Abstract
Many children with pervasive developmental disorders (PDD) exhibit behaviors and symptoms of attention-deficit/hyperactivity disorder (ADHD). We sought to determine the relative efficacy of medications for treating ADHD symptoms in children with PDD by identifying all double-blind, randomized, placebo-controlled trials examining the efficacy of medications for treating ADHD symptoms in children with PDD. We located seven trials involving 225 children. A random effects meta-analysis of four methylphenidate trials showed methylphenidate to be effective for treating ADHD symptoms in children with PDD (ES = .67). Several adverse events were greater for children were taking methylphenidate compared to placebo. An individual trial of clonidine and two trials of atomoxetine suggest these agents may also be effective in treating ADHD symptoms in children with PDD.
Keywords: autism, pervasive developmental disorder, attention deficit/hyperactivity disorder, methylphenidate, meta-analysis, atomoxetine, clonidine, ADHD, ASD, autism spectrum disorder, PDD, PDD-NOS
Currently, the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association; APA, 2000) prohibits a comorbid diagnosis of attention-deficit/hyperactivity disorder (ADHD) in individuals diagnosed with a pervasive developmental disorder (PDD; e.g., autism, Asperger’s disorder, pervasive developmental disorder, not otherwise specified). However, many individuals with PDDs exhibit behaviors and symptoms associated with ADHD. Comorbidity of ADHD symptoms in individuals with PDDs have generally been reported to be between 30- and 50% (Sinzig, 2009) (Leyfer et al., 2006; Simonoff, 2008) although even higher rates (e.g., in excess of 70%) have been reported in clinical samples (Frazier, 2001; Lee & Ousley, 2006). It remains a matter of debate whether ADHD symptoms in children with PDD should be regarded as a comorbid condition (i.e., PDD and ADHD) or as common symptoms of the underlying developmental disorder (van der Meer et al., 2012) (Gargaro, 2011; Grzadzinski; Hazell, 2007; Lecavalier, 2006; Sinzig, 2009). Regardless, ADHD symptoms in children with PDD cause significant obstacles to educational achievement, socialization, and behavioral management,(Holtmann, Bolte, & Poustka, 2007; Yerys, 2009), just as they do in children with typical development. Since the expression of ADHD symptoms in children with and without PDDs appear similar, children with PDD may benefit from the same systematically tested, evidence-based treatments that have proven successful in typically developing children with ADHD.
ADHD is one of the few child psychiatric conditions in which pharmacotherapies have outperformed systematically implemented behavioral treatment in terms of short-term efficacy (MTA Group, 1999). Several pharmacotherapies have demonstrated significant short-term efficacy for the treatment of ADHD including psychostimulant medications (i.e. methylphenidate and dextroamphetamine derivatives) and other medications such as atomoxetine, alpha-2 agonists and desipramine (Cheng, 2007; Connor, 1999; Schachter, 2001; Spencer, 1996). However, several clinical case series have suggested that ADHD symptoms in children with PDD might respond differently to medications. For instance, a large retrospective case series of 195 children with PDD treated with psychostimulants suggested that these medications might have decreased efficacy and worse side effect profile in children with PDD (Stigler, Desmond, Posey, Wiegand, & McDougle, 2004).
Medications are commonly used to treat behavioral symptoms, including ADHD symptomatology, of children with PDDs (Aman et al., 2005; Mandell et al., 2008; Rosenberg, 2010). Roughly one-quarter of children with PDD are prescribed psychostimulants to treat symptoms of ADHD (Oswald & Sonenklar, 2007). In recent years, there have been several randomized, placebo-controlled trials examining the efficacy of pharmacotherapies for the treatment of ADHD symptoms in children with PDD. A recent systematic review in this area has examined the evidence behind pharmacological treatments in PDD but did not conduct a quantitative synthesis of available data (McPheeters, Warren, et al., 2011). We conducted a meta-analysis to determine which medications have demonstrated efficacy in treating ADHD symptoms in children with PDD. We also set out to estimate the magnitude of treatment effects and rate of side effects of these medications in children with PDDs.
Method
Search Strategy for Identification of Studies
Two reviewers independently searched PubMed and ClinicalTrials.gov for all relevant studies in January 2013. The PubMed search was conducted using the term “Child Development Disorders, Pervasive”[Mesh] AND (“Methylphenidate”[Mesh] OR “Dextroamphetamine”[Mesh] OR atomoxetine OR clonidine OR guanfacine).” We used the PubMed filters to further limit the search to randomized control trials or meta-analyses. The ClincialTrials.gov search was conducted using a targeted search of the term “autism or pervasive developmental disorder” and an intervention filter for “Methylphenidate OR Dextroamphetamine OR atomoxetine OR clonidine OR guanfacine.” Finally, we reviewed the reference lists of all included articles, reviews, and meta-analyses for citations of published or unpublished studies not located in the database search.
Selection of Studies
Two reviewers (the first and last authors) independently evaluated the titles and abstracts of the located studies to determine eligibility for inclusion in this meta-analysis. Studies were included in this meta-analysis if they were (1) randomized, double-blind, placebo-controlled trials comparing a medication (e.g., methylphenidate-derivative, amphetamine-derivative, atomoxetine, alpha-2 agonist) with placebo; (2) medication lasted at least 1 week; and (3) trials examining ADHD symptoms as an outcome measure.
Outcome Measures
The primary outcome measures for our analyses were standardized measures of global ADHD symptomatology and adverse events. Acceptable measures of ADHD symptomatology were, in order of preference, the Conners’ Parent and Teacher Rating Scales (Conners, 2001), SNAP-IV Rating Scale (Swanson, 1992), ADHD Rating Scale (DuPaul, Anastopoulos et al., 1998) and generic DSM-IV ADHD symptom scales. We examined five adverse events: decreased appetite, depression, insomnia, irritability, and social withdrawal. We also examined three specific symptoms of ADHD symptoms (hyperactivity, stereotypies, and irritability). Hyperactivity, stereotypies and irritability were all reported using the corresponding Aberrant Behavior Checklist scale with one exception (the Nisonger Child Behavior Rating Form Parent Hyperactive subscale was used to report hyperactivity in one study; Ghuman et al., 2009).
Choice of Summary Statistics
We estimated the difference between treatment and placebo for each trial on ADHD symptomatology, hyperactivity, irritability, and stereotypies by calculating the standardized mean difference effect size with small sample correction (i.e., Hedges g; Hedges, 1985). The effect size estimate was calculated from the post-treatment scores and standard deviations provided in each study report. We chose the standardized mean difference effect size with small sample correction over the weighted mean difference because multiple measures with different scales were sometimes used to assess one outcome (e.g., ADHD symptomatology) and a majority of the studies we located had small sample sizes. We examined the difference between treatment and placebo for adverse events by calculating the absolute risk difference (ARD).
Meta-Analytic Procedure
We combined results for the studies examining methylphenidate in a random effects meta-analysis using an inverse-variance weighted mean effect size (ES). A random effects model was used for the meta-analysis because there was evidence of considerable heterogeneity between the trials. The meta-analyses of ADHD symptomatology, hyperactivity, irritability, and stereotypic behavior were conducted using the Hedge’s g effect size using Comprehensive Meta-Analysis 2 (Borenstein, 2005) and results were confirmed using Wilson’s MeanES macro for SPSS (Wilson, 2005). Adverse events were combined in a meta-analysis using Comprehensive Meta-Analysis 2 using the absolute risk difference (ARD) metric with a random effects model. Due to the small number of studies, moderator analyses were not deemed appropriate at this time. We also decided against conducting a meta-analysis of the atomoxetine studies given the small number of located studies.
Assessment of Heterogeneity
We conducted two statistical estimates of heterogeneity. The first estimate examined heterogeneity using the Q-statistic, (Hedges, 1985) which provides a test of statistical significance indicating whether the differences in effect sizes are due to subject-level sampling error alone or other sources. Because recent criticism has been raised about the validity of the Q-statistic as a test of homogeneity in meta-analyses, (Heudo-Medina, 2006) we also estimated heterogeneity using I2, which estimates the proportion of between-studies variance.
Assessment of Publication Bias
Publication bias occurs when there are unpublished studies with negative results (e.g., file-drawer problem), and is often a problem when conducting research syntheses. A funnel plot is often used to detect publication bias, which can be analyzed visually, although use of a funnel plot when a small number of studies are located is not recommended (Sterne, 2008).
Results
Included Studies
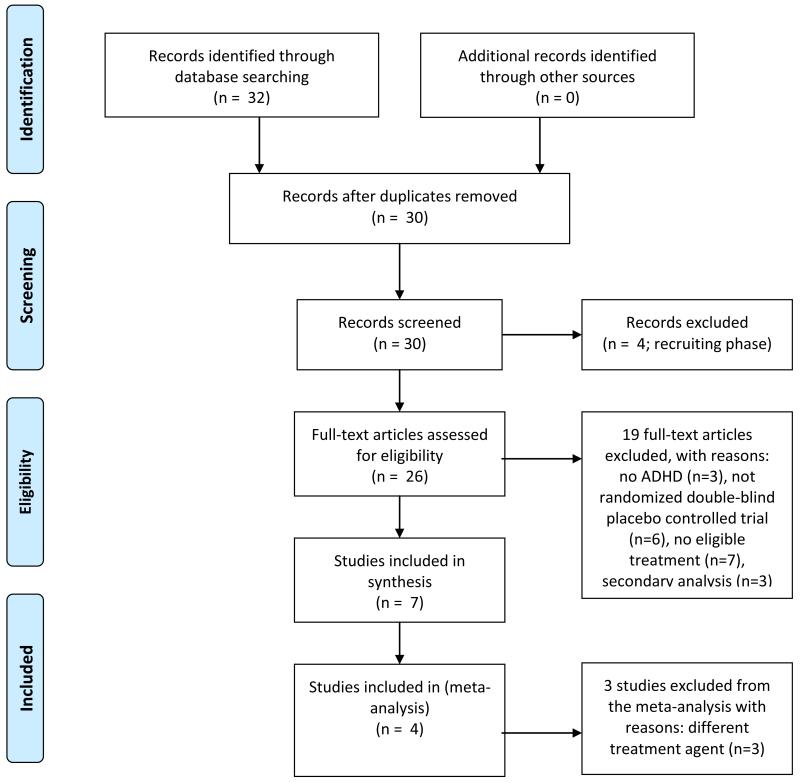
We located 32 studies in our search. Seven studies involving 225 participants were included in our analyses; the reasons for exclusion of the other 25 studies are provided in the flow diagram shown in Figure 1. Four studies involving 94 participants compared methylphenidate to placebo, one study involving 8 participants compared an alpha-2 agonist (clonidine) to placebo, and two studies involving 113 participants compared atomoxetine to placebo. The characteristics of these seven studies are shown in Table 1.
Figure 1.
Study inclusion decision tree (using PRISMA flow diagram, of Moher et al., 2009)
Table 1. Characteristics of Included Studies.
| Study | N | Mean age, y |
Sex (%M) |
Design | Length of Treatment |
JADAD | Medication | Mean dose |
|---|---|---|---|---|---|---|---|---|
| Arnold et al. (2006) | 16 | 9.3 | 75 | crossover | 6 weeks | 4 | ATOM | 44.2 mg/day |
| Harfterkamp et al. (2012) | 97 | 10.0 | 86 | parallel | 10 weeks | ATOM | 1.2 mg/kg.day | |
| Ghuman et al. (2009) | 12 | 4.8 | 93 | crossover | 2 weeks | 3 | MPH | .4 mg/kg/dose |
| Handen et al. (2000) | 13 | 7.4 | 77 | crossover | 1 week | 2 | MPH | .45 mg/kg/dose |
| Quintana et al. (1995) | 10 | 8.5 | 60 | crossover | 2 weeks | 3 | MPH | .40 mg/kg/dose |
| RUPP (2005) | 66 | 7.5 | 89 | crossover | 1 week | 5 | MPH | .29 mg/kg/dose |
| Jaselskis et al. (1992) | 8 | 8.1 | 100 | crossover | 6 weeks | 2 | α-2 agonist | .15-.20 mg/day |
Key: N – sample size; y – years; %M – percent male; MPH – methylphenidate; ATOM – atomoxetine; α-2 agonist (clonidine); mg/kg/dose – milligrams per kilograms per does; mg/day – milligrams per day
Methylphenidate Derivatives
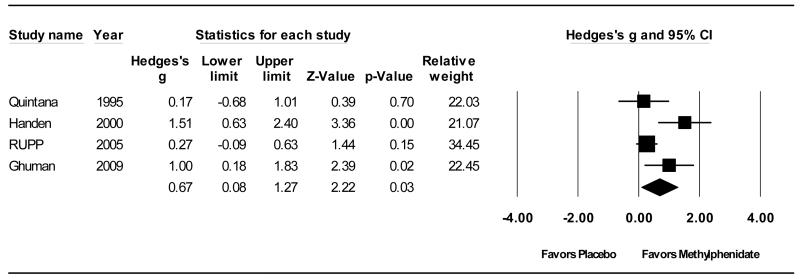
Four studies involving 94 participants comparing methylphenidate derivatives to placebo in children with PDD were located (Ghuman, 2009; Handen, Johnson, & Lubetsky, 2000; Quintana et al., 1995; RUPP, 2005). Figure 2 depicts the forest plot of the effect of methylphenidate on ADHD symptomatology. Methylphenidate was shown to be superior to placebo for the treatment of ADHD symptomatology in children with PDDs (ES = .67; 95% CI .08-1.27; z = 2.22, p < .05). There was a high degree heterogeneity for the use of methylphenidate to treat ADHD symptoms (Q(3) = 8.71, p < .05; I2 = 66%), however, due to the small sample of studies involving mostly small sample sizes, we deemed moderator analyses inappropriate. Likewise, the small number of studies located precluded our ability to use statistical methods or visual analysis to examine the presence or absence of publication bias, (Sterne, 2008) thus it cannot be ruled out and should be taken into consideration when interpreting these results.
Figure 2.
Forrest Plot of Effect Size Estimates for Differences in ADHD Symptomatology
All four studies also reported the effects of methylphenidate specifically on hyperactivity. When the results were combined, the results showed methylphenidate was effective in treating hyperactivity in children with PDDs (ES = .66; 95% CI .30-1.03; z = 3.57, p < .001). Two studies (Handen et al., 2000) and (Quintana et al., 1995) reported data on irritability and stereotypies. When the results of these studies were combined, methylphenidate was shown to have moderate, albeit not statistically significant, effects in treating irritability and stereotypies in children with PDDs (ES = .52; 95% CI -.06-1.10; z = 1.77, p = .08 and ES = .47; 95% CI -.11-1.05; z = 1.59, p = .11, respectively).
Adverse events were combined and analyzed using a weighted absolute risk difference, which calculates the difference in the percentage of cases reporting an adverse event during the treatment and placebo phases. Three studies reported adverse events; two studies (Ghuman et al., 2009; RUPP, 2005) reported on all five adverse events and (Handen et al., 2000) reported on all adverse events except insomnia). Overall, there were a greater number of adverse events reported during the methylphenidate phase than placebo. Children were more likely to have (a) decreased appetite (ARD = .17; 95% CI .03-.31; NNH=5.9; 95% CI: 3.2-33.3; z = 2.36, p < .05), (b) greater insomnia (ARD = .19; 95% CI .02-.36; NNH=5.3; 95%CI: 2.8-50; z = 2.21, p < .05), (c) more depressive symptoms (ARD = .07; 95% CI .004-.13; NNH=14.3; 95%CI: 7.7-250; z = 2.07, p < .05), (d) greater irritability (ARD = .14; 95% CI .05-.24; NNH=7.1; 95%CI: 4.2-20; z = 2.91, p < .01), and (e) higher levels of social withdrawal (ARD = .07; 95% CI .002-.15; NNH=14.3; 95% CI: 6.7-500; z = 2.02, p < .05).
Alpha-2 Agonists
One study (Jaselskis, Cook, et al., 1992) was located comparing clonidine to placebo in eight children with a PDD. No statistically significant findings were found in their study for our primary (ADHD symptoms) or secondary outcomes (improvements in irritability, stereotypic behaviors, and hyperactivity). However, our calculation of Hedge’s g for the primary outcome of improvement in ADHD symptoms and secondary outcome of improvements in irritability show differences favoring the clonidine group to be in the medium effect range; g = .51; 95%CI -.44-1.45; z = 1.1, p = .29, and g = .64; 95%CI -.36-1.65; z = 1.25, p = .21, respectively. Smaller improvements in stereotypic behaviors (g = .24; 95%CI -.74-1.23; z = .48, p = .63) and hyperactivity (g = .30; 95%CI -.63-1.24; z = .64, p = .53) were also shown. Data on the adverse events we measured for this report were not provided in this study, but the authors reported increased hypotension and drowsiness in some children while they were taking clonidine.
Atomoxetine
We located two studies (Arnold, Aman, et al., 2006) (Harfterkamp, van de Loo Neus, et al., 2012) comparing atomoxetine to placebo in 113 children with a PDD. As shown in Table 1, there is a large difference in sample size between these two studies; 16 participants participated in the Arnold study and 97 participants participated in the Harfterkamp study. Statistically significant findings favoring atomoxetine were found on our primary (ADHD symptoms) and one secondary outcome (hyperactivity) in the Harfterkamp study but no significant differences were found in the Arnold study. Our calculation of Hedge’s g for the primary outcome of improvement in ADHD symptoms shows atomoxetine made significant improvements in ADHD symptoms in the larger Harfterkamp study (g = .83; 95%CI .39-1.26; z = 3.73, p = .0002) and the secondary outcome of hyperactivity (g = .80; 95%CI .36-1.23; z = 3.61, p = .0003). The Arnold study showed moderate improvements, although not statistically significant, on overall improvement in ADHD symptoms (g = .51; 95%CI -.18-1.19; z = 14, p = .15) but little to no difference for our secondary outcome measures of stereotypic behaviors (g = .33; 95%CI -.37-1.03; z = .92, p = .36), hyperactivity (g = .23; 95%CI -.45-0.91; z = .67, p = .51), or irritability (g = .10; 95%CI -.59-0.80; z =.29, p = .77). The Harfterkamp study reported significantly increased rates of nausea, decreased appetite, and early morning awakening in the atomoxetine group compared to placebo; similar reports were made in the Arnold study although their comparisons were not statistically significant.
Discussion
Previous clinical reports have raised the possibility that psychostimulants have decreased efficacy and worse side-effect profile when utilized in children with PDD (Stigler et al., 2004). Our results suggest methylphenidate is effective in treating ADHD in children with PDD (ES = .67), however it is slightly lower than estimates from studies examining methylphenidate for ADHD symptoms in typically developing children with ADHD alone (e.g.,. ES =.78; 95% CI: .64-.91; (Schachter, 2001); ES = 1.03; (Faraone, 2010)). More studies with adequate power and sample sizes are needed before comparisons between the efficacy for children with PDD and ASD and uncomplicated PDD for alpha-2 agents and atomoxetine can be made.
Our meta-analysis also demonstrated significantly greater risk of side effects associated with methylphenidate use in children with PDD when compared to placebo. The higher rate of side effects in the Ghuman study, (Ghuman, 2009) which was conducted with preschool-aged children had the highest rate of side effects, thus, using methylphenidate with preschool-aged children should be closely monitored. Overall, methylphenidate was associated with a greater than 15% increase in the likelihood of experiencing both insomnia and decreased appetite when compared to placebo. These results translate into a number needed to harm (NNH) of approximately 6 for both side-effects when compared to placebo. These adverse event rates, despite being both clinically and statistically significant are similar to the adverse event rates reported for the treatment of ADHD with this medication in typically developing children. By comparison a meta-analysis examining the rate of these adverse events in children with ADHD (Schachter, 2001) reported the ARD for insomnia at .17 (95% CI: .08-.26) and decreased appetite at .30 (95% CI: .18-.43). However, we also found significantly increased risk of side effects that although identified on labeling as potential side effects of methylphenidate use, have either not been reported (social withdrawal) or not identified as being significantly increased (depression and irritability) with methylphenidate use in randomized, placebo-controlled trials in children with uncomplicated ADHD. It is unclear whether social withdrawal, depression and irritability are unique side effects of methylphenidate in the PDD population or due to diagnostic uncertainty inherent in the PDD population. For instance, less social engagement in a non-verbal child with PDD being treated with methylphenidate could be a sign of depression or social withdrawal as a side effect of medication use or be decreased impulsivity due to effective control of his ADHD symptoms. Further study of the effects of methylphenidate for the treatment of ADHD symptoms in children with PDD are needed before more confident conclusions can be made about its efficacy for children with PDD.
Evidence supporting the efficacy of clonidine and atomoxetine in the treatment of ADHD symptoms in children with PDD relies on few double-blind RCTs. Although the effect size estimates of our primary outcome measure appear to be similar for clonidine and atomoxetine used to treat children with ADHD alone (for comparison, see meta-analyses of clonidine (Connor, 1999) and atomoxetine (Cheng, 2007)), the small number of studies we located precludes our ability to use meta-analytic techniques for comparison. More evidence is needed before more confident conclusions can be reached regarding the efficacy of these two medications in treating ADHD symptoms in children with PDD. Further trials should focus on confirming the efficacy and establishing the safety profile of these medications in the special population of children with developmental disabilities using well conducted randomized controlled trials with adequate sample sizes.
It is important to note several possible limitations that might have influenced our findings. First, this meta-analysis was based on a small number of studies including a small number of participants. Although meta-analysis is a tool that can be used to pool results, the confidence in results with smaller number of studies with small sample sizes is not as precise as that which can be reached in meta-analyses including a large number of studies. Examination of 95% CI in our results, which are quite large, supports this, and suggests caution must be used until results from additional studies with a greater number of participants are reported. Furthermore, all meta-analyses are limited by the quality of the original studies, and the included studies were shown to have mixed quality. Due to the small number of included studies, we were unable to assess what, if any, impact study quality had on our findings. Third, our meta-analysis found large heterogeneity (e.g., I2 = 66%) for the treatment of ADHD symptomatology, which we were unable to investigate given the small number of studies. As more trials are conducted, investigation of possible moderators of effect at both the individual trial level and meta-analytic level will be valuable in helping identify which children are most likely to benefit from these medications. We were also unable to test for publication bias, which therefore must be considered a possible confound.
In conclusion, our meta-analysis demonstrated the efficacy of methylphenidate treatment of ADHD symptoms in children with PDD. Common side effects such as decreased appetite and insomnia associated with methylphenidate appear to occur at similar rates when used in the PDD population, and depression, irritability, and social withdrawal side effects that appear be more common in children with PDD. Due to the increased risk of side effects, children with PDD using methylphenidate should be carefully monitored. Two additional medications that have been studied in randomized double-blind placebo-controlled trials for the treatment of ADHD symptoms in the PDD population (clonidine and atomoxetine) also appear to have promising, albeit preliminary, evidence for their efficacy.
Acknowledgments
MHB was supported by the National Institute of Mental Health support of the Yale Child Study Center Research Training Program, National Institutes of Health 1K23MH091240-01, the AACAP/ Eli Lilly Junior Investigator Award, the Trichotillomania Learning Center, the National Alliance for Research on Schizophrenia and Depression, and UL1 RR024139 from the National Center for Research Resources, a component of the National Institutes of Health, and NIH roadmap for Medical Research. FRV was supported by NIMH P50 MH081756 and NICHD P01 HD003008.
References
- Aman MG, Arnold L, McDougle CJ, Vitiello B, Scahill L, Davies M, et al. Acute and long-term safety and tolerability of risperidone in children with autism. Journal of Child and Adolescent Psychopharmacology. 2005;15(6):869–884. doi: 10.1089/cap.2005.15.869. [DOI] [PubMed] [Google Scholar]
- Arnold LE, Aman MG, Cook AM, Witwer AN, Hall KL, Thompson S, Ramadan Y. Atomoxetine for hyperactivity in autism spectrum disorders: placebo-controlled crossover pilot trial. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(10):1196–1205. doi: 10.1097/01.chi.0000231976.28719.2a. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual. 4th ed., text revision Author; Washington, D.C.: 2000. [Google Scholar]
- Borenstein M,HL, Higgins J, Rothstein H. Comprehensive Meta-Analysis 2. Biostat; Englewood, NJ: 2005. [Google Scholar]
- Cheng JYW, Chen RYL, Ko JSN, Ng EML. Efficacy and safety of atomoxetine for attention-deficit/hyperactivity disorder in children and adolescents - meta-analytsis and meta-regression analysis. Pyschopharmacology. 2007;194(2):197–209. doi: 10.1007/s00213-007-0840-x. [DOI] [PubMed] [Google Scholar]
- Conners C. Conners’ rating scales-revised: Technical manual. Multi-Health Systems; North Tonawanda, NY: 2001. [Google Scholar]
- Connor D, Fletcher KE, Swanson JM. A meta-analysis of clonidine for symptoms of attention-deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(12):1551–1559. doi: 10.1097/00004583-199912000-00017. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Anastopoulos AD, Power TJ, Reid R, Ikeda MJ, McGoey KE. Parent ratings of attention-deficit/hyperactivity disorder symptoms: factor structure and normative data. Journal of Psychopathologic Behavioral Assessment. 1998;20:83–102. [Google Scholar]
- Faraone S, Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. European Child and Adolescent Psychiatry. 2010;19:353–364. doi: 10.1007/s00787-009-0054-3. [DOI] [PubMed] [Google Scholar]
- Frazier J, Biederman J, et al. Should the diagnosis of attention-deficit/hyperactivity disorder be considered in children with pervasive developmental disorder? Journal of Attention Disorder. 2001;41:203–211. [Google Scholar]
- Gargaro BA, Rinehart NJ, Bradshaw JL, Tonge BJ, Sheppard DM. Autism and ADHD: How far have we come in the comorbidity debate? Neuroscience and Biobehavioral Review. 2011;35:1081–1088. doi: 10.1016/j.neubiorev.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Ghuman J, Aman MG, et al. Randomized, placebo-controlled, cross-over study of methylphenidate for attention-deficit/hyperactivity disorder symptoms in preschoolers with developmental disorders. Journal of Child and Adolescent Psychopharmacology. 2009;19(4):329–339. doi: 10.1089/cap.2008.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MTA Group A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Archieves of General Psychiatry. 1999;56(12):1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- Grzadzinski R, di Martino A, et al. Examining autistic traits in children with ADHD: Does the autism spectrum extend to ADHD? Journal of Autism and Developmental Disorders. doi: 10.1007/s10803-010-1135-3. doi: 10.1007/s10803-010-1135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. Journal of Autism & Developmental Disorders. 2000;30(3):245–255. doi: 10.1023/a:1005548619694. [DOI] [PubMed] [Google Scholar]
- Harfterkamp M, van de Loo-Neus G, Minderaa RB, van der Gaag RJ, Escobar R, Schacht A, et al. A randomized double-blind study of atomoxetine versus placebo for attention-deficit/hyperactivity disorder symptoms in children with autism spectrum disorders. Jounral of ther American Academy of Child & Adolescent Psychiatry. 2012;51(7):733–741. doi: 10.1016/j.jaac.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Hazell P. Drug therapy for attention-deficit/hyperactivity disorder-like symptoms in autistic disorder. Journal of Paediatrics & Child Health. 2007;43(1-2):19–24. doi: 10.1111/j.1440-1754.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- Hedges L, Olkin I. Statistical methods for meta-analysis. Academic Press; New York, NY: 1985. [Google Scholar]
- Heudo-Medina T, Sanchez-Meca J, Marin-Marinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index. Psychological Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- Holtmann M, Bolte S, Poustka F. Attention deficit hyperactivity disorder symptoms in pervasive developmental disorders: association with autistic behavior domains and coexisting psychopathology. Psychopathology. 2007;40(3):172–177. doi: 10.1159/000100007. [DOI] [PubMed] [Google Scholar]
- Jaselskis CA, Cook EH, Jr., Fletcher KE, Leventhal BL. Clonidine treatment of hyperactive and impulsive children with autistic disorder. Journal of Clinical Psychopharmacology. 1992;12(5):322–327. [PubMed] [Google Scholar]
- Lecavalier L. Behavioral and emotional problems in young people with pervasive developmental disorders: relative prevalence, effects of subject characteristics, and empirical classification. Journal of Autism & Developmental Disorders. 2006;36(8):1101–1114. doi: 10.1007/s10803-006-0147-5. [DOI] [PubMed] [Google Scholar]
- Lee DO, Ousley OY. Attention-deficit hyperactivity disorder symptoms in a clinic sample of children and adolescents with pervasive developmental disorders. Journal of Child & Adolescent Psychopharmacology. 2006;16(6):737–746. doi: 10.1089/cap.2006.16.737. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, et al. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. Journal of Autism & Developmental Disorders. 2006;36(7):849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Mandell DS, Morales KH, Marcus SC, Stahmer AC, Doshi J, Polsky DE. Psychotropic medication use among Medicaid-enrolled children with autism spectrum disorders. Pediatrics. 2008;121(3):e441–448. doi: 10.1542/peds.2007-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPheeters M, Warren Z, et al. A systematic review of medical treatments for children with autism spectrum disorders. Pediatrics. 2011;127(5):e1312–e1321. doi: 10.1542/peds.2011-0427. [DOI] [PubMed] [Google Scholar]
- Oswald DP, Sonenklar NA. Medication use among children with autism spectrum disorders. Journal of Child & Adolescent Psychopharmacology. 2007;17(3):348–355. doi: 10.1089/cap.2006.17303. [DOI] [PubMed] [Google Scholar]
- Quintana H, Birmaher B, Stedge D, Lennon S, Freed J, Bridge J, Greenhill L. Use of methylphenidate in the treatment of children with autistic disorder. Journal of Autism & Developmental Disords. 1995;25(3):283–294. doi: 10.1007/BF02179289. [DOI] [PubMed] [Google Scholar]
- Rosenberg R, Mandell DS, Farmer JE, Law JK, Marvin AR, Law PA. Psychotropic medication use among children with autism spectrum disorders enrolled in a national registry, 2007-2008. Journal of Autism & Developmental Disorders. 2010;40:342–351. doi: 10.1007/s10803-009-0878-1. [DOI] [PubMed] [Google Scholar]
- Research Units on Peadiatric Psychopharmolcology Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Archieves of General Psychiatry. 2005;62(11):1266–1275. doi: 10.1001/archpsyc.62.11.1266. [DOI] [PubMed] [Google Scholar]
- Schachter H, Pham B, King J, Langford S, Moher D. How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis. CMAJ. 2001;2001;(165):11. [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(8):921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Sinzig J, Walter D, Doepfner M. Attention deficit/hyperactivity disorder in children and adolescents with autism spectrum disorder: Symptom or syndrome? Journal of Attention Disorder. 2009;13(2):117–126. doi: 10.1177/1087054708326261. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens T, Harding M, O’Donnell D, Griffin S. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35(4):409–432. doi: 10.1097/00004583-199604000-00008. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Egger M, Moher D. Addressing reporting biases. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; Chichester, UK: 2008. pp. 297–333. [Google Scholar]
- Stigler KA, Desmond LA, Posey DJ, Wiegand RE, McDougle CJ. A Naturalistic Retrospective Analysis of Psychostimulants in Pervasive Developmental Disorders. Journal of Child & Adolescent Psychopharmacology. 2004;14(1):49–56. doi: 10.1089/104454604773840481. [DOI] [PubMed] [Google Scholar]
- Swanson J, Nolan W, Pelham WE. The SNAP-IV rating scale. 1992. [Google Scholar]
- van der Meer JMJ, Oerlemans AM, van Stijn DJ, Lappenschaar MGA, de Sonneville LMJ, Buitelaar JK, Rommelse NNJ. Are autism spectrum disorder and attention-deficit/hyperactivity disorder different manifestations of one overarching disorder? Cognitive and symptom evidence from a clinical and population-based sample. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51(11):1160–1172. doi: 10.1016/j.jaac.2012.08.024. [DOI] [PubMed] [Google Scholar]
- Wilson D. Meta-analysis macros for SPSS. 2005. [Google Scholar]
- Yerys BD, Wallace GL, Sokoloff JL, Shook DA, James JD, Kenworthy L. Attention deficit/hyperactivity disorder symptoms moderate cognition and behavior in children with autism spectrum disorders. Autism Research. 2009;2(6):322–333. doi: 10.1002/aur.103. [DOI] [PMC free article] [PubMed] [Google Scholar]