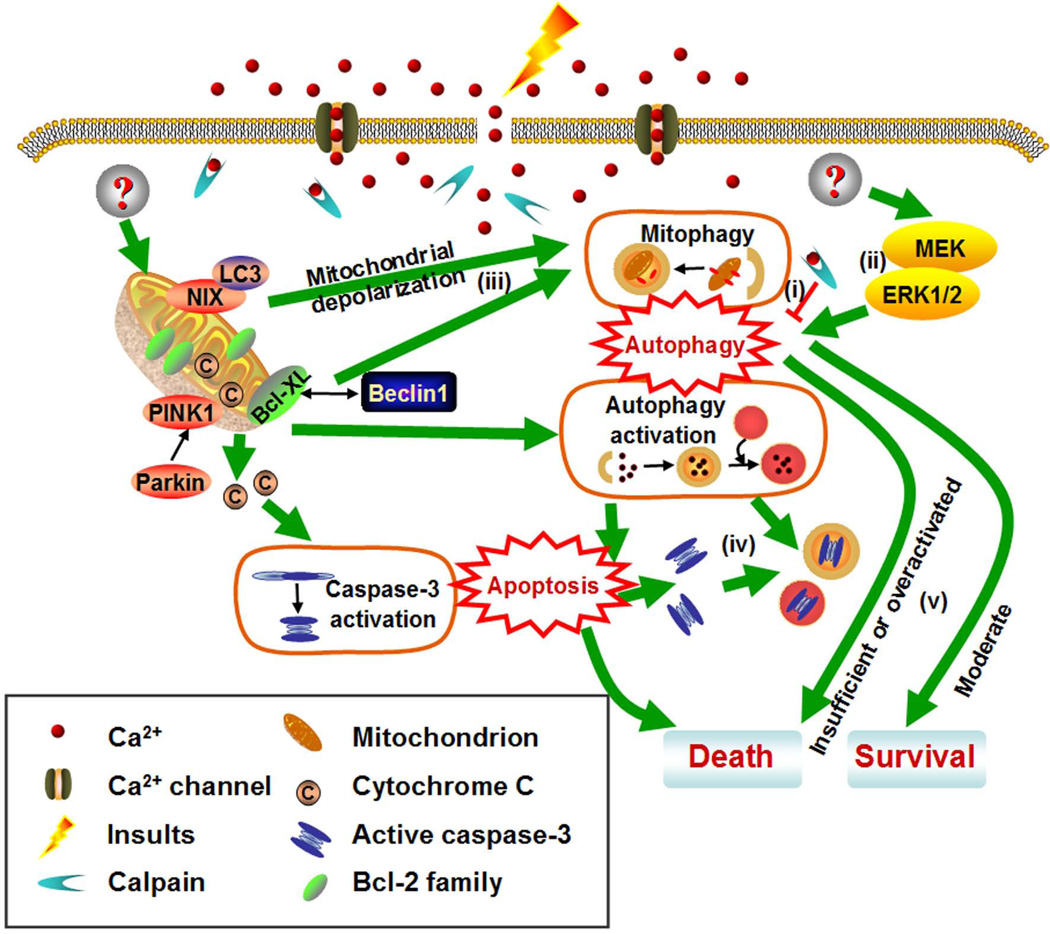
Injured mitochondria with depolarized membrane potential either occurring spontaneously or induced by pro-death signals can be targeted for selective elimination by autophagic processes [82]. In the axons of DRG neurons, approximately 90% of the depolarized mitochondria with high potential were transported towards the growth cone, while about 80% of the depolarized mitochondria with low potential were transported towards the cell body [83]. Emerging evidence has implicated dysfunctional mitophagy in the pathogenesis of AD and PD [84, 85]. For instance, several studies show the importance of mitophagy in the clearance of dysfunctional mitochondria during neuritic remodeling. A number of molecules, including Atg32, NIP3-like protein X (NIX), phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1) and parkin, participate in mitophagy [86] (Figure 3). Parkin was shown to mediate Beclin 1-dependent autophagic clearance of defective mitochondria and ubiquitinated amyloid β peptide (Aβ) in AD models [87]. Moreover, compared to mitophagy in non-neuronal cells, neuronal mitophagy is a slower process that may be compartmentally restricted or coupled with reduced anterograde mitochondrial transport [88]. Parkin-targeted mitochondria accumulate in the somatodendritic regions of cortical neurons where mature lysosomes are predominantly located [88]. In cortical neurons, overexpression of PD-linked LRRK2 mutants caused shortening dendrites, concomitant with enhanced mitophagy likely due to imbalanced calcium homeostasis [54, 55]. The evidence for axonal mitophagy was also reported. For example, in DRG neurons, autophagosomes move along axons toward the cell body carrying engulfed mitochondria cargo, although autophagosomes positive for mitochondrial fragments are scarce under basal condition [30]. Additionally, overexpression of PINK1 and Parkin was shown to decrease mitochondrial movement in rat hippocampal axons [89]. Despite the available evidence, the exact role of mitophagy in regulating the compartmentalized degeneration and regeneration of neurons requires further investigation.
Figure 3.
Putative molecular pathways involved in autophagy-associated neuritic degeneration. Although the underlying mechanism of autophagy-associated neuritic degeneration is still elusive, several pathways might contribute to this process: (i) Autophagy acts as a downstream target of calcium influx following mechanical axonal damage. Calpain may block autophagy activation by mediating the cleavage of Atg5. (ii) MEK/ERK may act as upstream signals of autophagic induction. (iii) Several molecules, including NIX, PINK1 and parkin, may participate in mitophagy. (iv) Autophagy may also mediate the clearance of cleaved caspase-3, reflecting the cross-talk between autophagy and apoptosis in the execution of cell death. (v) Moderate autophagic activation may contribute to neuritic survival, while insufficient or overactivated autophagy lead to neuritic degeneration. ERK, extracellular signal regulated protein kinase; LC3, microtubule-associated protein light chain 3; MEK, mitogen activated protein kinase/ERK kinase; NIX, NIP3-like protein X; PINK1, phosphatase and tensin homolog (PTEN)-induced putative kinase 1.
Potential molecular mechanisms underlying autophagy-regulated neuritic degeneration
Neuritic degeneration has been recognized as a self-destructive program distinct from the soma apoptosis [2]. Current evidence shows that autophagy acts as a downstream target of calcium influx following mechanical axonal damage [12] (Figure 3). In non-neural cells, calpain mediates the cleavage of Atg5, which is required for the formation of autophagosomes, and subsequently provokes apoptotic cell death [90]. However, further study is needed to clarify whether Atg5 cleavage by calpain activation occurs in, and contributes to, neuritic degeneration.
Although caspase activation may contribute to the cell body death, some types of neuritic degeneration do not involve local caspase activation because caspase inhibitors fail to block neuritic degeneration induced by various insults [68, 91]. However, in PS/APP mouse brain, impairment of lysosomal proteolysis by leupeptin enhances caspase-3 immunolabeling within autophagosomes of dystrophic neurites [72]. Hence, we propose that the absence of apoptotic activation in dystrophic axons and dendrites may reflect autophagic removal of local caspases via retrograde transport. In the spinal cord motor neurons of ALS model mice, rapamycin elevates the caspase-3 activity and induces the transfer of cleaved capase-3 into the nucleus [11]. Compared to the controls, rapamycin-treated ALS mice exhibit an elevated ratio of Bax/Bcl-2 (a marker of apoptosis activation), implying that autophagy-triggered apoptotic pathway may contribute to the motor neuron degeneration in ALS mice [11].
Mitogen activated protein kinase/extracellular signal regulated protein kinase (MAPK/ERK) kinase (MEK) pathway is thought to play crucial roles in regulating axonal degeneration [92]. Pharmacological inhibition of MEK pathway by U0126 reverts the protective effect of proteasome inhibition on axonal degeneration [92]. In contrast, U0126 has been shown to reduce LRRK2-induced neuritic autophagy and neuritic shortening, implicating the involvement of MAPK/ERK-related signaling pathway in autophagy-regulated neuritic degeneration.
Concluding remarks and future perspectives
Neuritic autophagy is a constitutive process consisting autophagosome formation at the distal end, transport and maturation of autophagosomes along the length of processes, and degradation and clearance of autophagosomes at the soma. Recent studies have revealed the dynamic autophagic activity in the axons and provided compelling evidence for the essential role of autophagy in the maintenance of axonal homeostasis and normal functions. Moreover, autophagy serves a self-defense mechanism in many disease conditions, notably in proteinopathies and specific axonal injuries, and contributes to the protection of the axons and dendrites. On the other hand, insufficient or excessive autophagy often causes axonal and dendritic degeneration through disrupting the homeostatic balance of critical proteins and membranes in the highly specialized compartments. A critical question is how exactly autophagy is altered and participates in diverse pathophysiological processes associated with axonal or dendritic degeneration (Box 1).
Box 1. Outstanding questions.
What are the molecular mechanisms that control axonal autophagosome biogenesis, autophagic cargo packing, transport, maturation and clearance?
By what mechanisms does autophagy contribute to neuritic protection and degeneration in diverse pathophysiological conditions?
What is the exact role of mitophagy in regulating the compartmentalized degeneration of neurons?
What is the cross-talk between autophagy and apoptosis in regulating axonal and dendritic degeneration?
How does autophagy participate in synaptic proteins or vesicles turnover and regulate synaptic activity?
In fact, many basic questions regarding axonal or dendritic autophagy remain unanswered (Box 1), such as molecular mechanisms that control axonal autophagosome biogenesis, cargo packing, transport, maturation and clearance. In addition, the source of the autophagosome membrane, the fusion with endosomes/lysosomes and the cross-talk with apoptotic machinery have yet to be characterized in the axons. Future experiments will also be needed to understand the difference in the signaling and regulation of autophagy between the soma and neurites (especially the axons). Furthermore, because of the known non-specific effects of the autophagy “enhancers” (e.g., rapamycin) [93] or “inhibitors” (e.g., 3-MA) [94], caution should be taken when interpreting the results in the studies using these compounds. Thus development of highly specific and potent small chemicals that stimulate or inhibit autophagy is desirable.
Finally, understanding how autophagy executes or protects against neuritic degeneration will be critical for the development of novel effective therapeutic or preventive interventions for neurodegenerative diseases. It remains to be determined whether altered autophagy is causative or secondary to the pathological processes of the axons. Specific autophagy-modifying tools should be developed to test the ideas that manipulation of autophagy offers opportunity to protect against axonal or dendritic degeneration in neurodegenerative diseases.
Highlights.
Neuritic degeneration is a pathological feature of many neurodegenerative diseases.
Autophagy regulates protein and organelle homeostasis in the axons and dendrites.
Insufficient or excessive autophagy contributes to neuritic degeneration.
Acknowledgements
This work was supported by National Basic Research Program of China (973 Program) (Grant No. 2011CB504400) (DS), National Natural Science Foundation of China (Grant No. 61031002) (ZX), the Zhejiang Provincial Natural Science Foundation (Grant No. LY12H31010) (ZL), Hangzhou Key Laboratory Research Program (Grant No. 20090233T12) (ZL), R01NS060123, R01NS060809, CHDI Foundation and the Michael J. Fox Foundation (ZY).
Glossary
- Autophagosome
A double-membrane vesicle that forms at an early stage of the autophagic pathway and fuses with endosomes and lysosomes for degradation of its contents [100].
- Autolysosome
A single-membrane vesicle that forms at a late stage of the autophagic pathway by fusion of autophagosomes with endosomes or lysosomes [101].
- Axonal dystrophy
characterized by either focal dilations that interrupt the continuity of axons or terminal end bulbs in dysfunctional or degenerating neurons.
- Dying back degeneration
degeneration initiates from the distal ends of axons, following by distal-to-proximal progression.
- Anterograde transport
from the cell body to the axonal or dendritic terminals, is carried out mostly by kinesin superfamilies, which are plus-end-directed motors [102].
- Retrograde transport
from the axonal or dendritic terminals to the cell body, is carried out mostly by dyneins, which are minusend-directed motors [102].
- Mitophagy
selective removal of mitochondria by autophagy [103].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coleman MP, Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 2002;25:532–537. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- 2.Raff MC, et al. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- 3.Mizushima N, et al. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 4.Kim J, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsen KE, et al. Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. J Neurosci. 2002;22:8951–8960. doi: 10.1523/JNEUROSCI.22-20-08951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadasivan S, et al. Acute NMDA toxicity in cultured rat cerebellar granule neurons is accompanied by autophagy induction and late onset autophagic cell death phenotype. BMC Neurosci. 2010;11:21. doi: 10.1186/1471-2202-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boland B, et al. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer's disease. J Neurosci. 2008;28:6926–6937. doi: 10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang QJ, et al. Induction of autophagy in axonal dystrophy and degeneration. J Neurosci. 2006;26:8057–8068. doi: 10.1523/JNEUROSCI.2261-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakrabarti L, et al. Autophagy activation and enhanced mitophagy characterize the Purkinje cells of pcd mice prior to neuronal death. Mol Brain. 2009;2:24. doi: 10.1186/1756-6606-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White KE, et al. OPA1 deficiency associated with increased autophagy in retinal ganglion cells in a murine model of dominant optic atrophy. Invest Ophthalmol Vis Sci. 2009;50:2567–2571. doi: 10.1167/iovs.08-2913. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, et al. Rapamycin treatment augments motor neuron degeneration in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Autophagy. 2011;7:412–425. doi: 10.4161/auto.7.4.14541. [DOI] [PubMed] [Google Scholar]
- 12.Knoferle J, et al. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc Natl Acad Sci U S A. 2010;107:6064–6069. doi: 10.1073/pnas.0909794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penas C, et al. Autophagy, and BiP level decrease are early key events in retrograde degeneration of motoneurons. Cell Death Differ. 2011;18:1617–1627. doi: 10.1038/cdd.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nixon RA, et al. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- 15.Ma JF, et al. Immunohistochemical evidence for macroautophagy in neurones and endothelial cells in Alzheimer's disease. Neuropathol Appl Neurobiol. 2010;36:312–319. doi: 10.1111/j.1365-2990.2010.01067.x. [DOI] [PubMed] [Google Scholar]
- 16.Kawai M, et al. Subcellular localization of amyloid precursor protein in senile plaques of Alzheimer's disease. Am J Pathol. 1992;140:947–958. [PMC free article] [PubMed] [Google Scholar]
- 17.Yu WH, et al. Macroautophagy--a novel Beta-amyloid peptide-generating pathway activated in Alzheimer's disease. J Cell Biol. 2005;171:87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dehay B, et al. Pathogenic lysosomal depletion in Parkinson's disease. J Neurosci. 2010;30:12535–12544. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarez-Erviti L, et al. Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol. 2010;67:1464–1472. doi: 10.1001/archneurol.2010.198. [DOI] [PubMed] [Google Scholar]
- 20.Shibata M, et al. Regulation of intracellular accumulation of mutant Huntingtin by Beclin 1. J Biol Chem. 2006;281:14474–14485. doi: 10.1074/jbc.M600364200. [DOI] [PubMed] [Google Scholar]
- 21.Nixon RA, Yang DS. Autophagy and neuronal cell death in neurological disorders. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viscomi MT, D'Amelio M. The "janus-faced role" of autophagy in neuronal sickness: focus on neurodegeneration. Mol Neurobiol. 2012;46:513–521. doi: 10.1007/s12035-012-8296-3. [DOI] [PubMed] [Google Scholar]
- 23.Harris H, Rubinsztein DC. Control of autophagy as a therapy for neurodegenerative disease. Nat Rev Neurol. 2012;8:108–117. doi: 10.1038/nrneurol.2011.200. [DOI] [PubMed] [Google Scholar]
- 24.Li H, et al. Huntingtin aggregate-associated axonal degeneration is an early pathological event in Huntington's disease mice. J Neurosci. 2001;21:8473–8481. doi: 10.1523/JNEUROSCI.21-21-08473.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komatsu M, et al. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci U S A. 2007;104:14489–14494. doi: 10.1073/pnas.0701311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yue Z, et al. A novel protein complex linking the delta 2 glutamate receptor and .autophagy: implications for neurodegeneration in lurcher mice. Neuron. 2002;35:921–933. doi: 10.1016/s0896-6273(02)00861-9. [DOI] [PubMed] [Google Scholar]
- 27.Sikorska B, et al. Autophagy is a part of ultrastructural synaptic pathology in Creutzfeldt-Jakob disease: a brain biopsy study. Int J Biochem Cell Biol. 2004;36:2563–2573. doi: 10.1016/j.biocel.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Yue Z. Regulation of neuronal autophagy in axon: implication of autophagy in axonal function and dysfunction/degeneration. Autophagy. 2007;3:139–141. doi: 10.4161/auto.3602. [DOI] [PubMed] [Google Scholar]
- 29.Kaasinen SK, et al. Autophagy generates retrogradely transported organelles: a hypothesis. Int J Dev Neurosci. 2008;26:625–634. doi: 10.1016/j.ijdevneu.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Maday S, et al. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J Cell Biol. 2012;196:407–417. doi: 10.1083/jcb.201106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S, et al. Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer's-like axonal dystrophy. J Neurosci. 2011;31:7817–7830. doi: 10.1523/JNEUROSCI.6412-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 33.Mizushima N, et al. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, et al. Microtubule and kinesin/dynein-dependent, bi-directional transport of autolysosomes in neurites of PC12 cells. Int J Biochem Cell Biol. 2011;43:1147–1156. doi: 10.1016/j.biocel.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Kimura S, et al. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- 36.Pankiv S, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 37.Zhou C, et al. Monitoring autophagic flux by an improved tandem fluorescent-tagged LC3 (mTagRFP-mWasabi-LC3) reveals that high-dose rapamycin impairs autophagic flux in cancer cells. Autophagy. 2012:8. doi: 10.4161/auto.20284. [DOI] [PubMed] [Google Scholar]
- 38.Xie R, et al. Acetylated microtubules are required for fusion of autophagosomes with lysosomes. BMC Cell Biol. 2010;11:89. doi: 10.1186/1471-2121-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fass E, et al. Microtubules support production of starvation-induced autophagosomes but not their targeting and fusion with lysosomes. J Biol Chem. 2006;281:36303–36316. doi: 10.1074/jbc.M607031200. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez-Varo R, et al. Abnormal accumulation of autophagic vesicles correlates with axonal and synaptic pathology in young Alzheimer's mice hippocampus. Acta Neuropathol. 2012;123:53–70. doi: 10.1007/s00401-011-0896-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baas PW, Lin S. Hooks and comets: The story of microtubule polarity orientation in the neuron. Dev Neurobiol. 2011;71:403–418. doi: 10.1002/dneu.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hollenbeck PJ, Bray D. Rapidly transported organelles containing membrane and cytoskeletal components: their relation to axonal growth. J Cell Biol. 1987;105:2827–2835. doi: 10.1083/jcb.105.6.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katsumata K, et al. Dynein- and activity-dependent retrograde transport of autophagosomes in neuronal axons. Autophagy. 2010;6:378–385. doi: 10.4161/auto.6.3.11262. [DOI] [PubMed] [Google Scholar]
- 44.Deinhardt K, et al. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006;52:293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 45.Hafezparast M, et al. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science. 2003;300:808–812. doi: 10.1126/science.1083129. [DOI] [PubMed] [Google Scholar]
- 46.LaMonte BH, et al. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron. 2002;34:715–727. doi: 10.1016/s0896-6273(02)00696-7. [DOI] [PubMed] [Google Scholar]
- 47.Chen S, et al. Autophagy dysregulation in amyotrophic lateral sclerosis. Brain Pathol. 2012;22:110–116. doi: 10.1111/j.1750-3639.2011.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stokin GB, et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer's disease. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- 49.Luo L, O'Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y, et al. Cellular Zn2+ chelators cause "dying-back" neurite degeneration associated with energy impairment. J Neurosci Res. 2007;85:2844–2855. doi: 10.1002/jnr.21411. [DOI] [PubMed] [Google Scholar]
- 51.Verma P, et al. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J Neurosci. 2005;25:331–342. doi: 10.1523/JNEUROSCI.3073-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matthews MR. An ultrastructural study of axonal changes following constriction of postganglionic branches of the superior cervical ganglion in the rat. Philos Trans R Soc Lond B Biol Sci. 1973;264:479–505. doi: 10.1098/rstb.1973.0002. [DOI] [PubMed] [Google Scholar]
- 53.Miller FD, Kaplan DR. Signaling mechanisms underlying dendrite formation. Curr Opin Neurobiol. 2003;13:391–398. doi: 10.1016/s0959-4388(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 54.Cherra SJ, 3rd., et al. Regulation of the autophagy protein LC3 by phosphorylation. J Cell Biol. 2010;190:533–539. doi: 10.1083/jcb.201002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cherra SJ, 3rd., et al. Mutant LRRK2 elicits calcium imbalance and depletion of dendritic mitochondria in neurons. Am J Pathol. 2013;182:474–484. doi: 10.1016/j.ajpath.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plowey ED, et al. Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J Neurochem. 2008;105:1048–1056. doi: 10.1111/j.1471-4159.2008.05217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramonet D, et al. Dopaminergic neuronal loss, reduced neurite complexity and autophagic abnormalities in transgenic mice expressing G2019S mutant LRRK2. PLoS One. 2011;6:e18568. doi: 10.1371/journal.pone.0018568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fuhrmann M, et al. Dendritic pathology in prion disease starts at the synaptic spine. J Neurosci. 2007;27:6224–6233. doi: 10.1523/JNEUROSCI.5062-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liberski PP, et al. Autophagy contributes to widespread neuronal degeneration in hamsters infected with the Echigo-1 strain of Creutzfeldt-Jakob disease and mice infected with the Fujisaki strain of Gerstmann-Straussler-Scheinker (GSS) syndrome. Ultrastruct Pathol. 2011;35:31–36. doi: 10.3109/01913123.2010.527038. [DOI] [PubMed] [Google Scholar]
- 60.Hernandez D, et al. Regulation of presynaptic neurotransmission by macroautophagy. Neuron. 2012;74:277–284. doi: 10.1016/j.neuron.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rowland AM, et al. Presynaptic terminals independently regulate synaptic clustering and autophagy of GABAA receptors in Caenorhabditis elegans. J Neurosci. 2006;26:1711–1720. doi: 10.1523/JNEUROSCI.2279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen W, Ganetzky B. Autophagy promotes synapse development in Drosophila. J Cell Biol. 2009;187:71–79. doi: 10.1083/jcb.200907109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Batlevi Y, et al. Dynein light chain 1 is required for autophagy, protein clearance, and cell death in Drosophila. Proc Natl Acad Sci U S A. 2010;107:742–747. doi: 10.1073/pnas.0907967107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haberman A, et al. The synaptic vesicle SNARE neuronal Synaptobrevin promotes endolysosomal degradation and prevents neurodegeneration. J Cell Biol. 2012;196:261–276. doi: 10.1083/jcb.201108088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tan Z, et al. Mutant ubiquitin found in Alzheimer's disease causes neuritic beading of mitochondria in association with neuronal degeneration. Cell Death Differ. 2007;14:1721–1732. doi: 10.1038/sj.cdd.4402180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takeuchi H, et al. Neuritic beading induced by activated microglia is an early feature of neuronal dysfunction toward neuronal death by inhibition of mitochondrial respiration and axonal transport. J Biol Chem. 2005;280:10444–10454. doi: 10.1074/jbc.M413863200. [DOI] [PubMed] [Google Scholar]
- 67.Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- 68.Yang Y, et al. Induction of autophagy in neurite degeneration of mouse superior cervical ganglion neurons. Eur J Neurosci. 2007;26:2979–2988. doi: 10.1111/j.1460-9568.2007.05914.x. [DOI] [PubMed] [Google Scholar]
- 69.Cheng HC, et al. Akt suppresses retrograde degeneration of dopaminergic axons by inhibition of macroautophagy. J Neurosci. 2011;31:2125–2135. doi: 10.1523/JNEUROSCI.5519-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang Y, et al. Transport of autophagosomes in neurites of PC12 cells during serum deprivation. Autophagy. 2008;4:243–245. doi: 10.4161/auto.5431. [DOI] [PubMed] [Google Scholar]
- 71.Koike M, et al. Participation of autophagy in storage of lysosomes in neurons from mouse models of neuronal ceroid-lipofuscinoses (Batten disease) Am J Pathol. 2005;167:1713–1728. doi: 10.1016/S0002-9440(10)61253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang DS, et al. Neuronal apoptosis and autophagy cross talk in aging PS/APP mice, a model of Alzheimer's disease. Am J Pathol. 2008;173:665–681. doi: 10.2353/ajpath.2008.071176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bednarski E, et al. Suppression of cathepsins B and L causes a proliferation of lysosomes and the formation of meganeurites in hippocampus. J Neurosci. 1997;17:4006–4021. doi: 10.1523/JNEUROSCI.17-11-04006.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sternberg C, et al. Caspase dependence of the death of neonatal retinal ganglion cells induced by axon damage and induction of autophagy as a survival mechanism. Braz J Med Biol Res. 2010;43:950–956. doi: 10.1590/s0100-879x2010007500082. [DOI] [PubMed] [Google Scholar]
- 75.Rodriguez-Muela N, et al. Autophagy promotes survival of retinal ganglion cells after optic nerve axotomy in mice. Cell Death Differ. 2012;19:162–169. doi: 10.1038/cdd.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whitmore AV, et al. Glaucoma: thinking in new ways-a role for autonomous axonal self-destruction and other compartmentalised processes? Prog Retin Eye Res. 2005;24:639–662. doi: 10.1016/j.preteyeres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 77.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng S, et al. Deletion of the huntingtin polyglutamine stretch enhances neuronal autophagy and longevity in mice. PLoS Genet. 2010;6:e1000838. doi: 10.1371/journal.pgen.1000838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Friedman LG, et al. Disrupted autophagy leads to dopaminergic axon and dendrite degeneration and promotes presynaptic accumulation of alpha-synuclein and LRRK2 in the brain. J Neurosci. 2012;32:7585–7593. doi: 10.1523/JNEUROSCI.5809-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ahmed I, et al. Development and characterization of a new Parkinson's disease model resulting from impaired autophagy. J Neurosci. 2012;32:16503–16509. doi: 10.1523/JNEUROSCI.0209-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McMahon J, et al. Impaired autophagy in neurons after disinhibition of Mammalian target of rapamycin and its contribution to epileptogenesis. J Neurosci. 2012;32:15704–15714. doi: 10.1523/JNEUROSCI.2392-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xue L, et al. Mitochondria are selectively eliminated from eukaryotic cells after blockade of caspases during apoptosis. Curr Biol. 2001;11:361–365. doi: 10.1016/s0960-9822(01)00100-2. [DOI] [PubMed] [Google Scholar]
- 83.Miller KE, Sheetz MP. Axonal mitochondrial transport and potential are correlated. J Cell Sci. 2004;117:2791–2804. doi: 10.1242/jcs.01130. [DOI] [PubMed] [Google Scholar]
- 84.Santos RX, et al. A synergistic dysfunction of mitochondrial fission/fusion dynamics and mitophagy in Alzheimer's disease. J Alzheimers Dis. 2010;20(Suppl 2):S401–S412. doi: 10.3233/JAD-2010-100666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Imai Y, Lu B. Mitochondrial dynamics and mitophagy in Parkinson's disease: disordered cellular power plant becomes a big deal in a major movement disorder. Curr Opin Neurobiol. 2011;21:935–941. doi: 10.1016/j.conb.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khandelwal PJ, et al. Parkin mediates beclin-dependent autophagic clearance of defective mitochondria and ubiquitinated Abeta in AD models. Hum Mol Genet. 2011;20:2091–2102. doi: 10.1093/hmg/ddr091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cai Q, et al. Spatial parkin translocation and degradation of damaged mitochondria via mitophagy in live cortical neurons. Curr Biol. 2012;22:545–552. doi: 10.1016/j.cub.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang X, et al. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yousefi S, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 91.Finn JT, et al. Evidence that Wallerian degeneration and localized axon degeneration induced by local neurotrophin deprivation do not involve caspases. J Neurosci. 2000;20:1333–1341. doi: 10.1523/JNEUROSCI.20-04-01333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.MacInnis BL, Campenot RB. Regulation of Wallerian degeneration and nerve growth factor withdrawal-induced pruning of axons of sympathetic neurons by the proteasome and the MEK/Erk pathway. Mol Cell Neurosci. 2005;28:430–439. doi: 10.1016/j.mcn.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 93.King MA, et al. Rapamycin inhibits polyglutamine aggregation independently of autophagy by reducing protein synthesis. Mol Pharmacol. 2008;73:1052–1063. doi: 10.1124/mol.107.043398. [DOI] [PubMed] [Google Scholar]
- 94.Xue L, et al. Autophagy is activated by apoptotic signalling in sympathetic neurons: an alternative mechanism of death execution. Mol Cell Neurosci. 1999;14:180–198. doi: 10.1006/mcne.1999.0780. [DOI] [PubMed] [Google Scholar]
- 95.Sasaki S. Autophagy in spinal cord motor neurons in sporadic amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2011;70:349–359. doi: 10.1097/NEN.0b013e3182160690. [DOI] [PubMed] [Google Scholar]
- 96.Seidel K, et al. Axonal inclusions in spinocerebellar ataxia type 3. Acta Neuropathol. 2010;120:449–460. doi: 10.1007/s00401-010-0717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nishiyama J, et al. Aberrant membranes and double-membrane structures accumulate in the axons of Atg5-null Purkinje cells before neuronal death. Autophagy. 2007;3:591–596. doi: 10.4161/auto.4964. [DOI] [PubMed] [Google Scholar]
- 98.Kim-Han JS, et al. The Parkinsonian mimetic, MPP+, specifically impairs mitochondrial transport in dopamine axons. J Neurosci. 2011;31:7212–7221. doi: 10.1523/JNEUROSCI.0711-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bajsarowicz K, et al. A brain aggregate model gives new insights into the pathobiology and treatment of prion diseases. J Neuropathol Exp Neurol. 2012;71:449–466. doi: 10.1097/NEN.0b013e3182544680. [DOI] [PubMed] [Google Scholar]
- 100.Dunn WA., Jr. Studies on the mechanisms of autophagy: maturation of the autophagic vacuole. J Cell Biol. 1990;110:1935–1945. doi: 10.1083/jcb.110.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dunn WA., Jr. Studies on the mechanisms of autophagy: formation of the autophagic vacuole. J Cell Biol. 1990;110:1923–1933. doi: 10.1083/jcb.110.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hirokawa N, Takemura R. Molecular motors and mechanisms of directional transport in neurons. Nat Rev Neurosci. 2005;6:201–214. doi: 10.1038/nrn1624. [DOI] [PubMed] [Google Scholar]
- 103.Tolkovsky AM. Mitophagy. Biochim Biophys Acta. 2009;1793:1508–1515. doi: 10.1016/j.bbamcr.2009.03.002. [DOI] [PubMed] [Google Scholar]