Abstract
Rationale
Excessive eating often leads to obesity. Although a variety of neurotransmitters and brain regions are involved in modulating food intake, a role of accumbal dopamine is thought to be critical for several aspects of this behavior. Since 18-methoxycoronaridine (18-MC), a selective antagonist of α3β4 nicotinic receptors, was previously shown to alter dopamine release in the nucleus accumbens in response to chronic injections of cocaine and morphine, this drug could be a promising therapy for abnormal eating behavior.
Objectives
Assess the effect of 18-MC on the consumption of sucrose (15%) vs. water in a self-administration paradigm and on the intake of freely available palatable fluids (i.e., 5% sucrose, 0.1% saccharin, and 0.6% saline solutions) as well as on water intake. Determine whether repeated administration of 18-MC (20 mg/kg i.p.) affects weight gain, food intake, and fat deposition in rats drinking 30% sucrose solution.
Results
Acute administration of 18-MC (10–40 mg/kg i.p.) reduced operant responding for sucrose and decreased ad libitum ingestion of sucrose, saccharin, and saline. The highest dose of 18-MC also reduced consumption of water when palatable fluids were not available. In rats having unlimited access to sucrose (30%), chronic treatment with 18-MC (20 mg/kg i.p.) prevented sucrose-induced increases in body weight, decreased fat deposition, and reduced consumption of sucrose while not altering food intake.
Conclusions
These data suggest that antagonism of α3β4 nicotinic receptors may be involved in the regulation of intake of palatable substances regardless of its caloric value and may participate in maintaining obesity.
Keywords: Palatable fluids, Feeding behavior, Nicotinic receptors, Obesity
Introduction
Obesity, a source of significant morbidity and increased mortality of the US population, is among the major public health problems in the country. Today’s epidemic of obesity and associated disorders shows no signs of slowing down and threatens to undo the improvements in American health statistics achieved by reductions in cardiovascular morbidity (Yach et al. 2006). Needless to say, the benefits from losing weight are both medical and cosmetic, and since the majority of obese patients are excellent candidates for pharmacological treatments, the “race is on for the pill to control obesity” (Yach et al. 2006). Despite these many efforts and a critical demand for safe and effective agents, there are only few agents currently approved by the FDA and available for clinical use in obese patients (Bray and Greenway 2007).
Considerable evidence exists that an increase in sugar consumption and drinking of sweetened substances can lead to obesity in humans (Bray et al. 2004, 1992). Thus, several animals models based on the consumption of palatable fluids have been developed to assess different aspects of excessive eating behavior and obesity (for review, see Speakman et al. 2007). For example, intermittent sugar intake models have been applied to study compulsive bingeing behavior (Avena et al. 2007), while continuous sugar intake models have been used to assess body weight dynamics and its hormonal regulation (Bock et al. 1995). Furthermore, operant administration of sucrose and other sweet solutions has been utilized to assess appetitive and motivational aspects of aberrant eating behavior (Sclafani 2006; Sclafani and Ackroff 2003).
Mesolimbic dopamine is critically involved in the mediation of food reward (Berridge 1996), satiety and expression of ingestive motor behavior (Berthoud 2002). Ingestion of sucrose or saccharin was shown to increase dopamine release in the nucleus accumbens (Hajnal and Norgren 2001; Mark et al. 1991; Rada et al. 2005), while cessation of chronic intake of glucose precipitated a withdrawal-like decrease of dopamine release similar to that observed in morphine-dependent rats (Colantuoni et al. 2002). In humans, the density of D2 receptors has been shown to be negatively correlated with the body mass index (Wang et al. 2001). The latter finding led to the conclusion that, in obese individuals, excessive eating represents a means of compensating for reduced dopamine function in the brain (Wang et al. 2001). Similarities between the neurochemical and behavioral consequences of excessive sugar consumption and addiction to drugs have led to the concept of “sugar addiction” (Avena et al. 2007).
18-MC, a congener of the naturally occurring alkaloid ibogaine, is a potential anti-addictive agent and a selective antagonist of α3β4 nicotinic receptors (for review, see Maisonneuve and Glick 2003). 18-MC has been previously shown to attenuate sensitized morphine-induced dopamine release in the nucleus accumbens of morphine-experienced rats. Furthermore, systemic pretreatment with 18-MC has been shown to reduce the intravenous self-administration of morphine and other drugs, while not affecting the responding for non-drug reinforcers (cf. Glick et al. 2000; Pace et al. 2004). 18-MC appears to decrease the reinforcing efficacies of drugs (Maisonneuve and Glick 1999). 18-MC has also been shown to alleviate several signs of opioid withdrawal in rats (Rho and Glick 1998). Given the important role of dopamine in compulsive eating behavior and in the development of obesity, the current studies were undertaken to assess the effects of 18-MC on operant self-administration of sucrose, consumption of palatable fluids, and intake of normal chow as well as weight gain of rats. 18-MC lacks significant side effects (for review, see Maisonneuve and Glick 2003).
Materials and methods
Animals
Naïve female Sprague–Dawley rats (230–270 g; Taconic, Germantown, NY, USA) were housed individually and maintained on a normal 12-h light cycle (lights on/off at 7 a.m./7 p.m.) in the colony room. For all experiments, food (normal chow) and water were provided ad libitum. The experiments were conducted in accordance with the “Guide for the Care and Use of Laboratory Animals” (National Research Council 1996). New groups of naïve animals were used for each experiment.
Drugs
18-MC (Albany Molecular Research, Albany, NY, USA) was dissolved in 0.01 M NaH2PO4 (vehicle). Sucrose (5%, 15%, or 30% wt/vol; MP Biomedicals, Solon, OH, USA), saccharin sodium hydrate (0.1% wt/vol; Sigma, St. Louis, MO, USA), and sodium chloride (0.6% wt/vol; Fisher Scientific, Fair Lawn, NJ, USA) were dissolved in water.
Operant sucrose self-administration procedure
Rats were water-deprived for 23 h so that shaping of the bar-press response could initially be accomplished by training rats to bar-press for water. Thereafter, in non-deprived rats, oral sucrose self-administration testing began with a 16-h nocturnal session followed by daily 1-h sessions, 5 days (Monday–Friday) a week. Rats were allowed access to two levers mounted 15 cm apart on the front wall of each operant conditioning chamber (Coulbourn Instruments, Allentown, PA, USA). A response on either of the two levers produced a sucrose reward (15% solution; 0.01 mL) on a fixed ratio 1 with a 20-s time-out (FR1 TO 20) schedule. Two levers were used so that endogenous side preferences would not impede acquisition of responding or interact with treatment effects (e.g., Carlson and Glick 1989; Glick and Hinds 1985). After rates of sucrose self-administration stabilized (±20% variation from 1 day to the next across 5 days), usually after 2 weeks of testing, 18-MC (10–40 mg/kg) or vehicle was administered i.p. 15 min before a test session. Each rat received all four different treatments (in randomized order, n=6/group) spaced at 1-week intervals.
Palatable liquid consumption
On the first day of the experiment, the regular cage tops were replaced with custom-made cage tops containing metal holders for two 100-mL graduated glass bottles (Lab Products, Seaford, DE, USA); the bottles were placed approximately 5 cm apart. Different groups of naive rats were allowed 19 h (3:30 p.m.–10:30 a.m.) unlimited access to water and 5% sucrose, water and 0.1% saccharin, water and 0.6% saline, or water and water. The concentrations were chosen in accordance with previous reports using similar two-bottle drinking paradigms (Dess 2000; Warwick and Weingarten 1996). The animals were maintained on normal chow during these sessions. The bottles with palatable fluids were removed at the end of the 19-h sessions, but water and chow remained always available. The baseline consumption levels of fluids were established during the initial two sessions; immediately before the third session, animals were injected with 18-MC (10, 20, or 40 mg/kg i.p.) or vehicle. To account for possible side preferences of rats, the bottle sides were switched daily; animals expressing strong side preferences during the baseline sessions were excluded from further analysis. The animals were excluded if they exhibited strong side preferences during baseline testing (i.e., amount on day 1 was <10% of amount on day 2). Animals who did not prefer palatable fluid to water (i.e., drink <7% of maximally available palatable fluid per day) were also excluded.
The consumption of palatable fluids and water during the sessions was recorded on a daily basis for four consecutive sessions: two initial baseline sessions, one post-injection session, and one recovery session. Each animal was used for 4 weeks, receiving three different dosages of 18-MC and vehicle in random order; the treatments were administered at 1-week intervals. The latter dosing intervals were chosen to ensure the complete elimination of the drug from the body prior to the next dose (Glick et al. 1999).
Weight gain paradigm in home cages
Animals were maintained on regular chow and water for the duration of the experiment (3 weeks) and were individually housed in their home cages. On the first day of the experiment, animals received an additional bottle containing 30% sucrose solution or water that remained available for 24 h for the following 3 weeks; the bottle sides were switched daily. The body weights of rats were recorded daily for the following 2 weeks immediately prior to the administration of 18-MC (20 mg/kg i.p.) or vehicle. After the last 18-MC or vehicle injection, weights were monitored for an additional week.
Weight gain paradigm in food intake monitor chambers
The rats were individually housed in cages equipped with a food intake monitor system (BioDAQ, Research Diets, New Brunswick, NJ, USA) and custom-made cage tops (see above) and were allowed to acclimate for 3 days, while consuming normal chow and water. The normal chow and water remained available for the entire 4 weeks of the study. On the first day of the study, rats received an additional bottle containing 30% sucrose solution. To control for possible side preferences, the bottle sides were switched daily. After a week of baseline measurements, animals were injected with 18-MC (20 mg/kg i.p.) daily for 2 weeks. After the last injection, rats were monitored in the chambers for an additional week. Body weights of rats were recorded daily 6 days a week.
Total food intake and both sucrose and water consumption were measured for a 23-h period 6 days a week for the entire 4 weeks. The food intake monitor was comprised of a custom-modified cage connected to a central controller. A peripheral sensor was coupled to a food hopper, which was weighed approximately 50 times per second; the mean and standard deviation of the mean were generated each second by a computer. A feeding bout was recorded when more than 0.15 g of food was removed from the hopper; the end of the bout was defined as a lack of weight fluctuation for 5 s.
Assessment of fat depots
On the eighth day after the last 18-MC injection, animals were euthanized in CO2 chambers and decapitated. Necropsies were performed to remove periovarian, perirenal, and inguinal fat pads, and the combined fat tissue was weighed. The experimenter was blinded as to the treatment status of the animals.
Statistical analysis
The sucrose self-administration data were analyzed by two-way ANOVA; the number of responses was the dependent variable, while reinforcer (sucrose vs. water) and dose of 18-MC were used as categorical variables. Subsequent post hoc tests (Newman–Keuls) compared the effects of 18-MC to baseline.
The average levels of fluid consumption (in milliliters) during the first two sessions (baseline), as well as consumption during the post-injection session and the recovery session, were compared by a repeated-measure within-subject ANOVA using treatment and session as main factors; this analysis was followed by one-way ANOVAs for each treatment group and post hoc tests when appropriate.
For the weight gain experiment (Fig. 6), the data for all four treatment groups from days 1 to 21 were analyzed using two-way ANOVA with treatment and time as main factors. Furthermore, the data for the first 2 weeks and the last week for water–vehicle, water–18-MC, and sucrose–18-MC were analyzed separately using two-way ANOVA with treatment and time as main factors. Post hoc comparison tests (Fisher LSD tests) were conducted when appropriate.
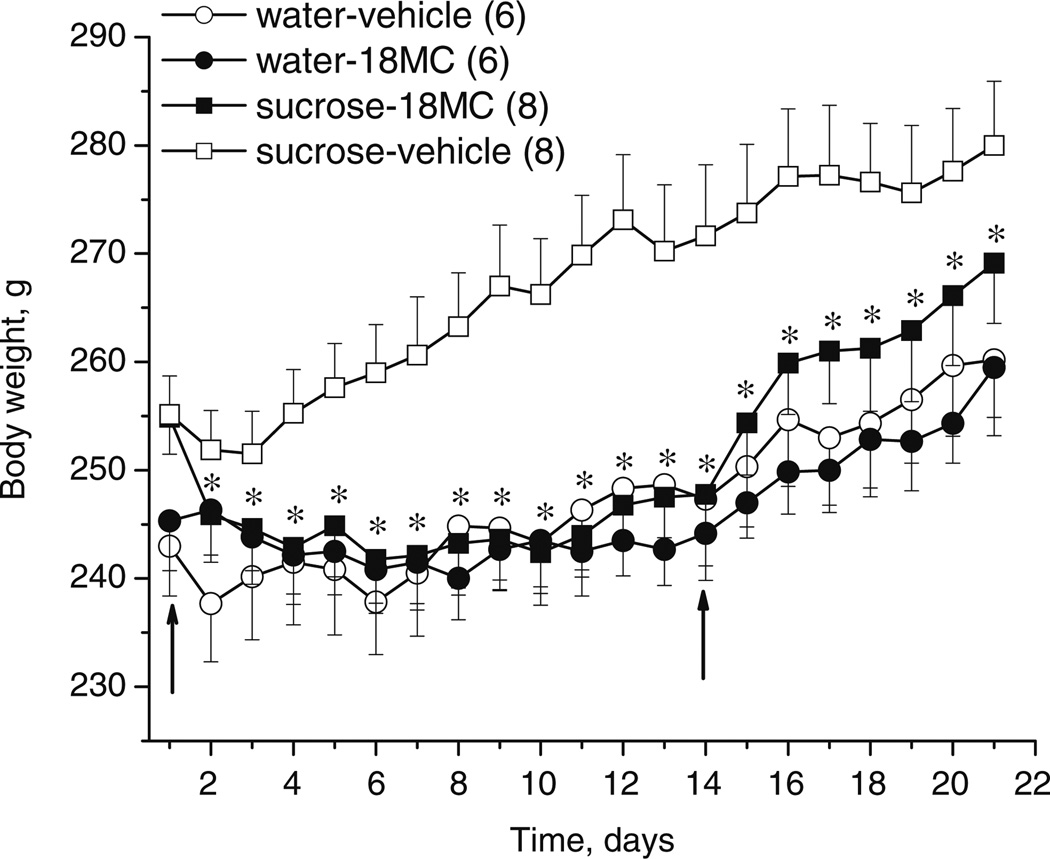
Fig. 6.
Effects of 18-MC (20 mg/kg i.p.) on weight gain of rats (mean±SEM) in home cages. Each data point represents the mean body weight (±SEM) of rats having access to water and chow (water–vehicle or water–18-MC groups, n=6) or sucrose (in addition to water) and chow (sucrose–vehicle or sucrose–18-MC groups, n= 8). Arrows indicate the beginning and the end of treatment with 18-MC.*p<0.05, significant difference between sucrose–vehicle vs. sucrose–18-MC, Fisher LSD tests
For the food monitor experiment (Figs. 7, 8, and 9), sucrose and water consumption data as well as food intake and weight gain data were analyzed using two-way ANOVA with treatment and time as main factors; Fisher LSD tests were applied when appropriate. The fat depots data (Fig. 10) were analyzed by one-way ANOVA.
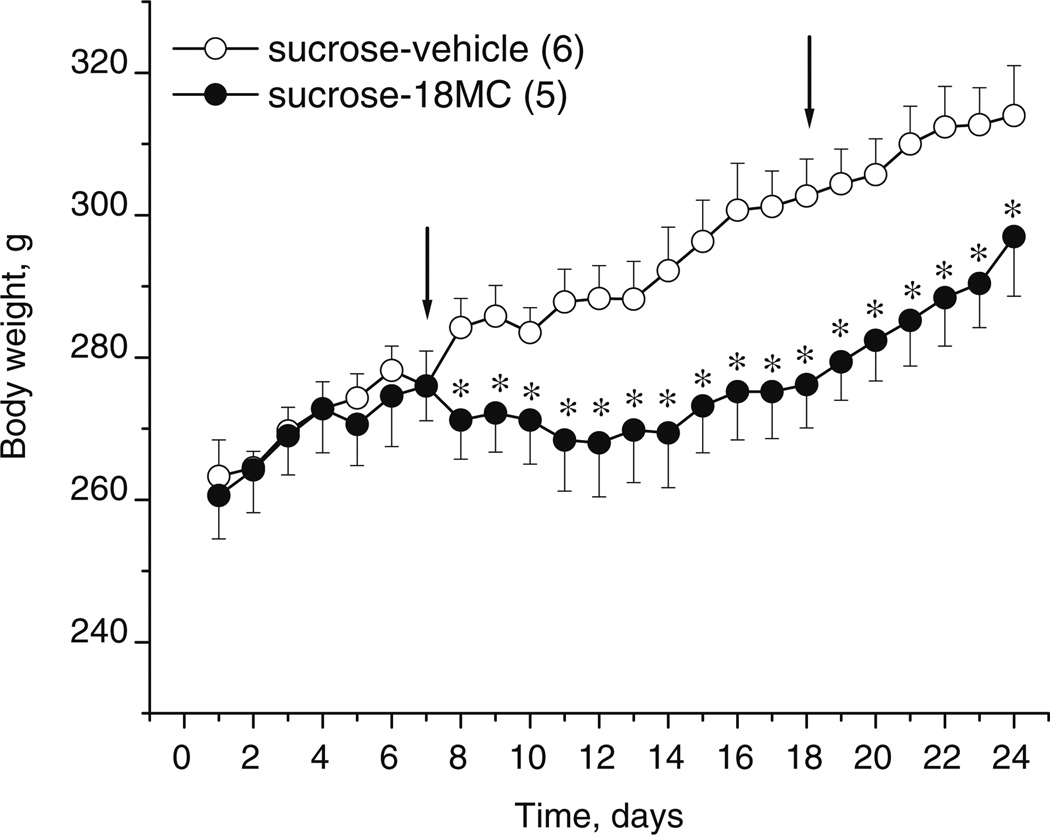
Fig. 7.
Effects of 18-MC (20 mg/kg i.p.) on weight gain of rats (mean±SEM) in food monitor chambers. Each data point represents the mean body weight (±SEM) of rats having access to water, sucrose, and chow and treated with 18-MC (n=5) or vehicle (n=6). Arrows indicate the beginning and the end of treatment with 18-MC. *p<0.05, significant difference between drug vs. vehicle, Fisher LSD tests
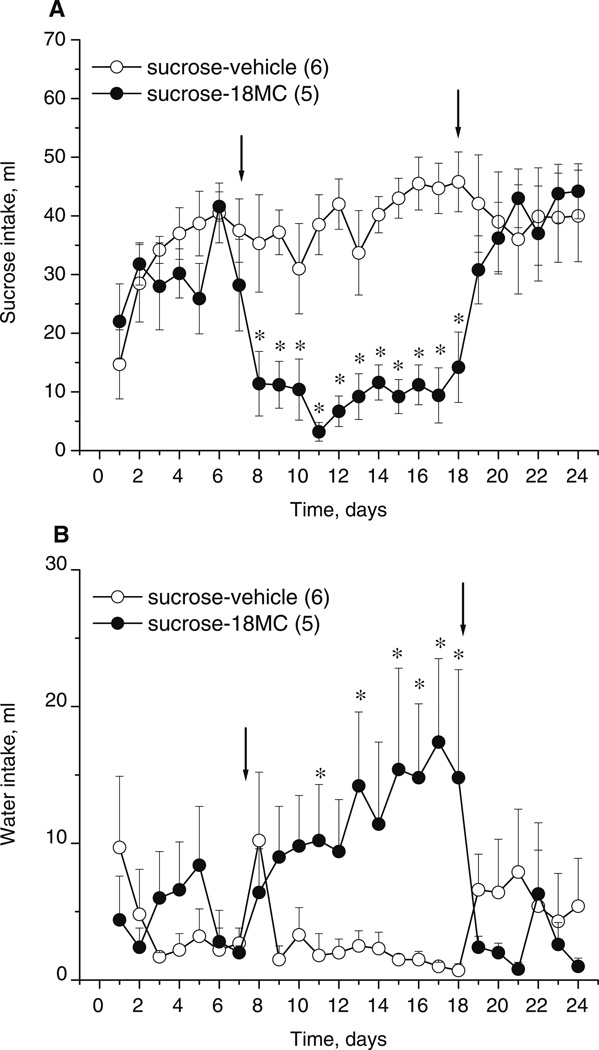
Fig. 8.
Effects of 18-MC (20 mg/kg i.p.) on sucrose consumption (a) and water consumption (b) in rats (mean±SEM) assessed in food monitor chambers. Each data point represents the mean sucrose intake for 23 h (±SEM) of rats having access to water, sucrose, and chow and treated with 18-MC (n=5) or vehicle (n=6). Arrows indicate the beginning and the end of treatment with 18-MC. *p<0.05, significant difference between drug vs. vehicle, Fisher LSD tests
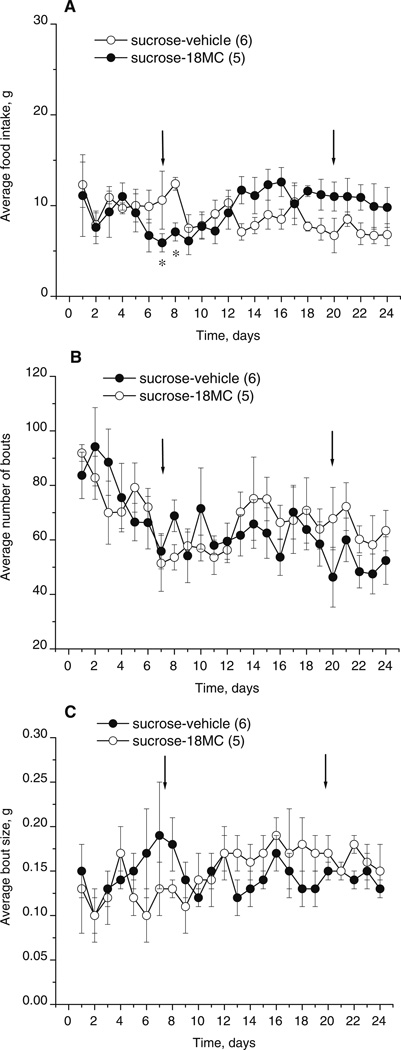
Fig. 9.
Effects of 18-MC (20 mg/kg i.p.) on food intake in rats assessed in food monitor chambers: a mean intake of chow (in grams), b average number of bouts, and c average bout size (in grams). Each data point represents the mean values for 23 h (±SEM) of rats having access to water, sucrose, and chow and treated with 18-MC (n=5) or vehicle (n=6). Arrows indicate the beginning and the end of treatment with 18-MC. *p<0.05, significant difference between drug vs. vehicle, Fisher LSD tests
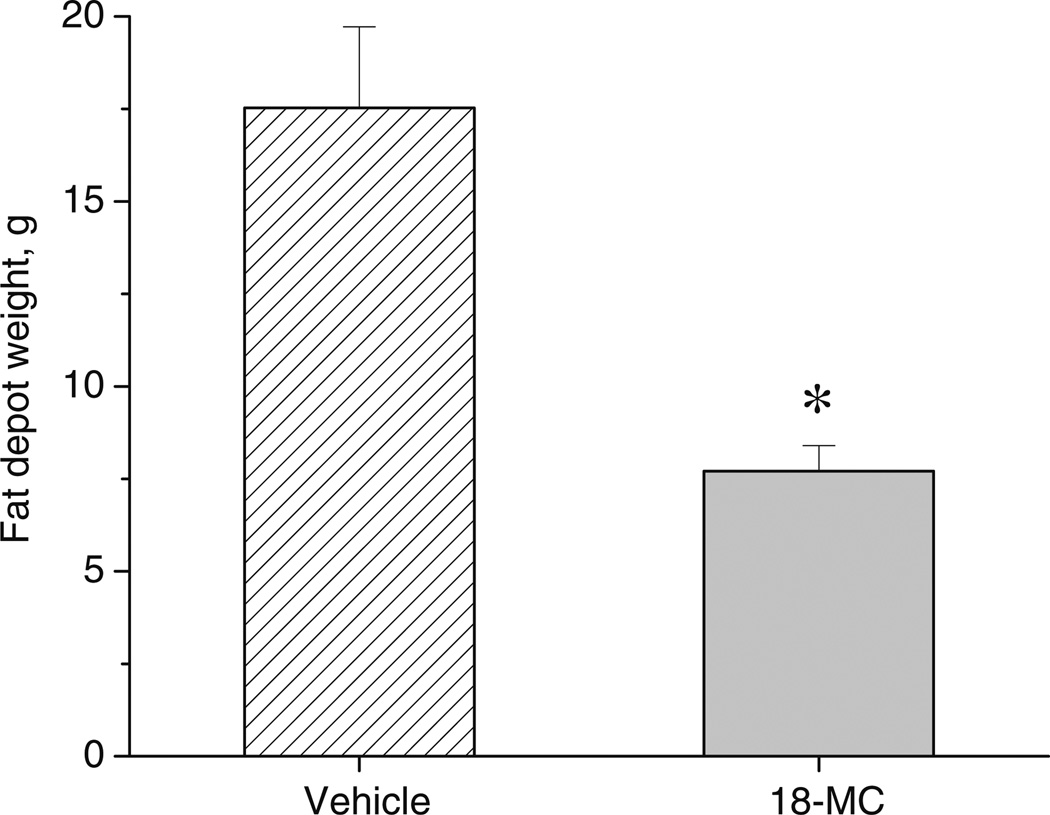
Fig. 10.
Weights of fat depot in rats treated with 18-MC (20 mg/kg i.p., n=5) or vehicle (n=6). Each bar represents the mean weight of periovarian, perirenal, and inguinal fat pads in rats. *p<0.05, significant difference between drug vs. vehicle, ANOVA
Results
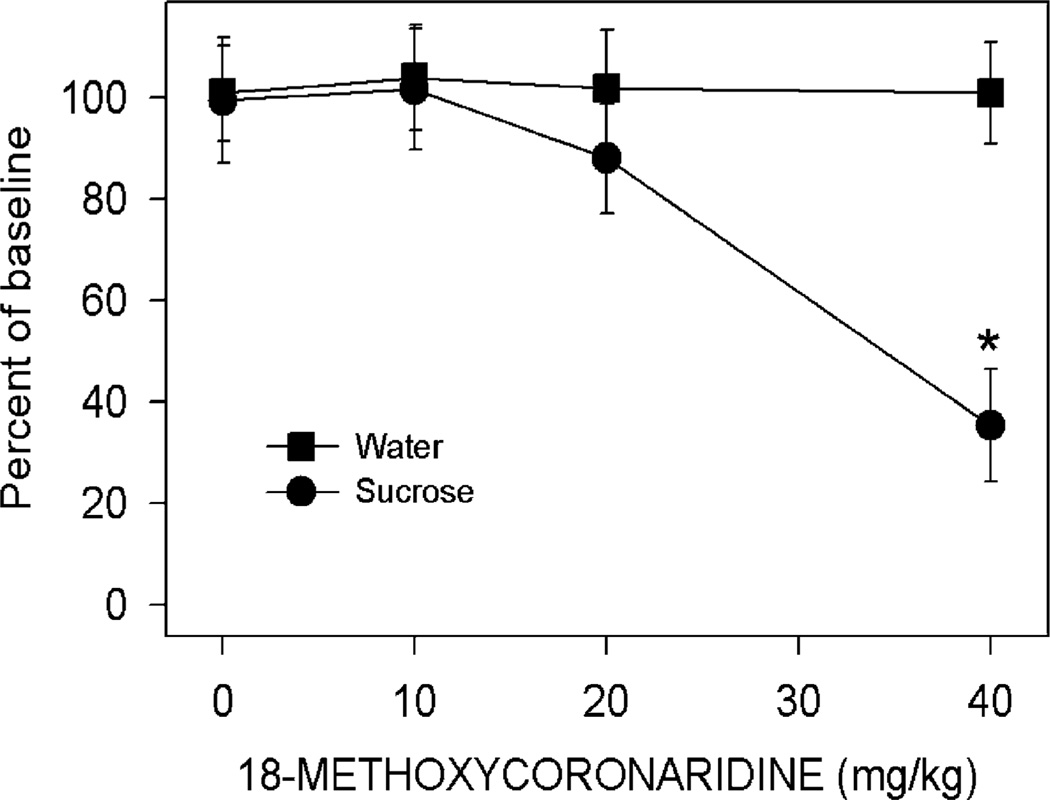
18-MC reduces operant sucrose self-administration
Analysis of the sucrose self-administration data revealed a significant reinforcer×treatment interaction (F3,20=4.30, p<0.02, Fig. 1). Post hoc tests showed that only one effect was significant: a dose of 40 mg/kg of 18-MC reduced operant responding for sucrose (p<0.005).
Fig. 1.
Effects of 18-MC (10–40 mg/kg) or vehicle on operant self-administration of sucrose and water. Baseline responses per hour for sucrose averaged (±SEM) 52.0±9.6 while baseline responses per hour for water averaged (±SEM) 792±121. Each data point represents the mean (±SEM) percent of baseline of six rats. *p<0.005, significant difference between drug vs. vehicle, Newman–Keuls test
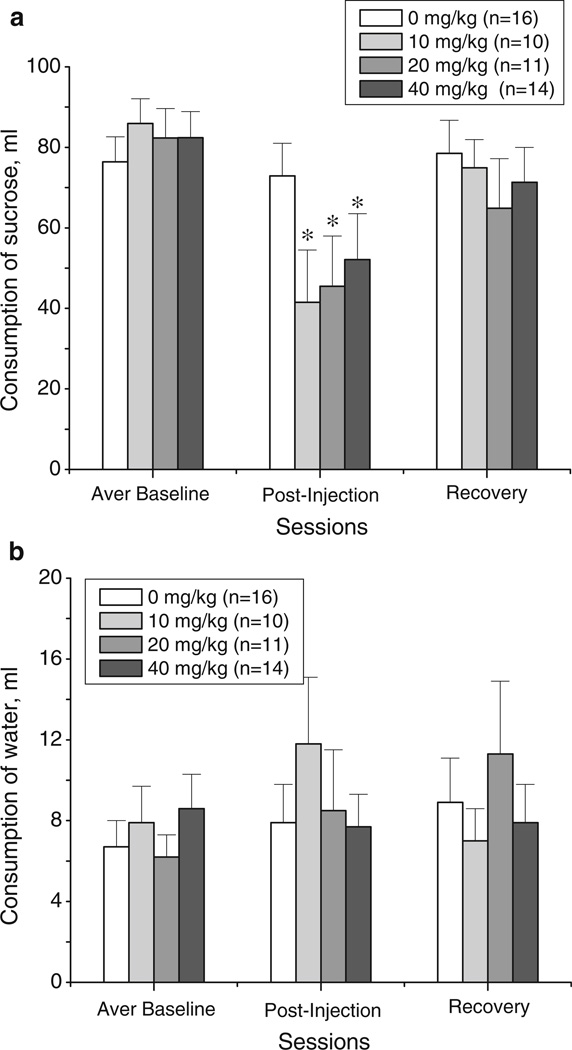
18-MC reduces sucrose consumption
The data for intake of 5% sucrose solution and water in the vehicle- and 18-MC-treated (10, 20, and 40 mg/kg) groups are shown on Fig. 2a–b. There were no significant differences in average basal levels of intake of sucrose solution or water among the four treatment groups.
Fig. 2.
Effects of 18-MC (10, 20, and 40 mg/kg i.p.) on the consumption of sucrose (a) and water (b) during the averaged baseline session, postinjection session, and a recovery session. “Averaged baseline” was calculated based on responses for 19 h sessions on day 1 and day 2; the averaged baselines were as follows (mean±SEM): 76.4±6.2, 85.9±6.2, 82.3±7.3, and 82.4±6.5 for 0, 10, 20, and 40 mg/kg of 18-MC, respectively. *p<0.05, significant difference between drug vs. baseline, Fisher LSD tests
The overall ANOVA of sucrose consumption revealed a significant main effect of session (session effect: F2,94= 18.35, p<0.00001). Further analysis of sucrose intake for each treatment group indicated that consumption of sucrose remained constant across sessions in the vehicle-injected group, but was significantly reduced in all 18-MC-treated groups (for vehicle: F2,30=0.23, p>0.79; for 10 mg/kg: F2,18=8.60, p<0.002; for 20 mg/kg: F2,20=4.66, p<0.02; for 40 mg/kg: F2,26=7.91, p<0.002). As shown in Fig. 2a, all 18-MC-injected animals decreased their sucrose intake levels during the 19-h session immediately following the treatment compared to their baseline levels (post hoc tests). Compared to baseline levels, the intake of sucrose was reduced on average by 44.4±13.1, 36.7±10.7, and 30.3± 8.9 mL by 10, 20, and 40 mg/kg, respectively; sucrose intake returned to baseline during the recovery session. The analysis of water consumption in the same animals indicated that levels of water intake remained stable across sessions in all treated animals (Fig. 2b).
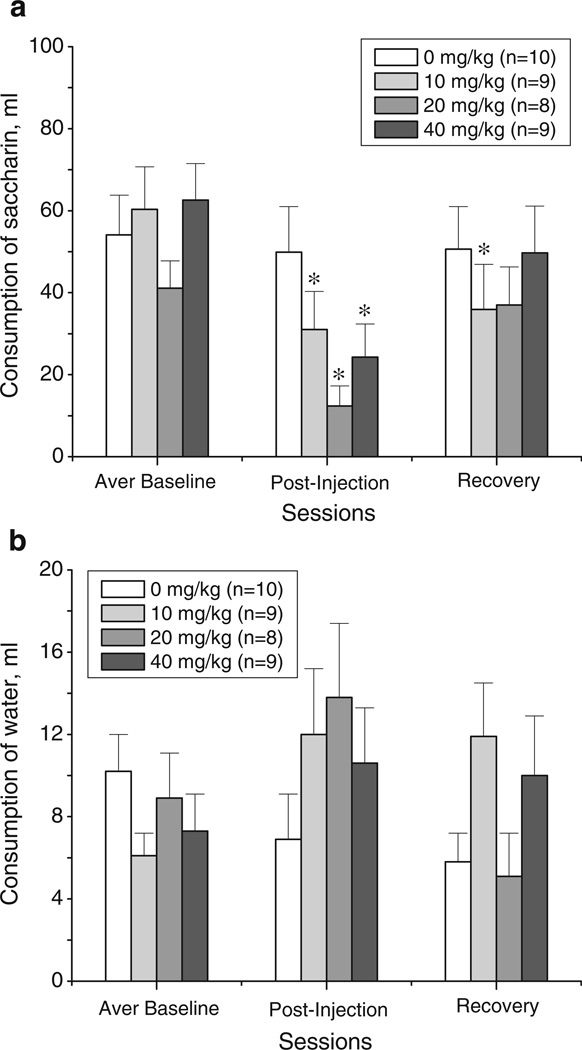
18-MC reduces saccharin consumption
The data for intake of 0.1% saccharin solution and water in the vehicle- and 18-MC-treated (10, 20, and 40 mg/kg) groups are shown on Fig. 3a–b. There were no significant differences in average basal levels of intake of saccharin or water among the four treatment groups.
Fig. 3.
Effects of 18-MC (10, 20, and 40 mg/kg i.p.) on the consumption of saccharin (a) and water (b) during the baseline session, postinjection session, and a recovery session (mean±SEM). “Averaged baseline” was calculated based on responses for 19 h sessions on day 1 and day 2; the averaged baselines were as follows (mean±SEM): 54.1±9.7, 60.3±10.4, 41.1±6.7, and 62.6±8.9 for 0, 10, 20, and 40 mg/kg of 18-MC, respectively. *p<0.05, significant difference between drug vs. baseline, Fisher LSD tests
The ANOVA of saccharin consumption across the sessions revealed a significant main effect of session and a significant session×treatment interaction (session effect: F2,64 =32.61, p<0.00001; session×treatment interaction: F6,64=4.26, p<0.001). Additional analysis for each treatment group indicated that all dosages of 18-MC resulted in significantly less saccharin intake during the postinjection session compared to baseline intake (for 10 mg/kg: F2,16=21.97, p<0.00003; for 20 mg/kg: F2,14=7.67, p<0.006; for 40 mg/kg: F2,16=15.97, p<0.0002; post hoc tests; Fig. 3a). Compared to baseline levels, the intake of saccharin was reduced on average by 29.3±5.9, 28.8±7.1, and 38.3± 5.6 mL by 10, 20, and 40 mg/kg, respectively; saccharin intake for the latter two groups returned to baseline during the recovery session. Interestingly, the group treated with the lowest dose of the drug (i.e., 10 mg/kg) also had reduced intake during the recovery session (24.4±4.8 compared to baseline levels). The consumption of saccharin in the vehicle-treated group remained unchanged across sessions (F2,18=0.35, p>0.71). The analysis of water consumption across sessions in the same animals showed that it was not altered by pretreatment with 18-MC (Fig. 3b).
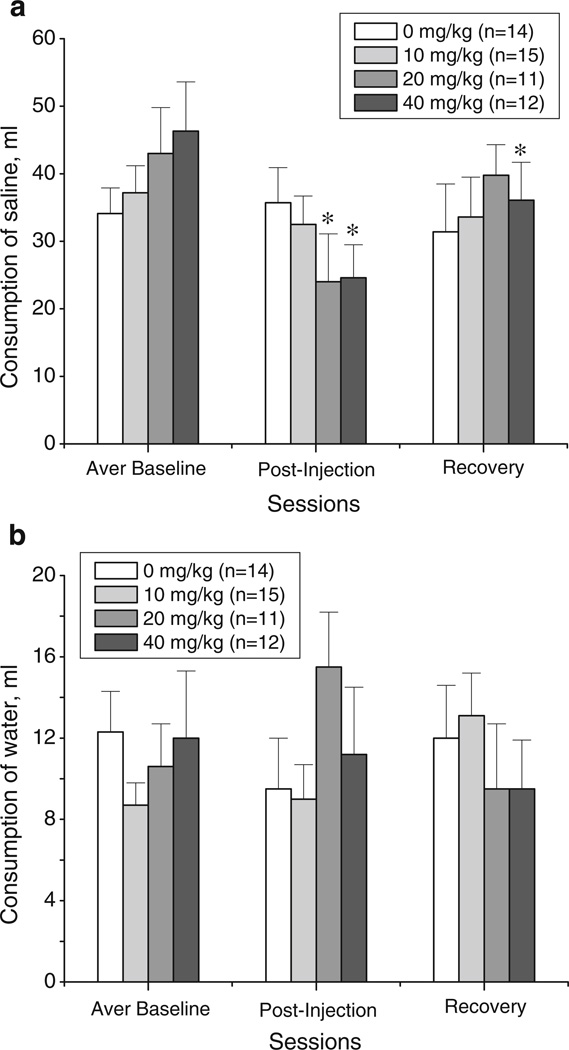
18-MC reduces saline consumption
The data for intake of 0.6% saline solution and water in the vehicle- and 18-MC-treated (10, 20, and 40 mg/kg) groups are shown on Fig. 4a–b. There were no significant differences in average basal levels of intake of saline or water among the four treatment groups.
Fig. 4.
Effects of 18-MC (10, 20, and 40 mg/kg i.p.) on the consumption of saline (a) and water (b) during the baseline session, postinjection session, and a recovery session (mean±SEM). “Averaged baseline” was calculated based on responses for 19 h sessions on day 1 and day 2; the averaged baselines were as follows (mean±SEM): 34.1±3.8, 37.2±4.0, 43.0±6.8, and 46.3±7.3 for 0, 10, 20, and 40 mg/kg of 18-MC, respectively. *p<0.05, significant difference between drug vs. baseline, Fisher LSD tests
The ANOVA of saline consumption revealed a significant main effect of session and a significant session×treatment interaction (session effect: F2,96=9.41, p<0.0002; treatment×session interaction: F6,96 =2.90, p<0.01). The analysis of the effect of session for each treatment group showed that consumption of saline was reduced (compared to the baseline) in the groups pretreated with 20 and 40 mg/kg of 18-MC but remained unchanged in the groups pretreated with 10 mg/kg of 18-MC and vehicle (for 20 mg/kg: F2,20=8.20, p<0.003; for 40 mg/kg: F2,22=9.91, p<0.001; for 10 mg/kg: F2,28=0.92, p>0.41; for vehicle: F2,26=0.24, p>0.79). As shown in Fig. 4a, the effects of 20 and 40 mg/kg of 18-MC were significant during the 19 h immediately following injection (post hoc tests). Compared to baseline levels, the intake of saline was reduced on average by 19.0±4.0 and 21.7±5.5 mL by 20 and 40 mg/kg of 18-MC, respectively; saline intake for the former group returned to baseline during the recovery session. For the group treated with 40 mg/kg of 18-MC, the intake of saline was also significantly reduced by 10.2±4.7 mL compared to baseline levels during the recovery session. The consumption of water in the same rats was not different across sessions in all treatment groups.
18-MC (40 mg/kg i.p.) reduces intake of water
The data for total water intake, when choice of a palatable fluid was not available, are shown on Fig. 5. There were no significant differences in average basal levels of water intake among the four treatment groups.
Fig. 5.
Effects of 18-MC (10, 20, and 40 mg/kg i.p.) on the consumption of water during the baseline session, postinjection session, and a recovery session (mean±SEM). “Averaged baseline” was calculated based on responses for 19 h sessions on day 1 and day 2; the averaged baselines were as follows (mean±SEM): 26.9± 1.7, 26.0±1.2, 23.8±1.4, and 25.6±1.6 for 0, 10, 20, and 40 mg/kg of 18-MC, respectively.*p<0.05, significant difference between drug vs. baseline, Fisher LSD tests
The analysis of water consumption revealed a significant session×treatment interaction (treatment×session interaction: F6,88=3.21, p<0.007). Further one-way ANOVAs for each treatment group showed that water consumption was significantly reduced in the group treated with 40 mg/kg of 18-MC, while it remained unchanged in all other treatment groups (for 40 mg/kg: F2,22=15.57, p<0.0001; for vehicle: F2,22=2.34, p>0.12; for 10 mg/kg: F2,22=1.14, p>0.34; for 20 mg/kg: F2,22=2.34, p>0.12; Fig. 5).
18-MC reduces body weight gain induced by intake of 30% sucrose solution
The average weights of rats immediately before the initiation of treatment (day 1) in water–vehicle, water–18-MC, sucrose–vehicle, and sucrose–18-MC groups were as follows (g±SEM): 243.0±4.6, 245.3±4.6, 255.1±3.6, and 254.9±3.4, respectively (Fig. 6). The average weights on day 1 were not significantly different among the treatment groups.
Analysis of data for all treatment groups for the entire 3-week period of observation revealed a significant main effect of treatment and a significant treatment×time interaction (treatment effect: F3,24=4.47, p<0.01; treatment×time interaction: F60,480=3.85, p<0.00001). Further analysis revealed that sucrose-drinking animals pretreated with 18-MC (40 mg/kg i.p.) had significantly lower body weights compared to the vehicle-treated animals at all time points of observation except for the first one (treatment effect: F1,14=6.33, p<0.025; treatment×time interaction: F20,280=5.60, p<0.00001; post hoc tests). Furthermore, during the 2 weeks of treatment, the average weights of rats in the former group were not significantly different from those in either vehicle-treated or 18-MC-treated groups maintained on water (treatment effect: F2,17=0.08, p>0.92). These results indicate that 18-MC reduces body weights of animals consuming a highly caloric sucrose solution, while it has no effect in animals maintained on a normal diet (treatment effect: F1,10=0.001, p>0.97). After cessation of treatment, the weights in the 18-MC-treated sucrose-drinking group were not significantly different from those in either the vehicle-treated group or the 18-MC-treated group maintained on water, indicating that recovery of the weight gain in the 18-MC-treated sucrose-drinking group did not occur for another week (treatment effect: F2,17=0.93, p>0.41; treatment×time interaction: F12,102=0.56, p>0.87).
18-MC prevents weight gain in rats by reducing their sucrose intake but not food intake
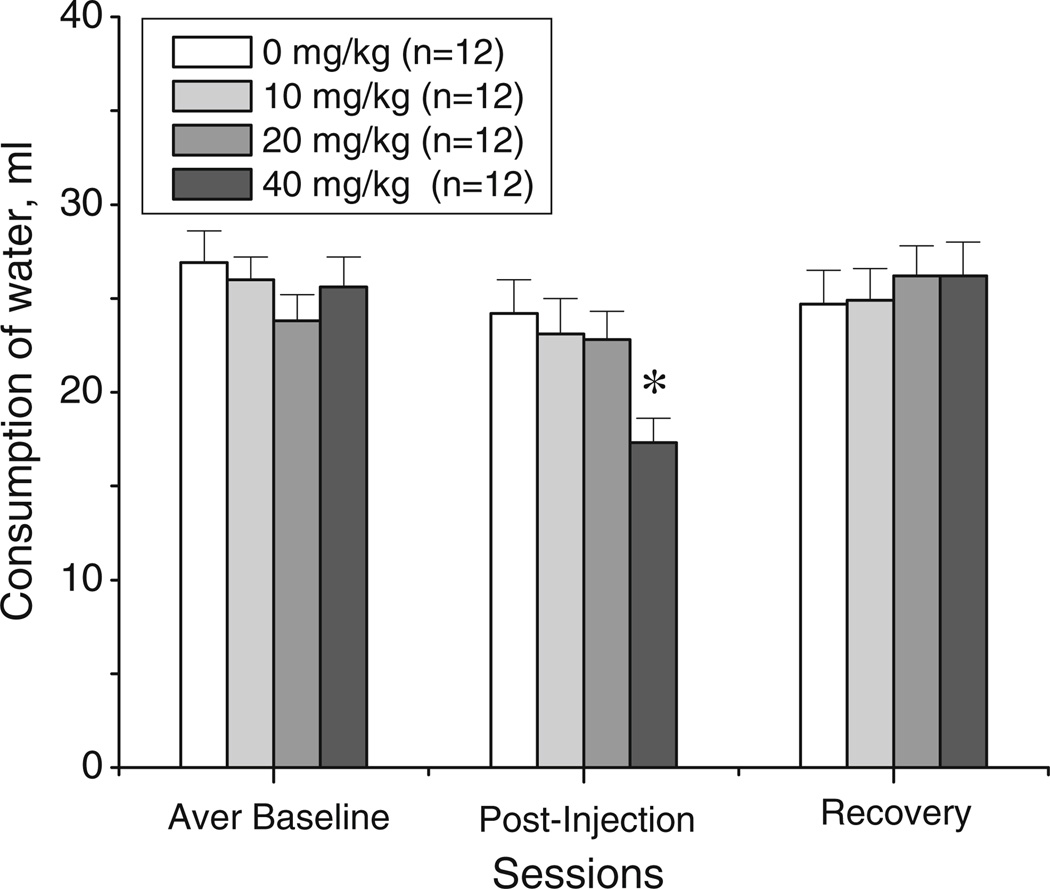
Prior to beginning 18-MC treatment, the average weights of rats on day 1 (baseline) in vehicle- and 18-MC-assigned groups were as follows (g±SEM): 265.0±3.9 and 266.6± 6.4, respectively; there was no significant difference between the two treatment groups. As apparent from Fig. 7, during the first baseline week, both treatment groups displayed similar rates of weight gain while drinking 30% sucrose solution; this rate was retained until the end of the experiment for the vehicle-treated group. Consistent with the data from the preceding experiment, during the 2-week injection period, the 18-MC-treated group displayed no weight gain while having access to 30% sucrose solution. After cessation of 18-MC treatment, the average weight of the latter group began to increase; however, at the end of the study, these rats still weighted significantly less than the vehicle-treated animals. Thus, 18-MC significantly reduced weight gain induced by sucrose-drinking, and recovery of weight did not occur for at least 1 week after the last 18-MC treatment (treatment×time interaction: F23,207=6.06, p<0.00001; post hoc tests).
The intakes of 30% sucrose solution and water in the vehicle- and 18-MC-treated (20 mg/kg i.p.) groups are shown on Fig. 8a–b. There were no significant differences in average basal levels of intake of either sucrose solution or water between the two treatment groups. 18-MC significantly reduced consumption of sucrose in rats (treatment×time interaction: F23,207 =4.83, p<0.0001; Fig. 8a). This effect was apparent on the second day of treatment and remained significant until the last day of injections (post hoc tests). 18-MC-treated rats also had compensatory increase in water intake relative to vehicle-treated rats (treatment×time interaction: F23,207=2.52, p<0.0003; post hoc tests).
The time-course of food intake in rats is shown in Fig. 9a. There was no significant difference in basal levels of food intake between the two treatment groups. Although ANOVA of food intake of both vehicle- and 18-MC-treated groups revealed a significant time×treatment interaction, an 18-MC effect was only apparent at two time points (treatment×time interaction: F23,207=1.61, p<0.04; post hoc tests). Furthermore, ANOVAs of data for each treatment group separately revealed no significant changes from baseline food intake (for vehicle: F23,115=1.47, p>0.09; for 18-MC: F23,92=1.06, p>0.41).
The average number of bouts per 23-h period is shown in Fig. 9b. There was no significant difference in basal levels between the two treatment groups, although the levels of both groups declined during the baseline (time effect: F5,45=2.59, p<0.04). 18-MC had no significant effects (treatment effect: F1,9=0.12, p>0.74; treatment× time interaction: F23,207=1.30, p>0.17).
The average bout sizes per 23-h period are shown in Fig. 9c. There was no significant difference in basal levels between the two treatment groups. 18-MC had no significant effect (treatment effect: F1,9=0.08, p>0.79; treatment×time interaction: F23,207=1.16, p>0.28).
18-MC reduces the weights of fat depots in rats drinking 30% sucrose solution
The analysis of animal’s fat depots, including periovarian, perirenal, and inguinal pads, revealed that 18-MC significantly reduced the weights of fat tissue in rats (F1,9=15.44, p<0.003) (Fig. 10). Interestingly, this effect was observed 1 week after the last 18-MC treatment.
Discussion
This study is the first to characterize the effects of treatment with 18-MC, a selective antagonist at α3β4 nicotinic receptors, on operant self-administration of sucrose, intake of palatable fluids, and normal chow as well as sucrose-induced body weight gain. The solutions tested in the two-bottle consumption studies were 5% sucrose, which is palatable and nutritive, as well as 0.1% saccharin and 0.6% saline, which are also tasteful for rats but are not nutritive (Fregly and Rowland 1992; Hausmann 1933; McHugh 1979; Stefurak and van der 1992; Warwick and Weingarten 1996). Consistent with previous reports (Fregly and Rowland 1992; Hausmann 1933; Hayward et al. 2006; Stewart et al. 1994), under basal conditions, all three palatable fluids were preferred to water; however, sucrose, at the concentrations used, was consumed more avidly than the two other solutions. Overall, the two highest dosages of 18-MC (i.e., 20 and 40 mg/kg i.p.) significantly reduced the consumption of all three palatable liquids without affecting water consumption in the same animals. The lowest dose of the drug was effective in reducing the consumption of the two sweet liquids (i.e., sucrose and saccharin) and had no effect on the consumption of water in the same groups of rats. Despite there being no changes in water consumption in the above groups, the total consumption of fluids was reduced (data not shown). The lack of compensation with water could be due to the fact that the animal’s intake of fluids greatly exceeded physiological requirements while drinking sucrose or saccharin. Consistent with this explanation, in a control experiment (Fig. 5), animals consumed no more on average than 27 mL of water over the same 19-h period; this was much less than their consumption of sucrose or saccharin solutions under the same conditions (Figs. 2 and 3). Taken collectively, these findings suggest that 18-MC reduces consumption of palatable fluids regardless of their caloric value. Since 18-MC has been shown not to produce an effect on locomotor activity (Maisonneuve and Glick 2003), its effect on palatable fluid intake is likely to be behaviorally specific.
There was no apparent dose–response effect of 18-MC on the consumption of sucrose and saccharin over the range of doses studied, suggesting a ceiling effect at 10 mg/kg. For saline consumption, the ceiling appeared at 20 mg/kg. The apparent long-lasting effects of the two treatments (see Figs. 3a and 4a) during the recovery session could possibly be due to an effect of 18-hydroxycoronaridine, an active metabolite present in plasma in low concentrations (Glick et al. 1999; Zhang et al. 2002).
As previously mentioned, the effect of 18-MC on water consumption alone was assessed in an identical experimental paradigm. The highest dose of 18-MC reduced the consumption of water, while the two other dosages had no significant effect (Fig. 5). Such an effect of 18-MC on water consumption was not present when a choice of another palatable fluid was present (Figs. 2, 3, and 4). This discrepancy could be explained by the fact that basal levels of water consumption were already very low in the latter groups. Nevertheless, the control experiment showed that there was at least fourfold selectivity of 18-MC for reducing the consumption of sucrose and saccharin relative to water. Importantly, 18-MC had no effect on responding for water in a sucrose self-administration paradigm (Fig. 1), consistent with previous reports from our laboratory (Glick et al. 1996, 1998).
The two approaches used in the present study (i.e., operant responding and the two-bottle drinking paradigms) allowed assessment of both the motivational and the consummatory aspects of feeding behavior in rats treated with 18-MC. 18-MC appeared to reduce sucrose reward and also attenuate ingestion of freely available sucrose. Interestingly, only the highest dose of 18-MC reduced operant responding for sucrose, while all three dosages were equally effective in reducing free intake of sucrose. These data suggest that operant responding may be less sensitive to the effects of 18-MC than is a consummatory response. Such an interpretation, however, should be applied with caution since the concentrations of sucrose solutions used for the operant procedure and for the free-drinking experiments were not the same.
The mechanism of 18-MC’s effects on consumption of palatable fluids may involve an alteration in taste perception or changes in central neurotransmission mediating reward. For example, 18-MC could enhance the expression of gustducin-IR cells on the taste buds (Wong et al. 1996), and thus increase sensitivity of the tongue to the sweet stimuli reducing sucrose and saccharin intake. Exposure to nicotine was previously shown to affect the same cells and increase appetite to salty and sweet substances in animals and humans (Jias and Ellison 1990; Sato et al. 2002; Tomassini et al. 2007). Alternatively, 18-MC could block nicotinic receptors located in the nucleus of the solitary tract, a brainstem structure responsible for the recognition of basic taste qualities in rats (Dhar et al. 2000; Roussin et al. 2007). This effect could increase sensitivity to sweet or salty taste and reduce the consumption of palatable fluids. 18-MC could also indirectly attenuate sucrose-induced dopamine release in the nucleus accumbens (Di Chiara 1999) and attenuate sucrose reward, thus preventing excessive intake of the tested substances. 18-MC could also attenuate the release of ghrelin in the hypothalamus where it participates in the transduction of feeding-related information from the nucleus of the solitary tract. Nicotinic receptors have been shown to mediate ghrelin-induced augmentation of dopamine release in the nucleus accumbens (Jerlhag et al. 2008).
Previous studies with intermittent access to sucrose showed that rats maintained on normal chow tend to decrease their chow intake in order to compensate for excess caloric intake from sucrose solution; this adjustment may result in unchanged body weights throughout an experiment (Avena and Hoebel 2003; Colantuoni et al. 2002). Thus, in order to better characterize the effects of 18-MC on weight gain, a highly concentrated sucrose solution was made available continuously throughout the 3 weeks of the experiment. Daily injection of 18-MC (20 mg/kg i.p. for 2 weeks) attenuated sucrose-induced weight gain in rats on normal chow, while it had no effect on the body weight of rats maintained on water and chow (Fig. 6). Since neither food nor sucrose intake was assessed in the former experiment, the effects of 18-MC on those parameters were additionally investigated in a related experiment with the use of a food intake monitoring system. In these animals, 18-MC prevented sucrose-induced weight gain by reducing their sucrose intake and only transiently altering food consumption (Figs. 7, 8, and 9). Further analysis revealed no effect of 18-MC on either the average frequencies of bouts or bout sizes during the 23-h period of observation (Fig. 9a–b) Collectively, these findings suggest that 18-MC appears to have little effect on the intake of substances with basic nutritive value (i.e., chow) regardless of whether the choice of sucrose is present or not. The mechanism may involve alterations of sucrose-induced reward. Such differential effects of the drug on the intake of sweet liquids vs. intake of ordinary chow could be due to the relatively low activation of the reward system by the latter (Kirkham and Williams 2001). The present findings might have an important implication for the treatment of obese patients who would be able to maintain their basic nutrition needs without the urge to consume highly palatable sugars. The lack of effects of 18-MC on bout frequencies and bout sizes further supports the behavioral selectivity of the effects of 18-MC. Interestingly, the 18-MC-induced reduction of adiposity in rats was long-lasting and remained significant as long as 1 week after the last drug injection.
The mechanism of 18-MC’s effect on sucrose consumption remains to be determined and may involve nicotinic receptor-mediated alteration of complex homeostatic systems of energy expenditure in the brain and in the periphery (Jo et al. 2002). For example, downstream effects of 18-MC’s antagonism at α3β4 nicotinic receptors could mimic those of prolonged exposure to nicotine and nicotine-induced desensitization of the same receptors (Alkondon and Albuquerque 2005; Giniatullin et al. 2005). Given the fact that nicotine-induced anorexia is linked to low body weight in smokers and in nicotine-exposed animals, 18-MC could reduce body weight by a similar mechanism (Blaha et al. 1998; Bray 2000). Consistent with this premise, administration of a selective agonist (WO03/062224) of the β4-subunit of the nicotinic receptor produced an attenuation of instrumental responding for food in rodents (Greenhalgh et al. 2008). Possible sites for 18-MC’s action may include the nucleus of the solitary tract, the area postrema, and parasympathetic ganglia of the gastrointestinal tract. These areas express high levels of α3β4 nicotinic receptors and are known to be involved in feeding behavior (Berthoud 2002; Di Angelantonio et al. 2003; Jo et al. 2002; Nguyen et al. 2003).
In conclusion, we have demonstrated that acute systemic administration of 18-MC reduces operant self-administration of sucrose and reduces ad libitum intake of sucrose, saccharin, and saline. Furthermore, repeated administration of 18-MC reduces sucrose-induced weight gain in rats with only transient attenuation of food intake. Although the precise mechanisms of these effects are not entirely clear at this time, 18-MC deserves further attention as a potential treatment for obesity.
Acknowledgments
This study was supported by NIDA grant DA016283. The authors would like to thank Michael Bryda for the technical assistance.
Contributor Information
Olga D. Taraschenko, Email: tarasco@mail.amc.edu, Center for Neuropharmacology and Neuroscience MC-136, Albany Medical College, 47 New Scotland Avenue, Albany, NY 12208, USA.
Heather Y. Rubbinaccio, Center for Neuropharmacology and Neuroscience MC-136, Albany Medical College, 47 New Scotland Avenue, Albany, NY 12208, USA
Isabelle M. Maisonneuve, Center for Neuropharmacology and Neuroscience MC-136, Albany Medical College, 47 New Scotland Avenue, Albany, NY 12208, USA
Stanley D. Glick, Center for Neuropharmacology and Neuroscience MC-136, Albany Medical College, 47 New Scotland Avenue, Albany, NY 12208, USA
References
- Alkondon M, Albuquerque EX. Nicotinic receptor subtypes in rat hippocampal slices are differentially sensitive to desensitization and early in vivo functional up-regulation by nicotine and to block by bupropion. J Pharmacol Exp Ther. 2005;313:740–750. doi: 10.1124/jpet.104.081232. [DOI] [PubMed] [Google Scholar]
- Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience. 2003;122:17–20. doi: 10.1016/s0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2007;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Blaha V, Yang ZJ, Meguid M, Chai JK, Zadak Z. Systemic nicotine administration suppresses food intake via reduced meal sizes in both male and female rats. Acta Medica (Hradec Kralove) 1998;41:167–173. [PubMed] [Google Scholar]
- Bock BC, Kanarek RB, Aprille JR. Mineral content of the diet alters sucrose-induced obesity in rats. Physiol Behav. 1995;57:659–668. doi: 10.1016/0031-9384(94)00312-2. [DOI] [PubMed] [Google Scholar]
- Bray GA. Reciprocal relation of food intake and sympathetic activity: experimental observations and clinical implications. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S8–S17. doi: 10.1038/sj.ijo.0801269. [DOI] [PubMed] [Google Scholar]
- Bray GA, Greenway FL. Pharmacological treatment of the overweight patient. Pharmacol Rev. 2007;59:151–184. doi: 10.1124/pr.59.2.2. [DOI] [PubMed] [Google Scholar]
- Bray GA, York B, DeLany J. A survey of the opinions of obesity experts on the causes and treatment of obesity. Am J Clin Nutr. 1992;55:151S–154S. doi: 10.1093/ajcn/55.1.151s. [DOI] [PubMed] [Google Scholar]
- Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- Carlson JN, Glick SD. Cerebral lateralization as a source of interindividual differences in behavior. Experientia. 1989;45:788–798. doi: 10.1007/BF01954054. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- Dess NK. Responses to basic taste qualities in rats selectively bred for high versus low saccharin intake. Physiol Behav. 2000;69:247–257. doi: 10.1016/s0031-9384(99)00246-2. [DOI] [PubMed] [Google Scholar]
- Dhar S, Nagy F, McIntosh JM, Sapru HN. Receptor subtypes mediating depressor responses to microinjections of nicotine into medial NTS of the rat. Am J Physiol Regul Integr Comp Physiol. 2000;279:R132–R140. doi: 10.1152/ajpregu.2000.279.1.R132. [DOI] [PubMed] [Google Scholar]
- Di Angelantonio S, Matteoni C, Fabbretti E, Nistri A. Molecular biology and electrophysiology of neuronal nicotinic receptors of rat chromaffin cells. Eur J Neurosci. 2003;17:2313–2322. doi: 10.1046/j.1460-9568.2003.02669.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Fregly MJ, Rowland NE. Comparison of preference thresholds for NaCl solution in rats of the Sprague–Dawley and Long–Evans strains. Physiol Behav. 1992;51:915–918. doi: 10.1016/0031-9384(92)90070-i. [DOI] [PubMed] [Google Scholar]
- Giniatullin R, Nistri A, Yakel JL. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci. 2005;28:371–378. doi: 10.1016/j.tins.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Glick SD, Hinds PA. Differences in amphetamine and morphine sensitivity in lateralized and non-lateralized rats: locomotor activity and drug self-administration. Eur J Pharmacol. 1985;118:239–244. doi: 10.1016/0014-2999(85)90134-7. [DOI] [PubMed] [Google Scholar]
- Glick SD, Kuehne ME, Maisonneuve IM, Bandarage UK, Molinari HH. 18-Methoxycoronaridine, a non-toxic iboga alkaloid congener: effects on morphine and cocaine self-administration and on mesolimbic dopamine release in rats. Brain Res. 1996;719:29–35. doi: 10.1016/0006-8993(96)00056-x. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Visker KE, Fritz KA, Bandarage UK, Kuehne ME. 18-Methoxycoronardine attenuates nicotine-induced dopamine release and nicotine preferences in rats. Psychopharmacology (Berl) 1998;139:274–280. doi: 10.1007/s002130050716. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Kuehne ME, Bandarage UK. CNS Drug Rev. 1. Vol. 5. Branford, CT: Neva Press; 1999. (±) 18-Metoxycoronaridine: a novel iboga alkaloid congener having potential anti-addictive efficacy; pp. 27–42. [Google Scholar]
- Glick SD, Maisonneuve IM, Dickinson HA. 18-MC reduces methamphetamine and nicotine self-administration in rats. NeuroReport. 2000;11:2013–2015. doi: 10.1097/00001756-200006260-00041. [DOI] [PubMed] [Google Scholar]
- Greenhalgh CE, Smith JW, Clifton PG. WO03/062224 is an in vivo selective agonist at nicotinic beta4 receptors. Pharmacol Biochem Behav. 2008 doi: 10.1016/j.pbb.2008.06.005. in press. [DOI] [PubMed] [Google Scholar]
- Guide for the Care and Use of Laboratory Animals. Institute of Laboratory Animal Resources Commission for Life Sciences, National Research Council. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- Hajnal A, Norgren R. Accumbens dopamine mechanisms in sucrose intake. Brain Res. 2001;904:76–84. doi: 10.1016/s0006-8993(01)02451-9. [DOI] [PubMed] [Google Scholar]
- Hausmann M. The behavior of albino rats in choosing foods: II. Differentiaion between sugar and saccharin. J Comp Psychol. 1933;15:419–428. [Google Scholar]
- Hayward MD, Schaich-Borg A, Pintar JE, Low MJ. Differential involvement of endogenous opioids in sucrose consumption and food reinforcement. Pharmacol Biochem Behav. 2006;85:601–611. doi: 10.1016/j.pbb.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Svensson L, Engel JA. Alpha-conotoxin MII-sensitive nicotinic acetylcholine receptors are involved in mediating the ghrelin-induced locomotor stimulation and dopamine overflow in nucleus accumbens. Eur Neuropsycho-pharmacol. 2008;18:508–518. doi: 10.1016/j.euroneuro.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Jias LM, Ellison G. Chronic nicotine induces a specific appetite for sucrose in rats. Pharmacol Biochem Behav. 1990;35:489–491. doi: 10.1016/0091-3057(90)90192-k. [DOI] [PubMed] [Google Scholar]
- Jo YH, Talmage DA, Role LW. Nicotinic receptor-mediated effects on appetite and food intake. J Neurobiol. 2002;53:618–632. doi: 10.1002/neu.10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM. Synergistic effects of opioid and cannabinoid antagonists on food intake. Psychopharmacology (Berl) 2001;153:267–270. doi: 10.1007/s002130000596. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Glick SD. Attenuation of the reinforcing efficacy of morphine by 18-methoxycoronaridine. Eur J Pharmacol. 1999;383:15–21. doi: 10.1016/s0014-2999(99)00560-9. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Glick SD. Anti-addictive actions of an iboga alkaloid congener: a novel mechanism for a novel treatment. Pharmacol Biochem Behav. 2003;75:607–618. doi: 10.1016/s0091-3057(03)00119-9. [DOI] [PubMed] [Google Scholar]
- Mark GP, Blander DS, Hoebel BG. A conditioned stimulus decreases extracellular dopamine in the nucleus accumbens after the development of a learned taste aversion. Brain Res. 1991;551:308–310. doi: 10.1016/0006-8993(91)90946-s. [DOI] [PubMed] [Google Scholar]
- McHugh PR. Aspects of the control of feeding: application of quantitation in psychobiology. Johns Hopkins Med J. 1979;144:147–155. [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. J Pharmacol Exp Ther. 2003;307:1090–1097. doi: 10.1124/jpet.103.056408. [DOI] [PubMed] [Google Scholar]
- Pace CJ, Glick SD, Maisonneuve IM, He LW, Jokiel PA, Kuehne ME, Fleck MW. Novel iboga alkaloid congeners block nicotinic receptors and reduce drug self-administration. Eur J Pharmacol. 2004;492:159–167. doi: 10.1016/j.ejphar.2004.03.062. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Rho B, Glick SD. Effects of 18-methoxycoronaridine on acute signs of morphine withdrawal in rats. NeuroReport. 1998;9:1283–1285. doi: 10.1097/00001756-199805110-00004. [DOI] [PubMed] [Google Scholar]
- Roussin AT, Victor JD, Chen JY, Di Lorenzo PM. Variability in responses and temporal coding of tastants of similar quality in the nucleus of the solitary tract of the rat. J Neurophysiol. 2007;99:644–655. doi: 10.1152/jn.00920.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Endo S, Tomita H. Sensitivity of three loci on the tongue and soft palate to four basic tastes in smokers and non-smokers. Acta Otolaryngol Suppl. 2002;546:74–82. doi: 10.1080/00016480260046445. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Sucrose motivation in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice measured by progressive ratio licking. Physiol Behav. 2006;87:734–744. doi: 10.1016/j.physbeh.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. Reinforcement value of sucrose measured by progressive ratio operant licking in the rat. Physiol Behav. 2003;79:663–670. doi: 10.1016/s0031-9384(03)00143-4. [DOI] [PubMed] [Google Scholar]
- Speakman J, Hambly C, Mitchell S, Krol E. Animal models of obesity. Obes Rev. 2007;8(Suppl 1):55–61. doi: 10.1111/j.1467-789X.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- Stefurak TL, van der KD. Saccharin’s rewarding, conditioned reinforcing, and memory-improving properties: mediation by isomorphic or independent processes? Behav Neurosci. 1992;106:125–139. doi: 10.1037//0735-7044.106.1.125. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Russell RN, Lumeng L, Li TK, Murphy JM. Consumption of sweet, salty, sour, and bitter solutions by selectively bred alcohol-preferring and alcohol-nonpreferring lines of rats. Alcohol Clin Exp Res. 1994;18:375–381. doi: 10.1111/j.1530-0277.1994.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Tomassini S, Cuoghi V, Catalani E, Casini G, Bigiani A. Long-term effects of nicotine on rat fungiform taste buds. Neuroscience. 2007;147:803–810. doi: 10.1016/j.neuroscience.2007.04.053. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Warwick ZS, Weingarten HP. Flavor-postingestive consequence associations incorporate the behaviorally opposing effects of positive reinforcement and anticipated satiety: implications for interpreting two-bottle tests. Physiol Behav. 1996;60:711–715. doi: 10.1016/0031-9384(96)00087-x. [DOI] [PubMed] [Google Scholar]
- Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006;12:62–66. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ramamoorthy Y, Tyndale RF, Glick SD, Maisonneuve IM, Kuehne ME, Sellers EM. Metabolism of 18-methoxy-coronaridine, an ibogaine analog, to 18-hydroxycoronaridine by genetically variable CYP2C19. Drug Metab Dispos. 2002;30:663–669. doi: 10.1124/dmd.30.6.663. [DOI] [PubMed] [Google Scholar]