Abstract
Background & Aims
New-onset diabetes in patients with pancreatic cancer is likely to be a paraneoplastic phenomenon caused by tumor-secreted products. We aimed to identify the diabetogenic secretory product(s) of pancreatic cancer
Methods
Using microarray analysis, we identified adrenomedullin as a potential mediator of diabetes in patients with pancreatic cancer. Adrenomedullin was up-regulated in pancreatic cancer cell lines, in which supernatants reduced insulin signaling in beta cell lines. We performed quantitative reverse-transcriptase polymerase chain reaction and immunohistochemistry on human pancreatic cancer and healthy pancreatic tissues (controls) to determine expression of adrenomedullin messenger RNA and protein, respectively. We studied the effects of adrenomedullin on insulin secretion by beta cell lines and whole islets from mice and on glucose tolerance in pancreatic xenografts in mice. We measured plasma levels of adrenomedullin in patients with pancreatic cancer, patients with type 2 diabetes mellitus, and individuals with normal fasting glucose levels (controls)
Results
Levels of adrenomedullin messenger RNA and protein were increased in human pancreatic cancer samples compared with controls. Adrenomedullin and conditioned media from pancreatic cell lines inhibited glucose-stimulated insulin secretion from beta cell lines and islets isolated from mice; the effects of conditioned media from pancreatic cancer cells were reduced by small hairpin RNA-mediated knockdown of adrenomedullin. Conversely, overexpression of adrenomedullin in mice with pancreatic cancer led to glucose intolerance. Mean plasma levels of adrenomedullin (femtomoles per liter) were higher in patients with pancreatic cancer compared with patients with diabetes or controls. Levels of adrenomedullin were higher in patients with pancreatic cancer who developed diabetes compared those who did not.
Conclusions
Adrenomedullin is up-regulated in patients with pancreatic cancer and causes insulin resistance in β cells and mice.
Keywords: Pancreas, Mechanisms, Mouse Model, Tumor
The association between diabetes mellitus (DM) and pancreatic cancer (PaC) has long been recognized. While long-standing DM may be a risk factor for development of PaC, new-onset DM is a manifestation of the cancer. When fasting blood glucose or oral glucose tolerance tests are performed in patients with PaC, 40% to 65% meet criteria for DM.1–4 In a majority (52%–88%) of these patients, the DM is new, having developed during the 36 months preceding the diagnosis of PaC.1,3,4 In addition, there is a high prevalence (5.2%–13.6%) of PaC in patients with recent DM.5,6 In a population-based study, our group reported that patients with new-onset DM had an 8-fold higher risk of PaC than the general population.7 Thus, patients with new-onset DM are a high-risk population that could be targeted for screening to detect early PaC.
Although the association between new-onset DM and PaC is well established, the pathogenesis of PaC-associated DM (PaCDM) is not well understood. Based on epidemiologic, clinical, and in vitro studies, it has been postulated that PaCDM is a paraneoplastic phenomenon caused by diabetogenic tumor-secreted product(s).4 In addition, studies have also shown resolution of new-onset DM in PaC following cancer resection.4,8–10 Pannala et al, for example, showed that DM resolved following pancreaticoduodenectomy in 57% of patients with new-onset DM compared with none in the group with long-standing DM.4
There are limited data on glucose metabolism in animal models of PaC. Although impaired insulin release has been shown in N-nitrosobis(2-oxopropyl)amine (BOP)-treated hamsters that develop PaC,11 there are no studies on DM in the more recently developed genetically engineered mouse models of PaC. In vitro studies have shown that conditioned media from PaC cell lines not only impair glucose metabolism in peripheral tissues12–14 but also inhibit insulin release from beta cell lines.15,16 Permert et al showed insulin resistance in subjects with PaC that resolved following PaC resection.2,10,17,18 When compared with controls, skeletal muscle from patients with PaC has impaired glucose transport and phosphatidylinositol 3-kinase activity but there are no differences in the initial steps of insulin signaling (insulin receptor binding, tyrosine kinase activity, and insulin receptor substrate 1 content).19 Others have shown an impaired beta cell response to oral glucose load, hyperglycemic clamp, and glucagon stimulation.17,20–23 In studies using homeostasis model assessment, we observed that beta cell function was markedly diminished in PaC with impaired fasting glucose when insulin resistance was only modestly increased.24
Our goal was to identify the mediator(s) of beta cell dysfunction in PaCDM so as to improve understanding of its pathophysiology and to identify potential biomarkers to screen patients with new-onset DM for PaC. We adopted a translational approach in which we used basic science tools to first understand the biological basis of the epidemiologic relationship between new-onset DM and PaC. Our search identified that adrenomedullin (AM), a 52–amino acid peptide that is up-regulated in PaC cell lines, impairs insulin secretion from beta cells and contributes to the insulin inhibitory effect of PaC cells in vitro and in vivo. We then evaluated these findings in human PaC, where we found marked AM up-regulation at the gene level, at the protein level, and in the plasma of patients with PaC, especially those with DM.
Materials and Methods
Cell Culture
Rat insulinoma cells (INS1) were cultured in RPMI (with 10% fetal bovine serum, 10 mmol/L HEPES, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, and 50 μxmol/L β-mercaptoethanol). PaC cell lines were obtained from American Type Culture Collection (Manassas, VA) and cultured following the manufacturer's protocols. The L3.6 cells were kindly provided by Dr Fidler (MD Anderson Cancer Center, Houston, TX) and HPDE6 cells by Dr Tsao (University of Toronto, Toronto, Ontario, Canada).
Microarray Analysis
Expression profiles of PaC cell lines (PANC1, HPAFII, SU86.86, L3.6) and a control cell line, HPDE6 (immortalized human pancreatic ductal epithelial cells), were generated using the Affymetrix (Santa Clara, CA) Human U133 Plus 2.0 microarray. Data were analyzed using the dChip software package (Boston, MA). Genes were selected if there was at least a 3× higher expression compared with the control cell line. All expression values less than 100 were set to a floor of 100 to account for cross-hybridization and nonspecific array expression. Protein products encoded by genes from the microarray were identified through a search of the GeneCards Database. Cellular localization (secreted, membrane, other, or unknown) was assigned according to Swiss-Prot annotation. In silico prediction was used for proteins without annotated localization.25
Islet Isolation
Mouse islet cells were isolated from perfusion of the pancreas and digested using collagenase (Sigma-Aldrich, St Louis, MO) solution in Hank's balanced salt solution. Islet cells were handpicked (∼10 to 15 islets per well) and placed in a 96-well plate containing RPMI 1640 (Gibco, Grand Island, NY) media.
Insulin Secretion Assay
Media on INS1 cells and mouse islets was replaced after 24 hours of incubation with Krebs' buffer (in mmol/L: 120 NaCl, 5 NaHCO3, 5 KCl, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, 10 HEPES, 0.2% bovine serum albumin; pH 7.4) containing varying concentrations of AM (Phoenix Pharmaceuticals, Belmont, CA). After 1 to 2 hours, INS1 cells and islets were stimulated by the addition of glucose. After 1 hour, supernatants were assayed for rat insulin (Crystal Chem, Downers Grove, IL). Insulin values were normalized to cellular protein. Proteins were quantitated using the Bio-Rad protein assay (Bio-Rad, Hercules, CA).
AM Knockdown
PANC1 cells were transfected with scramble or ADM small hairpin RNA (shRNA; sc-39273SH; Santa Cruz Biotechnology, Santa Cruz, CA) using Lipofectamine (Invitrogen, Carlsbad, CA). After 24, 48, 72, and 96 hours, cells and supernatants were collected. Cells were lysed and Western blotted for AM (Phoenix Pharmaceuticals Inc), and β-actin antibody was obtained from Sigma-Aldrich. INS1 cells and islets were exposed to supernatants for 1 to 2 hours followed by glucose stimulation as described previously.
In Vivo Assays
Subcutaneous and orthotopic tumors were developed with MPanc96 luciferase cells, and wild-type mice served as control. Orthotopic tumors were developed using Chinese hamster ovary (CHO) luciferase cells overexpressing AM, and control vector-bearing cells served as control.26 We have previously reported that MPanc96 cells secrete AM and this can be inhibited by AM shRNA26 In the same study,26 we reported that CHO cells (misidentified as PANC1 cells) do not secrete AM,26 but ectopic expression of an AM complementary DNA leads to significant AM secretion in those cells.26 Cells (0.25 million cells) were suspended in phosphate-buffered saline at a volume of 50 μL and injected either subcutaneously or orthotopically in athymic nude mice (n = 10), and the tumor was allowed to progress. Mice were measured every week for tumor growth by bioluminescence imaging using the Xenogen IVIS 100 Imaging System (Caliper Life Science, Hopkinton, MA) and were size matched. Glucose tolerance test was performed by injecting 1.5 mg glucose/g body wt. Blood glucose levels were measured on each mouse using a glucometer (Elite; Bayer Inc, Pittsburgh, PA) before and after the glucose load at different time points (0–150 minutes at 15-minute intervals) every week. Data shown are mean ± SEM.
Immunohistochemistry
Immunohistochemistry was performed on 30 PaC resection specimens (age, 65.1 ± 12.1 years; 13 men) and 4 histologically normal pancreatic specimens. Among patients with PaC, 8 had early-stage disease (I/II) and the remaining 22 had late-stage disease (III/IV). Unstained 5-μm sections were deparaffinized with xylene and rehydrated. Primary antibody against AM (Phoenix Pharmaceuticals Inc) diluted 1:500 in Dako diluent (Dako, Carpinteria, CA) was applied for 1 hour, followed by EnVision + Dual Link System-HRP (Dako) for 15 minutes. Slides were developed with 3,3-diaminobenzidine substrate and counter-stained with hematoxylin. All slides were reviewed by an expert pathologist (T.S.), and stain intensity was scored semiquantitatively as none, mild, moderate, or marked.
RNA Isolation and Real-Time Polymerase Chain Reaction
RNA was isolated from frozen samples using the miRNeasy Mini Kit (Qiagen, Valencia, CA). AM transcript expression was determined using real-time reverse-transcriptase polymerase chain reaction (RT-PCR) using TaqMan fluorescence methodology and ABI 7900 (Applied Biosystems, Foster City, CA). A predesigned primer/probe set (#Hs 00969450_g1) for AM was purchased from Applied Biosystems. RNA was reverse transcribed using the High Capacity cDNA Synthesis Kit (Applied Biosystems). The coding region of the AM gene (NCBI reference sequence NM_001124.1) was cloned27 into p3XFLAG-CMV (Sigma-Aldrich) and was used to generate the standard curves. A total of 121 samples from 75 patients were analyzed using qRT-PCR, among which were 37 PaC samples (age, 67 ± 10.6 years; 21 men; 16 with DM), 36 benign samples (25 from patients with cystic lesions of the pancreas and 11 from normal tissue surrounding PaC; age, 63.2 ± 9.5 years; 22 men; 10 with DM), 31 chronic pancreatitis samples (64.7 ±11 years; 21 men; 18 with DM), 11 intraductal papillary mucinous neoplasm (IPMN) samples (73.5 ± 11.3 years; 10 men; 6 with DM), and 6 pancreatic intraepithelial neoplasia (PaNIN) samples (59.2 ± 7.2 years; 4 men; 2 with DM).
Measurement of Plasma AM
We measured fasting plasma AM levels in 55 subjects without PaC and 61 patients with PaC. Among the 55 subjects without PaC, 27 had new-onset type 2 DM (age, 67.2 ± 7.7 years; 17 men) and 28 had normal fasting glucose levels (age, 65.6 ± 11.4 years; 16 men). Among the 61 patients with PaC, 30 had new-onset DM (age, 67.9 ± 8.3 years; 17 men) and 31 had normal fasting glucose levels (age, 67 ± 11.9 years; 17 men). All 4 groups were age and sex matched. The DM and non-DM groups were matched for fasting glucose levels (±20 mg/dL). Among the patients with PaC, 23 had early-stage disease (I/II) and 38 had late-stage disease (III/IV). Plasma AM levels were measured using the AM RIA Shionogi Kit (Shionogi, Osaka, Japan), an immunoradiometric assay in which AM molecules are sandwiched between biotinylated antibody specific to the intramolecular ring structure and 125I-labeled antibody specific to the C-terminal portion of human AM. We verified the use of this kit by spiking 10 and 100 fmol/L and observed recoveries of 83% and 89%, respectively. In the same cohort, plasma levels of CA 19-9 were measured as well.
Ascertainment of DM Status
Subjects were defined as having DM using the 1997 American Diabetes Association criteria (ie, a fasting blood glucose level ≥ 126 mg/dL, random blood glucose level ≥200 mg/dL, or hemoglobin A1c ≥6.5%).28
Statistical Analyses
Statistical comparisons between 2 groups were performed using 2-sample t tests. Wilcoxon rank sum tests were used for small sample sizes or non-normally distributed data. Comparisons across more than 2 groups were performed using an analysis of variance or Kruskal–Wallis test (small sample size or non-normally distributed data). All analyses were performed using SAS V8 or SAS.JMP (SAS Institute Inc, Cary, NC). P values less than .05 were considered statistically significant.
Results
Identification of AM as a Candidate Mediator of PaCDM
When INS1 cells were exposed to conditioned media from PaC cell lines (PANC1, L3.6, HPAFII, SU86.86), glucose-stimulated insulin secretion from INS1 cells was reduced by ∼30% as compared with the control cell line (HPDE) (P = .02) (Figure 1A). Microarray analysis on these PaC cell lines identified 241 probe sets, representing 182 genes. The microarray data have been loaded into the GEO database (NCBI GEO study GSE40096). Based on Swiss-Prot annotation and in silico prediction, 18 genes encoded secreted proteins (Supplementary Table 1). These 18 proteins were studied to identify candidates with an established role in insulin secretion. Our analysis identified AM, a 52–amino acid peptide that has been reported to inhibit insulin secretion.29,30 Marked AM overexpression (∼7-fold higher) was seen in PANC1 cells (and to a lesser degree in Su86.86 cells) as compared with HPDE cells (Figure 1B). AM expression data from microarray studies were validated by PCR and Western blot. AM messenger RNA levels were significantly higher only in PANC1 cells compared with the control immortalized human duct cell line (HPDE6). However, all pancreatic cancer cell lines showed higher protein levels of AM than control (HPDE6) cells in Western blot studies (Supplementary Figure 1).
Figure 1.
Analysis of gene expression PaC cell lines led to the identification of AM, a candidate mediator of the diabetogenic effect of these cell lines. (A) Effect of PaC cell supernatants on insulin secretion in INS1 cells showed inhibition of insulin secretion with supernatants from PANC1, L3.6, HPAFII, and SU86.86 as compared with HPDE6 cells. Data pooled from at least 3 experiments with each condition in triplicate. Error bars represent the SEM. (B) Microarray data using the Affymetrix Human U133 Plus 2.0 microarray in PaC cell lines showed a 7-fold overexpression of AM in PANC1 cells and to a lesser degree in SU86.86 compared with HPDE6 cells.
AM From PaC Cells Impairs Insulin Secretion
To study the effect of AM on glucose-stimulated insulin secretion, we first determined 15 mmol/L to be the optimal concentration of glucose for stimulation of insulin secretion in INS1 cells using a dose-response experiment (data not shown). When INS1 cells were stimulated with 15 mmol/L glucose following exposure to AM at concentrations of 1 and 20 pmol/L, insulin secretion was reduced to 55% and 52%, respectively (P = .02) (Figure 2A). The effect was more pronounced in isolated islets from mouse pancreas, where glucose stimulation (optimal concentration, 16.7 mmol/L) following exposure to AM at concentrations of 1 and 20 pmol/L led to a reduction of insulin secretion to 41.6% and 4.3%, respectively (P = .009) (Figure 2C).
Figure 2.
AM inhibits insulin secretion and contributes to the insulin inhibitory effect of PaC cells. (A) AM inhibits glucose-stimulated (15 mmol/L) insulin secretion in INS1 cells. (B) AM knockdown using AM shRNA ameliorates the inhibitory effect of PANC1 supernatants on insulin secretion in INS1 cells as compared with scramble (Scr) shRNA. (C) AM inhibits glucose-stimulated (16.7 mmol/L) insulin secretion in isolated mouse islets in a dose-dependent manner. (D) AM knockdown using AM shRNA ameliorates the inhibitory effect of PANC1 supernatants on insulin secretion in isolated mouse islets as compared with scramble (Scr) shRNA. Error bars represent the SEM.
Because AM was overexpressed in PANC1 cells, we used AM shRNA to knock down AM in PANC1 cells. Following transfection, knockdown was first confirmed using Western blotting (Figure 2B and D). Once knockdown of AM was confirmed, the diabetogenic effect of these PANC1 supernatants on glucose-stimulated insulin secretion from INS1 cells and isolated mouse islets was studied. Following shRNA-mediated knockdown of AM, the inhibitory effect of PANC1 cells on insulin secretion from INS1 cells was ameliorated (174% AM shRNA vs 100% scramble; P = .04) (Figure 2B). A similar effect was seen in isolated mouse islets (146% AM shRNA vs 100% scramble; P = .0002) (Figure 2D).
Glucose tolerance tests on athymic nude mice injected subcutaneously and orthotopically with MPanc96 luciferase cells showed a significant increase in blood glucose levels at 4 weeks as compared with control mice (P < .05) (Figure 3A). To test the diabetogenic effect of AM in an in vivo model, we developed PaC tumors that overexpressed AM. To avoid the proliferative effects of AM, tumors were size matched at the time of the glucose tolerance tests. Mice with PaC that overexpressed AM showed a significant increase in glucose intolerance as compared with mice bearing the tumor with the control vector (P < .05) (Figure 3B).
Figure 3.
AM overexpression in PaC in vivo leads to the development of glucose intolerance. (A) MPanc96 luciferase cells were injected subcutaneously and orthotopically in athymic nude mice (n = 10) and pancreatic tumor was developed. Wild-type mice served as control. By bioluminescence imaging, tumors were size matched, mice were subjected to glucose tolerance tests, and blood glucose levels were measured using a glucometer at different time points. Mice bearing both subcutaneous and orthotopic tumors showed a significant increase (P < .05) in blood glucose levels as compared with control mice at 15, 30, 60, and 90 minutes. (B) Orthotopic tumors were developed with and without CHO luciferase cells expressing AM in SCID mice (n = 10). By bioluminescence imaging, tumors were size matched, and mice were subjected to glucose tolerance tests and blood glucose levels were measured using a glucometer at different time points. Mice bearing tumors expressing AM showed a significant increase (P < .05) in blood glucose levels as compared with control tumor-bearing mice at 30, 45, 60, and 90 minutes.
AM as a Candidate Biomarker of PaCDM
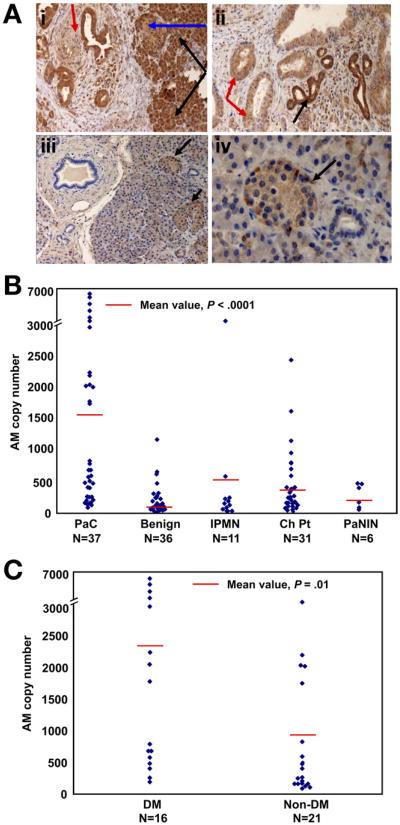
On immunohistochemistry, only the islets stained peripherally for AM in the normal pancreata. In comparison, all 30 PaC specimens showed moderate to marked AM overexpression (Figure 4A). AM expression was seen not only in cancer cells but also neighboring ducts, acini, and islets. Islets adjacent to the cancer also showed overexpression throughout the islet. Although ducts surrounding the PaC had a membranous staining pattern, the PaC showed diffuse cytoplasmic staining.
Figure 4.
AM is overexpressed in human PaC, especially in patients with DM, compared with control patients with and without other pancreatic diseases. (A) Immunohistochemistry for AM. (i) PaC with perineural invasion showing diffuse cytoplasmic staining (red arrow). Normal peritumoral acini (black arrow) and peritumoral islet (blue arrow) also show intense staining. (ii) AM stain shows darker, membranous staining in normal ducts (black arrow) whereas PaC has diffuse, cytoplasmic staining (red arrow). (iii and iv) In normal pancreas, only islets stain for AM (black arrows). (B) Quantitative RT-PCR on pancreatic tissue from human PaC tissue and controls shows marked AM overexpression in PaC compared with controls. (C) Quantitative RT-PCR on RNA isolated from human PaC tissue shows higher AM expression in patients with DM compared with patients without DM.
A total of 121 samples from 75 patients were analyzed using qRT-PCR. There were 37 PaC samples, 36 benign samples (25 from patients with cystic lesions of the pancreas and 11 from normal tissue surrounding PaC), 31 chronic pancreatitis samples, 11 IPMN samples, and 6 PaNIN samples. Patients with PaC had the highest mean AM copy number (1550.4 ± 1897.2) as compared with controls with benign lesions (190.4 ± 231.2), IPMN (502 ± 1082.9), chronic pancreatitis (449.6 ± 524), and PaNIN lesions (282.2 ± 192.1) (P < .0001) (Figure 4B). Further, when all patients with PaC were considered, the AM level was higher in DM cases as compared with non-DM cases (2335.9 ± 2393.8 vs 952 ± 1145; P= .01) (Figure 4C). A similar difference was not seen between AM copy number in patients with chronic pancreatitis with and without DM (536.4 ± 621.7 vs 329.2 ± 334.4; P = .30).
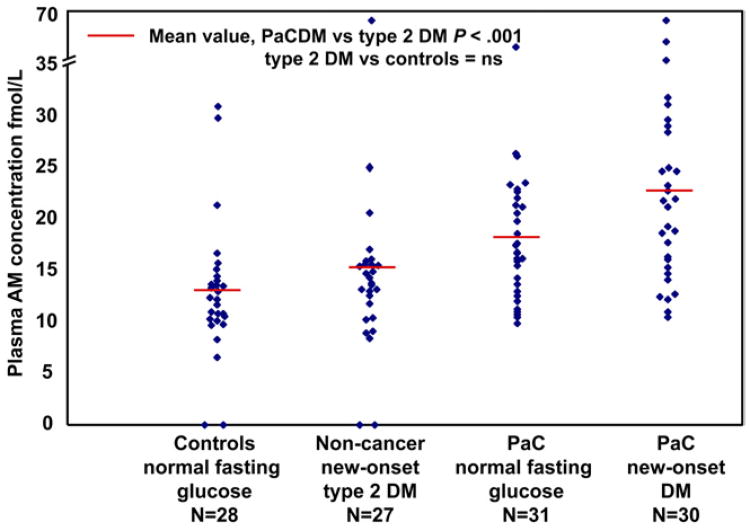
A scatterplot of AM levels in the 4 groups is shown in Figure 5. Mean plasma levels (fmol/L) were higher in 30 patients with PaCDM (age, 67.9 ± 8.3 years; 17 men; AM level, 22.9 ± 10.7) compared with 31 patients with PaC who had normal fasting glucose levels (age, 67 ± 11.9 years; 17 men; AM level, 18.3 ± 7.0; P = .057), 27 non-cancer subjects with new-onset type 2 DM (age, 67.2 ± 7.7 years; 17 men; AM level, 14.8 ± 10.7; P < .001), and 28 noncancer subjects with normal fasting glucose levels (age, 65.6 ± 11.4 years; 16 men; AM level, 12.9 ± 6.6; P < .001). Noncancer subjects with and without DM had similar AM values. Patients with early-stage (I/II, n = 23) and late-stage (III/IV, n = 38) PaC had similar AM values. The area under the receiver operating characteristic curve was 0.77. At a cutoff of 16 fmol/L, AM had a sensitivity of 69% and a specificity of 81% to distinguish cases from noncancer controls. The sensitivity of AM for early-stage PaC (69.6%) was not different from sensitivity for late-stage PaC (65.8%). At a cutoff of the upper limit of normal (37 IU/mL), CA 19-9 had a sensitivity of 75.4% and 100% specificity with an area under the receiver operating characteristic curve of 0.94.
Figure 5.
AM as a candidate biomarker of PaCDM. Fasting plasma AM levels (measured using radioimmunoassay) in 61 patients with PaC (31 with normal fasting glucose levels and 30 with new-onset type 2 DM) and 55 age- and sex-matched controls (28 patients with normal fasting glucose levels and 27 with new-onset type 2 DM). Mean AM levels are higher in patients with PaCDM (22.9 ± 10.7) compared with patients with PaC with normal fasting glucose levels (18.3 ± 7.0, P = .057), noncancer subjects with new-onset type 2 DM (14.8 ± 10.7; P < .001), and noncancer subjects with normal fasting glucose levels (12.9 ± 6.6; P < .001).
Discussion
PaCDM has both similarities and significant differences compared with the more common type 2 DM. Subjects with PaCDM and type 2 DM have similar risk factors for DM, including premorbid body mass index and family history of DM.4,31 On the other hand, type 2 DM is associated with weight gain, whereas PaCDM is associated with weight loss, in the absence of cachectic symptoms (eg, anorexia and decreased exercise tolerance).31 In type 2 DM, weight loss results in improved fasting glucose levels whereas PaCDM is associated with a paradoxical worsening of fasting glucose levels even as there is ongoing weight loss.31 Finally, although resection of PaC with new-onset (presumably cancer-induced) DM results in amelioration of DM, PaC resection in subjects with long-standing DM (presumably type 2 DM) does not improve DM status.4 These observations suggest that PaCDM has a unique pathogenetic mechanism and is caused by the cancer. We show that AM is overexpressed in human PaC tissue at both RNA and protein levels, is found at elevated levels in the blood of patients with PaC, impairs insulin release from beta cell lines as well as isolated pancreatic islets, and induces glucose intolerance in SCID mice. Our data strongly suggest that AM is a candidate mediator of PaCDM.
Because the identification of the putative mediator(s) of PaCDM would have implications for screening, various strategies have been used to identify such mediators. Basso et al showed that derangements in human myoblasts exposed to PaC-conditioned media resulted from changes in mitochondrial energy due to proteins with a molecular weight of <10,000 daltons.12,13 There has also been enthusiasm for the role of amylin (IAPP) in the development of PaCDM, because amylin induces insulin resistance in skeletal muscle.32 Although an initial study of 124 patients found elevated amylin levels in patients with PaCDM, subsequent studies showed that the marker had low sensitivity for PaC.32,33
We used a genomics approach to identify the mediator of beta cell dysfunction in PaC-induced DM. Our search yielded AM, a 52-amino acid, pluripotent hormone that bears homology with amylin. Although ubiquitously distributed in different tissues, AM is found exclusively in the F cells of the islets of Langerhans.34 AM receptors are found on beta cells, which appear to be its main target in normal pancreas.35 In our studies, AM was markedly overexpressed in patients with PaC when compared with controls with benign/cystic pancreatic diseases. Further, the expression of AM was higher in patients with PaC and DM as compared with those without DM. In vitro, AM inhibited glucose-stimulated insulin secretion and AM knockdown ameliorated the diabetogenic effects of PaC cell line supernatants on insulin secretion, whereas in vivo AM overexpression in orthotopically implanted PaC led to the development of glucose intolerance, supporting a role for AM in the pathogenesis of PaCDM.
Although the role of AM in PaCDM has not been studied before, our results are consistent with the effects of AM described by other investigators. Martinez et al showed that a monoclonal antibody against AM increases insulin release 5-fold in isolated rat islets.29 Sekine et al showed a reduction in insulin secretion from beta cells following AM exposure.30 AM injection in diabetic SHR/ N-cp rats decreases serum insulin levels with a concomitant increase in circulating glucose levels.36
Plasma AM levels, normally in low concentrations (femtomoles per liter), are not affected by prandial state, sex, and body mass index.36–38 The highest levels of AM are reported in pregnancy and sepsis.39,40 Previous studies have found elevated AM levels in renal failure and in subjects with diabetic microangiopathic complications but not in patients with type 2 or type 1 DM without these complications.38,41–44
In our study, plasma AM levels were significantly higher in patients with PaC compared with non-PaC controls (20.5 ± 9.2 vs 13.8 ± 8.8; P < .001). The sensitivity (69%) and specificity (81%) of AM in distinguishing PaC cases from non-PaC controls was inferior to that of CA 19-9 (75.4 and 100%, respectively). However, CA 19-9 is a late marker of PaC and has poor sensitivity in early asymptomatic PaC.45 Because PaCDM develops an average of 13 months before the diagnosis of PaC,1 CA 19-9 would not be useful in patients with new-onset DM. By comparison, plasma AM levels could distinguish patients with PaCDM from those with type 2 DM (22.9 ± 10.7 vs 14.8 ± 10.7; P < .001), suggesting the potential utility of AM as a biomarker in patients with PaCDM.
Our studies identified AM as a candidate mediator of PaCDM. However, DM is a complex disorder, and it is very likely that PaCDM represents a heterogeneous population in which other pathogenetic mechanisms may be involved as well. This might be an explanation for the finding that while all PaC cell lines inhibited insulin secretion from INS1 cells, AM overexpression was only seen in 2 of these cell lines (PANC1 and SU86.86). Likewise, translating experimental data from mice to human PaC can be challenging, especially because glycemic criteria for DM in animals are not established. Despite these obvious pitfalls, the consistency of results seen in isolated cells, islets, animal experiments, and human PaC argues for exploring the role of AM, at the very least, as a mediator of PaCDM.
Patients with new-onset DM provide a high-risk population that could be screened for PaC at a time when the cancer is asymptomatic. However, the success of the strategy to use new-onset DM as a marker of PaC will depend on our ability to distinguish PaCDM from the more common type 2 DM. Identification of the mediator(s) of PaCDM could serve as an important tool for screening in this population. Further studies to investigate the role of AM as well as identify other candidate mediator(s) of PaCDM are needed to develop a discriminating panel of biomarkers that can identify PaCDM among subjects with new-onset DM.
Supplementary Material
Supplementary Table 1. Secreted Points Overexpressed in Diabetogenic Cell Lines
Supplementary Figure 1. AM expression in immortalized pancreatic duct cell line and PaC cell lines. (Top panel) AM messenger RNA expression levels were determined by real-time PCR expression in HPDE6, PANC1, L3.6, HPAFII, and SU86.86. AM messenger RNA levels are expressed and normalized to 18S housekeeping control. Briefly, total RNA was extracted from cultured cells (HPDE6, L3.6, PANC1, HPAFI, SU86.86) using TRIzol reagent (Invitrogen). A total of 1 to 2 μg of RNA was reverse-transcribed using the High Capacity cDNA Synthesis Kit (Applied Biosystems). AM transcript expression was determined using real time RT-PCR with TaqMan fluorescence methodology and ABI 7900 (Applied Biosystems). The amount of AM transcript was calculated and expressed as the difference relative to the control gene 18S (2ΔCt, where ΔCt represents the difference in threshold cycles between the target and control gene). (Lower panel) The levels of AM and β-actin were determined by Western blot in the indicated cell lines as previously described in Materials and Methods.
Acknowledgments
Drs Gaurav Aggarwal and Vijaya Ramachandran contributed equally to this paper.
Funding: Dr Chari was supported by grants from the National Institutes of Health (R01 CA 100685) and the Mayo Clinic Pancreas Cancer SPORE (P50 CA 102701); Dr Fernandez-Zapico, Dr George Klee, and Dr Petersen were supported by the Mayo Clinic Pancreas Cancer SPORE (P50 CA 102701); and Dr Logsdon was supported by funds from the Lockton Endowment and the Lustgarten Foundation. Dr Mukhopadhyay and Shamit Dutta were supported by funding from NIH (R01 CA150190).
Abbreviations used in this paper
- AM
adrenomedullin
- CHO
Chinese hamster ovary
- DM
diabetes mellitus
- IPMN
intraductal papillary mucinous neoplasm
- PaC
pancreatic cancer
- PaCDM
pancreatic cancer– associated diabetes mellitus
- PaNIN
pancreatic intraepithelial neoplasia
- RT-PCR
reverse-transcriptase polymerase chain reaction
- shRNA
small hairpin RNA
Footnotes
Supplementary Material: Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2012.08.044.
Conflicts of interest: The authors disclose no conflicts.
References
- 1.Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134:95–101. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Permert J, Ihse I, Jorfeldt L, et al. Pancreatic cancer is associated with impaired glucose metabolism. Eur J Surg. 1993;159:101–107. [PubMed] [Google Scholar]
- 3.Chari ST, Klee GG, Miller LJ, et al. Islet amyloid polypeptide is not a satisfactory marker for detecting pancreatic cancer. Gastroenterology. 2001;121:640–645. doi: 10.1053/gast.2001.27210. [DOI] [PubMed] [Google Scholar]
- 4.Pannala R, Leirness JB, Bamlet WR, et al. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134:981–987. doi: 10.1053/j.gastro.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogawa Y, Tanaka M, Inoue K, et al. A prospective pancreato-graphic study of the prevalence of pancreatic carcinoma in patients with diabetes mellitus. Cancer. 2002;94:2344–2349. doi: 10.1002/cncr.10493. [DOI] [PubMed] [Google Scholar]
- 6.Damiano J, Bordier L, Le Berre JP, et al. Should pancreas imaging be recommended in patients over 50 years when diabetes is discovered because of acute symptoms? Diabetes Metab. 2004;30:203–207. doi: 10.1016/s1262-3636(07)70111-8. [DOI] [PubMed] [Google Scholar]
- 7.Chari ST, Leibson CL, de Andrade M, et al. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129:504–511. doi: 10.1053/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chari ST. Detecting pancreatic cancer early: problems and prospects. Semin Oncol. 2007;34:284–294. doi: 10.1053/j.seminoncol.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gullo L, Pezzilli R, Morselli-Labate AM. Diabetes and the risk of pancreatic cancer. Italian Pancreatic Cancer Study Group. N Engl J Med. 1994;331:81–84. doi: 10.1056/NEJM199407143310203. [DOI] [PubMed] [Google Scholar]
- 10.Permert J, Ihse I, Jorfeldt L, et al. Improved glucose metabolism after subtotal pancreatectomy for pancreatic cancer. Br J Surg. 1993;80:1047–1050. doi: 10.1002/bjs.1800800841. [DOI] [PubMed] [Google Scholar]
- 11.Ahren B, Andren-Sandberg A. Glucose tolerance and insulin secretion in experimental pancreatic cancer in the Syrian hamster. Res Exp Med. 1993;193:21–26. doi: 10.1007/BF02576207. [DOI] [PubMed] [Google Scholar]
- 12.Basso D, Millino C, Greco E, et al. Altered glucose metabolism and proteolysis in pancreatic cancer cell conditioned myoblasts: searching for a gene expression pattern with a microarray analysis of 5000 skeletal muscle genes. Gut. 2004;53:1159–1166. doi: 10.1136/gut.2003.024471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basso D, Valerio A, Seraglia R, et al. Putative pancreatic cancer-associated diabetogenic factor: 2030 MW peptide. Pancreas. 2002;24:8–14. doi: 10.1097/00006676-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Valerio A, Basso D, Brigato L, et al. Glucose metabolic alterations in isolated and perfused rat hepatocytes induced by pancreatic cancer conditioned medium: a low molecular weight factor possibly involved. Biochem Biophys Res Commun. 1999;257:622–628. doi: 10.1006/bbrc.1999.0521. [DOI] [PubMed] [Google Scholar]
- 15.Wang F, Adrian TE, Westermark G, et al. Dissociated insulin and islet amyloid polypeptide secretion from isolated rat pancreatic islets cocultured with human pancreatic adenocarcinoma cells. Pancreas. 1999;18:403–409. doi: 10.1097/00006676-199905000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Wang F, Larsson J, Abdiu A, et al. Dissociated secretion of islet amyloid polypeptide and insulin in serum- free culture media conditioned by human pancreatic adenocarcinoma cell lines. Int J Pancreatol. 1997;21:157–164. doi: 10.1007/BF02822387. [DOI] [PubMed] [Google Scholar]
- 17.Permert J, Adrian TE, Jacobsson P, et al. Is profound peripheral insulin resistance in patients with pancreatic cancer caused by a tumor-associated factor? Am J Surg. 1993;165:61–66. doi: 10.1016/s0002-9610(05)80405-2. [DOI] [PubMed] [Google Scholar]
- 18.Permert J, Larsson J, Fruin AB, et al. Islet hormone secretion in pancreatic cancer patients with diabetes. Pancreas. 1997;15:60–68. doi: 10.1097/00006676-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Isaksson B, Strommer L, Friess H, et al. Impaired insulin action on phosphatidylinositol 3-kinase activity and glucose transport in skeletal muscle of pancreatic cancer patients. Pancreas. 2003;26:173–177. doi: 10.1097/00006676-200303000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Cersosimo E, Pisters PW, Pesola G, et al. Insulin secretion and action in patients with pancreatic cancer. Cancer. 1991;67:486–493. doi: 10.1002/1097-0142(19910115)67:2<486::aid-cncr2820670228>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Schwarts SS, Zeidler A, Moossa AR, et al. A prospective study of glucose tolerance, insulin, C-peptide, and glucagon responses in patients with pancreatic carcinoma. Am J Dig Dis. 1978;23:1107–1114. doi: 10.1007/BF01072886. [DOI] [PubMed] [Google Scholar]
- 22.Fox JN, Frier BM, Armitage M, et al. Abnormal insulin secretion in carcinoma of the pancreas: response to glucagon stimulation. Diabet Med. 1985;2:113–116. doi: 10.1111/j.1464-5491.1985.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 23.Basso D, Plebani M, Fogar P, et al. β-cell function in pancreatic adenocarcinoma. Pancreas. 1994;9:332–335. doi: 10.1097/00006676-199405000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Chari ST, Zapiach M, Yadav D, et al. β-cell function and insulin resistance evaluated by HOMA in pancreatic cancer subjects with varying degrees of glucose intolerance. Pancreatology. 2005;5:229–233. doi: 10.1159/000085276. [DOI] [PubMed] [Google Scholar]
- 25.Klee EW, Finlay JA, McDonald C, et al. Bioinformatics methods for prioritizing serum biomarker candidates. Clin Chem. 2006;52:2162–2164. doi: 10.1373/clinchem.2006.072868. [DOI] [PubMed] [Google Scholar]
- 26.Ramachandran V, Arumugam T, Hwang RF, et al. Adrenomedullin is expressed in pancreatic cancer and stimulates cell proliferation and invasion in an autocrine manner via the adrenomedullin receptor, ADMR. Cancer Res. 2007;67:2666–2675. doi: 10.1158/0008-5472.CAN-06-3362. [DOI] [PubMed] [Google Scholar]
- 27.Martínez A, Vos M, Guédez L, et al. The effects of adrenomedullin overexpression in breast tumor cells. J Natl Cancer Inst. 2002;94:1226–1237. doi: 10.1093/jnci/94.16.1226. [DOI] [PubMed] [Google Scholar]
- 28.Engelgau MM. Diabetes diagnostic criteria and impaired glycemic states: evolving evidence base. Clin Diabetes. 2004;22:69–70. [Google Scholar]
- 29.Martinez A, Weaver C, Lopez J, et al. Regulation of insulin secretion and blood glucose metabolism by adrenomedullin. Endocrinology. 1996;137:2626–2632. doi: 10.1210/endo.137.6.8641217. [DOI] [PubMed] [Google Scholar]
- 30.Sekine N, Takano K, Kimata-Hayashi N, et al. Adrenomedullin inhibits insulin exocytosis via pertussis toxin-sensitive G protein-coupled mechanism. Am J Physiol Endocrinol Metab. 2006;291:E9–E14. doi: 10.1152/ajpendo.00213.2005. [DOI] [PubMed] [Google Scholar]
- 31.Hart PA, Kamada P, Rabe KG, et al. Weight loss precedes cancer-specific symptoms in pancreatic cancer-associated diabetes mellitus. Pancreas. 2011;40:768–772. doi: 10.1097/MPA.0b013e318220816a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Permert J, Larsson J, Westermark GT, et al. Islet amyloid polypeptide in patients with pancreatic cancer and diabetes. N Engl J Med. 1994;330:313–318. doi: 10.1056/NEJM199402033300503. [DOI] [PubMed] [Google Scholar]
- 33.Chari ST, Klee GG, Miller LJ, et al. Islet amyloid polypeptide is not a satisfactory marker for detecting pancreatic cancer. Gastroenterology. 2001;121:640–645. doi: 10.1053/gast.2001.27210. [DOI] [PubMed] [Google Scholar]
- 34.Hong Y, Hay DL, Quirion R, et al. The pharmacology of Adre-nomedullin 2/Intermedin. Br J Pharmacol. 2012;166:110–120. doi: 10.1111/j.1476-5381.2011.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zudaire E, Cuttitta F, Martinez A. Regulation of pancreatic physiology by adrenomedullin and its binding protein. Regul Pept. 2003;112:121–130. doi: 10.1016/s0167-0115(03)00030-2. [DOI] [PubMed] [Google Scholar]
- 36.Martinez A, Elsasser TH, Bhathena SJ, et al. Is adrenomedullin a causal agent in some cases of type 2 diabetes? Peptides. 1999;20:1471–1478. doi: 10.1016/s0196-9781(99)00158-8. [DOI] [PubMed] [Google Scholar]
- 37.Ong KL, Tso AW, Leung RY, et al. A genetic variant in the gene encoding adrenomedullin predicts the development of dysglycemia over 6.4 years in Chinese. Clin Chim Acta. 2011;412:353–357. doi: 10.1016/j.cca.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Nishikimi T, Horio T, Kohmoto Y, et al. Molecular forms of plasma and urinary adrenomedullin in normal, essential hypertension and chronic renal failure. J Hypertens. 2001;19:765–773. doi: 10.1097/00004872-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 39.Lenhart PM, Caron KM. Adrenomedullin and pregnancy: perspectives from animal models to humans. Trends Endocrinol Metab. 2012 Mar 16; doi: 10.1016/j.tem.2012.02.007. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibbons C, Dackor R, Dunworth W, et al. Receptor activity-modifying proteins: RAMPing up adrenomedullin signaling. Mol Endocrinol. 2007;21:783–796. doi: 10.1210/me.2006-0156. [DOI] [PubMed] [Google Scholar]
- 41.Lim SC, Morgenthaler NG, Subramaniam T, et al. The relationship between adrenomedullin, metabolic factors, and vascular function in individuals with type 2 diabetes. Diabetes Care. 2007;30:1513–1519. doi: 10.2337/dc06-1899. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki Y, Horio T, Nonogi H, et al. Adrenomedullin as a sensitive marker for coronary and peripheral arterial complications in patients with atherosclerotic risks. Peptides. 2004;25:1321–1326. doi: 10.1016/j.peptides.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Herder C, Karakas M, Koenig W. Biomarkers for the prediction of type 2 diabetes and cardiovascular disease. Clin Pharmacol Ther. 2011;90:52–66. doi: 10.1038/clpt.2011.93. [DOI] [PubMed] [Google Scholar]
- 44.Hayashi M, Shimosawa T, Isaka M, et al. Plasma adrenomedullin in diabetes. Lancet. 1997;350:1449–1450. doi: 10.1016/s0140-6736(05)64211-0. [DOI] [PubMed] [Google Scholar]
- 45.Humphris JL, Chang DK, Johns AL, et al. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann Oncol. 2012;23:1713–1722. doi: 10.1093/annonc/mdr561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Secreted Points Overexpressed in Diabetogenic Cell Lines
Supplementary Figure 1. AM expression in immortalized pancreatic duct cell line and PaC cell lines. (Top panel) AM messenger RNA expression levels were determined by real-time PCR expression in HPDE6, PANC1, L3.6, HPAFII, and SU86.86. AM messenger RNA levels are expressed and normalized to 18S housekeeping control. Briefly, total RNA was extracted from cultured cells (HPDE6, L3.6, PANC1, HPAFI, SU86.86) using TRIzol reagent (Invitrogen). A total of 1 to 2 μg of RNA was reverse-transcribed using the High Capacity cDNA Synthesis Kit (Applied Biosystems). AM transcript expression was determined using real time RT-PCR with TaqMan fluorescence methodology and ABI 7900 (Applied Biosystems). The amount of AM transcript was calculated and expressed as the difference relative to the control gene 18S (2ΔCt, where ΔCt represents the difference in threshold cycles between the target and control gene). (Lower panel) The levels of AM and β-actin were determined by Western blot in the indicated cell lines as previously described in Materials and Methods.