Abstract
Recently, we showed that post cyclophosphamide (CTX) microenvironment benefits the function of transferred T cells. Analysis of the kinetics of cellular recovery after CTX treatment showed that a single 4 mg/mouse CTX treatment decreased the absolute number of leukocytes in the peripheral blood (PBL) at days 3-15, and in the spleen and bone marrow (BM) at days 3-6. The absolute numbers of CD11c+CD11b− and CD11c+CD11b+ dendritic cells (DCs), CD11b+ and Ly6G+ myeloid cells, T and B cells, CD4+CD25+ T regulatory (Treg) cells, and NK1.1+ cells also decreased. The cell numbers returned to control levels during the recovery phase. The absolute numbers of B cells remained low for 3 weeks. The numbers of DCs increased in PBL and spleen at day 9 but returned to control levels at day 15. These data indicate that CTX alters the cellular microenvironment in kinetics that might be precisely targeted to benefit the host.
Keywords: Cyclophosphamide, Chemotherapy, Dendritic cells, Lymphocytes, Regulatory T cells, Natural Killer cells, Blood, Bone marrow, Spleen
Introduction
Cyclophosphamide (CTX) is a chemotherapeutic agent widely used to treat various types of malignancies as well as lymphoproliferative and autoimmune disorders (1, 2). Besides its direct cytotoxic effect as a deoxyribonucleic acid (DNA) alkylating agent, CTX has been used as an immunomodulatory agent in protocols for cancer vaccination (3, 4) and adoptive cell transfer (ACT) therapy (2). Several reports have shown a strong synergistic effect of combined therapy with CTX and adoptively transferred T cells in inhibiting tumor growth in different murine (5, 6) and human (7-9) tumors. In this regard, CTX can facilitate adoptive immunotherapy by depleting and inhibiting the suppressive function of T regulatory (Treg) cells (10-13). An alternative hypothesis that the increased production of type I interferon (IFN) (14, 15) (along with the induction of granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-1β, IL-2, IL-7, IL-15, IL-21, and IFN-γ (6), therefore sustaining the proliferation, survival, and activity of transferred T cells. CTX has also been shown to activate cells of the innate immune system, in particular dendritic cells (DCs), and increase the number and persistence of CD44high CD4+ and CD8+ T cells (15-17). Moreover, the immunopoteniating activity of CTX has been found to be mediated by a reduction in T cell-derived IL-10 and transforming growth factor-β (TGF-β production (18) and a shift in T helper (Th) 2 (IL-10 and TGF- β)/Th1 (IL2 and IFN-γ) cytokine production (19).
In spite of the many studies mentioned above, the specific mechanisms by which CTX conditioning can enhance the activity of adoptively transferred T cells are still a matter of debate. Moreover, these mechanisms have mostly been investigated during the lymphopenic phase, and only a few studies have addressed the role of the cellular components that might be altered as the host recovers from the induced lymphopenia (the recovery phase; days 5-18). Because most of the beneficial effects of CTX for ACT therapy could be mediated by its effects on the endogenous cells, exploring how endogenous immune cells in a recipient host are altered by CTX treatment can help shed light on what mechanisms are most relevant to lymphopenic immunomodulation. Therefore, this study aimed to determine the alterations in the relative and absolute numbers of different types of immune cells in lymphoid and non-lymphoid compartments at multiple time points during the lymphopenic and rebounding (recovery) phases. In summary, our data showed that CTX treatment resulted in alteration within the leukocyte compartments in different organs. These changes in the host cellularity post CTX treatment can be manipulated in a way that can benefit the application of CTX in immunotherapy.
Materials and Methods
Mice
C57BL/6 (Ly5.2; B6) were purchased from the Jackson Laboratory (Bar Harbor, ME). All animals were housed under specific pathogen-free conditions in accordance with institutional and federal guidelines at the Medical University of South Carolina, USA.
Cell lines, antibodies and reagents
Anti-CD16/CD32, and FITC-, PE-, and cychrome-conjugated monoclonal antibodies (mAbs) against CD11c, CD11b/CD18, Gr-1 (Ly6G, CD3, CD4, CD8, CD25, B220, and NK1.1 were purchased from BD Pharmingen (San Diego, CA). CTX (Sigma, St. Louis, MO) was reconstituted in PBS and frozen until used.
Preparation of immune cells
Mice were injected intraperitoneally (i.p.) with PBS or 4 mg/mouse CTX as described previously (15). At the indicated time points, mice were bled from the orbital sinus to harvest peripheral blood (PBL) and sacrificed to harvest spleen and bone marrow (BM). The total number of leucocytes in PBL was enumerated using an automated instrument for complete blood counts (CBC) (VetScan HM2™ Hematology System, Abaxis®, Union City, CA).
Erythrocytes were then depleted with ammonium chloride-potassium chloride (ACK; Invitrogen, Carlsbad, CA) buffer (20). Spleen and BM suspensions were prepared and counted using a hemocytometer with trypan blue dye exclusion (20).
Flow cytometry
Fresh single-cell suspensions of leukocytes from PBL, spleen, and BM were prepared as described above. About 1 × 106 cells were treated with anti-CD16/CD32 for 5 min on ice. Cells were then stained with the indicated conjugated mAbs and incubated for 30 min on ice. Cells were washed twice and re-suspended in 0.3 ml of 0.5% BSA/0.02% sodium azide solution. Cells were acquired on a FACSCalibur™ (BD Biosciences, San Jose, CA) and analyzed using CellQuest™ software (BD Biosciences). The absolute numbers of different cell populations in each compartment were calculated as: % cells from flow cytometry × total number of cells/100.
Statistics
Numerical data obtained from each experiment were expressed as mean ± SD, and the statistical differences between experimental and control groups were assessed using the Student t test. The P values < 0.05 were considered statistically significant
Results
Effect of CTX on the total cell number of PBL, spleen, and BM
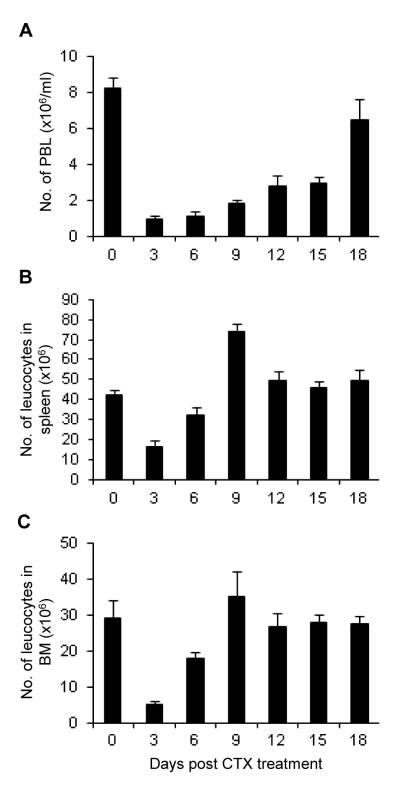
To understand the effect of CTX therapy on cellularity in the host lymphoid and non-lymphoid compartments, B6 mice were i.p. Injected with a single dose of CTX (4 mg/mouse). The results showed that CTX induced a rapid lymphopenia (days 1-6) in PBL (Fig. 1A), spleen (Fig. 1B), and BM (Fig. 1C), followed by a recovery phase, in which the cellular components started to rebound from lymphopenia. By day 18, the total number of PBL returned to the normal level; spleen and BM cell numbers, however, reached the recovery phase by day 9. In all experiments, control mice were treated with PBS (indicated as day 0 in the figures).
Fig. 1. Kinetics of the total cell number of PBL, spleen, and bone marrow after CTX treatment.
C57BL/6 mice were injected i.p. with PBS or CTX (4 mg/mouse). The average of the data from PBS-treated mice (control group) is depicted as day 0 in the figure. At the indicated time points, mice were bled to collect PBL and then sacrificed to harvest spleen and BM. The total number of leucocytes was determined in the PBL (A), spleen (B), and BM (C). Data represent the mean ± SD (n = 4).
CTX induced alteration in the levels of DCs in different organs
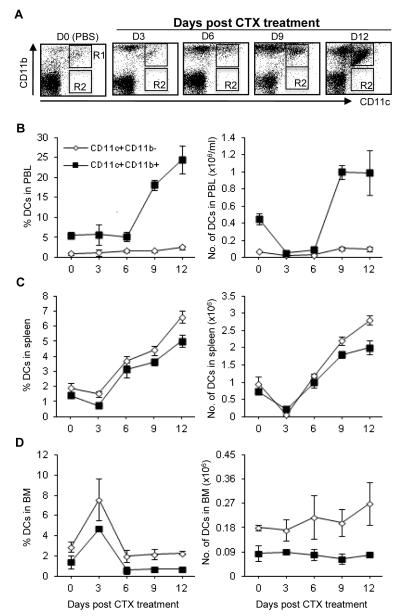
CTX treatment induced a modest but significant increase in the relative and absolute numbers of CD11c+CD11b− DCs in PBL (Fig. 2A) and spleen (Fig. 2B) during the recovery phase, peaking on day 12 post treatment. However, the relative and absolute numbers of CD11c+CD11b+ DCs increased markedly in PBL (Fig. 2A) and spleen (Fig. 2B) during the recovery phase, peaking on day 12 post treatment; These cells recovered to normal by day 15 of treatment (data not depicted). Compared to PBL and spleen, BM showed an increase in the relative, but not the absolute, number of CD11c+CD11b+ DCs on day 3 and recovered to the control level by day 6 after treatments (Fig. 2C).
Fig. 2. Effect of CTX treatment on the relative and absolute numbers of CD11c+CD11b− and CD11c+CD11b+ cells.
C57BL/6 mice were injected i.p. with PBS or CTX (4 mg/mouse). The average of the data from PBS-treated mice (control group) is depicted as day 0 in the figure. At the indicated time points, mice were bled to collect PBL and then sacrificed to harvest spleen and BM. Leucocytes were counted, stained with mAbs against the indicated cell markers, and then analyzed by flow cytometry. The total leukocytes were gated after exclusion of dead cells and from which the frequency of CD11c+CD11b+ (R1) and CD11c+CD11b− (R2) was analyzed in the blood as a representative dot plot analysis as shown in (A). The relative and absolute numbers in PBL (B), spleen (C), and BM (D) are depicted. The absolute numbers were calculated as (total cell number × %) /100. Data represent the mean ± SD (n = 4).
Effect of CTX on the numbers of myeloid cells
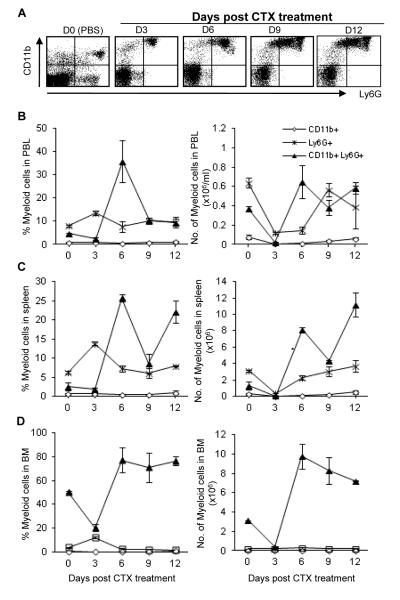
As shown in Fig. 3, CTX treatment did not result in any significant changes in the frequency of CD11b+ myeloid cells in PBL, spleen and BM. The absolute number of these cells decreased significantly during the lymphopenic phase and was back to the normal level by day 12 after treatment.
Fig. 3. Effect of CTX treatment on the relative and absolute numbers of CD11b+, Ly6G+, and CD11b+Ly6G+ cells.
C57BL/6 mice were injected i.p. with PBS or CTX (4 mg/mouse). The average of the data from PBS-treated mice (control group) is depicted as day 0 in the figure. At the indicated time points, mice were bled to collect PBL and then sacrificed to harvest spleen and BM. Leucocytes were counted, stained with mAbs against the indicated cell markers, and then analyzed by flow cytometry. The total leukocytes were gated after exclusion of dead cells and from which the frequency of Ly6G+CD11b+ (upper right), Ly6G+CD11b− (lower right), and Ly6G−CD11b+ (upper right) was analyzed in the blood as a representative dot plot analysis shown in (A). The relative and absolute numbers depicted in PBL (A), spleen (B), and BM (C) as described in the legend of Fig. 2. Data represent the mean ± SD (n = 4).
The frequency of Ly6G+ myeloid cells in PBL (Fig. 3A, left panel), spleen (Fig. 3B, left panel), and BM (Fig. 3C, left panel) increased significantly on day 3 and reached the pretreatment level by day 6 after treatment. In PBL, the absolute number of this cell population decreased significantly on days 3 and 6 and considerably recovered to normal by day 9 (Fig. 3A, right panel). In spleen, however, their absolute number decreased significantly on day 3 and recovered by day 6 (Fig. 3B, right panel). Compared to those in PBL and spleen, CTX did not induce significant alteration in the relative and absolute numbers of Ly6G+ cells in BM (Fig. 3C, right panel).
Of interest, PBL, spleen, and BM from CTX-treated mice showed a remarkable increase in the relative and absolute numbers of CD11b+Ly6G+ (the phenotype of myeloid-derived suppressor cells; MDSCs, peaking on day 6 post treatment (Fig. 3). A considerable recovery was observed in PBL (Fig. 3A) by day 12, whereas the cell number continued to increase in spleen (Fig. 3B) and BM (Fig. 3C); MDSCs levels returned to control level by day 15 of treatment (data not depicted).
Effect of CTX on the numbers of T and B lymphocytes
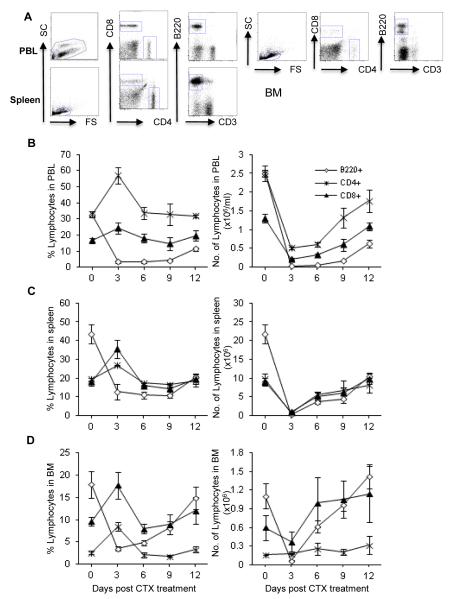
CTX treatment significantly induced a modest increase in the percentage of CD4+ T cells in PBL (Fig. 4A, left panel), spleen (Fig. 4B, left panel), and BM (Fig. 4C, left panel) on day 3. However, their absolute numbers in PBL (Fig. 4A, right panel) and spleen (Fig. 4B, right panel) dropped sharply on day 3, with a gradual return to pre-treatment levels range by day 12 after treatment. In contrast, the cell number in BM did not change significantly as compared with control (Fig. 4C, right panel).
Fig. 4. Effect of CTX treatment on the relative and absolute numbers of CD4+ and CD8+ T cells and B220+ B cells.
C57BL/6 mice were injected i.p. with PBS or CTX (4 mg/mouse). The average of the data from PBS-treated mice (control group) is depicted as day 0 in the figure. At the indicated time points, mice were bled to collect PBL and then sacrificed to harvest spleen and BM. Leucocytes were counted, stained with mAbs against the indicated cell markers, and then analyzed by flow cytometry. The total leukocytes were gated after exclusion of dead cells and from which the frequency of the indicated cell populations was analyzed in the blood of control mice treated with PBS as a representative dot plot analysis shown in (A). The relative and absolute numbers depicted in PBL (A), spleen (B), and BM (C) as described in the legend of Fig. 2. Data represent the mean ± SD (n = 4).
As shown in Fig. 4, CTX treatment induced similar changes in the relative and absolute numbers of CD8+ T cells to those observed with CD4+ T cells. The frequency, in parallel with the total number, of B220+ B cells decreased sharply in PBL (Fig. 4A), spleen (Fig. 4B), and BM (Fig. 4C) in response to CTX treatment. By day 12 after treatment, B cell number in bone marrow (Fig. 4C) was restored, whereas PBL (Fig. 4A) and spleen (Fig. 4B) remained affected.
Effect of CTX on the numbers of Treg cells
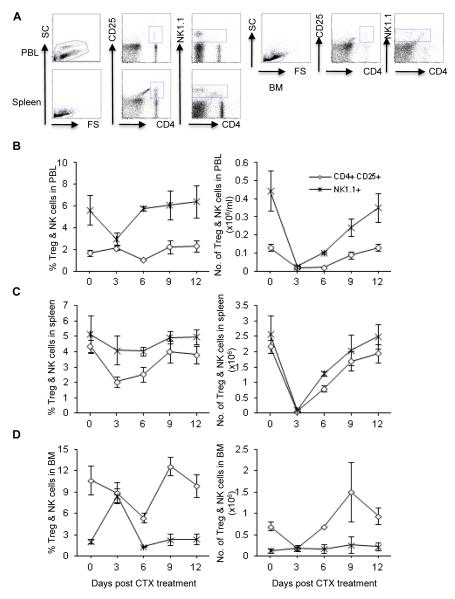
CTX treatment induced a marked decrease in the percentage of CD4+CD25+ Treg cells on day 6 in PBL (Fig. 5A, left panel) and BM (Fig. 5C, left panel) and on days 3 and 6 in spleen (Fig. 5B, left panel). Congruent with the effect of CTX on the relative number of these cells, it also induced decreases in their absolute number, which returned to its control level by day 12 in PBL (Fig. 5A, right panel), day 9 in spleen (Fig. 5B, right panel), and day 6 in BM (Fig. 5C, right panel).
Fig. 5. Effect of CTX treatment on the relative and absolute numbers of Treg (CD4+CD25+) and NK1.1+ cells.
C57BL/6 mice were injected i.p. with PBS or CTX (4 mg/mouse). The average of the data from PBS-treated mice (control group) is depicted as day 0 in the figure. At the indicated time points, mice were bled to collect PBL and then sacrificed to harvest spleen and BM. Leucocytes were counted, stained with mAbs against the indicated cell markers, and then analyzed by flow cytometry. The total leukocytes were gated after exclusion of dead cells and from which the frequency of the indicated cell populations was analyzed in the blood of control mice treated with PBS as a representative dot plot analysis shown in (A). The relative and absolute numbers depicted in PBL (A), spleen (B), and BM (C) as described in the legend of Fig. 2. Data represent the mean ± SD (n = 4).
Effect of CTX on the numbers of NK cells
The percentage of NK1.1+ cells did not change substantially in PBL (Fig. 5A, left panel) and spleen (Fig. 5B, left panel) by CTX treatment. There was, however, a significant increase in the percentage of these cells in BM on day 3 after CTX treatment (Fig. 5C, left panel). The absolute number of NK1.1+ cells in PBL (Fig. 5A, right panel) and spleen (Fig. 5B, right panel) decreased remarkably, and the recovery was evident by days 12 in PBL and 9 in spleen. Conversely, the cell number in BM remained unchanged during all time points (Fig. 5C, right panel).
Discussion
Several reports have shown a strong synergistic effect of combined therapy with CTX and adoptively transferred T cells in inhibiting tumor growth in mice (25, 28). The specific mechanisms of the beneficial effects of CTX, however, remained unclear. Moreover, these mechanisms have mostly been investigated during the lymphopenic phase, and few studies addressed the role of the cellular components that might be altered at the recovery phase (11, 13, 21, 22). In this study, we analyzed the kinetics of the alteration in different cell populations at different time points during both the cytoreductive and rebounding phases.
The data showed that CTX treatment induced lymphopenia in PBL (days 3-15), spleen, and BM (days 3-6). This lymphopenia resulted in decreases in the absolute numbers of DCs (CD11c+CD11b− and CD11c+CD11b+), myeloid cells (CD11b+, Ly6G+, and CD11b+Ly6G+), lymphocytes (B cells [B220+] and T cells [CD4+ and CD8+]), Treg cells (CD4+CD25+), and natural killer cells (NK1.1+). The data further showed that different immune cell populations were back to normal levels during the recovery phase (day 9 onward). However, B cells, MDSCs, and DCs were different in their pattern of recovery form CTX-induced lymphopenia. The absolute numbers of B cells remained low for 3 weeks after CTX treatment. In keeping with reports that demonstrated a transient accumulation of myeloid cells with the phenotype of MDSCs (CD11b+Ly6G+ population) in the spleen following CTX treatment (15, 23, 24), a significant increase in these cells was seen in PBL, spleen, and BM by day 6 and returned back to their normal level by day 15 after treatment. The numbers of CD11c+CD11b− and CD11c+CD11b+ DCs increased significantly in blood and spleen by day 9 and recovered to normal by day 15 after treatment. The increase in the circulating levels of DCs would be of paramount significance since DC maturation stimuli can be administered in vivo at these time points to induce full maturation of DCs and their migration to lymph nodes. Indeed, we tested this hypothesis recently using poly(I:C), the typical TLR3 agonist, to mature DCs and induce their migration to lymph nodes to enhance antigen-specific T cell responses (25). Our observation of post CTX DC expansion is consistent with the recent clinical studies that showed increases in the number of DCs in PBL of cancer patients treated with CTX and G-CSF (26, 27). Although these reports did not examine the sole effect of CTX or G-CSF on DC mobilization, the current results showed that CTX per se was capable of inducing systemic DC expansion. Our recent reports suggested that CTX-induced expansion of DCs was associated with proliferation of DCs in BM during the lymphopenic phase and in blood and spleen during the recovery phase. In addition, CTX induced a dynamic surge in the expression of growth factors and chemokines in BM, where CCR2 and Flt3 signaling pathways were critical for DC expansion (28).
In immunocomptetent (lymphoreplete) hosts, endogenous cells, such as T, B, and NK, compete for homeostatic and survival cytokines, particularly IL-7 and IL-15, with the transferred T cells. Such competition is known as the cytokine sink effect (29, 30). Conversely, lymphodepletion reduces competing cytokine sinks (31). Using pmel-1 adoptive transfer mouse model, it has been demonstrated that, in mice deficient for both IL-7 and IL-15, the antitumor efficacy of tumor-reactive CD8+ T cells adoptively transferred into lymphodeplete hosts was completely abrogated. On the contrary, the antitumor responses were restored when these cytokines were exogenously administered or when the host cells competing for these cytokines were removed by using mice lacking both Rag2 and γc (these mice lack B cells, T cells, and NK cells) (29). Therefore, the depletion of endogenous B220+ B cells, CD4+ and CD8+ T cells, and NK1.1+ cells as shown in our study can explain the beneficial effects of CTX preconditioning to the survival and homeostatic-driven and antigen-driven expansion of adoptively transferred T cells. The delayed recovery of B cells (B220+) after CTX treatment would explain the beneficial effects of CTX treatment in autoimmune diseases (2).
Besides decreasing the circulating levels of lymphocytes and NK cells, CTX also significantly decreased the numbers Treg cells, explaining another mechanism for the beneficial effects of CTX to adoptive T cell therapy. In fact, studies conducted by Antony et al. have persuasively shown that Treg cells suppress the antitumor activity of tumor-reactive CD8+ T cells transferred into lymphoreplete hosts (32), where the antitumor responses were enhanced by the adoptive transfer of tumor-reactive CD8+ T cells into mice deficient in CD4+ T cells, but not in mice deficient in CD8+ T cells. They showed further that transfer of CD4+CD25+ Treg cells alone or together with CD4+CD25− helper T cells into CD4+ T cell-deficient mice prevented the efficacy of the adoptive cell therapy. By contrast, transfer of these helper T cells alone induced autoimmunity and regression of established tumor, indicating to the restriction of the suppressive activity to the CD25+ T cell population (32).
The overall decreases in the relative and absolute numbers of Treg cells in PBL and spleen at the lymphopenic phase (day 3-6) is expected due to the induced leucopenia. On the other hand, the return of Treg cell number to normal values in spleen and to slightly higher values over the normal levels in PBL could be attributed to the steady state of mobilization of lymphoid cell precursors from BM to periphery. The decreases in frequency of Treg cells in BM on days 3 and 6 support this concept. The decreases in the numbers of Treg cells during the early lymphopenic phase post CTX treatment (Fig. 5) would explain the enhanced T cell responses after peptide vaccination 1 day after adoptive transfer of OT-1 CD8+ T cells into CTX-treated mice as we reported previously (15). The T cell responses can also benefit from the higher numbers of DCs (Fig. 2) at the recovery phase even in the presence of normal numbers of Treg cells at this stage especially after provision of DC-stimulant such as poly(I:C) as we reported recently (25).
Besides the inhibitory effects of Treg cells, MDSCs are also another arm of the immunosuppressive cells. It has been observed in mice that reducing the numbers of MDSCs after treatment with gemcitabine can increase the antitumor activity of CD8+ T cells and activated NK cells (33). Similar to our results in the mouse model, we have recently reported that cancer patients treated with CTX-containing chemotherapy harbor a high number of MDSC, which are capable of suppressing T cell responses in vitro (34).
Our initial published studies (15) demonstrated that vaccination at the lymphopenic phase post CTX treatment, at which MDSC are decreased in numbers, enhanced expansion of OT-1 CD8+ T cells to peptide vaccination and that this enhanced expansion continued during the recovery phase, at which high number of MDSC exist (Fig. 3). Furthermore, our recent studies showed that peptide vaccination with OVA peptide (SIINFEKL) or melanoma peptide (gp100) at the recovery phase can enhance expansion of CD8+ T cells in vivo (25). Coupling the results of these studies with the results of the current study, it can be suggested that high numbers of MDSC don’t abrogate CD8+ T cell responses to vaccination in particular if a potent adjuvant system such poly(I:C) is co-administered. The host microenvironment created post effective vaccination could favor blocking of MDSC suppressive activity or induce their differentiation into a beneficial cell subset. In line with this, treatment of CD11b+Ly6G+ cells isolated from immune compromised animals or patients with agents such as GM-CSF/IL-4 and all-trans-retinoic acid blocked their suppressive activity (35, 36). Rather, the suppressive function of MDSCs could be blocked by targeting their regulatory pathways (37). Indeed, a recent study has clearly shown that adoptive transfer of CD8+ T cells (pmel-1) engineered to secrete the inflammatory cytokine IL-12 into lymphodepleted tumor-bearing recipients can trigger the differentiation of MDSC into beneficial host cells that enhanced the anti-tumor responses of the transferred cells in IFN-γ-mediated mechanisms (38). Studies are currently investigated by our group to address the effects of post vaccination environment on MDSC phenotype and functions. In conclusion, the expansion of cells with MDSC phenotype post CTX treatment at certain time points as shown in our studies can be targeted to benefit the host.
In summary, our data suggest that CTX therapy leads to dynamic alterations in the host celluarity in different organs at certain time points. It is feasible that application of strategies to manipulate these post CTX phases in vivo taking into consideration their dynamic nature and organ specificity, could remarkably enhance the beneficial effects of CTX applications in immunotherapeutic regimens in general and anti-cancer immunotherapy in particular.
Highlights.
The kinetics of changes in myeloid and lymphoid cells after CTX treatment was investigated.
CTX treatment decreased the absolute numbers of myeloid and lymphoid, regulatory, and NK cells.
The leukocyte levels returned to normal levels during the recovery phase except for B cells.
The cellular changes post CTX treatment can be targeted to benefit the use of CTX in immunotherapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bass KK, Mastrangelo MJ. Immunopotentiation with low-dose cyclophosphamide in the active specific immunotherapy of cancer. Cancer Immunol Immunother. 1998;47:1. doi: 10.1007/s002620050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brode S, Cooke A. Immune-potentiating effects of the chemotherapeutic drug cyclophosphamide. Crit Rev Immunol. 2008;28:109. doi: 10.1615/critrevimmunol.v28.i2.20. [DOI] [PubMed] [Google Scholar]
- 3.Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, Okoye FI, Jaffee EM. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689. [PubMed] [Google Scholar]
- 4.Hermans IF, Chong TW, Palmowski MJ, Harris AL, Cerundolo V. Synergistic effect of metronomic dosing of cyclophosphamide combined with specific antitumor immunotherapy in a murine melanoma model. Cancer Res. 2003;63:8408. [PubMed] [Google Scholar]
- 5.Proietti E, Greco G, Garrone B, Baccarini S, Mauri C, Venditti M, Carlei D, Belardelli F. Importance of cyclophosphamide-induced bystander effect on T cells for a successful tumor eradication in response to adoptive immunotherapy in mice. J Clin Invest. 1998;101:429. doi: 10.1172/JCI1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bracci L, Moschella F, Sestili P, La Sorsa V, Valentini M, Canini I, Baccarini S, Maccari S, Ramoni C, Belardelli F, Proietti E. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin Cancer Res. 2007;13:644. doi: 10.1158/1078-0432.CCR-06-1209. [DOI] [PubMed] [Google Scholar]
- 7.Abrams JS, Eiseman JL, Melink TJ, Sridhara R, Hiponia DJ, Bell MM, Belani CP, Adler WH, Aisner J. Immunomodulation of interleukin-2 by cyclophosphamide: a phase IB trial. J Immunother Emphasis Tumor Immunol. 1993;14:56. doi: 10.1097/00002371-199307000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Gold JE, Ross SD, Krellenstein DJ, LaRosa F, Malamud SC, Osband ME. Adoptive transfer of ex vivo activated memory T-cells with or without cyclophosphamide for advanced metastatic melanoma: results in 36 patients. Eur J Cancer. 1995;31A:698. doi: 10.1016/0959-8049(94)00523-8. [DOI] [PubMed] [Google Scholar]
- 9.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 11.Ikezawa Y, Nakazawa M, Tamura C, Takahashi K, Minami M, Ikezawa Z. Cyclophosphamide decreases the number, percentage and the function of CD25+ CD4+ regulatory T cells, which suppress induction of contact hypersensitivity. J Dermatol Sci. 2005;39:105. doi: 10.1016/j.jdermsci.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4+25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 13.Motoyoshi Y, Kaminoda K, Saitoh O, Hamasaki K, Nakao K, Ishii N, Nagayama Y, Eguchi K. Different mechanisms for anti-tumor effects of low- and high-dose cyclophosphamide. Oncol Rep. 2006;16:141. [PubMed] [Google Scholar]
- 14.Schiavoni G, Mattei F, Di Pucchio T, Santini SM, Bracci L, Belardelli F, Proietti E. Cyclophosphamide induces type I interferon and augments the number of CD44(hi) T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer. Blood. 2000;95:2024. [PubMed] [Google Scholar]
- 15.Salem ML, Kadima AN, El-Naggar SA, Rubinstein MP, Chen Y, Gillanders WE, Cole DJ. Defining the Ability of Cyclophosphamide Preconditioning to Enhance the Antigen-specific CD8+ T-cell Response to Peptide Vaccination: Creation of a Beneficial Host Microenvironment Involving Type I IFNs and Myeloid Cells. J Immunother. 2007;30:40. doi: 10.1097/01.cji.0000211311.28739.e3. [DOI] [PubMed] [Google Scholar]
- 16.Phipps RP, Mandel TE, Schnizlein CT, Tew JG. Anamnestic responses induced by antigen persisting on follicular dendritic cells from cyclophosphamide-treated mice. Immunology. 1984;51:387. [PMC free article] [PubMed] [Google Scholar]
- 17.Limpens J, Van Meijer M, Van Santen HM, Germeraad WT, Hoeben-Schornagel K, Breel M, Scheper RJ, Kraal G. Alterations in dendritic cell phenotype and function associated with immunoenhancing effects of a subcutaneously administered cyclophosphamide derivative. Immunology. 1991;73:255. [PMC free article] [PubMed] [Google Scholar]
- 18.Matar P, Rozados VR, Gervasoni SI, Scharovsky OG. Down regulation of T-cell-derived IL-10 production by low-dose cyclophosphamide treatment in tumor-bearing rats restores in vitro normal lymphoproliferative response. Int Immunopharmacol. 2001;1:307. doi: 10.1016/s1567-5769(00)00028-x. [DOI] [PubMed] [Google Scholar]
- 19.Matar P, Rozados VR, Gervasoni SI, Scharovsky GO. Th2/Th1 switch induced by a single low dose of cyclophosphamide in a rat metastatic lymphoma model. Cancer Immunol Immunother. 2002;50:588. doi: 10.1007/s00262-001-0237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salem ML, Kadima AN, Cole DJ, Gillanders WE. Defining the antigen-specific T-cell response to vaccination and poly(I:C)/TLR3 signaling: evidence of enhanced primary and memory CD8 T-cell responses and antitumor immunity. J Immunother. 2005;28:220. doi: 10.1097/01.cji.0000156828.75196.0d. [DOI] [PubMed] [Google Scholar]
- 21.Miyauchi A, Hiramine C, Tanaka S, Hojo K. Differential effects of a single dose of cyclophosphamide on T cell subsets of the thymus and spleen in mice: flow cytofluorometry analysis. Tohoku J Exp Med. 1990;162:147. doi: 10.1620/tjem.162.147. [DOI] [PubMed] [Google Scholar]
- 22.Brode S, Raine T, Zaccone P, Cooke A. Cyclophosphamide-induced type-1 diabetes in the NOD mouse is associated with a reduction of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177:6603. doi: 10.4049/jimmunol.177.10.6603. [DOI] [PubMed] [Google Scholar]
- 23.Angulo I, de las Heras FG, Garcia-Bustos JF, Gargallo D, Munoz-Fernandez MA, Fresno M. Nitric oxide-producing CD11b(+)Ly-6G(Gr-1)(+)CD31(ER-MP12)(+) cells in the spleen of cyclophosphamide-treated mice: implications for T-cell responses in immunosuppressed mice. Blood. 2000;95:212. [PubMed] [Google Scholar]
- 24.Pelaez B, Campillo JA, Lopez-Asenjo JA, Subiza JL. Cyclophosphamide induces the development of early myeloid cells suppressing tumor cell growth by a nitric oxide-dependent mechanism. J Immunol. 2001;166:6608. doi: 10.4049/jimmunol.166.11.6608. [DOI] [PubMed] [Google Scholar]
- 25.Salem ML, Diaz-Montero CM, Al-Khami AA, El-Naggar SA, Naga O, Montero AJ, Khafagy A, Cole DJ. Recovery from cyclophosphamide-induced lymphopenia results in expansion of immature dendritic cells which can mediate enhanced prime-boost vaccination antitumor responses in vivo when stimulated with the TLR3 agonist poly(I:C) J Immunol. 2009;182:2030. doi: 10.4049/jimmunol.0801829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radcliff FJ, Caruso DA, Koina C, Riordan MJ, Roberts AW, Tang ML, Baum CM, Woulfe SL, Ashley DM. Mobilization of dendritic cells in cancer patients treated with granulocyte colony-stimulating factor and chemotherapy. Br J Haematol. 2002;119:204. doi: 10.1046/j.1365-2141.2002.03717.x. [DOI] [PubMed] [Google Scholar]
- 27.Vuckovic S, Kim M, Khalil D, Turtle CJ, Crosbie GV, Williams N, Brown L, Williams K, Kelly C, Stravos P, Rodwell R, Hill GR, Wright S, Taylor K, Gill D, Marlton P, Bradstock K, Hart DN. Granulocyte-colony stimulating factor increases CD123hi blood dendritic cells with altered CD62L and CCR7 expression. Blood. 2003;101:2314. doi: 10.1182/blood-2002-03-0973. [DOI] [PubMed] [Google Scholar]
- 28.Salem ML, Al-Khami AA, El-Naggar SA, Diaz-Montero CM, Chen Y, Cole DJ. Cyclophosphamide induces dynamic alterations in the host microenvironments resulting in a Flt3 ligand-dependent expansion of dendritic cells. J Immunol. 2010;184:1737. doi: 10.4049/jimmunol.0902309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gattinoni L, Powell DJ, Jr., Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;26:111. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, Rosenberg SA, Restifo NP. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 34.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MR. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res. 1995;1:95. [PubMed] [Google Scholar]
- 36.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 37.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, Leonardi AJ, Morgan RA, Wang E, Marincola FM, Trinchieri G, Rosenberg SA, Restifo NP. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 2011;121(12):4746–57. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]