Abstract
A spontaneous ameloblastic fibroma was found in a 9-week-old guinea pig. Histopathologically, neoplastic cells consisted of two components: an odontogenic epithelium and odontogenic mesenchyme. The odontogenic epithelium formed strands, nests and islands that were interspersed within the odontogenic mesenchyme. In the marginal region, odontoblasts and scant dysplastic eosinophilic material were seen between these two components. Immunohistochemically, the odontogenic epithelium was positive for cytokeratin AE1/AE3, and the odontogenic mesenchyme and odontoblast were positive for vimentin, in the same manner as in the normal tooth germ (control). We could not obtain conclusive data suggesting that the eosinophilic material was dental hard tissue because the eosinophilic material was not stained specifically by any methods. Based on these histological characteristics, the tumor in the present case was diagnosed as an ameloblastic fibroma. This is the first report of ameloblastic fibroma in guinea pigs.
Keywords: ameloblastic fibroma, guinea pig, rodent, odontogenic tumor, spontaneous, amelogenin
An ameloblastic fibroma is a rare odontogenic tumor consisting of both odontogenic epithelial and mesenchymal components. Histologically, this tumor is primarily composed of a compact, loose, collagen-poor and primitive stroma resembling dental pulp. The characteristic features include interconnected cords and sheets of odontogenic epithelium, which sometimes resembles a less differentiated enamel organ epithelium1. In the field of veterinary pathology, ameloblastic fibroma is the most common odontogenic neoplasm in cattle2 but is rare in other species. In laboratory animals, odontogenic tumors are also rarely encountered but may be induced by carcinogens3. There is a case report of an ameloblastic fibroma-like lesion in rats. However, the lesion was experimentally induced by aflatoxin4. To the best of our knowledge, no report has been published on odontogenic tumors in guinea pigs. In the present study, we report the first case of spontaneous ameloblastic fibroma in guinea pigs and describe its histological and immunohistochemical features.
A 4-week-old male Hartley guinea pig was purchased from Japan SLC Inc. (Shizuoka, Japan) for a toxicity study. The animal protocol was reviewed and approved by the Institutional Animal Care and Ethics Committee of Otsuka Pharmaceutical Factory. The tumor was firstly noticed as a small intraoral nodule on the maxilla when the animal was 7 weeks old, and it then rapidly became larger. The animal was euthanized and necropsied at 9 weeks due to feeding disorder. On macroscopic examination, the tumor was a spherical mass that was 2 cm in diameter and protruded from the upper incisor in the oral cavity (Fig. 1). The surface was rough and dark red to brown with hemorrhage. After fixation, the cut surface of the tumor was mottled pale yellow and dark red in color. No other remarkable changes were noted at necropsy.
Fig. 1.
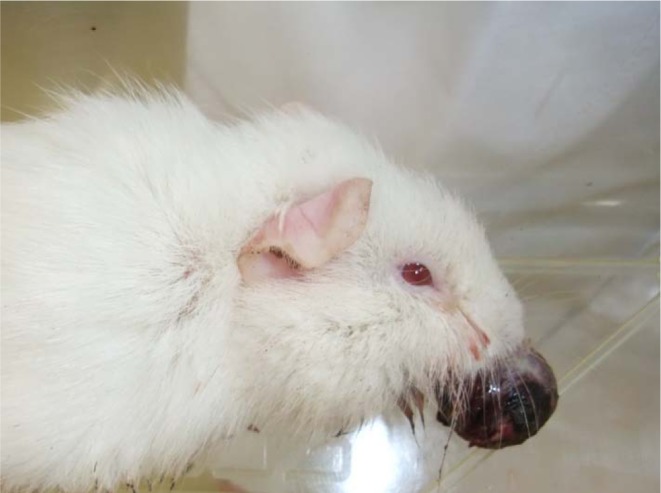
Gross appearance of the tumor. The tumor protruded from the oral cavity.
Tissue samples of the tumor were fixed in 10% neutral phosphate buffered formalin, decalcified in 5% formic acid solution and processed by routine methods. The samples were then embedded in paraffin, cut to 5 μm and stained with hematoxylin and eosin for histopathological examination. Undecalcified samples were stained with Congo red stain for amyloid, and calcified tissue was stained with Von Kossa stain. In addition, for immunohistochemical staining, endogenous peroxidase was blocked in deparaffinized sections by incubation in 3% H2O2 for 10 minutes. The primary antibodies used were cytokeratin AE1/AE3 (mouse monoclonal, 1:2500, Dako, Japan), vimentin V9 (mouse monoclonal, 1:2500, Dako, Japan) and amelogenin (rabbit polyclonal, 1:5000, Hokudo, Sapporo, Japan). All sections were incubated with these primary antibodies at 4°C overnight and then incubated with an EnVision system (EnVisionTM + System-HRP labelled polymer, Dako, Japan) for 30 minutes at room temperature. Visualization was performed with DAB, and the samples were counterstained lightly with hematoxylin. As a control section, the tooth germ tissue from a normal guinea pig fetus (gestational age was 45 to 49 days, SLC:Hartley, Japan SLC Inc.) was used.
Histologically, the tumor was not encapsulated and showed a biphasic growth pattern in areas of epithelial and mesenchymal components. The epithelium formed strands, nests and islands that were interspersed within the loose mesenchymal tissue resembling dental pulp (Fig. 2A). In epithelial components, cuboidal or columnar ameloblast-like cells were palisaded marginally, whereas central cells were connected loosely by long intercellular bridges that resembled the enamel organ. The nucleus was located at the apical pole of the palisaded cells. Although mitosis was often seen, cellular atypia was less dominant (Fig. 2B). Mesenchymal components consisted of immature fibrous tissues comprising poorly differentiated spindle or stellate cells. Furthermore, many mitotic figures, nuclear atypia and pleomorphism were seen (Fig. 2C). Focally, the invasion into the existing maxilla (Fig. 2D) and secondary reactive ossification were seen. Collagen production was not observed. In the margin of the tumor, odontoblast-like cells arranged themselves between the ameloblast and dental pulp. Dysplastic eosinophilic material with partial calcification formed on the odontoblast-like cells (Fig. 2E, arrow).
Fig. 2.
H&E, immunohistochemical and Von Kossa staining of the tumor. A: Biphasic growth pattern consisting of the epithelial and mesenchymal components. The epithelial components formed strands, nests and islands that were interspersed within the loose mesenchymal tissue resembling dental pulp. Bar= 1 mm. B: Ameloblast-like cells were palisaded marginally, whereas central cells were connected loosely by long intercellular bridges that resembled the enamel organ. Mitosis was often seen, but cellular atypia was less dominant. Bar= 50 μm. C: The mesenchymal components consisted of immature fibrous tissues comprising poorly differentiated spindle or stellate cells. Many mitotic figures, nuclear atypia and pleomorphism were seen. Bar= 50 μm. D: The mesenchymal components invaded into the existing maxilla. Bar= 100 μm. E: Odontoblast-like cells and dysplastic eosinophilic materials were partly calcified and arranged themselves between the ameloblast and dental pulp (arrow). Bar= 200 μm. F: Cytokeratin AE1/AE3 labeled the cytoplasm of the epithelial component. Bar= 100 μm. G: With vimentin antibody, the cytoplasm of the mesenchymal component and odontoblast-like cells was positive. Bar= 100 μm. H: Only the calcified part in eosinophilic materials was positive with Von Kossa stain. Bar= 100 μm.
Immunohistochemically, the epithelial component of the tumor was stained positive for cytokeratin AE1/AE3 (Fig. 2F). Also, the ameloblast and enamel organ in normal fetal teeth were positive.
For vimentin, the cytoplasm of the mesenchymal component and odontoblast-like cells was intensely positive (Fig. 2G). Also positive were the dental pulp and odontoblast in normal fetal teeth (Table 1). These results indicate that the epithelial components originated from the odontogenic epithelium and that the mesenchymal components originated from the odontogenic mesenchyme. The eosinophilic material was negative for Congo red staining. However, only the calcified part was positive with Kossa stain (Fig. 2H). In the normal fetal tooth, the dentin and enamel matrix were positive, whereas the predentin was negative. There was no labeling for amelogenin antibody on the eosinophilic material, but the enamel matrix in the normal fetal tooth was positive.
Table 1. Immunohistochemical Reactivities of Antigens in the Tumor of the Present Case and Tissues from a Normal Guinea Pig Fetus.

In mammals, tooth development is regulated by interactions between the epithelial and underlying mesenchymal tissues5.
The first morphological sign of tooth development is the formation of the dental lamina, a thickening of the oral epithelium. Subsequently, the dental lamina grows into the underlying mesenchyme of the first branchial arch, forming epithelial buds (bud stage). During this process, mesenchymal cells accumulate around the buds and form the dental papilla, which later gives rise to the dental pulp and dentin, secreting odontoblasts. After the bud stage, the epithelial compartment eventually gives rise to ameloblasts that deposit enamel6. Ameloblasts do not become secretory until odontoblasts lay down a thin layer of predentin and start to mineralize7.
Amelogenin, named by Eastoe (1965), is an enamel protein that contains high concentrations of proline, glutamine, leucine and histidine, which are synthesized and secreted from ameloblasts8. Amelogenin accounts for 90% of the enamel matrix constituents9.
In our study, the dysplastic eosinophilic material was negative for amelogenin and only partly positive for Kossa stain. These stain results suggest the possibility that the eosinophilic material was not an enamel matrix but predentin-like and dentin-like materials. However, we could not make a definitive conclusion because there was no specific result of staining indicative of predentin and dentin.
In the WHO histological classification of odontogenic tumors1, tumors of the odontogenic epithelium with an odontogenic mesenchyme are categorized into 5 types. In this classification, ameloblastic fibroma is defined as a tumor whose structure includes both the epithelial and mesenchymal components, whereas ameloblastic fibro-odontoma additionally contains deposition of the dentin matrix or enamel matrix.
In our study, the histological figures showed biphasic neoplasia in the odontogenic epithelium and odontogenic mesenchyme. However, dentinoid (hard tissue) formation was relatively scanty in the entire mass. Therefore, we diagnosed this tumor as an ameloblastic fibroma with dentinoid formation, ruling out ameloblastic fibro-odontoma.
There is one malignant case report of an odontogenic tumor in a dog that metastasized to multiple distal organs10. However, no distinction between benign and malignant tumor has been made in this classification.
According to the human histological classification11, odontogenic tumors are well subdivided into several groups based on the degree of odontogenesis and stage of dental hard tissue formation. Benign and malignant criteria are also included in the classification. If the epithelial component, odontogenic mesenchyme and dysplastic dentin are found, ameloblastic fibro-dentinoma (AFD) is diagnosed. Furthermore, ameloblastic fibro-dentinosarcoma (AFDS) is known as the malignant counterpart of AFD. Histologically, AFDS shows a benign epithelial component and a malignant mesenchymal component. The mesenchymal component displaying mitotically active cells surrounds the epithelial component. Additionally, an AFDS is a highly locally aggressive tumor. Based on these characteristics, this tumor may be diagnosed as AFDS, assuming that a specific result indicative of hard tissues is obtained.
In conclusion, on the basis of morphological and immunohistochemical characteristics, the tumor that developed in a guinea pig in our facility was diagnosed as an ameloblastic fibroma with dentinoid formation. To the best of our knowledge, ameloblastic fibroma is rare in rodents, and no study on ameloblastic fibroma in guinea pigs has been reported. Our study is the first case report of ameloblastic fibroma in guinea pigs. We hope that this report will contribute to the classification of this uncommon tumor in rodents.
Acknowledgments
We would like to thank Drs. Katsuhiko Yoshizawa, Susan Elmore, Dick Dubielzig and Cynthia Bell for their scientific advice. Also, we would like to show our appreciation to Mr. Yasutaka Hayami for his technical assistance.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (by-nc-nd) License <http://creativecommons.org/licenses/by-nc-nd/3.0/>.
References
- 1.Head KW, Cullen JM, Dubielzig RR, Else RW, Misdorp W, Patnaik AK, Tateyama S, and Van Der Gaag I. Histological classification of tumors of the alimentary system of domestic animals. Second Series. WHO Armed Forces Institute of Pathology, Washington D.C. 2003 [Google Scholar]
- 2.Head KW, Else RW, and Dubielzig RR. Tumors of the alimentary tract. In: Tumors in Domestic Animals, 4th ed. DJ Meuten (ed). Iowa State Press, Iowa. 401–481. 2003 [Google Scholar]
- 3.Klaus W. Induced and spontaneous lesions in teeth of laboratory animals. J Toxicol Pathol. 20: 203–213 2007 [Google Scholar]
- 4.Cullen JM, Ruebner BH, Hsieh DP, and Burkes EJ., Jr Odontogenic tumors in Fischer rats. J Oral Pathol. 16: 469–473 1987 [DOI] [PubMed] [Google Scholar]
- 5.Jernvall J, and Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev. 92: 19–29 2000 [DOI] [PubMed] [Google Scholar]
- 6.Peters H, and Balling R. Teeth: Where and how to make them. Trends Genet. 15: 59–65 1999 [DOI] [PubMed] [Google Scholar]
- 7.Salmela E, Sahlberg C, Alaluusua S, and Lukinmaa PL. Tributyltin impairs dentin mineralization and enamel formation in cultured mouse embryonic molar teeth. Toxicol Sci. 106: 214–222 2008 [DOI] [PubMed] [Google Scholar]
- 8.Yamada Y, Fuangtharnthip P, Tamura Y, Takagi Y, and Ohya K. Gene expression and immunolocalization of amelogenin in enamel hypoplasia induced by successive injections of bisphosphonate in rat incisors. Arch Oral Biol. 45: 207–215 2000 [DOI] [PubMed] [Google Scholar]
- 9.Bleicher F, Couble ML, Farges JC, Couble P, and Magloire H. Sequential expression of matrix protein genes in developing rat teeth. Matrix Biol. 18: 133–143 1999 [DOI] [PubMed] [Google Scholar]
- 10.Ueki H, Sumi A, Takaishi H, Ito H, Oyamada T, and Yoshikawa H. Malignant ameloblastic fibro-odontoma in a dog. Vet Pathol. 41: 183–185 2004 [DOI] [PubMed] [Google Scholar]
- 11.Barnes L, Eveson JW, Reichart P, and Sidransky D. World Health Organization classification of tumours. Pathology and genetic of head and neck tumours. IARC Press, Lyon. 2005 [Google Scholar]



