Abstract
Purpose.
To investigate the efficacy of a combination treatment composed of the cationic, membrane-penetrating peptide G2, and acyclovir (ACV) in both in vitro and ex vivo models of herpes simplex virus 1 (HSV-1) ocular infection.
Methods.
The antiviral activity of a combined G2 peptide and ACV therapy (G2-ACV) was assessed in various treatment models. Viral entry, spread, and plaque assays were performed in vitro to assess the prophylactic efficacy of G2, G2-ACV, and ACV treatments. In the ex vivo model of HSV-1 infection, the level of viral inhibition was also compared among the three treatment groups via Western blot analysis and immunohistochemistry. The potential change in expression of the target receptor for G2 was also assessed using immunohistochemistry and RT-PCR.
Results.
Statistically significant effects against HSV-1 infection were seen in all treatment groups in the viral entry, spread, and plaque assays. The greatest effects against HSV-1 infection in vitro were seen in the G2-ACV group. In the ex vivo model, statistically significant anti–HSV-1 effects were also noted in all control groups. At 24 hours, the greatest inhibitory effect against HSV-1 infection was seen in the ACV group. At 48 hours, however, the G2-ACV–treated group demonstrated the greatest antiviral activity. Syndecan-1, a target of G2, was found to be upregulated at 12-hours postinfection.
Conclusions.
This study shows that G2-ACV may be an effective antiviral against HSV-1 (KOS) strain when applied as single prophylactic applications with or without continuous doses postinfection.
Keywords: herpes simplex keratitis, heparan sulfate, acyclovir
In this study, we demonstrate an investigative therapy to control ocular herpes infection. A cationic peptide called G2 when combined with acyclovir generates better protective effects than G2 or acyclovir alone.
Introduction
Herpes simplex virus is an enveloped, double-stranded DNA virus and a member of Alphaherpesvirinae, a subfamily of Herpesviridae. Of the three members of this subfamily, which include herpes simplex virus 1 (HSV-1), herpes simplex virus 2 (HSV-2), and varicella zoster virus (VZV), HSV-1 has the greatest association with ocular infection.1 With approximately 8.4 to 13.2 new cases per 100,000 people per year, HSV-1 is actually the main cause of infectious blindness in developed countries.2 In the United States, approximately 500,000 individuals are afflicted with HSV ocular infection, with treatment costs rising to US$ 17.7 million annually for initial onset and recurring cases.2,3 Ocular manifestations of HSV-1 include iridocyclitis, acute retinal necrosis, conjunctivitis, and keratitis.1
Current mainstay treatment options against ocular HSV-1 infection include a dual regimen consisting of topical antivirals and topical corticosteroids.4 The nucleoside analog trifluridine (TFT) can also be used as treatment against ocular HSV-1 infections. However, none of these current treatments are without limitations. Corticosteroids, for instance, in addition to suppressing the immune system, can lead to ocular complications such as steroid-induced glaucoma and cataracts. Trifluridine is known to pose toxicity at high doses and prolonged use.5,6 Additionally, topical antivirals such as acyclovir (ACV) can inhibit viral replication, but cannot always preclude viral latency. Thus, although effective, the shortcomings of current treatment options against ocular HSV-1 infection highlight the potential for new therapies to succeed in areas where the previous generation of medications has not.
Prior work in our laboratory has yielded two 12-mer peptides with antiviral activity against HSV-1. The mechanism of action of these peptides rely on binding specifically to heparan sulfate (HS) and a modified form of HS, 3-O-sulfated HS (3-OS HS), both of which serve as entry receptors for HSV-1 on many different cell lines, including human corneal epithelial (HCE) cells. The peptides G1 (LRSRTKIIRIRH) and G2 (MPRRRRIRRRQK) both have high positive charge densities, and specific arginine and lysine residues are necessary to inhibit virus-cell binding and virus-induced membrane fusion.7 In addition to inhibitory effects on HSV-1 entry into primary cultures of human corneal fibroblasts, both peptides have been demonstrated to effectively serve as prophylactic eye drops in an in vivo murine corneal model.7 In both the cell culture and live animal models, these peptides have also been shown to have low cytotoxicity at the applicable concentrations.7
Studies in other laboratories have also demonstrated the antiviral potential of highly cationic peptides. Jose et al.8 have shown the efficacy of a cationic peptide, TAT-CD0, which is able to inhibit HSV-1 ocular infection in vivo, and Brandt et al.9 have shown the antiviral efficacy of another highly cationic molecule, a modified theta defensin, RC-2, in a murine model of HSV-1 keratitis. With our own peptide work, we suggested that coupling an agent that inhibits viral entry with a nucleoside analogue, such as ACV, that inhibits viral replication, would target the viral infection in two distinct ways. The proposed hypothesis is that combining our G2 peptide and ACV into one combination therapy, G2-ACV, would offer more effective viral inhibition both at the cell surface for entry and cell nucleus for replication.
In our report, we show the efficacy of our G2-ACV combined therapy in both in vitro and ex vivo studies. In all cases, HSV-1 infection was significantly reduced. In the ex vivo model, prophylactic administration of G2-ACV reduced the disease at all time points indicated. The results suggest that a G2-ACV combination therapy has significant antiviral activity both in vitro and ex vivo, and warrants further investigation.
Materials and Methods
Peptide Synthesis
Selection of phages against 3-OS HS was done as described previously.7 G2 (MPRRRRIRRRQK) was synthesized at the University of Illinois at Chicago Research Resources Center with percent purity exceeding 99%, as verified by high performance liquid chromatography. The correct mass was confirmed by mass spectrometry.
Cells, Viruses, and Treatment Preparations
The African green monkey kidney (Vero) cell line was obtained (Patricia G. Spear, Northwestern University, Chicago, IL) and cultured as previously described.10 The HCE cell line (RCB1834 HCE-T) was obtained from Kozaburo Hayashi (National Eye Institute, Bethesda, MD)11 and was cultured as previously described.12
The viruses used in the study—HSV-1 (KOS), HSV-1 (KOS) gL86, and HSV-1 (KOS) GFP—were also provided by Patricia G. Spear.10 All virus stocks were kept at a low multiplicity of infection (MOI), propagated in Vero cell lines, and stored at −80°C. Titers for all three viruses were determined using Vero cells.
The synthetic peptides were stored at −20°C and resuspended in PBS supplemented with glucose and calf serum (PBS-GCS) at pH 8.4 for use. G2 was used at a concentration of 0.5 mM, which has previously been shown to be effective with low toxicity.7 Acyclovir was used as 3% in solution.
Viral Entry Assay
Viral entry assays were performed using the HCE cell line. Cells were plated on a 96-well tissue culture dish at a density of 2 × 104 cells per well. Upon confluency, cells were treated with G2, G2-ACV, ACV, or mock treatment for 30 minutes at 37°C. Cells were then infected with serial dilutions of β-galactosidase expressing HSV-1 (KOS) gL86 virus at a maximum MOI of 10 for 6 hours at 37°C. After 6 hours of infection, cells were washed with PBS and then incubated with soluble substrate o-nitrophenyl-β-D-galactopyranoside (ImmunoPure ONPG; Pierce, Rockford, IL), which allowed for quantification of β-galactosidase expressing HSV-1 (KOS) gL86 viral entry into each of the cell types. Enzymatic activity was measured by a microplate reader (TECAN GENios Pro; Tecan Systems, Inc., San Jose, CA) at 405 nm.
Virus Spread Assay
A virus spread assay was performed as described previously.13 Briefly, a monolayer of HCE cells were treated with G2, G2-ACV, ACV, or mock treatment, and challenged with HSV-1(KOS) K26 GFP virus strain at a MOI of 0.001. After 2-hours postinfection, the inoculum was removed. Cells were washed once with PBS and overlaid with 1% methylcellulose (Sigma-Aldrich, St. Louis, MO) in Minimum Essential Media (MEM). Seventy-two–hours postinfection the spread of HSV-1(KOS) K26 GFP amongst HCE cells was assessed by capturing images of the green virally infected cell clusters using a 10× objective fluorescent microscope (Zeiss Axiovert 200; Carl Zeiss Microscopy, LLC, Thornwood, NY).
Plaque Assay
Viral entry and replication were also confirmed using a plaque assay. Monolayers of HCE cells were plated on a 24-well tissue culture dish. Cells were treated with G2, G2-ACV, ACV, or mock treated for 30 minutes at 37°C. Cells were infected at 0.0001 MOI with HSV-1 (KOS) virus or mock infected for 2 hours. After primary incubation, the viral or mock solution was replaced with MEM with low glucose with 10% fetal bovine serum (FBS) and penicillin/streptomycin additives with 1% methylcellulose to prevent secondary plaque formation. Cells were then incubated at 37°C for 72 hours. At the end of the incubation, cells were then fixed using 100% methanol for 20 minute at room temperature and subsequently stained with crystal violet. Plaques were counted and imaged at 10× objective (Zeiss Axiovert 200; Carl Zeiss Microscopy, LLC).
Organotypic Cornea Culture, Treatment, and Viral Infection
Corneal tissues were obtained from donated pig eyes (Park Packing, Inc., Chicago, IL) and incubated in MEM with low glucose supplemented with 5% antibiotic-antimycotic and 1% insulin-transferrin-sodium-selenite (Sigma-Aldrich) in a method as previously described.14 Following 1 hour of disinfection in the culture medium, sclerocorneal discs were prescarified on the corneal epithelium using a 19-gauge needle. Tissues were then inverted, epithelial side down, and filled with 2% agarose solution of the culture medium to function as a support scaffold. Tissues were then treated with either mock treatment (PBS-GCS) alone, G2, G2-ACV, or ACV for 30 minutes at 37°C. Following removal of treatment solutions, corneal tissues were then infected topically with HSV-1 (KOS) at 105 plaque forming units (PFU) for 2 hours. Tissues were then washed in 2× PBS and cultured in medium with an air–liquid interface for 24 and 48 hours. Following the initial prophylactic dose, all treatments were applied topically to the corneal tissues every 5 hours.
For assessing changes in expression of syndecan-1, no treatment was applied. Instead, cultured corneas were infected topically with HSV-1 at 105 PFU and infected for up to 12 hours.
Immunohistochemistry
Scleral rims were cut using scissors and remaining corneal tissues were embedded in optimal cutting temperature (OCT) compound (Sakura Finetek USA, Torrance, CA) and snap frozen in liquid nitrogen and stored at −80°C. Following microtome sectioning, frozen tissues at a thickness of 5 μm were fixed in acetone followed by quenching of endogenous peroxidase with 0.3% H2O2. Following 1-hour blocking with 10% normal goat serum, tissues were incubated with rabbit polyclonal anti–HSV-1 antibodies at a dilution of 1:100 (Dako, Carpenteria, CA) overnight at 4°C. Probes against primary antibody were applied (MACH3 Rabbit AP-Polymer Detection; Biocare Medical, Concord, CA) and staining detected using 3,3′-diaminobenzidine tetrahydrochloride (DAB) chromagen with hematoxylin counterstain. Images were obtained using light microscopy at 10× objective (Zeiss Axiovert 200; Carl Zeiss Microscopy, LLC). Staining without primary antibody was used as a negative control. For assessing syndecan-1 expression, an anti–syndecan-1 antibody (catalog # ab60199; Abcam, Cambridge, MA) was used as the primary antibody. A semiquantitative scoring system, 0 to III (0: no staining, I: mild staining, II: moderate staining, III: intense staining), was implemented to assess differences in staining among the different treatment groups.
Western Blot Analysis
Proteins from infected corneas were extracted in Radio-Immunoprecipitation Assay (RIPA) buffer (Sigma-Aldrich) with protease and phosphatase inhibitor and incubated on ice for 1 hour. Lysates were centrifuged at 13,000 rpm for 20 minutes at 4°C and the supernatants were collected. Protein concentrations were determined using spectrophotometer and equal amounts were mixed with sample buffer, denatured by heating at 85°C, and subjected to gel electrophoresis. Following transfer to nitrocellulose membrane and 1-hour blocking at room temperature, the membranes were incubated with mouse monoclonal anti-HSV1 ICP0 antibody at a dilution of 1:4000 (Abcam) at 4°C overnight. The membranes were washed and then incubated with horseradish peroxidase-conjugated anti-mouse IgG antibody at a dilution of 1:2000 (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 hour at room temperature. Protein bands were visualized using ImageQuant LAS 4000 imager (GE Healthcare Life Sciences, Pittsburgh, PA) after the addition of SuperSignal West Femto maximum sensitivity substrate (Pierce). The densities of the bands were quantified using ImageQuant TL image analysis software (GE Healthcare Life Sciences, Pittsburgh, PA). Beta-actin was measured as a loading control.
Real-Time PCR
To examine the gene expression by quantitative RT–PCR, 10 μg of the RNA samples from HCE cells were converted to first strand cDNAs using the High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Foster City, CA) following the manufacturer's protocol. The cDNA was added into the Platinum SYBR Green qPCR SuperMix-UDG Master Mixes (Invitrogen, Carlsbad, CA) containing SYBR Green I fluorescent dye and Taq DNA polymerase. The mixtures were aliquoted into 96-well plate containing gene specific primer sets. The qRT–PCR reactions were performed using ABI Prism 7500 system (Applied Biosystems) with the following parameters: repeat 1 (1 cycle), 2 minutes at 50°C; 2 minutes at 95°C and repeat 2 (40 cycles), 15 seconds at 95°C, and followed by 30 seconds at 60°C, and the data were collected at each 60°C.
Statistical Analysis
Graph Pad Prism software (version 4.0; GraphPad Software, Inc., La Jolla, CA) was used for statistical analysis of each group. All data are expressed as means ± SD. Statistical comparison between G2, G2-ACV, or ACV, and mock treated groups was performed by Student's t-test. P values less than 0.05 were considered as the significant differences among mock treated and treated groups.
Results
Effect of G2, G2-ACV, ACV Treatment on HSV-1 Entry and Replication in HCE Cells
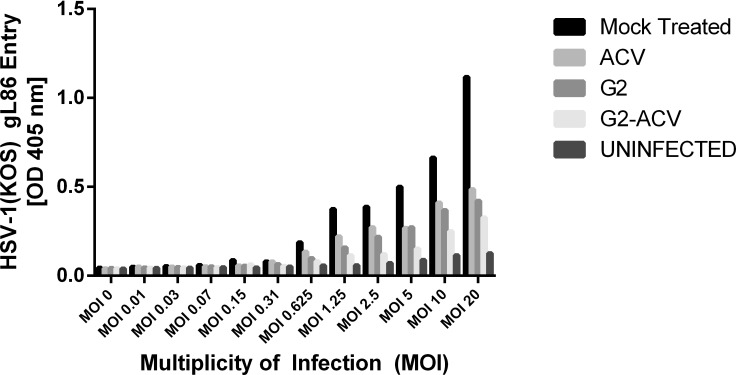
Herpes simplex virus type 1 entry into HCE cells was reduced in all treatment groups compared with that seen in the mock treated group (Fig. 1). These differences were statistically significant and represented almost a 3-fold decrease in viral entry at the highest MOIs. The greatest antiviral activity was noted in the presence of G2-ACV. Viral entry was also found to be decreased to a greater extent in the G2 group when compared with the ACV group. However, there was no statistically significant difference in the level of entry inhibition observed among the G2-, G2-ACV–, and ACV-treated groups.
Figure 1.
Effect of G2, G2-ACV, ACV, and mock treatments on HSV-1 cell entry. Cultured HCE cells were plated in 96-well tissue culture dishes and, upon confluency, were treated with G2, G2-ACV, ACV, or mock treatment for 30 minutes at 37°C. Cells were then infected with serial dilutions of β-galactosidase expressing HSV-1 (KOS) gL86 virus at a maximum MOI of 10 for 6 hours at 37°C. After 6 hours of infection, cells were washed with PBS and then incubated with ONPG substrate. Viral entry was measured using a microplate reader, which measured β-galactosidase activity at an optical density of 405 nm. Values in the figure were plotted as the means of two independent experiments.
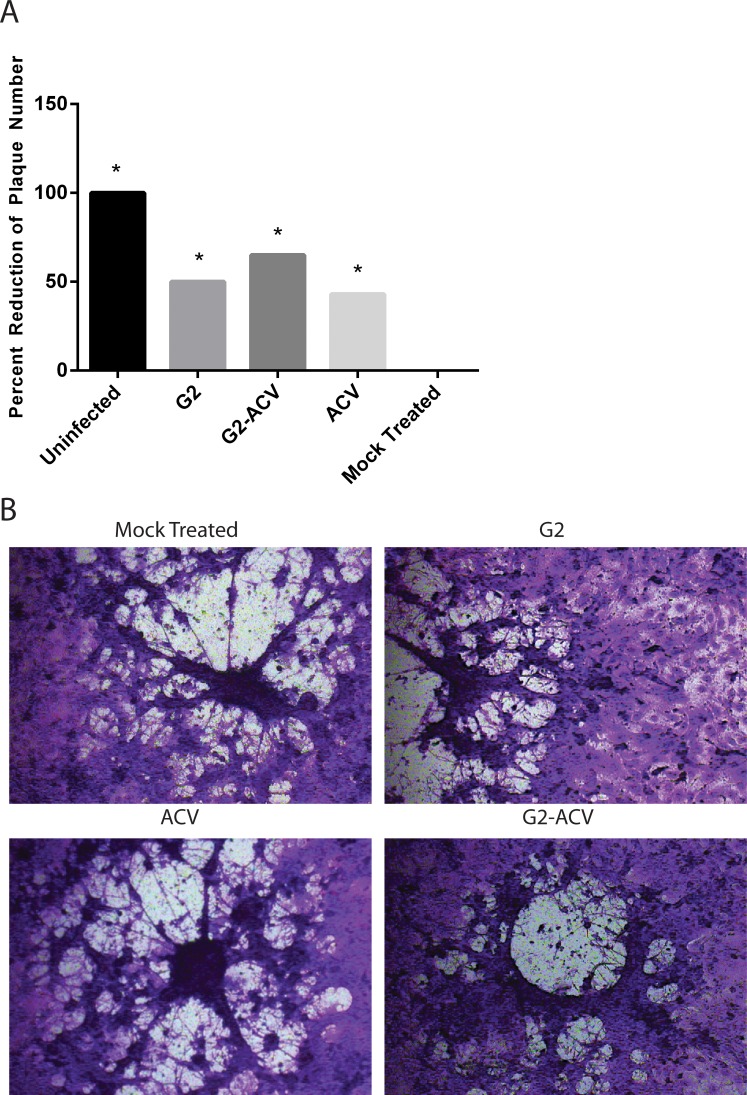
After establishing the effects of the different treatment groups on viral entry, we used a viral plaque assay to assess the ability of HSV-1 successfully replicate within the cells and induce cell-to-cell spread. The percent reduction of plaque formation was statistically significant in all treatment groups when compared with the mock treated condition (Fig. 2A). This percent reduction in plaque number is highest in the G2-ACV–treated group. This is followed by decreased reductions in the G2-treated group, followed by that in the ACV group. Image confirmation of plaque formation after 72-hours postinfection is shown in Figure 2B.
Figure 2.
Effect of G2, G2-ACV, ACV, or mock treatments on HSV-1 replication and cell-to-cell spread. Confluent monolayers of HCE cells were treated with G2, G2-ACV, ACV, or mock treatment for 30 minutes at 37°C, and subsequently infected at 0.0001 MOI with HSV-1 (KOS) virus or mock infected for 2 hours. Inoculums were harvested, fixed, and stained at 72-hours postinfection. Percent reduction of plaque formation is shown in (A). Data represents the mean of two independent experiments (±SD). Asterisks indicate a statistically significant difference compared to mock treatment. Images of plaque formation are shown in (B).
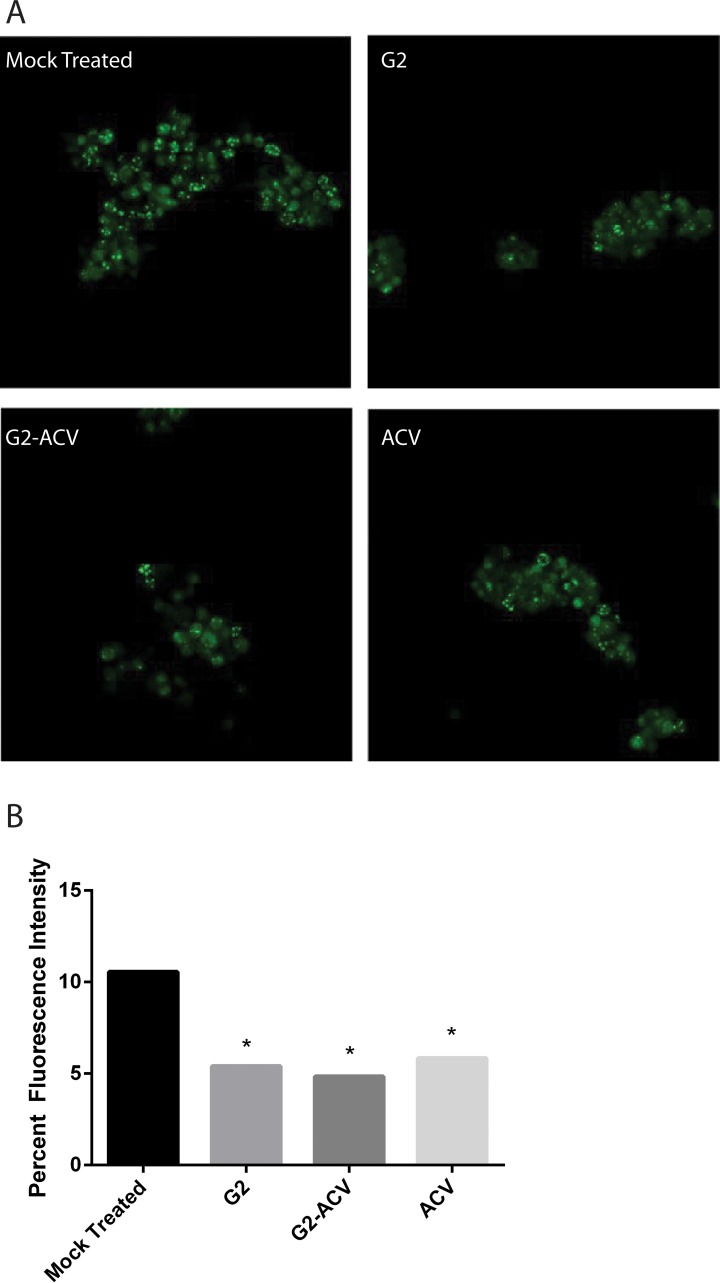
The ability of HSV-1 to replicate within HCE cells was further assessed through detection of infection clusters with HSV-1 (KOS) GFP. Infection clusters are able to highlight the actual presence of a fluorescently labeled HSV-1 virion particle within the cell. As shown in Figure 3A, the fluorescence intensity of infectious clusters is highest in the mock-treated group, with statistically significant reductions in fluorescence intensity seen in the ACV, G2, G2-ACV group, in that order. Representative confocal images of fluorescently labeled virus clusters are shown in Figure 3B.
Figure 3.
G2, G2-ACV, and ACV reduce infectious cell cluster formation. Herpes simplex virus type 1 (KOS) GFP was used to determine the effect of various treatments on viral replication and cell-to-cell spread. (A) Cellular expression of GFP and its distribution mark the ability of HSV-1 to form clusters of infected cells under various treatment conditions as indicated. (B) Relative sizes of infected cell cultures were determined by measurement of clusters under a fluorescent microscope. Asterisks indicate a statistically significant difference compared to mock treatment. Images are representative of two independent experiments.
Effect of G2, G2-ACV, ACV Treatment in an Ex Vivo Model of HSV-1 Ocular Infection
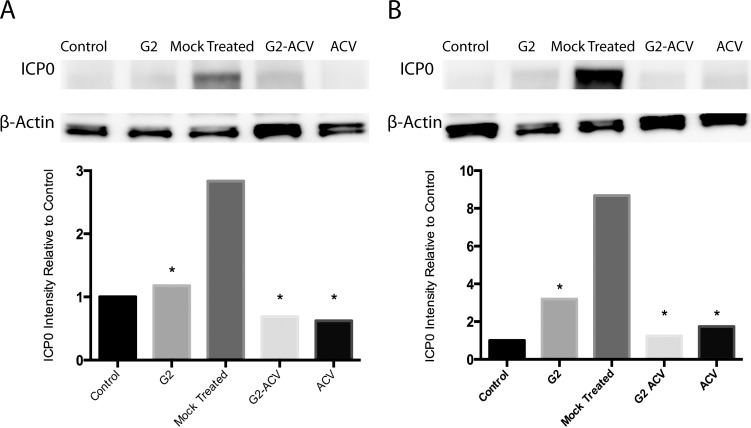
To study the effects of G2, G2-ACV, and ACV treatment in tissue, we utilized an ex vivo organotypic porcine corneal model. Ex vivo corneal models have previously served as practical, cost-effective methods to study corneal viral infection. Advantages include the ability to study corneal infection in the absence of a host immune system and other nonlocal factors. Organotypic cornea cultures have also previously been employed to study a multitude of corneal diseases, including HSV-1 infection.15,16 After HSV-1 entry, the immediate early protein ICP0 plays an important role in both lytic and latent infection,17 and, thus, can provide an alternative mechanism to verify HSV-1 internalization. As shown in the top panel of Figure 4A, Western blot detection of HSV-1 ICP0 at 24 hours indicated decreased presence of this viral protein in all of the treatment groups. Similar results are seen at 48-hours postinfection (Fig. 4B, top panel). Densitometry analysis of the protein bands showed that the decreased detection of HSV-1 ICP0 is statistically significant at both 24- and 48-hours postinfection when comparing any of the treatment to the mock-treated groups (Figs. 4A, 4B, bottom panels). At 24-hours postinfection, it appears that ACV is the most effective, although the difference between this group and G2-ACV is not statistically significant. At 48-hours postinfection, G2-ACV appears to have the greatest antiviral effect, but yet again, the difference between this group and ACV is not statistically significant.
Figure 4.
Effect of G2, G2-ACV, ACV, or mock treatment on HSV-1 internalization. Western blot analysis of ICP0 expression was performed to determine the effect of various treatment conditions on HSV-1 internalization. As indicated, ICP0 protein expression was determined for G2-, G2-ACV–, ACV-, or mock-treated corneal tissues infected with HSV-1(KOS). The lysates were prepared at (A) 24- and (B) 48-hours postinfection and Western blots were performed. ICP0 expression and relative protein intensity relative to control conditions are shown. βeta-actin was measured as a loading control. Results are representative of multiple independent experiments. Asterisk indicates statistical significance when compared with the mock treatment group.
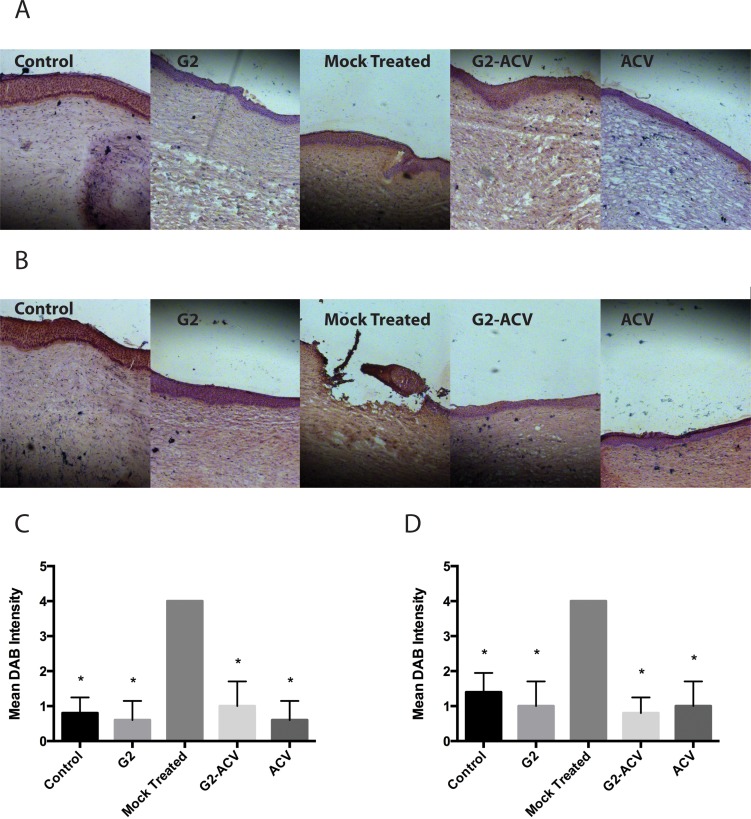
Corresponding immunohistochemistry images of frozen corneal tissue sections show similar increases of HSV-1 viral antigens in the case of the untreated group at both 24 and 48 hours (Figs. 5A, 5B). In the G2-, G2-ACV–, and ACV-treated groups, there appears to be decreased detection of DAB staining in both the corneal epithelium and stroma. In addition, at 48-hours postinfection, there is marked DAB staining at an area of probable corneal ulceration. There were no significant differences noted by the semiquantitative scoring system among the different treatment groups (Figs. 5C, 5D).
Figure 5.
Frozen corneal tissue sections at 24 hours (A) and 48 hours (B) were incubated with anti–HSV-1 antibody, stained with DAB, and assessed via a seminquantitative scoring system. Scores are plotted for both 24 (C) and 48 (D) hours. Asterisks indicate a statistically significant difference compared to mock treatment.
Effect of HSV-1 Infection on the Expression of a Heparan Sulfate Proteoglycan
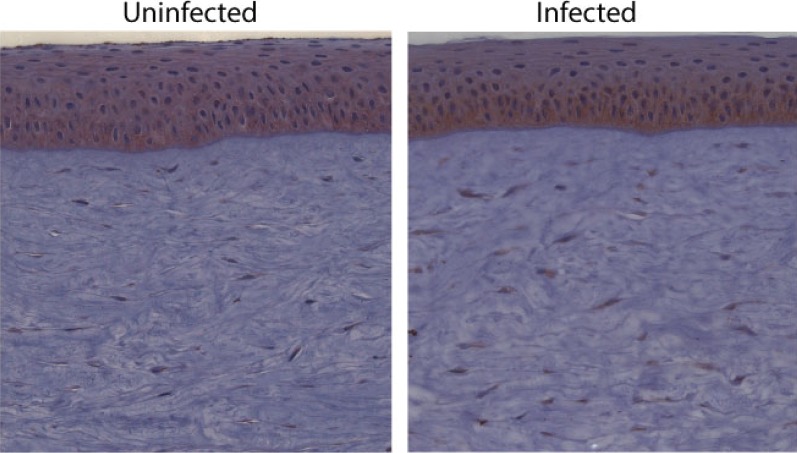
Since our G2 peptide is known to bind to modified forms of HS, we next decided to see if there is any change in the expression of any HS proteoglycans in HSV-1 infected corneal tissues to see whether treatments consisting of G2 might have varied efficacy at different times postinfection. In this case, we looked at the expression of syndecan-1, a HS proteoglycan, in the corneal epithelium. Syndecan-1 has been implicated in HSV-1 entry into various cell types.18 As shown in Figure 6, the uninfected cornea showed that syndecan-1 is expressed abundantly in the corneal epithelium, with some accentuation in the basal cells. There was also some staining of cells in the stroma. The infected section also demonstrated staining in the epithelium, but there appeared to be an increase in the basal layers. Interestingly, there was also greater expression in stromal cells.
Figure 6.
Frozen corneal tissue sections, either uninfected or at 12-hours postinfection with HSV-1, were incubated with anti–syndecan-1 antibody, stained with DAB, and counted in a blind fashion.
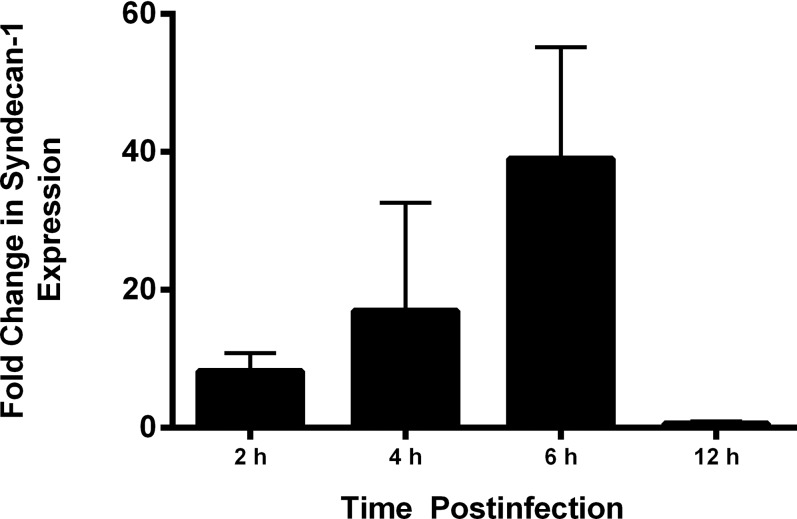
We also used RT-PCR analysis to assess for any changes in syndecan-1 expression. As shown in Figure 7, studies performed in porcine corneal tissue suggested a rise in expression of syndecan-1, with a peak increase occurring at 6-hours postinfection that is roughly 40-fold.
Figure 7.
Reverse transcription-PCR analysis for changes in syndecan-1 expression. Total RNA was isolated from porcine corneal tissues at the various time points indicated and converted to cDNA and analyzed by PCR for expression of syndecan-1. Densitometry analysis was performed using the National Institutes of Health (NIH) ImageJ software (Version 1.43; NIH) and data for relative intensity was plotted.
Discussion
Despite the existing high prevalence and incidence rates of HSV-1 ocular infection worldwide, there has been a lack of major advancements in anti–HSV-1 therapy since the development of ACV in the 1980s.19 Among the leading reasons highlighting the need for new therapeutic developments against HSV-1 include the rising resistance to this virus in specific populations. This resistance has been shown to occur via mutations in sequences in the viral genome that encode for either DNA polymerase or thymidine kinase.20–22 Although in the general population the incidence rate of HSV-1 resistance to ACV is relatively low, only 0.1 to 0.7%, resistance rates in immunocompromised populations increases significantly to a range of 4% to 14%. In those individuals with herpetic keratitis, the resistance rate of HSV-1 to ACV is 6.4%.23 An additional concern with current therapies is viral latency. In clinical situations, viral replication inhibitors such as ACV are only able to exert their actions after the virus has infected the host cell. This renders drugs such as ACV with the ability to only suppress recurrent HSV-1 infections, not to fully eradicate the virus.
We have previously shown that prophylactic application of the membrane-penetrating peptide G2 in an in vivo murine corneal model can effectively inhibit HSV-1 ocular infection at effective concentrations that demonstrate low toxicity.7 The ability of G2 to exhibit antiviral activity in cell culture at nontoxic concentrations has also been previously demonstrated.7 Here, we demonstrate a potential use for this peptide in combination therapies in conjunction with ACV. In all treatment scenarios, a G2-ACV combination therapy was able to significantly inhibit HSV-1 activity. Although there were no significant differences among G2, G2-ACV, and ACV in terms of antiviral activity, G2-ACV was found to have the greatest antiviral effect in all cell culture studies in this report. A couple points should be noted regarding the implications of the cell culture data. First, while ACV alone would not be expected to have any effect on blocking HSV-1 entry, our surrogate entry assay requires both viral cell entry and the initial viral replication in order for the encoded β-galactosidase to show strong signals. Since the assay takes up to 8 hours to complete, the loss of entry signal occurs as the initial viral replication is inhibited by ACV. This could explain the decreased β-galactosidase detection in the ACV only group. Second, although both application of G2 and ACV prophylactically inhibited plaque formation, the mechanism of each compound differs. While the loss of plaque formation in the presence of G2 is due to its inhibitory effect on virion particles from entering the cell, the reduced plaque formation in the ACV-pretreated group is due to inability of the entered virion particles to subsequently undergo replication.
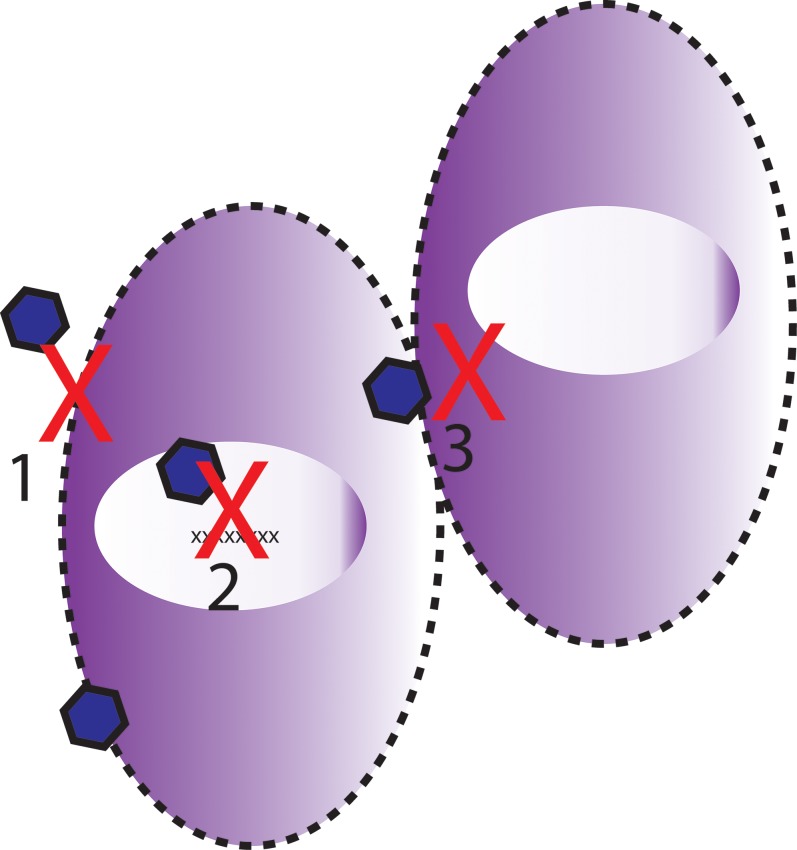
Examining the mechanism of action of each drug, then, suggests a potential synergistic role for a G2-ACV combined therapy (Fig. 8). While G2 prevents the binding of HSV-1 glycoproteins to the host cell membrane and effectively reduces viral-cell fusion, ACV works in the nucleus of the host cell. In the absence of 100% efficacy by G2, some HSV-1 virion particles will invariably enter the host cell and proceed to the nucleus to undergo viral replication. With the addition of G2, our hypothesis is that the viral burden on ACV would be decreased. We should note, however, that while the individual antiviral effects of G2 and ACV alone each represent approximately 50% of the control group, the combined efficacy is not a pure additive net effect. Ideally, we hypothesized that since G2 and ACV work at different steps of the viral infection, the net effect of a combined therapy should be additive. However, in reality, the inhibitory concentration to block 50% of infection (IC50) requires significantly less amount of an inhibitor than blocking 100% of the same infection, which may be the reason why we do not see 100% inhibition upon combining the two inhibitors at the original concentrations used.
Figure 8.
Schematic depicting proposed mechanism of action of G2-ACV. Both entry and viral replication steps are inhibited. G2 is proposed to inhibit both initial viral cell entry (1) and also viral cell-to-cell spread (3). Acyclovir is known to inhibit viral replication within the host cell nucleus (2).
Additionally, G2 is also a membrane-penetrating peptide. Previous work in our laboratory has demonstrated, through the use of a FITC-conjugated G2 peptide, the ability of G2 to bind to the host cell membrane and form pores in this membrane as the peptide-receptor complex is internalized into the cell (data not shown). With the pore formation and membrane penetration, G2 in the presence of ACV, may allow for better cell internalization by ACV. The possible delivery of this peptide to the nucleus upon internalization may also be a likely possibility.24 Taking this observation into account, additional studies are needed to further investigate the optimal conditions under which both drugs can be introduced simultaneously to the affected tissues. Some possibilities would include possible fusion of the two molecules, a technique that may more efficiently deliver ACV to the cell nucleus and render the combination more effective.
In the tissue model of HSV-1 infection, we found similar antiviral results among the different treatment groups. Although ACV was the most effective of the treatment groups at 24-hours postinfection, G2-ACV demonstrated the greatest antiviral effect at 48-hours postinfection. This latter difference was not statistically significant, but the observation might suggest that the initial entry inhibition provided by G2 against HSV-1 might decrease the viral burden on ACV. Although the difference in ICP0 expression between the G2-ACV– and ACV-treatment groups at 48 hours was not statistically significant, it cannot exclude possible effects on the clinical outcome of disease these differences might have. Additionally, differences in the efficacy between in vitro and ex vivo treatment models may point to the need for optimization of the delivery vehicle for our drugs of interest. To date, we have only tested G2 and G2-ACV in a PBS-based solution medium.
Finally, we looked at changes in expression of HS in cases of HSV-1 infection. The rationale behind this was to assess whether G2 peptide, either individually or combined with ACV, would have more target receptors as the viral infection progressed. Since HS is a viral entry receptor, we hypothesized that a viral infection may induce mediators to upregulate HS expression, possibly to promote viral entry. The increased expression of HS noted at a peak of 12-hours postinfection may be suggestive of a possible local mediator phenomenon, which results in the upregulation of this HS proteoglycan. The RT-PCR data is also an indication that there is likely an increase in syndecan-1 transcription that is signaled by HSV-1, and supports our immunohistochemical data demonstrating increased expression at 12-hours postinfection. The fact that there is an increase in the expression of HS supports our data in this study that therapies involving G2 continue to have antiviral effects at least up to 48-hours postinfection. In fact, it seems as the viral infection progresses, virally infected cells would be more susceptible to succumbing to the antiviral properties of a peptide like G2, whose target receptor is increased under these conditions. Although in this study there was one initial prophylactic dose, this trend may explain why the postinfection doses involving G2 are effective. It is also a promising sign for potential uses of our peptide, whether alone or combined with ACV, in pure postinfection models.
In summary, the data presented in this study support our hypothesis that a combination therapy involving an entry inhibitor, such as G2, and a viral nucleotide analogue, such as ACV, can demonstrate antiviral activity that is greater than or on par to that with ACV alone. The fact that these treatments involved a prophylactic dose, however, decreases the current potential clinical utility of G2-ACV in terms of countering an initial, primary infection. However, this may prove suitable for recurrent infections and this preliminary data warrants further investigation.
Acknowledgments
Supported by National Institutes of Health Grants AI057860, AI081869 (both to DS), EY01792 (core grant), and a seed grant from the Illinois Society for the Prevention of Blindness and a Fight for Sight Summer Student Fellowship (both to PJP).
Disclosure: P.J. Park, None; T.E. Antoine, None; A.V. Farooq, None; T. Valyi-Nagy, None; D. Shukla, None
References
- 1. Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012; 57: 448–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liesegang TJ, Melton LJ, 3rd,, Daly PJ, Ilstrup DM. Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989; 107: 1155–1159 [DOI] [PubMed] [Google Scholar]
- 3. Lairson DR, Begley CE, Reynolds TF, Wilhelmus KR. Prevention of herpes simplex virus eye disease: a cost-effectiveness analysis. Arch Ophthalmol. 2003; 121: 108–112 [DOI] [PubMed] [Google Scholar]
- 4. Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001; 20: 1–13 [DOI] [PubMed] [Google Scholar]
- 5. Imperia PS, Lazarus HM, Dunkel EC, Pavan-Langston D, Geary PA, Lass JH. An in vitro study of ophthalmic antiviral agent toxicity on rabbit corneal epithelium. Antiviral Res. 1988; 9: 263–272 [DOI] [PubMed] [Google Scholar]
- 6. Lass JH, Langston RH, Foster CS, Pavan-Langston D. Antiviral medications and corneal wound healing. Antiviral Res. 1984; 4: 143–157 [DOI] [PubMed] [Google Scholar]
- 7. Tiwari V, Liu J, Valyi-Nagy T, Shukla D. Anti-heparan sulfate peptides that block herpes simplex virus infection in vivo. J Biol Chem. 2011; 286: 25406–25415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jose GG, Larsen IV, Gauger J, et al. A cationic peptide, TAT-Cd0, inhibits herpes simplex virus type 1 ocular infection in vivo. Invest Ophthalmol Vis Sci. 2013; 54: 1070–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brandt CR, Akkarawongsa R, Altmann S, et al. Evaluation of a theta-defensin in a Murine model of herpes simplex virus type 1 keratitis. Invest Ophthalmol Vis Sci. 2007; 48: 5118–5124 [DOI] [PubMed] [Google Scholar]
- 10. Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996; 87: 427–436 [DOI] [PubMed] [Google Scholar]
- 11. Araki-Sasaki K, Ohashi Y, Sasabe T, et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995; 36: 614–621 [PubMed] [Google Scholar]
- 12. Shah A, Farooq AV, Tiwari V, Kim MJ, Shukla D. HSV-1 infection of human corneal epithelial cells: receptor-mediated entry and trends of re-infection. Mol Vis. 2010; 16: 2476–2486 [PMC free article] [PubMed] [Google Scholar]
- 13. Roller RJ, Herold BC. Characterization of a BHK(TK-) cell clone resistant to postattachment entry by herpes siplex virus types 1 and 2. J Virol. 1997; 71: 5805–5813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kabosova A, Kramerov AA, Aoki AM, Murphy G, Zieske JD, Ljubimov AV. Human diabetic corneas preserve wound healing, basement membrane, integrin and MMP-10 differences from normal corneas in organ culture. Exp Eye Res. 2003; 77: 211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alekseev O, Tran AH, Azizkhan-Clifford J. Ex vivo organotypic corneal model of acute epithelial herpes simplex virus type 1 infection. J Vis Exp. 2012; 69: e3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xiao J, Natarajan K, Rajala MS, Astley RA, Ramadan RT, Chodosh J. Vitronectin: a possible determinant of adenovirus type 19 tropism for human corneal epithelium. Am J Ophthalmol. 2005; 140: 363–369 [DOI] [PubMed] [Google Scholar]
- 17. Boutell C, Everett RD. Regulation of alphaherpesvirus infections by the ICP0 family of proteins. J Gen Virol. 2013; 94: 465–481 [DOI] [PubMed] [Google Scholar]
- 18. Karasneh GA, Ali M, Shukla D. An important role for syndecan-1 in herpes simplex virus type-1 induced cell-to-cell fusion and virus spread. PLoS One. 2011; 6: e25252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilson SS, Fakioglu E, Herold BC. Novel approaches in fighting herpes simplex virus infections. Expert Rev Anti Infect Ther. 2009; 7: 559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boyd MR, Gustafson KR, McMahon JB, et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother. 1997; 41: 1521–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frobert E, Ooka T, Cortay JC, Lina B, Thouvenot D, Morfin F. Herpes simplex virus thymidine kinase mutations associated with resistance to acyclovir: a site-directed mutagenesis study. Antimicrob Agents Chemother. 2005; 49: 1055–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frobert E, Ooka T, Cortay JC, Lina B, Thouvenot D, Morfin F. Resistance of herpes simplex virus type 1 to acyclovir: thymidine kinase gene mutagenesis study. Antiviral Res. 2007; 73: 147–150 [DOI] [PubMed] [Google Scholar]
- 23. Bacon TH, Levin MJ, Leary JJ, Sarisky RT, Sutton D. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin Microbiol Rev. 2003; 16: 114–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. WuDunn D, Spear PG. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989; 63: 52–58 [DOI] [PMC free article] [PubMed] [Google Scholar]