Abstract
Background and Aims. Fecal S100A12 is shown to be a useful noninvasive marker of gut inflammation. However, the studies to date have not characterised the patterns of expression in healthy young children. This study aimed to determine S100A12 levels in infants and children without symptoms of underlying gut disease. Methods. Stool samples were collected from healthy infants (<12 months) and children without gastrointestinal symptoms. Faecal S100A12 was measured by immunoassay. Results. Fifty-six children were recruited. Serial samples were obtained from seven term infants over the first 6 months of life. Single samples were obtained from 49 healthy children ranging from 0.16 to 13.8 years of age. Median S100A12 levels were 0.5 mg/kg (ranging from 0.39 to 25) in the healthy children, with high values (>10 mg/kg) in five infants only. There was no variation between gender. Median S100A12 levels in healthy infants remained below the established normal cut-off from birth to six months of age. Conclusion. S100A12 levels in well infants and children are almost exclusively lower than the standard cut-off. Transiently higher levels may be seen in early infancy. An elevated level of S100A12 in children older than 12 months of age is likely to represent organic gut disease.
1. Introduction
S100A12 (calgranulin C) is a cytosolic protein constitutively found in human neutrophils but also produced by other cell types such as monocytes [1, 2]. S100A12 is released by activated neutrophils or by damaged cells under stress as a damage-associated molecular pattern molecule (DAMP) [3]. Extracellular S100A12 has proinflammatory and chemotactic properties attracting inflammatory cells: it activates mast cells, stimulating release of cytokines including tumour necrosis factor- (TNF-) α [4]. S100A12 may bind to the receptor for advanced glycation end products (RAGE), resulting in activation of the nuclear factor- (NF-) κB signalling pathway and subsequent expression of various proinflammatory cytokines [5].
Elevated levels of S100A12 are seen in a number of inflammatory conditions. Serum levels are raised in diseases such as rheumatoid arthritis, Kawasaki's disease, and cystic fibrosis [6]. In addition, high levels of S100A12 are seen in the serum and colonic mucosa of children with inflammatory bowel disease (IBD) [7].
Furthermore, recent studies have determined S100A12 levels in stool samples [8, 9]. Sidler et al. [9] showed that fecal S100A12 levels at a cut-off of 10 mg/kg had sensitivity and specificity of 97% to distinguish between IBD and other noninflammatory conditions in a group of children presenting with gut symptoms. Other studies have shown higher levels of fecal S100A12 in adults with IBD than in subjects with irritable bowel syndrome (IBS) [10]. The available data suggests that fecal S100A12 measurement can be very useful in the ascertainment of gastrointestinal inflammation.
Calprotectin, a heterocomplex of two S100 proteins related to S100A12, is gaining acceptance as a valid and useful measure of gastrointestinal inflammation. However, calprotectin has been reported to be elevated in the feces at birth and can remain elevated up to 5 years of age where levels settle and are equivalent to levels found in adults [11]. Therefore assessment of fecal calprotectin levels in children younger than 5 years of age may be problematic.
To date, the pediatric studies of fecal S100A12 have included only small numbers of normal healthy children, predominantly older children. The aim of this study was to establish the patterns of fecal S100A12 measurement in normal healthy infants from birth and young children.
2. Methods
2.1. Healthy Term Infants
A group of infants born at term was recruited in the days following their birth at Royal Women's Hospital, Sydney, Australia. This cohort comprised part of a larger group to define the development of the intestinal flora in early life [12]. Exclusion criteria were gastrointestinal symptoms such as diarrhea, vomiting, or abdominal pain. After collection of a meconium sample, parents were asked to collect further samples on days 7, 14, 21, 28, 60, and 180. Details of mode of delivery were recorded. Details of early breast-feeding history were noted when possible.
2.2. Healthy Children
A healthy control group of children were enrolled from outpatient clinics, pediatric wards, or families of hospital staff at Christchurch Hospital, Christchurch, New Zealand. Children older than 1 month and younger than 18 years were included. Exclusion criteria were acute infectious gastroenteritis or recent/current gastrointestinal symptoms such as diarrhoea, vomiting, and abdominal pain. Children with known non-GI conditions associated with gut inflammation (e.g., rheumatological conditions or cystic fibrosis) or those born prematurely were excluded. Ongoing treatment with nonsteroidal anti-inflammatory agents (NSAID) or corticosteroids was also an exclusion criterion. Each child was asked to provide one stool sample. No additional routine investigations were conducted on the stool samples collected from these children.
2.3. Ethical Approval
Informed consent was obtained from each patient or one of his or her caregivers. The study was approved by the Upper South Regional Ethics Committee, New Zealand, and the South East Sydney Illawarra Ethics Committee, Sydney, Australia.
2.4. Sample Collection and Preparation
Stool samples were collected on the ward or at home by parents. After collection samples were first stored in a refrigerator at 4°C for up to 7 days prior to aliquoting into multiple eppendorf tubes. Samples were then stored at −20°C until required for analysis.
2.5. Fecal Extraction from Samples
For each measurement, approximately 100 mg of frozen stool was taken from one of the stored eppendorf tubes and measured to an exact weight. Fecal extraction buffer (0.25 M Tris, 0.25 M citric acid, 0.025 M CaCl2, 2.5 M urea, and 1.25% bovine serum albumin) was added at the ratio of 49 μL buffer per mg of stool to produce a dilution factor of 1 : 50. Sample dilutions were vortexed for 30 sec and then more vigorously shaken for 30 minutes on a shaker table for homogenization. 1.0 mL of homogenized feces was then transferred to an eppendorf tube and centrifuged for 5 minutes at 13000 g. After centrifugation, 0.7 mL of clear supernatant was transferred to a new clean eppendorf tube. The samples were stored at 2–8°C overnight prior to the assay.
2.6. Measurement of S100A12 Levels with Enzyme-Linked Immunosorbent Assay (ELISA)
Levels of S100A12 were measured by an Enzyme-Linked Immunosorbent Assay (ELISA) method as previously described [9]. 96-well ELISA plates (MaxiSorp, Nunc, Roskilde, Denmark) were used. Each plate was coated with 100 μL coating antibody (0.5 μg/mL of Rabbit polyclonal S100A12, Abcam, Cambridge, UK) in carbonate buffer (0.1 M Na2CO3 and 0.1 M NaHCO3 adjusted to pH 9.6.) and incubated at 4°C overnight. Plates were washed twice with wash solution (phosphate-buffered saline, 0.05% Tween 20), and 100 μL/well blocking buffer (wash solution with 1% skim milk powder) was added for incubation for 2 hrs at room temperature. The washing procedure was then repeated. Fecal extractions diluted in blocking buffer to three dilutions (1 : 2, 1 : 20, and 1 : 200) were added (100 μL/well). Dilutions of recombinant S100A12 (Recombinant Human EN-RAGE/S100A12, R&D Systems, Minneapolis, MN, USA) were added (100 μL/well) in order to create a standard curve. Plates were incubated for 1 hour at room temperature followed by three further wash cycles. A secondary antibody (goat anti-human EN-RAGE polyclonal antibody, Biotin Conjugate, R&D Systems) was added, and plates were incubated for 1 hour at room temperature. Three wash cycles were followed by incubation with streptavidin-horseradish peroxidase (Strep-HRP, BD Biosciences, San Jose, CA, USA) for 30 minutes at room temperature. Plates were washed three further times before TMB substrate (ThermoFisher Scientific, Rockford, IL, USA) was added (100 μL/well). The color reaction developed instantly, and a stop solution (2 M H2SO4, 50 μL/well) was added after approximately 2 minutes. Plates were read within 15 minutes after stopping the reaction in an ELISA plate reader (SpectraMax 190, Molecular Devices, Sunnyvale, CA,USA) using softMax Pro V5.3.
2.7. Statistical Analysis
GraphPad Prism 5 for Windows (GraphPad Software, La Jolla, CA, USA) was used for the statistical analysis and formation of graphs. Levels of S100A12 in different groups were compared with Kruskal- Wallis test. Significance was accepted at values of P < 0.05.
3. Results
3.1. Infants and Children
Samples from seven healthy term infants (29% male) were collected: 40 samples were available for measurement. Forty-nine healthy children (60% male) were recruited: these children ranged in age from 2 months to 13.8 years, with median age of 2.16 years. Twenty-two of these children were recruited as children of staff, whilst 27 children were recruited from paediatric wards or outpatient clinics.
3.2. Fecal S100A12 in Healthy Term Infants
The overall median for all 37 samples collected from term infants was 0.76 mg/kg (ranging from 0.39 to 28.70 mg/kg). The median fecal S100A12 level at each time point from birth to six months was below the adult cut-off of 10 mg/kg (Table 1). Of the 37 serial stools collected, only 3 samples (from 3 infants) were above the 10 mg/kg cut-off with 28.7 mg/kg being the highest value measured (Figure 1). Further, each elevated sample was both preceded and followed by samples below the 10 mg/kg cut-off.
Table 1.
Faecal S100A12 concentrations in 56 healthy infants and children. Serial stools collected from the first day of life (meconium) to 6 months of age from 7 healthy infants (Population 1) and single stools collected from 49 children (Population 2) were utilised to measure faecal S100A12 concentrations by immunoassay.
| Population 1 | Population 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Mec | D7 | D14 | D21 | D28 | M2 | M6 | <1 yr | 1-2 yrs | 2–5 yrs | >5 yrs |
| Number of values | 6 | 4 | 7 | 6 | 4 | 6 | 4 | 13 | 10 | 9 | 17 |
| Minimum | 0.39 | 0.39 | 0.39 | 0.39 | 0.39 | 0.39 | 0.39 | 0.40 | 0.39 | 0.40 | 0.39 |
| 25% | 0.30 | 0.52 | 0.39 | 0.67 | 0.39 | 0.39 | 0.39 | 0.65 | 0.48 | 0.40 | 0.39 |
| Median | 0.39 | 2.71 | 0.96 | 0.95 | 0.39 | 0.63 | 0.57 | 1.6 | 0.75 | 0.40 | 0.40 |
| 75% | 2.27 | 22.65 | 3.80 | 1.15 | 1.63 | 4.20 | 1.06 | 5.05 | 1.1 | 0.60 | 0.40 |
| Maximum | 7.8 | 28.7 | 13.3 | 1.46 | 2.04 | 12.7 | 1.16 | 25.0 | 2.50 | 4.60 | 3.10 |
| Mean | 1.63 | 8.62 | 3.01 | 0.93 | 0.80 | 2.69 | 0.67 | 4.70 | 0.95 | 0.92 | 0.59 |
| SD | 3.02 | 13.5 | 4.67 | 0.36 | 0.83 | 4.92 | 0.37 | 7.40 | 0.70 | 1.38 | 0.66 |
| SEM | 1.23 | 6.75 | 1.77 | 0.15 | 0.41 | 2.01 | 0.18 | 2.05 | 0.22 | 0.46 | 0.16 |
| Lower 95% CI | −1.5 | −12.9 | −1.3 | 0.55 | −0.51 | −2.48 | 0.09 | 0.23 | 0.45 | −0.14 | 0.25 |
| Upper 95% CI | 4.8 | 30.1 | 7.33 | 1.30 | 2.1 | 7.85 | 1.25 | 9.17 | 1.45 | 1.98 | 0.93 |
S100A12 concentrations expressed as mg/kg. Mec: meconium; D: day; M: month; Yrs: years; SD: standard deviation; SEM: standard error of the mean.
Figure 1.
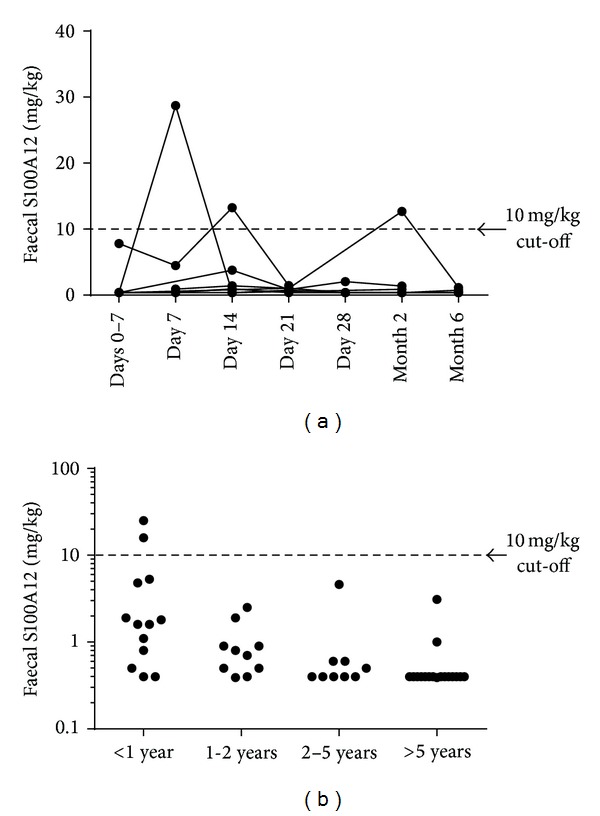
Measurement of fecal S100A12 infants and children. Repeated fecal samples were collected from seven term infants over the first six months of life (a). Single stool samples were collected from 49 healthy infants and children (b). S100A12 concentrations were measured by immunoassay. Only five samples (all in infants) were above the cut-off of 10 mg/kg.
Initial feeding information was available for 5 of the 7 term infants. Three term infants received breast milk for a minimum of 5 months. One of these infants had an elevated S100A12 (12.7 mg/kg) at month 2 of age. None of the samples from the other 2 infants showed elevated levels of S100A12. Two infants were wholly formula fed from the 2nd week of life: neither of these infants had an elevated S100A12 throughout the 6-month collection period. Feeding data was not available for the remaining 2 infants who both had one sample with elevated S100A12. Three of the infants were delivered by caesarean section. The mode of delivery did not influence faecal S100A12 levels (data not shown).
3.3. Fecal S100A12 Concentrations in Healthy Children
Within the 49 children, the median S100A12 level was 0.5 mg/kg (Table 1). Values ranged from 0.39 to 25: just 2 values were above the cut-off of 10 mg/kg. There were no differences in fecal S100A12 levels between males and females (data not shown: P > 0.05). In addition, there were no differences between the group of healthy children of staff and the group recruited from paediatric wards (data not shown: P > 0.05).
Faecal S100A12 levels were assessed across age bands (Table 1). All children above 12 months of age had levels below the cut-off. The two high values noted were both seen in infants—one aged 2 months and one aged 5 months. The concentrations in the infant group were higher than those in the group aged 5–10 years (P = 0.007).
3.4. Fecal S100A12 in Infants Compared to Values in Children Older than One Year
The median fecal S100A12 level in all samples collected from infants (n = 50, comprising 37 repeated samples from seven term infants and 13 single samples from infants) was not different to the values from the 36 children older than one year of age (P > 0.05: data not shown).
4. Discussion
This study indicated low levels of S100A12 present in stool samples collected from healthy infants and children. A transient increase of faecal S100A12 was seen in a small number of infants.
S100A12 is becoming established as a sensitive and specific indictor of gut inflammation. An initial pediatric study showed high levels in children with IBD compared to healthy asymptomatic controls [8], and a subsequent study demonstrated that this marker was able to discriminate between children with IBD and those with gut symptoms not due to IBD [9]. Several adult studies also demonstrate that this marker is useful in the assessment of gut inflammation [10, 13].
S100A12 has been compared to calprotectin, a more established fecal inflammatory marker. Calprotectin, a heterodimer of two other proteins from the S100 family (A8 and A9), was initially demonstrated to be a valid marker of gut inflammation more than three decades ago [14]. Subsequently, studies in various settings, including children and adults [15, 16], have led to widespread use of this test. Studies evaluating calprotectin in young children have shown that the normal range in infants and young children differs from that in older children [17]. Namely, these younger children have more variable calprotectin levels, with a higher normal range. It is not clear if this is due to differences in intestinal permeability or consequent to more frequent intercurrent infections in this age group. However, this feature of calprotectin gives rise to more difficulty in interpretation of the results in this age group, with more false positive test results.
One study reports that fecal calprotectin (FC) is higher in children between 2 and 9 years than in children older than 10 years [18]. Another study showed that levels can be high in healthy infants and that wide interindividual variation of FC is normal in infants [19]. In that study, the levels of FC were lower in infants whose mothers had received antibiotics before or at delivery, suggesting a link between FC excretion and the development of gut bacteria. They also suggest that a great inter individual variation in healthy infants. Another possibility is that FC levels vary accordingly to dietary variations. One study shows that exclusively breastfed infants have higher levels of FC [20].
An important finding in the current study was that the interindividual variations of S100A12 were less pronounced than those previously observed for levels of FC in healthy infants, where levels of FC could be much higher than the expected cut-off. The consistently low levels in normal children may give S100A12 better potential to be a reliable biomarker of gut inflammation than FC in young children.
In the current report, the only recordings above the cut-off of 10 mg/kg were in infants. It is feasible that these changes could be consequent to a subclinical gastrointestinal condition. In the infants with a single sample, a subsequent sample may have been helpful. The elevations in the infants with serial samples were isolated and not persistent, suggesting a transient cause. Further, the parents of these infants reported that the infants remained well, and there were no cases of subsequent symptoms or disease.
Overall, these data provide novel information of the performance of this biomarker in healthy children. Limitations of this study were the small number of patients across the age bands and the lack of serial samples in the toddlers and older children. In addition, further assessment (such as an endoscopic assessment or stool culture) was not available at the time of sample collection. This would have provided confirmation of the absence of inflammation in the majority of the cohort, whilst clarifying any underlying cause in the children with transient elevations in S100A12.
In conclusion, this study showed consistently low levels of fecal S100A12 in healthy infants and children. These data suggest that the adult cut-off of 10 mg/kg may be also of suitable utility in the assessment of intestinal inflammation in young children and even infants from birth. S100A12 measurement for assessing intestinal inflammation may have greater utility in infants and children than calprotectin.
References
- 1.Goyette J, Geczy CL. Inflammation-associated S100 proteins: new mechanisms that regulate function. Amino Acids. 2011;41(4):821–842. doi: 10.1007/s00726-010-0528-0. [DOI] [PubMed] [Google Scholar]
- 2.Leach ST, Day AS. S100 proteins in the pathogenesis and diagnosis of inflammatory bowel disease. Expert Review of Clinical Immunology. 2006;2(3):471–480. doi: 10.1586/1744666X.2.3.471. [DOI] [PubMed] [Google Scholar]
- 3.Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. Journal of Leukocyte Biology. 2007;81(1):28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- 4.Yang Z, Tao T, Raftery MJ, Youssef P, Di Girolamo N, Geczy CL. Proinflammatory properties of the human S100 protein S100A12. Journal of Leukocyte Biology. 2001;69(6):986–994. [PubMed] [Google Scholar]
- 5.Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97(7):889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 6.Meijer B, Gearry RB, Day AS. The role of S100A12 as a systemic marker of inflammation. International Journal of Inflammation. 2012;2012:6 pages. doi: 10.1155/2012/907078.907078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leach ST, Yang Z, Messina I, et al. Serum and mucosal S100 proteins, calprotectin (S100A8/S100A9) and S100A12, are elevated at diagnosis in children with inflammatory bowel disease. Scandinavian Journal of Gastroenterology. 2007;42(11):1321–1331. doi: 10.1080/00365520701416709. [DOI] [PubMed] [Google Scholar]
- 8.de Jong NSH, Leach ST, Day AS. Fecal S100A12: a novel noninvasive marker in children with Crohn’s disease. Inflammatory Bowel Diseases. 2006;12(7):566–572. doi: 10.1097/01.ibd.0000227626.72271.91. [DOI] [PubMed] [Google Scholar]
- 9.Sidler MA, Leach ST, Day AS. Fecal S100A12 and fecal calprotectin as noninvasive markers for inflammatory bowel disease in children. Inflammatory Bowel Diseases. 2008;14(3):359–366. doi: 10.1002/ibd.20336. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser T, Langhorst J, Wittkowski H, et al. Faecal S100A12 as a non-invasive marker distinguishing inflammatory bowel disease from irritable bowel syndrome. Gut. 2007;56(12):1706–1713. doi: 10.1136/gut.2006.113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rugtveit J, Fagerhol MK. Age-dependent variations in fecal calprotectin concentrations in children. Journal of Pediatric Gastroenterology and Nutrition. 2002;34(3):323–324. doi: 10.1097/00005176-200203000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Hallab JC, Leach ST, Zhang L, et al. Molecular characterization of bacterial colonization in the preterm and term infant's intestine. Indian Journal of Pediatrics. 2013;80:1–5. doi: 10.1007/s12098-012-0753-5. [DOI] [PubMed] [Google Scholar]
- 13.Foell D, Kucharzik T, Kraft M, et al. Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut. 2003;52(6):847–853. doi: 10.1136/gut.52.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roseth AG, Fagerhol MK, Aadland E, Schjonsby H. Assessment of the neutrophil dominating protein calprotectin in feces: a methodologic study. Scandinavian Journal of Gastroenterology. 1992;27(9):793–798. doi: 10.3109/00365529209011186. [DOI] [PubMed] [Google Scholar]
- 15.Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology. 2011;140(6):1817–1826. doi: 10.1053/j.gastro.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson P, Anderson NH, Wilson DC. The diagnostic accuracy of fecal calprotectin during the investigation of suspected pediatric inflammatory bowel disease: a systematic review and meta-analysis. The American Journal of Gastroenterology. 2013 doi: 10.1038/ajg.2013.131. [DOI] [PubMed] [Google Scholar]
- 17.Rugtveit J, Fagerhol MK. Age-dependent variations in fecal calprotectin concentrations in children. Journal of Pediatric Gastroenterology and Nutrition. 2002;34(3):323–324. doi: 10.1097/00005176-200203000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Joshi S, Lewis SJ, Creanor S, Ayling RM. Age-related faecal calprotectin, lactoferrin and tumour M2-PK concentrations in healthy volunteers. Annals of Clinical Biochemistry. 2010;47(3):259–263. doi: 10.1258/acb.2009.009061. [DOI] [PubMed] [Google Scholar]
- 19.Rougé C, Butel M-J, Piloquet H, et al. Fecal calprotectin excretion in preterm infants during the neonatal period. PloS ONE. 2010;5(6):p. e11083. doi: 10.1371/journal.pone.0011083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savino F, Castagno E, Calabrese R, Viola S, Oggero R, Miniero R. High faecal calprotectin levels in healthy, exclusively breast-fed infants. Neonatology. 2010;97(4):299–304. doi: 10.1159/000255161. [DOI] [PubMed] [Google Scholar]


