Abstract
Purpose
Radiation is a common mode of cancer therapy whose outcome is often limited due to normal tissue toxicity. We have shown previously that radiation-induced late reactive oxygen species (ROS) accumulation precedes cell death, suggesting that metabolic oxidative stress could regulate cellular radiation response. The purpose of this study is to investigate if selenoprotein P (SEPP1), a major supplier of selenium to tissues and an antioxidant, regulates late ROS accumulation and toxicity in irradiated normal human fibroblasts (NHFs).
Methods and Materials
Flow cytometry analysis of cell viability, cell cycle phase distribution and DHE-oxidation as well as clonogenic assays were used to measure oxidative stress and toxicity. Human Antioxidant Mechanisms Array (Applied Biosystems) and Q-RT-PCR assays were used to measure gene expression during late ROS accumulation in irradiated NHFs. Sodium selenite addition and sepp1 overexpression were used to determine the causality of SEPP1 regulating late ROS accumulation and toxicity in irradiated NHFs.
Results
Irradiated NHFs exhibit late ROS accumulation (4.5-fold increase from control; p<0.05) that occurs after the activation of the cell cycle checkpoint pathways and precedes cell death. mRNA levels of CuZn- and Mn-superoxide dismutase, catalase, peroxiredoxin 3, and thioredoxin reductase 1 increased approximately 2-to-3-fold, while mRNA levels of cold shock domain containing E1 and SEPP1 increased more than 6-fold (p<0.05). Addition of sodium selenite prior to the radiation treatment suppressed toxicity (45%; p<0.05). SEPP1 overexpression suppressed radiation-induced late ROS accumulation (35%; p<0.05) and protected NHFs from radiation-induced toxicity (58%; p<0.05).
Conclusion
SEPP1 mitigates radiation-induced late ROS accumulation and normal cell injury.
Introduction
Normal tissue toxicity is one of the most important factors limiting radiation therapy outcome [1]. Radiation causes normal tissue damage leading to early (e.g. erythema) and late effects (e.g. fibrosis and atrophy) [2]. Traditionally, it is thought that the initial generation of ROS (within milliseconds of radiation exposure) regulates toxicity. However, the amount of ROS generated from these primary ionization events is significantly lower than that generated from cellular metabolism [3]. Therefore, the initial production of ROS might not be entirely responsible for the long-term biological effects of radiation exposure. In fact, we have shown a later and more significant generation of ROS that may regulate the toxicity of radiation [4,5]. Consistent with this hypothesis, manipulations with antioxidants long after the initial exposure have been shown to suppress radiation-induced late effects [5,6].
We have previously shown that N-acetyl-L-cysteine (NAC), a thiol antioxidant widely used as a modulator of intracellular redox state, increases MnSOD activity [7]. MnSOD is a nuclear encoded and mitochondria matrix-localized redox enzyme that is well known to suppress oxidative stress and radiation-induced transformation [4,8]. Amifostine, a sulfhydryl compound [9] that is currently in Phase III clinical trials for ameliorating radiation-induced normal tissue toxicity [10–12], is believed to confer its radioprotective effects by inducing hypoxia inducible factor 1α (HIF-1α) that is well known to regulate the transcription of numerous genes that are involved in glycolysis [13]. Selenium is another compound that is believed to regulate cellular metabolism protecting normal cells from free radical-induced toxicity, including radiation damage [14–16]. Selenium is known to increase mitochondrial respiration, which is accompanied by an increase in mitochondria-biogenesis associated transcription factors, peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α) and nuclear respiratory factor 1 (NRF1) [15]. There are 25 selenoproteins in humans including selenoprotein P (SEPP1). SEPP1 is unique because of its antioxidant and selenium transport functions [17,18]. SEPP1 is an extracellular glycoprotein that contains 10 selenocysteines with the N-terminal selenocysteine having an antioxidant function and the C-terminal, with nine selenocysteines, serving as the major supplier of selenium to tissues [19]. Our results identified SEPP1 as a previously unrecognized antioxidant gene regulating radiation-induced late ROS accumulation and toxicity in normal human fibroblasts.
Methods and Materials
Cell culture
Human normal skin fibroblasts (AG01522D; Coriell Cell Repositories) from a 3-day-old male of non-fetal origin were cultured and cell population doubling time was calculated following our previously published protocol [20]. Exponential cultures were irradiated using a cesium-137 gamma radiation source (dose rate: 0.71 Gy/min). A clonogenic assay was used to measure cell survival. Control and irradiated normal human skin fibroblasts (NHFs) were plated on monolayers of feeder cells and cultured for 14 d followed by ethanol fixation and staining with Coomassie blue G250. Surviving fraction was calculated after correction for plating efficiency. Sodium selenite and NAC were purchased from Sigma Chemical Co. (St. Louis).
Human Antioxidant Mechanisms Array
Total cellular RNA was extracted using TRIzol reagent (Invitrogen) and quantified using a NanoDrop-1000 Spectrophotometer (Thermo Scientific). RNA was reverse-transcribed using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) and used in the Taqman Human Antioxidant Mechanism Array (Applied Biosystems). The arrays were run on an Applied Biosystems StepOne Plus® machine and the results were analyzed using the StepOne Plus and DataAssist® software v3.0 (Applied Biosystems).
Quantitative Real Time Polymerase Chain Reaction (PCR)
Real-time PCR amplification was performed using the Power SYBR Green kit and StepOne Plus™ System (Applied Biosystems). Primer sequence for individual genes used in the PCR assay is shown in Supplementary Table E1. The cycle threshold (CT) was determined according to our previously published protocol [21]. Fold-change in individual mRNA levels was calculated relative to corresponding mRNA levels in unirradiated cells.
Immunoblotting
Because SEPP1 is a secreted protein, its levels were analyzed both in cell lysates and in proteins precipitated by acetone treatment of cell culture media that were collected from unirradiated and irradiated cultures. Equal amounts of proteins were separated by SDS-PAGE and proteins were transferred to nitrocellulose membrane. Blots were incubated with 1:200 of primary antibodies to SEPP1 (Santa Cruz Biotechnology). Immunoreactive polypeptide was visualized using ECL Plus reagent and Typhoon 7000 Phosphorimager (GE Healthcare). Blots were re-probed with antibodies to actin (Santa Cruz Biotechnology). Results were quantitated using Image J software.
Flow Cytometry Assays
DHE-Oxidation Analysis
Flow cytometry measurements of dihydroethidium (DHE) oxidation were used to measure cellular ROS levels following our previously published method [20]. The specificity of the assay for measurements of cellular superoxide and hydrogen peroxide levels was determined by incubating cells with PEG-SOD and PEG-CAT prior to the flow cytometry assay.
Analysis of Cell Viability
Cell viability was assayed by propidium iodide (PI) dye exclusion and flow cytometry assay following our previously published protocol [22].
Analysis of cell cycle phases
Ethanol-fixed cells were treated with RNase and PI (35 µg/ml), and DNA content was analyzed following our previously published protocol [22].
SEPP1 overexpression in NHFs
Human sepp1 cDNA containing the ORF and two selenocysteine insertion sequences in its 3'-untranslated region was cloned into the pShooter™ mammalian expression vector (Invitrogen). Metafectene®pro (Biontex) was used to transfect NHFs. Cells were cultured in media supplemented with 30 nM sodium selenite. Quantitative RT-PCR and immunoblotting assays were used to measure SEPP1 mRNA and protein levels. Details of the cloning and transfection protocols are included in our recent publication [23].
Statistical analysis
Statistical analysis was done using Student’s t-test. Results are presented as average and standard deviation. Results from at least 3 independent experiments with p < 0.05 were considered significant.
Results
Radiation induces dose- and duration-dependent late ROS accumulation that occurs after cell cycle checkpoint activation and precedes cell death
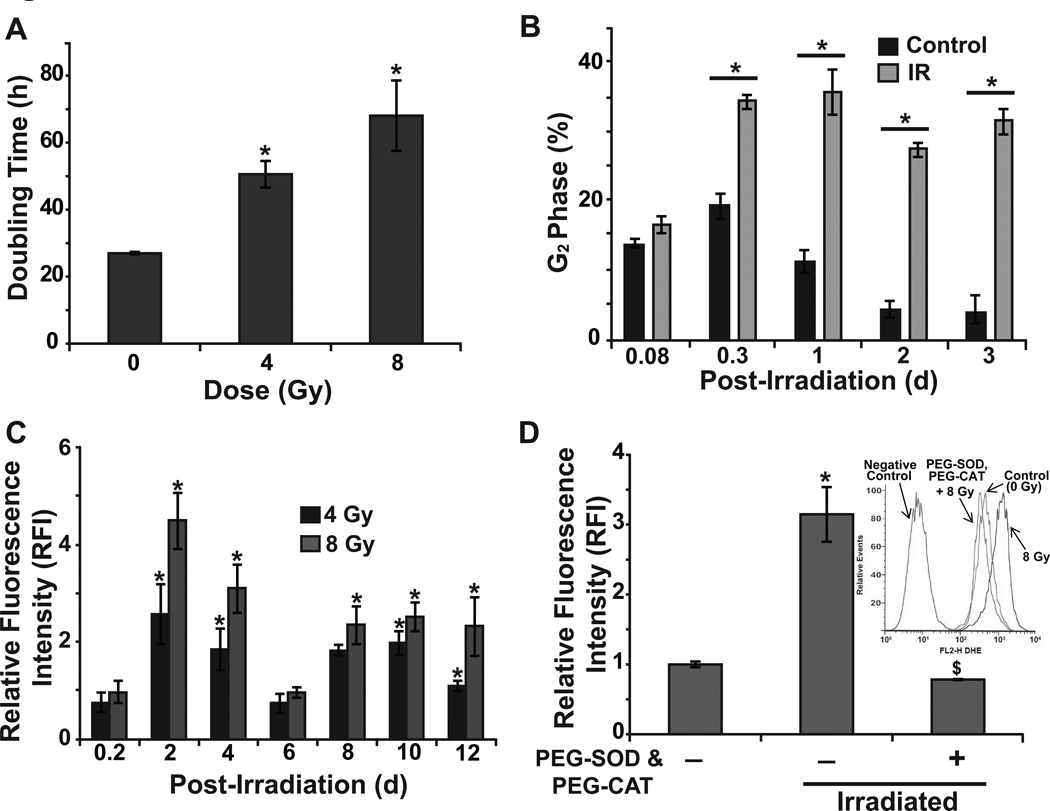
Normal tissue toxicity is one of the major complications limiting the effectiveness of radiation therapy [1]. To better understand the radiation-induced late ROS accumulation influencing radiation response of normal cells, NHFs were irradiated with 4 and 8 Gy and cell doubling time was calculated by counting cell numbers at different days post-irradiation. A delay in cell growth was observed in irradiated NHFs in a dose-dependent manner with a doubling time of 27 h for unirradiated, 48 h for 4 Gy, and 70 h for 8 Gy irradiated NHFs, respectively (Fig. 1A). The increase in cell doubling time was associated with radiation-induced activation of the cell cycle checkpoints. The percentage of cells in G2-phase increased approximately 2-fold at 8 h after radiation; 15% in unirradiated control compared to 35% in 8 Gy irradiated NHFs (Fig. 1B). This increase in the percentage of G2-cells persisted at 72 h after radiation. A decrease in the percentage of S-phase cells indicated the activation of the G1-checkpoint (Supplemental Fig. E1). These results suggest that the radiation-induced increase in cell doubling time could be the result of delays in cell cycle progression.
Figure 1. Radiation induces dose- and duration-dependent late ROS accumulation.
Control and 8 Gy irradiated NHFs were analyzed for (A) Cell doubling time, (B) percentage of G2 cells, and RFI of DHE oxidation in absence (C) and presence (D) of PEG-SOD and PEG-CAT (100 U/mL); inset shows representative histograms. Error bars represent standard deviation of the mean. Asterisks represent statistical significance relative to unirradiated cells; $ represents statistical significance relative to untreated, irradiated cells; p<0.05 as determined by a one-tailed students t-test; n=3.
We have previously shown that while the radiation treatment did not change cancer cell ROS levels within 30 min of radiation, there was a significant increase in cellular ROS levels at 24–48 h after radiation [24,25]. To further determine if late ROS accumulation also occurs in irradiated normal cells, cellular ROS levels were determined by flow cytometry measurements of DHE oxidation in control and irradiated NHFs at the indicated times (Fig. 1C). Consistent with our earlier observation [4,5,25], radiation treatment did not significantly change cellular ROS levels at 4 h after radiation. However, a dose-dependent late ROS accumulation was observed at 2 d after radiation: 4.5-fold increase in 8 Gy and 2.5-fold increase in 4 Gy irradiated cells (Fig. 1C). Cellular ROS levels decreased to control levels at 6 d post-irradiation and then increased approximately 2-fold at 8, 10, and 12 d post-irradiation. Radiation-induced increase in cellular ROS levels were suppressed in cells pre-treated with PEG-SOD and PEG-CAT (Fig. 1D), suggesting that the increase in cellular ROS levels could be due to an increase in the steady state levels of cellular superoxide and hydrogen peroxide. Because the percentage of cells with sub-G1 DNA content did not change in irradiated compared to unirradiated cells at 48 h post-irradiation, it is unlikely that the late ROS accumulation is a consequence of cells undergoing apoptosis (Supplemental Fig. E2).
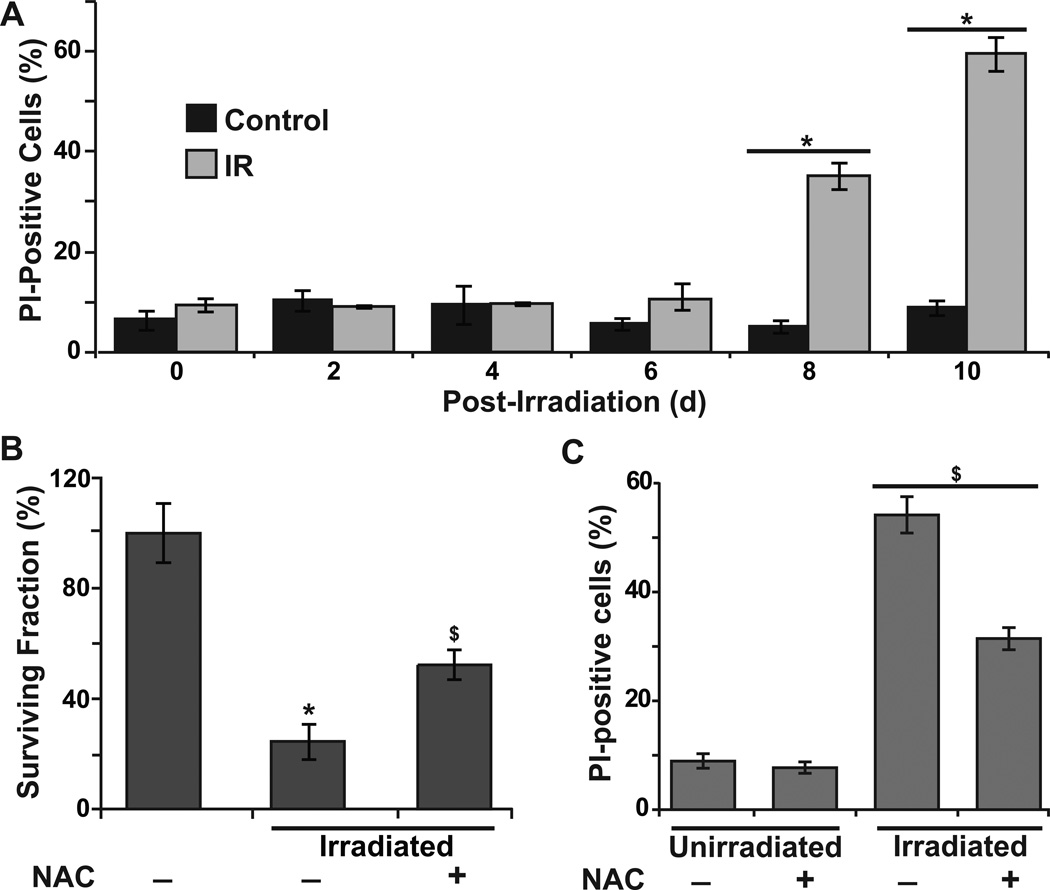
To determine if the biphasic nature of cellular ROS accumulation in irradiated NHFs could be related to radiation-induced toxicity, cell survival was determined using flow cytometry measurements of PI-positive (nonviable) and PI-negative (viable) cells (Fig. 2A). The percentage of PI-positive cells in 4 Gy irradiated NHFs was approximately 40% and 60% at 8 and 10 d post-irradiation, respectively (Fig. 2A). These results were also comparable to results obtained from a clonogenic assay (Fig. 2B). It is interesting to note that NHFs pre-treated with 4 mM NAC were resistant to radiation-induced cell death (Fig. 2B and 2C), suggesting that oxidative stress could regulate radiation-induced toxicity. Taken together, these results show that the radiation-induced late ROS accumulation occurs after cell cycle checkpoint activation and precedes cell death.
Figure 2. Radiation-induced late ROS accumulation precedes cell death.
(A) The percentage of PI-positive (nonviable) cells in time-matched control and 4 Gy irradiated NHFs; (B) A clonogenic assay was used to measure cell survival in 2 Gy irradiated NHFs in absence and presence of 4 mM NAC; (C) The percentage of PI-positive cells in 4 Gy irradiated NHFs at 10 d post-irradiation. Error bars represent standard deviation of the mean. Asterisks represent statistical significance relative to untreated, unirradiated cells; $ represents statistical significance relative to untreated, irradiated cells; p<0.05 as determined by a one-tailed students t-test; n=3.
Radiation-induced late ROS accumulation is associated with changes in the expression of specific oxidative stress response genes
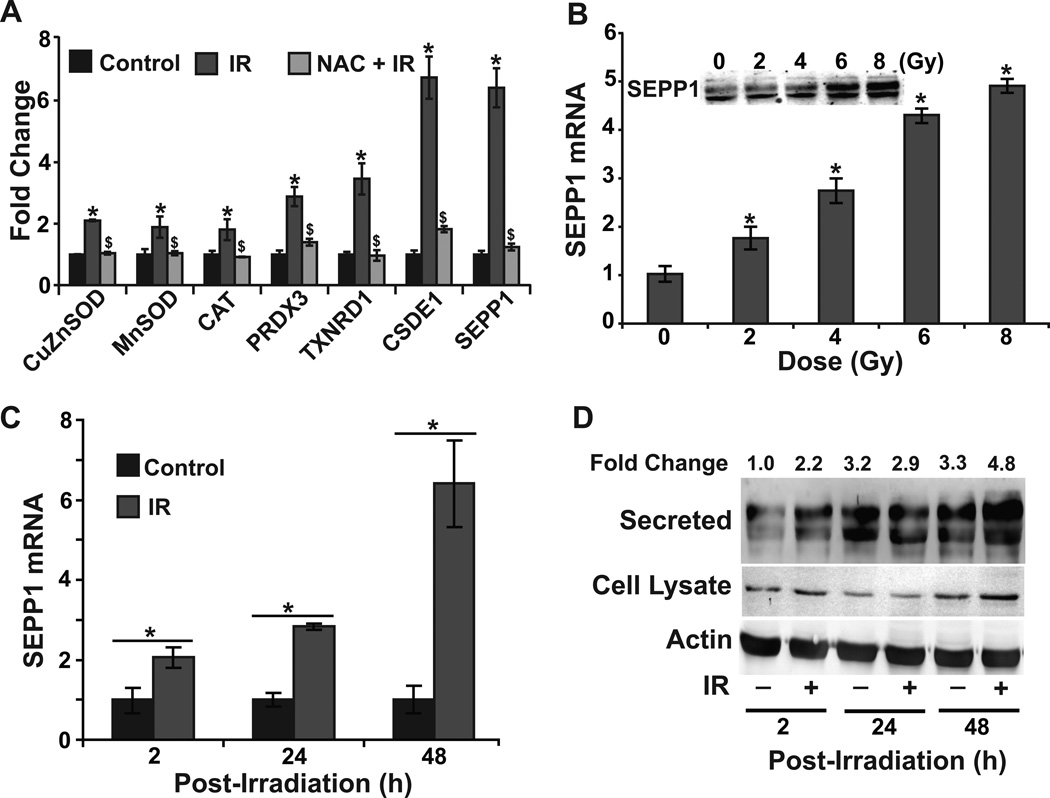
To determine if the radiation-induced late ROS accumulation is associated with changes in the expression of specific oxidative stress response genes, a Human Antioxidant Mechanisms PCR array was used to measure mRNA levels of 92 of the most commonly studied oxidative stress response genes. Although the radiation treatment did not show any significant change in the expression of the majority of the oxidative stress response genes, expression of CuZnSOD, MnSOD, CAT, PRDX3, and TXNRD1 increased approximately 4-fold while expression of CSDE1 and SEPP1 increased more than 6-fold (data not shown). These results obtained from the PCR array were further verified by performing a Q-RT-PCR assay (Fig. 3A). Interestingly, prior treatment with NAC suppressed radiation-induced enhancement in the expression of these genes indicating an oxidative stress response.
Figure 3. Radiation induces dose- and duration-dependent increase in SEPP1 expression.
(A) A quantitative RT-PCR assay was used to measure mRNA levels of selected oxidative stress response genes (based on PCR-array results) at 48 h post-irradiation in time-matched control and 8 Gy irradiated NHFs in absence and presence of 4 mM NAC. Q-RT-PCR and immunoblotting assays were used to measure dose and duration-dependent expression of SEPP1: (B) 0–8 Gy, 48 h post-irradiation, inset shows SEPP1 protein levels; (C and D) 8 Gy, 2–48 h post-irradiation. Error bars represent standard deviation of the mean. Asterisks represent statistical significance relative to untreated, unirradiated cells; $ represents statistical significance relative to untreated, irradiated cells; p<0.05 as determined by a one-tailed students t-test; n=3.
SEPP1 regulates radiation-induced late ROS accumulation and toxicity in NHFs
Our efforts were focused on studying SEPP1 because (a) SEPP1 is a major selenoprotein that has both antioxidant and selenium transport functions [17–19]; and (b) radiation effects on SEPP1 expression and subsequent cellular responses are currently unknown. Results show a dose-dependent increase in SEPP1 mRNA and protein levels in irradiated NHFs (Fig. 3B). Furthermore, SEPP1 expression (mRNA and protein levels) peaked at 48 h post-irradiation (Fig. 3C and 3D) coinciding with the maximal increase in cellular ROS levels (Fig. 1C). These results identified SEPP1 as an additional oxidative stress response gene whose expression is perturbed in irradiated NHFs.
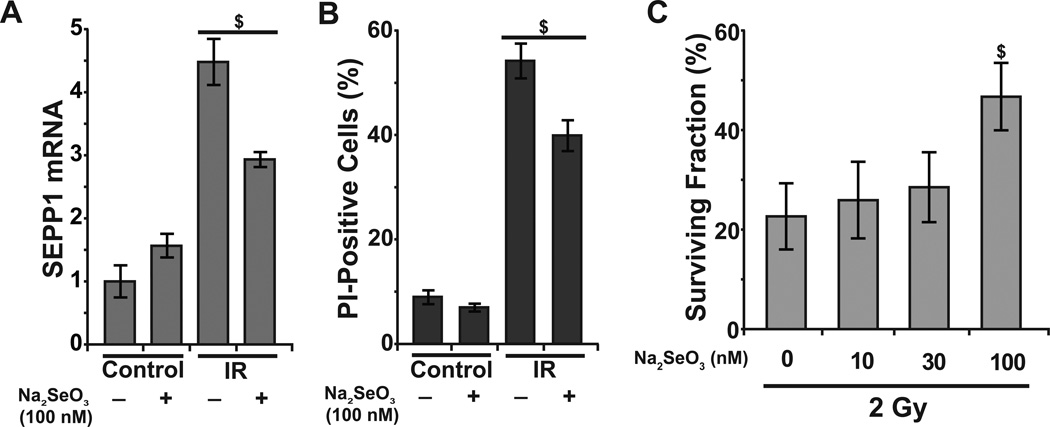
To determine the causality of SEPP1 regulating radiation-induced late ROS accumulation and toxicity, we applied both pharmacological and molecular approaches. NHFs were cultured in 100 nM sodium selenite supplemented media prior to the radiation treatment and SEPP1 expression was measured in control and 8 Gy irradiated cells. As shown before (Fig. 3), radiation treatment increased SEPP1 mRNA levels approximately 5-fold (Fig. 4A). However, prior treatment with sodium selenite significantly suppressed radiation-induced increase in SEPP1 expression. Sodium selenite treatment also significantly suppressed radiation-induced toxicity that was determined by flow cytometry measurements of PI-positive cells (Fig. 4B) as well as performing a clonogenic assay (Fig. 4C).
Figure 4. Sodium-selenite supplementation suppresses radiation-induced increase in SEPP1 mRNA levels and toxicity.
NHFs were treated with 0–100 nM of sodium-selenite for 24 h prior to radiation: (A) Q-RT-PCR analysis of SEPP1 mRNA levels in 8 Gy irradiated NHFs at 48 h post-irradiation; (B) percentage of PI-positive (nonviable) cells in 4 Gy irradiated NHFs at 10 d post-irradiation; (C) a clonogenic assay was used to measure survival in 2 Gy irradiated NHFs. Error bars represent standard deviation of the mean; $ represents statistical significance relative to untreated, irradiated cells; p<0.05 as determined by a one-tailed students t-test; n=3.
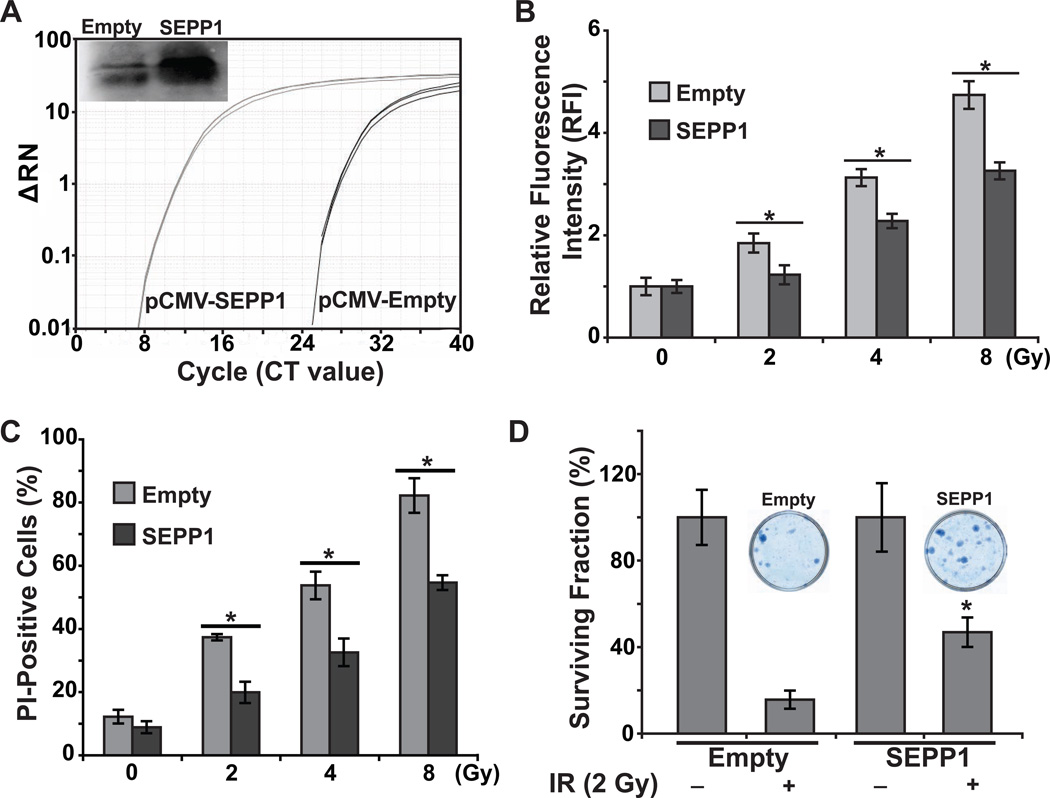
The causality of SEPP1 regulating radiation-induced cytotoxicity was further investigated in SEPP1-overexpressing cells. NHFs were transfected with plasmid DNA containing a CMV promoter-driven human sepp1 cDNA that encompasses the ORF and two SECIS sequences [23]. Results from Q-RT-PCR and immunoblotting assays showed a significant increase in SEPP1 mRNA and protein levels (Fig. 5A). Overexpression of SEPP1 did not affect cell cycle phase distributions (Supplemental Fig. E3). Interestingly, SEPP1 overexpression suppressed radiation-induced late ROS accumulation in 2, 4, and 8 Gy irradiated NHFs (Fig. 5B), which was associated with a significant inhibition in toxicity as determined by flow cytometry measurements of PI-positive cells (Fig. 5C) and performing a clonogenic assay (Fig. 5D). These results show that SEPP1 overexpression suppressed late ROS accumulation and toxicity in irradiated NHFs.
Figure 5. Overexpression of SEPP1 prior to radiation suppresses radiation-induced late ROS accumulation and toxicity.
NHFs were transfected with pShooter plasmid DNA carrying human SEPP1 cDNA (SEPP1) [23]. NHFs transfected with plasmid DNA without an insert sequence (empty) were used as a control. (A) SEPP1 expression was assayed by measuring both mRNA and secreted protein (inset) levels; (B) DHE oxidation at 48 h post-irradiation; cell survival was assessed by measuring (C) the percentage of PI-positive (nonviable) cells, and (D) a clonogenic assay. Representative dishes with colonies are shown in (D) for comparison. Error bars represent standard deviation of the mean. Asterisks represent statistical significance relative to irradiated Empty vector transfected cells; p<0.05 as determined by a one-tailed students t-test; n=3.
Discussion
Radiation is one of the most commonly used therapies in cancer treatment. In fact, about half of all cancer patients receive radiation therapy as part of their treatment regimen [26]. Unfortunately, normal tissue toxicity limits the effectiveness of radiation therapy, e.g. radiation-induced fibrosis in lung, skin, heart and liver [2]. Our results show that radiation induces late ROS accumulation in NHFs that occurs after cell cycle checkpoint activation and precedes cell death. Furthermore, these results identified SEPP1 as a previously unrecognized antioxidant gene that regulates radiation-induced late ROS accumulation and toxicity in NHFs.
Radiation is a classical generator of ROS which are known to cause cellular macromolecular damage resulting in cell death. However, it is also known that the ROS generated from the primary ionization events are significantly lower than ROS generated from cellular metabolism [3], suggesting that the radiation-induced increase in the initial ROS levels may not be entirely responsible for the cellular response to radiation. This premise is consistent with our earlier observation and results shown in Figure 1C demonstrating a minimal change in cellular ROS levels within 30 min – 4 h post-irradiation (early response) [4,5,25]. However, when these measurements were extended to 2–12 d post-irradiation then cellular ROS levels (late response) showed a biphasic response (Fig. 1C): approximately 4- to -5-fold increase at 2 and 4 d that decreased to basal levels at 6 d and then increased approximately 2- to -4-fold at 8–12 d post-irradiation. Whereas the increase in cellular ROS levels at 2 and 4 d post-irradiation precedes cell death, increases in cellular ROS levels at 8–12 d were associated with cell death (Fig. 2A). Although the mechanisms regulating radiation -induced late ROS accumulation are currently unknown, we observed a significant increase in glucose consumption rate: 0.5 pmol cell−1 h−1 in unirradiated control cells compared to 1.7 pmol cell−1 h−1 in 8 Gy radiated cells (Supplemental Fig. E4). This observation and our earlier published results [4,5,25] suggest that the radiation-induced late ROS accumulation could result from a shift in cellular metabolic processes. As such, intervention of radiation-induced “metabolic oxidative stress” could be an attractive approach to mitigate radiation-induced normal cell injury.
To better understand the mechanisms regulating radiation-induced late ROS accumulation and cellular responses, an Antioxidant Mechanisms Array and Q-RT-PCR assays were performed at 48 h post-irradiation. Consistent with previous reports [27–30], CuZnSOD, MnSOD, CAT, PRDX3, and TXNRD1 expression increased in irradiated cells (Fig. 3A). However, these results also identified SEPP1 as the new radiation-response antioxidant gene whose mRNA levels showed the largest increase (6-fold). SEPP1 is known to have both antioxidant and selenium transport functions [18]. It is an extracellular glycoprotein that contains ten selenocysteine residues which allows it to serve as the major supplier of selenium to tissues [19]. A knockdown of SEPP1 resulted in an increase in oxidative stress and decreased viability in myofibroblasts [31], demonstrating its antioxidant property. Our results show NHFs overexpressing SEPP1 mitigate radiation-induced late ROS accumulation and toxicity (Fig. 5), suggesting that selenoproteins are critical regulators of cellular radiation response. This hypothesis is further supported by results demonstrating suppression of radiation toxicity in sodium selenite treated NHFs (Fig. 4).
In summary, results from this study show that radiation induces a late ROS-accumulation (presumably due to metabolic oxidative stress) in NHFs, which is independent of cell cycle checkpoint activation and precedes cell death. SEPP1 is a newly identified radiation-responsive antioxidant gene that regulates late ROS accumulation and toxicity in irradiated NHFs. These results will be of significance in developing additional clinical approaches to mitigate radiation-induced normal tissue toxicity (e.g. mucositis, fibrosis, etc.) as well as potential countermeasures to radiation-induced injury.
Supplementary Material
Summary.
Normal tissue injury limits radiation therapy outcome. In this study, we observed that radiation induces a late accumulation of ROS (4.5-fold; p<0.05) that occurs after the activation of cell cycle checkpoints and precedes cell death. Selenoprotein P (SEPP1) is identified as a previously unrecognized antioxidant enzyme that inhibits late ROS accumulation (35%; p<0.05) and toxicity (58%; p<0.05) in irradiated NHFs. We conclude that SEPP1 needs to be considered to mitigate radiation-induced normal tissue injury.
Acknowledgements
We thank Professors Raymond Burk and Kristina Hill at the Vanderbilt University for the 7C1 pBluescript DNA. This study was supported by NIH 2R01CA111365, T32CA078586, and P42ES013661.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: none
References
- 1.Anscher MS, Vujaskovic Z. Mechanisms and potential targets for prevention and treatment of normal tissue injury after radiation therapy. Semin. Oncol. 2005;32:S86–S91. doi: 10.1053/j.seminoncol.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Stone HB, Coleman CN, Anscher MS, et al. Effects of radiation on normal tissue: Consequences and mechanisms. Lancet Oncol. 2003;4:529–536. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 3.Ward JF. DNA damage as the cause of ionizing radiation-induced gene activation. Radiat. Res. 1994;138:S85–S88. [PubMed] [Google Scholar]
- 4.Du C, Gao Z, Venkatesha VA, et al. Mitochondrial ros and radiation induced transformation in mouse embryonic fibroblasts. Cancer Biol. Ther. 2009;8:1962–1971. doi: 10.4161/cbt.8.20.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Z, Sarsour EH, Kalen AL, et al. Late ros accumulation and radiosensitivity in sod1-overexpressing human glioma cells. Free Radic. Biol. Med. 2008;45:1501–1509. doi: 10.1016/j.freeradbiomed.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petkau A, Chelack WS, Pleskach SD. Letter: Protection of post-irradiated mice by superoxide dismutase. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1976;29:297–299. doi: 10.1080/09553007614550341. [DOI] [PubMed] [Google Scholar]
- 7.Menon SG, Sarsour EH, Kalen AL, et al. Superoxide signaling mediates n-acetyl-lcysteine-induced g1 arrest: Regulatory role of cyclin d1 and manganese superoxide dismutase. Cancer Res. 2007;67:6392–6399. doi: 10.1158/0008-5472.CAN-07-0225. [DOI] [PubMed] [Google Scholar]
- 8.Miriyala S, Spasojevic I, Tovmasyan A, et al. Manganese superoxide dismutase, mnsod and its mimics. Biochim. Biophys. Acta. 2012;1822:794–814. doi: 10.1016/j.bbadis.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorr RT. Radioprotectants: Pharmacology and clinical applications of amifostine. Semin. Radiat. Oncol. 1998;8:10–13. [PubMed] [Google Scholar]
- 10.Vacha P, Fehlauer F, Mahlmann B, et al. Randomized phase iii trial of postoperative radiochemotherapy +/− amifostine in head and neck cancer. Is there evidence for radioprotection? Strahlenther. Onkol. 2003;179:385–389. doi: 10.1007/s00066-003-1016-1. [DOI] [PubMed] [Google Scholar]
- 11.Brizel DM, Wasserman TH, Henke M, et al. Phase iii randomized trial of amifostine as a radioprotector in head and neck cancer. J. Clin. Oncol. 2000;18:3339–3345. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 12.Wasserman TH, Brizel DM, Henke M, et al. Influence of intravenous amifostine on xerostomia, tumor control, and survival after radiotherapy for head-and-neck cancer: 2-year follow-up of a prospective, randomized, phase iii trial. Int. J. Radiat. Oncol. Biol. Phys. 2005;63:985–990. doi: 10.1016/j.ijrobp.2005.07.966. [DOI] [PubMed] [Google Scholar]
- 13.Koukourakis MI, Giatromanolaki A, Chong W, et al. Amifostine induces anaerobic metabolism and hypoxia-inducible factor 1 alpha. Cancer Chemother. Pharmacol. 2004;53:8–14. doi: 10.1007/s00280-003-0691-z. [DOI] [PubMed] [Google Scholar]
- 14.Mehta SL, Kumari S, Mendelev N, et al. Selenium preserves mitochondrial function, stimulates mitochondrial biogenesis, and reduces infarct volume after focal cerebral ischemia. BMC Neurosci. 2012;13:79. doi: 10.1186/1471-2202-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendelev N, Mehta SL, Idris H, et al. Selenite stimulates mitochondrial biogenesis signaling and enhances mitochondrial functional performance in murine hippocampal neuronal cells. PLoS One. 2012;7:e47910. doi: 10.1371/journal.pone.0047910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sieber F, Muir SA, Cohen EP, et al. Dietary selenium for the mitigation of radiation injury: Effects of selenium dose escalation and timing of supplementation. Radiat. Res. 2011;176:366–374. doi: 10.1667/rr2456.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mostert V. Selenoprotein p: Properties, functions, and regulation. Arch. Biochem. Biophys. 2000;376:433–438. doi: 10.1006/abbi.2000.1735. [DOI] [PubMed] [Google Scholar]
- 18.Arteel GE, Mostert V, Oubrahim H, et al. Protection by selenoprotein p in human plasma against peroxynitrite-mediated oxidation and nitration. Biol Chem. 1998;379:1201–1205. [PubMed] [Google Scholar]
- 19.Burk RF, Hill KE. Selenoprotein p-expression, functions, and roles in mammals. Biochim. Biophys. Acta. 2009;1790:1441–1447. doi: 10.1016/j.bbagen.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarsour EH, Venkataraman S, Kalen AL, et al. Manganese superoxide dismutase activity regulates transitions between quiescent and proliferative growth. Aging Cell. 2008;7:405–417. doi: 10.1111/j.1474-9726.2008.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhuri L, Sarsour EH, Kalen AL, et al. Polychlorinated biphenyl induced ros signaling delays the entry of quiescent human breast epithelial cells into the proliferative cycle. Free Radic. Biol. Med. 2010;49:40–49. doi: 10.1016/j.freeradbiomed.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarsour EH, Agarwal M, Pandita TK, et al. Manganese superoxide dismutase protects the proliferative capacity of confluent normal human fibroblasts. The Journal of biological chemistry. 2005;280:18033–18041. doi: 10.1074/jbc.M501939200. [DOI] [PubMed] [Google Scholar]
- 23.Xiao W, Zhu Y, Sarsour EH, et al. Selenoprotein p regulates 1-(4-chlorophenyl)-benzo-2,5-quinone induced oxidative stress and toxicity in human keratinocytes. Free Radical Biology and Medicine. doi: 10.1016/j.freeradbiomed.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher CJ, Goswami PC. Mitochondria-targeted antioxidant enzyme activity regulates radioresistance in human pancreatic cancer cells. Cancer Biol. Ther. 2008;7:1271–1279. doi: 10.4161/cbt.7.8.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalen AL, Sarsour EH, Venkataraman S, et al. Mn-superoxide dismutase overexpression enhances g2 accumulation and radioresistance in human oral squamous carcinoma cells. Antioxid. Redox Signal. 2006;8:1273–1281. doi: 10.1089/ars.2006.8.1273. [DOI] [PubMed] [Google Scholar]
- 26.Baskar R, Lee KA, Yeo R, et al. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012;9:193–199. doi: 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otero G, Avila MA, Emfietzoglou D, et al. Increased manganese superoxide dismutase activity, protein, and mrna levels and concurrent induction of tumor necrosis factor alpha in radiation-initiated syrian hamster cells. Mol. Carcinog. 1996;17:175–180. doi: 10.1002/(SICI)1098-2744(199612)17:4<175::AID-MC1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 28.Bravard A, Luccioni C, Moustacchi E, et al. Contribution of antioxidant enzymes to the adaptive response to ionizing radiation of human lymphoblasts. Int. J. Radiat. Biol. 1999;75:639–645. doi: 10.1080/095530099140285. [DOI] [PubMed] [Google Scholar]
- 29.Otomo T, Hishii M, Arai H, et al. Microarray analysis of temporal gene responses to ionizing radiation in two glioblastoma cell lines: Up-regulation of DNA repair genes. J. Radiat. Res. 2004;45:53–60. doi: 10.1269/jrr.45.53. [DOI] [PubMed] [Google Scholar]
- 30.Kato K, Takahashi K, Monzen S, et al. Relationship between radiosensitivity and nrf2 target gene expression in human hematopoietic stem cells. Radiat. Res. 2010;174:177–184. doi: 10.1667/RR2146.1. [DOI] [PubMed] [Google Scholar]
- 31.Kabuyama Y, Oshima K, Kitamura T, et al. Involvement of selenoprotein p in the regulation of redox balance and myofibroblast viability in idiopathic pulmonary fibrosis. Genes Cells. 2007;12:1235–1244. doi: 10.1111/j.1365-2443.2007.01127.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.