Abstract
Objectives
This study aims to develop a high-sensitivity antibody diagnostic kit that will enable a rapid and accurate detection of Cryptospofidium parvum and Giardia lamblia in patients with diarrhea.
Methods
The cultivated C. parvum oocysts and G. lamblia cysts in each calf and dog were injected to mice to obtain antibodies, which were titrated. Spleen cells of the immunized mouse were separated and blended with myelomas to produce hybrid cell lines that form monoclonal antibodies. Using ELISA method, antibodies that specifically respond to C. parvum and G.lamblia were then selected. The cells were injected into the abdominal cavity of a BALB/c mouse to isolate hydrops abdominis containing high level of antibodies. The IgG antibody was purified using protein G gel.
Results
The detection limit of monoclonal antibodies for Cryptosporidium parvum and Giardia lamblia was 125 oocysts/mL and 1250 cysts/mL, respectively. In addition, during testing they did not show cross-reactivity to viruses (n = 15), bacteria (n =17), and parasites (n = 9).
Conclusion
The rapid diagnostic antibody kit developed in this study, which specifically responds to C. parvum and G. lamblia, will be useful in detecting and monitoring diarrheal infections.
Keywords: Cryptosporidium parvum, Giardia lamblia, immunochromatography, rapid diagnostic kit
1. Introduction
Cryptosporidium parvum and Giardia lamblia have been recognized as the causative agents of diarrhea in humans worldwide [1]. These protozoans are transmitted by the fecal–oral route and most commonly by the consumption of contaminated food and water [2]. Infections are mostly seen in young children and immunocompromised patients. These infections are seen in both developing countries and developed countries. However, especially in developing countries, there is an increased risk of transmission, due to urban crowding and poor sanitation facilities [3].
Among the Korean population, C. parvum and G. lamblia account for less than 1% of diarrheal cases; however, the rate of C. parvum infection has been on the rise, and one case of infection by G. lamblia was reported in Jinan-gun, Jeollabuk-do Province, among people who drank water from a nearby valley [4,5].
Traditional diagnostic methods for treating these parasitic infections include testing fecal samples for the pathogen and must include concentration procedures along with specific staining techniques for proper microscopic detection and identification of the parasite [6]. These methods are laborious, take a long time, and require specialized and trained personnel. Although other techniques such as immunofluorescence microscopy improve sensitivity, they are expensive and laborious, and are not routinely available in all laboratories [7]. In addition, molecular techniques to detect Cryptosporidium, Giardia include polymerase chain reaction (PCR) and real-time PCR that provide high sensitivity and specificity, but these techniques are time consuming and require expensive specialized equipment [8,9].
Therefore, there is a need for a simple yet accurate method of detection for rapid and effective treatment of diarrheal infection. The aim of this study was to develop a new antigen diagnostic kit and evaluate its efficiency in detecting C. parvum and G. lamblia infections. In addition, the usefulness of this rapid diagnostic kit was compared with enzyme-linked immunosorbent assay (ELISA) and other diagnostic kits that are commercially available.
2. Materials and Methods
2.1. Preparation of immunogen
C. parvum oocyst was purchased from MEGACOR (MEGACOR Diagnostik GmbH, Hoerbranz, Vorarlberg, Austria) and orally injected to a month-old calf. From the 2nd day onward, the stool samples were examined with a Crypto-Strip (Coris BioConcept, Gembloux, Belgium). The samples were floated on saline solution, kept still for 2 hours, and the upper layer was extracted. The collected fluid was centrifuged and the precipitation was cleansed three times with sterilized saline solution to retrieve C. parvum oocysts.
G. lamblia cyst was purchased from American Type Culture Collection (Manassas, VA, USA; catalog number: PRA-242) and orally injected to a 2-month-old beagle dog. From the 2nd day onward, the stool samples were examined with a Giardia-Strip (Coris BioConcept). The cysts were retrieved similar to the procedure described for C. parvum.
2.2. Administration of adjuvant emulsions
Samples of C. parvum and G. lamblia were separately mixed with complete Freund’s adjuvant (Sigma Aldrich). Approximately 200 μg of the emulsion was injected four times into the tail vein of a mouse at a 2-week interval. While complete adjuvant was used for the first injection, incomplete adjuvant was used for the rest of the injections. Overall, three intravenous injections were administered into the tail vein of the mouse.
2.3. Serum collection, titration, and cell fusion
A small amount of blood was drawn from the tail of the immunized mouse; subsequently, the serum was separated and ELISA was used for titration of the serum sample. The immunogen was adhered to the ELISA plate at a concentration of 1 μg/mL. An antiserum was then diluted in ten stages (10, 100, 1000 times, and so on) by adding 1% bovine serum albumin for reactivity test. Secondary reactivity test was conducted using the goat antimouse immunoglobulin G (IgG) peroxidase, and 3,3′,5,5′-tetramethylbenzidine substrate (Thermo Fisher Scientific Inc., Rockford, IL, USA) was added for color development. The cut-off rate was set at three times the absorbance level of a normal mouse serum. At a dilution factor greater than 1000, if the sample shows antibody titer above the cut-off value, cell fusion was performed. Spleen cells of the immunized mouse were separated and blended with myelomas to produce hybrid cell lines that form monoclonal antibodies. Using ELISA method, antibodies that specifically respond to C. parvum were then selected. The cell lines were cultured in a large quantity, and the cells were injected into the abdominal cavity of a BALB/c mouse to isolate hydrops abdominis containing high level of antibodies. The IgG antibody was purified using protein G gel.
2.4. Pharmaceutical engineering test
A solution containing 40-nm gold nanoparticles was prepared. To 1 mL of this solution, 20 μg of the antibody was added. The pH levels were properly adjusted for each antibody and at the end of the preset time, a solution of polyethylene glycol was added to stop the reaction and obtain gold conjugates. A matching test was then carried out for the purified antibody and the gold conjugates to identify the optimal pair of antibodies for the test and the gold conjugates.
3. Results
3.1. Development of C. parvum-specific antibody
From the serum of the immunized mouse, three antibodies with the highest level of anti-Cryptosporidium titers were selected for fusion (data not shown); among the hybridomas produced, seven cells (numbers 1–7) with the highest anti-Cryptosporidium titers in the supernatant were selected to test detection limit by antibody dilution. According to the test results, cell number 1 (1E6) and cell number 2 (5D2) were selected as antibodies for the final test and the gold conjugates, respectively (Figure 1).
Figure 1.

Selection of optimal pair for anti-Cryptosporidium parvum by the detection limit test.
3.2. Development of G. lamblia-specific antibody
From the serum of the immunized mouse, five antibodies with the highest level of anti-Cryptosporidium were selected for fusion (data not shown); among the hybridomas produced, eight cells (numbers 1–8) with the highest anti-Cryptosporidium titer in the supernatant were selected to test detection limit by antibody dilution. According to the test results, cell number 3 (4E12) and cell number 5 (2D5) were selected as antibodies for the final test and the gold conjugates, respectively (Figure 2).
Figure 2.

Selection of optimal pair for anti-Giardia lamblia by the detection limit test.
3.3. Detection limit test for C. parvum and G. lamblia
In order to test the detection limit of C. parvum, the samples were tested with two imported rapid detection kits, namely, Crypto-Strip and Cryptosporidium Antigen Detection ELISA (Diagnostics Automation, Inc.). The results of the comparative test were that the solution developed in this study, Crypto-Strip, and Cryptosporidium Antigen Detection ELISA showed positive concentration for 12.5 oocysts/0.1 mL, 50 oocysts/0.1 mL, and 12.5 oocysts/0.1 mL, respectively (data not shown). In case of G. lamblia, the detection limit was 125 cysts/0.1 mL, 250 cysts/0.1 mL, and 125 cysts/0.1 mL, respectively (data not shown).
3.4. Sensitivity and specificity test for field sample
The sensitivity and specificity tests for the field samples obtained from Professor Dr Yeong-Min Lee (Department of Internal Medicine, Veterinary College of Jeju National University) were also conducted. The antibody developed in this study showed the same level of sensitivity and specificity when compared with the imported kits (tested using cow stool samples for C. parvum and dog stool samples for G. lamblia) (Table 1).
Table 1.
Comparison of imported detection kits and antibody developed in this study
| CORIS BioConcept Crypto-Strip |
CORIS BioConcept Giardia-Strip |
Cryptosporidium antigen detection ELISA |
Giardia lamblia antigen detection ELISA |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | ||
| Antibody developed in this study | Positive | 8 | 0 | 9 | 0 | 8 | 0 | 9 | 0 |
| Negative | 0 | 30 | 0 | 30 | 0 | 30 | 0 | 30 | |
ELISA = enzyme-linked immunosorbent assay.
3.5. Cross-reactivity test
A cross-reactivity test was conducted for 15 viruses, 17 bacteria, and nine parasites. The samples were diluted 100 times by extracting the stool samples. The samples did not show any cross reactivity (Tables 2–4).
Table 2.
Results of cross-reactivity testing for Cryptosporidium parvum and Giardia lamblia antigen rapid kit against 15 viruses
| Panel | Culture | Strain | Concentration (mg/mL) | Result | Reference |
|---|---|---|---|---|---|
| Virus | Adenovirus 3 | GB | 1 | — | ATCC VR3 |
| Adenovirus 6 | Tonsil 99 | 1.2 | — | ATCC VR6 | |
| Adenovirus 21 | AV-1645/128 | 1 | — | ATCC VR256 | |
| Cytomegalovirus | Towne | 1.5 | — | ATCC VR977 | |
| Echovirus 2 | Cornelis | 1.3 | — | ATCC VR1039 | |
| Echovirus 5 | Noyce | 0.9 | — | ATCC VR1043 | |
| Echovirus 11 | Gregory | 1 | — | ATCC VR41 | |
| Herpes simplex virus 1 | F | 0.25 | — | ATCC VR733 | |
| Herpes simplex virus 2 | G | 0.115 | — | ATCC VR734 | |
| Mumps virus | Enders | 1.8 | — | ATCC VR1379 | |
| Parainfluenza virus 1 | Sendai | 1.5 | — | ATCC VR105 | |
| Parainfluenza virus 2 | CA/Greer | 3.9 | — | ATCC VR92 | |
| Parainfluenza virus 3 | C243 | 1.93 | — | ATCC VR93 | |
| Respiratory syncytial virus | A-2 | 1 | — | ATCC VR1540 | |
| Respiratory syncytial virus | Long | 0.42 | — | ATCC VR26 |
ATCC = American Type Culture Collection.
Table 3.
Results of cross-reactivity testing for Cryptosporidium parvum and Giardia lamblia Ag rapid test kit against 17 different bacteria
| Panel | Culture | Concentration (cells/mL) | Result (dilution ratio 1:100) | Reference |
|---|---|---|---|---|
| Bacteria | Bordetella pertussis | 1 × 108 | — | ATCC 8467 |
| Enterococcus faecalis | 1 × 108 | — | ATCC 4079 | |
| Escherichia coli | 1 × 108 | — | ATCC 25922 | |
| Haemophilus influenzae | 1 × 108 | — | ATCC 8142 | |
| Klebsiella pneumoniae | 1 × 108 | — | ATCC 4208 | |
| Legionella pneumophila | 1 × 108 | — | ATCC 33153 | |
| Mycobacterium avium | 1 × 108 | — | ATCC 15769 | |
| Mycobacterium intracellulare | 1 × 108 | — | KTCC 9514 | |
| Mycobacterium tuberculosis | 1 × 108 | — | ATCC 27294 | |
| Mycoplasma pneumoniae | 1 × 108 | — | ATCC 15492 | |
| Neisseria gonorrhoeae | 1 × 108 | — | ATCC 31953 | |
| Neisseria meningitidis | 1 × 108 | — | ATCC 6250 | |
| Proteus vulgaris | 1 × 108 | — | ATCC 6361 | |
| Pseudomonas aeruginosa | 1 × 108 | — | ATCC 27853 | |
| Staphylococcus aureus | 1 × 108 | — | ATCC 29213 | |
| Streptococcus pneumoniae | 1 × 108 | — | ATCC 19615 | |
| Streptococcus pyogenes | 1 × 108 | — | ATCC 4012 |
ATCC = American Type Culture Collection; KTCC = Korean Type Culture Collection.
Table 4.
Results of cross-reactivity testing for the Cryptosporidium parvum and Giardia lamblia Ag rapid test kit against nine different parasites
| Panel | Culture | Concentration (parasite/μL) | Result (dilution ratio 1:100) | Reference |
|---|---|---|---|---|
| Parasite | Entamoeba histolytica | 1 × 104 | — | ATCC 30190 |
| Microsporidia | 1 × 104 | — | ATCC 50040 | |
| Cyclospora cayetanensis | 1 × 104 | — | Positive sample in this study | |
| Plasmodium vivax | 1 × 104 | — | Positive sample in this study | |
| Plasmodium falciparum | 1 × 104 | — | Positive sample in this study | |
| Toxoplasma gondii | 1 × 104 | — | ATCC 50174 | |
| Trypanosoma cruzi | 1 × 104 | — | ATCC 50791 | |
| Leishmania donovani | 1 × 104 | — | ATCC 50127 | |
| Leishmania infantum | 1 × 104 | — | ATCC 50134 |
ATCC = American Type Culture Collection.
4. Discussion
The World Health Organization ranks diarrheal disease as the second most common cause of morbidity and mortality in children in the developing world. The etiological agents of diarrhea are viruses, bacteria, and parasites. Among parasites, Entamoeba histolytica, G. lamblia, and Cryptosporidium spp. are considered to be the most common and important [3].
G. lamblia is considered as one of the main nonviral causes of diarrhea in developed countries [10]. Cryptosporidiosis is a frequent cause of diarrheal disease in humans. In developing countries, Cryptosporidium spp. infections occur mostly in children younger than 5 years of age, with a peak in children younger than 2 years of age [11]. In immunodeficient humans, especially individuals with human immunodeficiency virus infection/acquired immunodeficiency syndrome, cryptosporidiosis can be associated with chronic, potentially life-threatening diarrhea [11].
In Korea, C. parvum and G. lamblia infection is on the rise, which was not the case before [4,5,12]. And yet, there is no standard test method for the detection and diagnosis of the protozoan that causes acute diarrhea.
For these two kinds of parasites, diagnosis by analyzing microscopic results is neither sensitive nor specific. Individual antigen detection tests and PCR tests are now available for these parasites, but multiplex antigen detection tests are still under development [13]. The PCR assay for the detection of G. lamblia and Cryptosporidium spp. developed in this study is a useful alternative for diagnosis of these parasites.
In recent years, antigen-detection assays, such as enzyme immunoassays, various PCR methods, and immunochromatography, to detect Cryptosporidium and Giardia have been developed [13–15]. A number of products with a good range of sensitivity and specificity are commercially available. For the diagnosis of Cryptosporidium, discrepancies found between microscopy results and PCR can be interpreted as follows: in samples that were positive by microscopy and negative by PCR, this technique will fail because of the low number of oocysts present in the samples and/or because of the presence of PCR-inhibitory substances. By contrast, in samples that were negative by microscopy, with a low number of oocysts, and without the presence of PCR-inhibitory substances, the greater sensitivity of this technique permits the detection of Cryptosporidium [8].
In the case of Giardia detection, microscopy was the reference technique, but PCR was found to be 100 times more sensitive than ELISA in detecting this parasite [9].
In this study, an antibody diagnostic kit for C. parvum and G. lamblia was developed and compared with commercial kits. The kit will enable early detection and diagnosis of diarrheal infections, thereby preventing collective waterborne protozoans.
Appendix
For the testing method, stool (feces) samples were used. The testing steps are as follows (Figure 3):
-
•
Open the tube containing the solution.
-
•
Draw the assay diluents up to the fill line marked on the dropper, and transfer them to the sample-collection tube.
-
•
Take a portion of the sample with a swab and dip it into the tube; swirl the swab at least 10 times.
-
•
Discard the swab.
-
•
Cap the tube.
-
•
Take the test device out of the silver foil, and place it on an even surface.
-
•
Add four to five drops of the diluted sample into the sample well.
-
•
Analyze the outcome after 10–20 minutes. A positive reactivity can be seen immediately in some cases.
Figure 3.
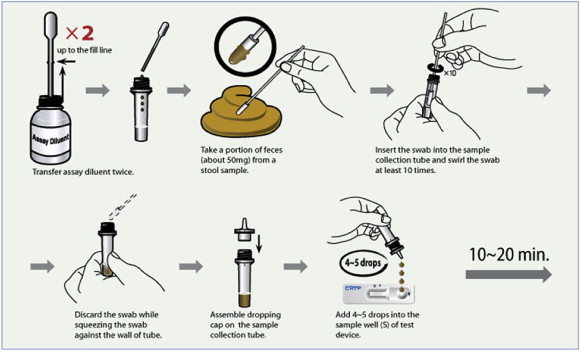
Test procedure for Cryptosporidium parvus and Giardia lamblia rapid test kit.
Acknowledgments
This work was supported by a grant from the Korea National Institute of Health (Grant No. NIH-091-4800-4847-300), National Research and Development Program, Ministry of Health and Welfare, the Republic of Korea.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Marshall M.M., Naumovitz D., Ortega Y., Sterling C.R. Waterborne protozoan pathogens. Clin Microbiol Rev. 1997 Jan;10(1):67–85. doi: 10.1128/cmr.10.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleckenstein J.M., Bartels S.R., Drevets P.D. Infectious agents of food- and water-borne illnesses. Am J Med Sci. 2010 Sep;340(3):238–246. doi: 10.1097/MAJ.0b013e3181e99893. [DOI] [PubMed] [Google Scholar]
- 3.Savioli L., Smith H., Thompson A. Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasitol. 2006 May;22(5):203–208. doi: 10.1016/j.pt.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Lee M.Y., Cho E.J., Lee J.H. A survey of Cryptosporidium oocysts in water supplies during a 10-year period (2000–2009) in Seoul. Korean J Parasitol. 2010 Sep;48(3):219–224. doi: 10.3347/kjp.2010.48.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee M.Y., Cho E.J., Lee J.H. A ten-year survey of Giardia cysts in drinking water supplies of Seoul, the Republic of Korea. Korean J Parasitol. 2011 Mar;49(1):9–15. doi: 10.3347/kjp.2011.49.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia L.S. ASM Press; Washington, DC: 1997. Diagnostic medical parasitology. [Google Scholar]
- 7.Garcia L.S., Shimizu R.Y. Evaluation of nine immunoassay kits (enzyme immunoassay and direct fluorescence) for detection of Giardia lamblia and Cryptosporidium parvum in human fecal specimens. J Clin Microbiol. 1997 Jun;35(6):1526–1529. doi: 10.1128/jcm.35.6.1526-1529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kehl K.S., Cicirello H., Havens P.L. Comparison of four different methods for detection of Cryptosporidium species. J Clin Microbiol. 1995 Feb;33(2):416–418. doi: 10.1128/jcm.33.2.416-418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J.H., Lee J., Park S.J. Detection and genotyping of Giardia intestinalis isolates using intergenic spacers(IGS)-based PCR. Korean J Parasitol. 2006 Dec;44(4):343–353. doi: 10.3347/kjp.2006.44.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali S.A., Hill D.R. Giardia intestinalis. Curr Opin Infect Dis. 2003 Oct;16(5):453–460. doi: 10.1097/00001432-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Dillingham R.A., Lima A.A., Guerrant R.L. Cryptosporidiosis: epidemiology and impact. Microbes Infect. 2002 Aug;4(10):1059–1066. doi: 10.1016/s1286-4579(02)01630-1. [DOI] [PubMed] [Google Scholar]
- 12.Park J.H., Kim H.J., Guk S.M. A survey of cryptosporidiosis among 2,541 residents of 25 coastal islands in Jeollanam-Do (Province), Republic of Korea. Korean J Parasitol. 2006 Dec;44(4):367–372. doi: 10.3347/kjp.2006.44.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goñi P., Martín B., Villacampa M. Evaluation of an immunochromatographic dip strip test for simultaneous detection of Cryptosporidium spp, Giardia duodenalis, and Entamoeba histolytica antigens in human faecal samples. Eur J Clin Microbiol Infect Dis. 2012 Aug;31(8):2077–2082. doi: 10.1007/s10096-012-1544-7. [DOI] [PubMed] [Google Scholar]
- 14.Haque R., Roy S., Siddique A. Multiplex real-time PCR assay for detection of Entamoeba histolytica, Giardia intestinalis, and Cryptosporidium spp. Am J Trop Med Hyg. 2007 Apr;76(4):713–717. [PubMed] [Google Scholar]
- 15.Minak J., Kabir M., Mahmud I. Evaluation of rapid antigen point-of-care tests for detection of Giardia and Cryptosporidium species in human fecal specimens. J Clin Microbiol. 2012 Jan;50(1):154–156. doi: 10.1128/JCM.01194-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


