Abstract
The corneal epithelial basement membrane (BM) is positioned between basal epithelial cells and the stroma. This highly specialized extracellular matrix functions not only to anchor epithelial cells to the stroma and provide scaffolding during embryonic development but also during migration, differentiation, and maintenance of the differentiated epithelial phenotype. Basement membranes are composed of a diverse assemblage of extracellular molecules, some of which are likely specific to the tissue where they function; but in general they are composed of four primary components—collagens, laminins, heparan sulfate proteoglycans, and nidogens—in addition to other components such as thrombospondin-1, matrilin-2, and matrilin-4 and even fibronectin in some BM. Many studies have focused on characterizing BM due to their potential roles in normal tissue function and disease, and these structures have been well characterized in many tissues. Comparatively few studies, however, have focused on the function of the epithelial BM in corneal physiology. Since the normal corneal stroma is avascular and has relatively low keratocyte density, it is expected that the corneal BM would be different from the BM in other tissues. One function that appears critical in homeostasis and wound healing is the barrier function to penetration of cytokines from the epithelium to stroma (such as transforming growth factor β-1), and possibly from stroma to epithelium (such as keratinocyte growth factor). The corneal epithelial BM is also involved in many inherited and acquired corneal diseases. This review examines this structure in detail and discusses the importance of corneal epithelial BM in homeostasis, wound healing, and disease.
Keywords: basement membrane, myofibroblasts, corneal epithelium, wound healing
The corneal epithelial basement membrane serves critical functions in development, homeostasis, wound healing, and disease. The basement membrane is composed of many components arranged precisely to serve these critical functions. Variations in the components are noted in the conjunctiva, limbal cornea, and central cornea.
Introduction
Basement membranes (BM) are highly specialized extracellular matrices that form thin acellular layers underlying cells and separate them from, as well as connect them to, their interstitial matrix.1,2 Basement membranes function not only in anchoring adjacent cells and providing scaffolding during embryonic development, but also in migration, differentiation, and maintenance of the differentiated phenotype of associated epithelial or endothelial cells.3,4 In addition, BM control cellular functions by binding and modulating the local concentrations of growth factors and cytokines,5,6 and are able to regulate cell polarity,7,8 cell adhesion, spreading, and migration via their effects on the cytoskeleton.9–11
Basement membranes are highly divergent depending on their tissues of localization.3,4 Many studies have focused on characterizing BM due to their potential role in normal tissue function and disease, and this structure has been well characterized in many organs.1,4,12,13 Relatively fewer studies, however, have characterized the role of the epithelial BM in the cornea. This review focuses on the structure and importance of corneal epithelial BM in homeostasis, wound healing, and disease.
Development and Structure of Corneal Epithelial Basement Membrane
The corneal epithelial BM is positioned between basal epithelial cells and the stroma. It is first detected at 8 to 9 weeks of gestation in the human,14 and after the fourth month the corneal epithelium is separated from the stroma by a continuous BM.15 Limited evidence has been provided for a stromal cell origin for some epithelial BM components in the cornea.16–18 In adult humans, rabbits, mice, and many other species, the BM ultrastructure (Fig. 1) at the transmission electron microscopic level using standard fixation methods reveals adjacent layers termed the lamina lucida (layer between basal epithelial cell membrane and lamina densa) and the lamina densa.19–21
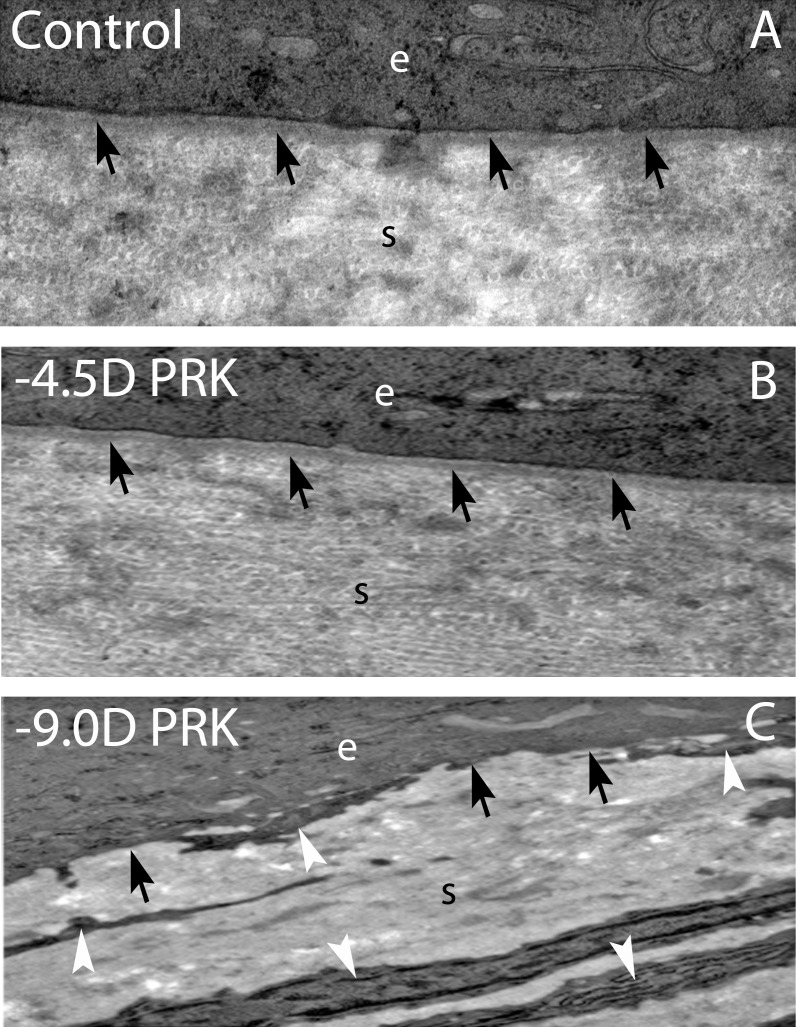
Figure 1. .
Transmission electron microscopy of the central corneal epithelial basement membrane of rabbits after photorefractive keratectomy and in controls. (A) A control corneal section showing a sharp epithelial basement membrane that includes the lamina densa (arrows) and lamina lucida (the less dense band between lamina densa and basal epithelial cells) present between the epithelium (e) and the stroma (s). (B) A −4.5-diopter (D) PRK cornea that healed without haze at 1 month after surgery showing a regenerated epithelial basement membrane (arrows) similar to the control cornea in (A). The extracellular matrix in the stroma (s) also demonstrates a similar structural pattern compared to the control cornea. (C) A −9.0-D PRK cornea that healed with dense subepithelial haze at 1 month after PRK. A large number of myofibroblasts (arrowheads) with large amounts of rough endoplasmic reticulum are surrounded by disorganized extracellular matrix in the stroma (s) beneath the epithelium (e). There is no evidence of a lamina densa-like or lamina lucida-like structure between the epithelium and the stroma. Magnification for all: ×30,000.
Since the corneal stroma is avascular and has a relatively low keratocyte density, it is likely that the corneal BM is different in composition from the BM in other tissues. Corneal epithelial BM undergoes considerable change during development and appears to have regional heterogeneity from central cornea to limbus to conjunctiva.22 In general terms, corneal epithelium BM is assembled from four primary components: collagens, laminins, heparan sulfate proteoglycans (HSPGs), and nidogens,1,4 although many other components such as fibronectin are also present—some of which may be tissue specific. The presence of collagen type IV was at one time controversial,4 with some reports failing in detect type IV collagen in the corneal BM.23–26 However, several immunohistochemical studies localized type IV collagen beneath the human corneal epithelium.27–30 One study31 found type IV collagen to be abundantly present in the conjunctival and limbal BM but noted that immunoreactivity disappeared within a short distance of the start of Bowman's layer. It appears that the reason for disparity between different studies is the spatial variability (“horizontal” heterogeneity) in the BM composition between the central cornea, limbus, and conjunctiva.32 It is also now recognized that type IV collagen has six α chains that can assemble into different heterotrimers, such as [α1(IV)2α2(IV)], [α3α4α5(IV)], [α3(IV)2α4(IV)], or [α5(IV)2α6(IV)].33,34 This variability could also have contributed to early confusion about the presence of collagen IV in the corneal epithelial BM. Ljubimov and coworkers32 showed in adult human corneas that central BM had type IV collagen α3 through α6 chains, whereas only limbal and conjunctival BM contained α1 and α2 chains. In addition, limbal BM had collagen IV α5 and α6 chains. Limbal and conjunctival epithelial BM also had laminin α2 and β2 chains whereas central cornea BM did not. Laminin-332, perlecan, fibronectin, entactin/nidogen, and type VII collagen were detected in the entire ocular surface BM—central cornea, limbus, and conjunctiva. These authors suggested that these shifts in collagen IV chains and the appearance of additional laminins in the limbus may be related to the differentiation state of corneal cells contributing to BM formation. The different distribution of BM components in adult corneal epithelial BM is summarized in the Table. Some studies have found other collagens in the corneal epithelial BM, including type VII collagen as a primary structural element in anchoring fibrils,35 type XV and type XVIII collagens as active molecules in corneal wound healing36–38 and perhaps involved in the coneal avascularity,39,40 type XVII collagen as an adhesion molecule present in hemidesmosomes,41,42 and the long form of type XII collagen.43–45
Table. .
Distribution of Basement Membrane Components in Adult Corneal Epithelial Basement Membrane
|
Central BM |
Limbus BM |
|
| Type IV collagen chains | α3–α6 | α1, α2, α5, α6 |
| Type VII collagen | + | + |
| Type XII collagen | + | + |
| Type XV collagen | − | + |
| Type XVII collagen | + | + |
| Type XVIII collagen | + | + |
| Laminin isoforms | 311, 332, 411, 511 | 211, 213, 221, 311, 321, 323, 332, 333, 411, 421, 423, 511, 521, 522, 523 |
| Perlecan | + | + |
| Nidogens-1 and −2 | + | + |
| Fibronectin | + | + |
| Thrombospondin-1 | + | − |
| Tenascin-C | − | + |
| Fibrillin-1 | − | + |
| Matrilin-2 | + | + |
| Matrilin-4 | + | + |
Table modified from Kabosova et al.22
Laminins are the most abundant noncollagenous proteins in BM.46 Laminins are heterotrimeric glycoproteins that are composed of three chains, including one α, one β, and one γ chain. At present, five α, three β, and three γ peptides coded by different genes are known for mice and humans.46 The trimers were previously designated laminin-1 to -15 in order of their discovery, with no relationship to chain composition. According to the previous nomenclature,47 a trimer could be identified by either an Arabic numeral (e.g., 10) or its chains (e.g., α5β1γ1). Aumailley et al.48 proposed an abbreviated form, for example, 511 for α5β1γ1, which better identifies the peptide composition of individual laminins. Laminins have been shown to influence tissue development, and laminin gene defects have potential roles in diseases in many organs, including keratoconus, Fuchs' dystrophy, and bullous keratopathy in the eye.49–52 The expression of laminin chains is regulated both spatially and temporally,46 suggesting that different laminin isoforms might have distinct roles. Laminins are vital for the assembly of BM and interact with collagen networks via nidogens and other extracellular matrix molecules.49 In vivo and in vitro studies have suggested that laminins are principally responsible for initial organizing of BM assembly since they uniquely self-assemble into sheet-like structures on cell surfaces without the contribution of other components required for the assembly of a fully functional BM, such as type IV collagens bound to nidogen-1 and nodogen-2, the HSPGs agrin and perlecan, and many other components.13,53 It has been demonstrated in Drosophilia melanogaster that the complete absence of laminin results in disorganized extracellular matrix and abnormal accumulations of major BM components.54 Most laminin subunit knockouts in the mouse model are lethal, for example, laminin γ1,55 due to the lack of BM formation. If laminin knockout mice do survive, they develop severe disease, depending on the subunits deleted and their tissue distributions.1
Another BM component, perlecan, is the most prevalent HSPG in this structure. It is a complex, multidomain protein with a number of discrete binding partners.56 The protein's core consists of five domains that share homology with other molecules involved in nutrient metabolism, cell proliferation, and adhesion, including laminin, the low-density lipoprotein (LDL) receptor, epithelial growth factor (EGF), and the neural cell adhesion molecule (N-CAM).1,12 Perlecan, a typical proteoglycan, mediates the migration, proliferation, and differentiation of a variety of cells by mediating cell signaling events.57 Perlecan mediates these functions mainly by controlling the availability of fibroblast growth factors (FGF), bone morphogenic proteins (BMP), platelet-derived growth factor (PDGF), vascular endothelial growth factors (VEGF), transforming growth factor β-1 (TGF-β1), and insulin-like growth factors (IGF)58–62 to bind receptors on the cells they modulate. In vertebrates, perlecan functions in a diverse range of developmental and biological processes—from the development of cartilage to the regulation of wound healing.63–66 Sher et al.67 found that perlecan regulates both the survival and terminal differentiation steps of keratinocytes and that it is critical for the formation of normal epidermis. Another study68 reported that perlecan expression is upregulated after corneal stromal injury, as well as after an artificial increase in intraocular pressure. Inomata et al.69 investigated the role of perlecan in the structure of corneal epithelium by use of perlecan-deficient (Hspg2−/−-TG) mice. In that study, perlecan was identified in corneal epithelial BM, and the epithelium was shown to be thin and poorly differentiated in perlecan-deficient mice (Hspg2−/−-TG) and accompanied by downregulation of Ki67, cytokeratin12, connexin43, Notch 1, and Pax6. These findings revealed that BM perlecan is likely critical for normal epithelial formation and terminal differentiation in the cornea.
Nidogen-1 and nidogen-2, other major BM components, are sulfated glycoproteins. Both nidogens consist of three globular domains separated by link-like and rod-like regions,70,71 and they have similar distribution within the corneal epithelial BM. Due to their strong affinity to laminin and collagen IV, nidogens are considered to be link proteins in BM.72 Genetic deletion of either NID gene in mouse did not produce detectible alterations in tissue and BM architecture.73–75 Redistribution and upregulation of the more restrictively expressed nidogen-2- in nidogen-1-deficient mice suggested compensatory functions of the two nidogens.74,76 One study77 reported predominantly nidogen-2 accumulation around corneal INTACS stromal implants, along with other known fibrotic extracellular matrix components. Studies in mice lacking both nidogen isoforms showed that this is indeed the case, since the double knockouts had severe abnormalities in lungs, heart, and limbs that were directly related to BM defects.78,79 Surprisingly, however, ultrastructurally normal BM were seen in many other tissues—demonstrating that the other BM components may still assemble and form BM structures without nidogens in some tissues. This may indicate tissue-specific requirements for nidogens. Nan et al.80 reported the nidogen-1 gene as a locus for nevogenesis and melanoma development, with decreased expression levels of nidogen-1 in benign nevi and in primary melanomas compared with the normal skin in individuals of European ancestry. Moreover, abnormal expression and distribution of nidogen were described in Hirschsprung's disease (congenital colonic aganglionosis).81
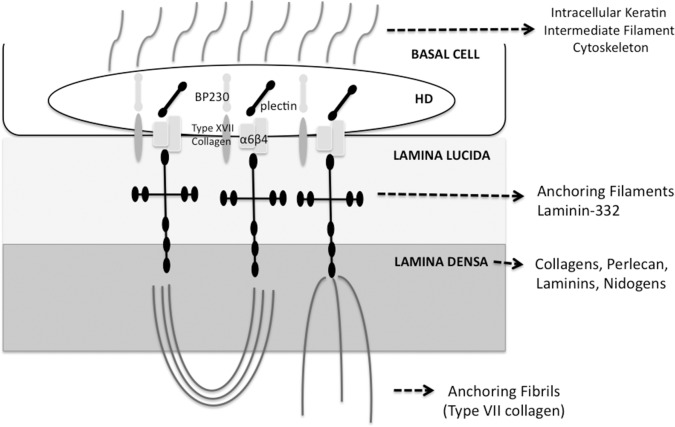
A better appreciation of the structure and function of the BM is provided by understanding of the interactions between its layers and structures in the overlying basal epithelial cells and the underlying anterior stroma (Fig. 2). Corneal basal epithelial cells contain small stud-like structures called hemidesmosomes (HD) at their basal cell membrane,82 with which they firmly attach themselves to the underlying extracellular matrix—in the case of the cornea, the BM and underlying stroma, including Bowman's layer in humans and some other species.83 The combined HD, anchoring fibril, and anchoring filament complex is referred to as the hemidesmosome-stable adhesion complex or the anchoring complex. The anchoring complex forms an uninterrupted structural link between the intracellular keratin intermediate filament cytoskeleton of the basal epithelial cell and the underlying BM and stroma that firmly adheres the cell to its underlying substratum. The anchoring complex is composed of more than 10 component molecules that themselves often vary in isoform between different epithelia.83,84
Figure 2. .
Schematic diagram of the basement membrane, overlying basal cell, and underlying stroma in the cornea interconnected by the hemidesmosome-anchoring filament complex. It is important to note that other component molecules are present in corneal epithelial basement membrane that are not included in this simplified diagram. HD, hemidesmosome; BP230, bullous pemphigoid antigen 230.
Another frequently encountered term in the literature is the basement membrane zone (BMZ), ultrastructurally composed of the HD, the upper lamina lucida, the lower lamina lucida, the lamina densa, and the sublamina densa, which is the uppermost region of the stroma.85
The molecular organization of the HD is based on three classes of proteins: the cytoplasmic plaque proteins acting as linkers for elements of the cytokeleton at the cytoplasmic surface of the plasma membrane, the transmembrane proteins serving as cell receptors connecting the cell interior to the extracellular matrix, and the BM-associated proteins on the extracellular matrix.86 The cytoplasmic constituents include the bullous pemphigoid antigen 230 (BP230),87,88 plectin,89,90 and other less characterized proteins. BP230 and plectin are proteins with related sequences belonging to the plakin family of proteins implicated in the organization of the cytoskeleton architecture.91,92 BP230 was first recognized as a target antigen in bullous pemphigoid, an autoimmune blistering disorder of the skin.93 The transmembrane constituents of the HD include the α6β4 integrin and type XVII collagen. In contrast to most of the integrins associated with the actin cytoskeleton, the α6β4 integrin is unique in that it is found in HD at sites where keratin intermediate filaments attach.94 The extracellular domain of α6β4 integrin is crucial for cell adhesion. Several different antibodies directed against the α6β4 integrin prevent the assembly of HD and induce dermo–epidermal separation in vivo.95 In addition, natural mutations of the α6 and β4 genes in humans or their corresponding mutations in mice result in extensive blistering of the skin and mucous membranes of the digestive and respiratory tracts.96,97
Preliminary in vitro binding studies suggest that type XVII collagen interacts with β3 chain of laminin-332 (formerly termed laminin-5). Laminin-332 also supports cell binding and spreading and is a major adhesive ligand for the α6β4 integrin. Laminin-332 is concentrated below the HD plaque linking the α6β4 integrin (and probably type XVII collagen) to type VII collagen.98 In order for type VII collagen to function as an anchoring molecule, its NC1 domain must bind to other structural molecules of extracellular matrix.99,100 It has been demonstrated that this NC1 domain binds to laminin-332 and collagen IV in a site-specific interaction.101,102 Villone et al.103 also have reported direct and strong covalent cross-links between collagens VII and I. The critical role of collagen VII in stabilizing the structure of the BMZ is demonstrated by the discovery that mutations in collagen VII found in patients with dystrophic epidermolysis bullosa lead to pathological changes in structure of the dermal–epidermal junction104–106 and often affect the eye. The ocular abnormalities may include corneal erosions and blisters, corneal scarring, symblepharon formation, ectropion, impaired vision, and corneal perforation.107,108 Epidermolysis bullosa-like disorders also occur in patients with mutations in genes encoding for keratins 4 and 14, type VII and XVII collagens, plectin, α6 and β4 integrin subunits, and each of the three chains of laminin-332.107
Anchoring fibrils (Fig. 3)—commonly detected via immuno-identification of their major component collagen VII—traverse the lamina lucida and insert into the electron-dense lamina densa, where the anchoring filaments are located, and may extend further into underlying stroma into extracellular matrix structures called anchoring plaques.83 One study showed that the average depth of penetration of anchoring fibrils into the corneal stroma was 0.60 and 0.54 μm in human and rabbit corneas, respectively.83 Interestingly, no obvious differences in anchoring fibril structure or distribution were noted between human corneas, which have a Bowman's layer, and rabbit corneas, which do not have a Bowman's layer.83
Figure 3. .
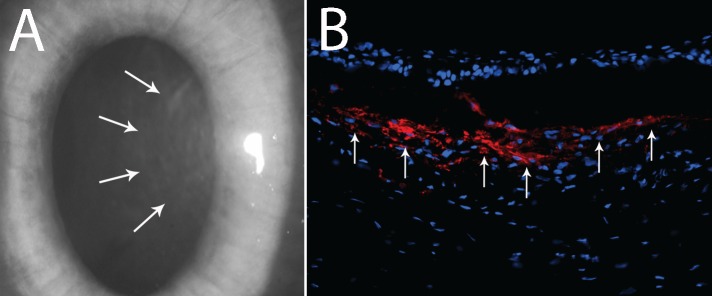
Corneal haze and myofibroblasts. (A) Slit-lamp photo of rabbit cornea at 1 month after −9.0-D PRK corneal ablation surgery. Note the dense subepithelial haze (arrows). Magnification: ×20. (B) Immunocytochemistry for alpha-smooth actin (αSMA)+ myofibroblast cells (red) shows a high density of αSMA+ myofibroblasts (arrows) in the subepithelial stroma at 1 month after −9.0-D PRK. Magnification: ×400.
It is clear from studies of mutant model organisms12 that the BM plays diverse roles in regulating early embryonic development, organogenesis, tissue differentiation, and adult homeostasis. This is reflected in a broad range of inherited diseases, such as Alport and Fraser syndromes, caused by perturbation of genes that contribute to the structure or regulatory properties of the BM.82
Human corneal epithelial BM undergoes significant compositional changes between the infant and adult phases.22 In adult corneas, basal epithelial precursor cells are thought to be localized primarily in the limbus, although in embryonic and newborn corneas they have been detected in the central cornea.109–111 Epithelial BM heterogeneity between the limbal and central cornea becomes more pronounced during embryonic and early postnatal life, and this could reflect a requirement for limbal stem cells to maintain a specific BM composition to preserve the undifferentiated state of these cells.22,101
Epithelial Basement Membrane in Corneal Wound Healing
Several studies have demonstrated the importance of epithelial BM in corneal wound healing.19,20,112,113 For example, Pal-Ghosh and coworkers113 demonstrated that removal of the epithelial BM enhances many wound healing processes in the cornea, including keratocyte apoptosis and nerve death. Corneal surgery, injury, or infection frequently triggers the appearance of stromal myofibroblasts (Fig. 3) associated with persistent corneal opacity (haze).21 The opacity develops as a result of diminished transparency of the cells themselves and the production of disordered extracellular matrix components by stromal cells.114–116 Singh et al.117 reported that the normally functioning epithelial BM critically modulates myofibroblast development through its barrier function preventing penetration of epithelial TGF-β1 and PDGF into the stroma at sufficient levels to drive myofibroblast development and maintain viability once mature myofibroblasts are generated. This hypothesis holds that stromal surface irregularity after photorefractive keratectomy (PRK) or other cornea injury leads to structural and functional defects in the regenerated epithelial BM, which increases and prolongs penetration of epithelial TGF-β1 and PDGF into the anterior corneal stroma to promote myofibroblast development from either keratocyte-derived or bone marrow–derived precursor cells118 (Singh V, Wilson SE, unpublished data, 2013).
Recent studies of epithelial BM ultrastructure using transmission electron microscopy21 have demonstrated defects in the normal regeneration of epithelial BM in rabbit corneas with haze at 1 month after high-correction PRK (−9.0-diopter PRK). Moreover, highly disorganized extracellular matrix and prominent myofibroblasts were observed in these rabbit corneas with haze and not in corneas with moderate correction (−4.5 diopter PRK) without haze or unwounded control corneas (Fig. 1)—although in one −4.5-diopter PRK cornea with localized haze there were also numerous myofibroblasts, disorganized extracellular matrix deposition, and defective epithelial BM regeneration in the area of the cornea with haze. The working hypothesis under investigation is that prominent mature myofibroblast generation and resulting disorganized extracellular matrix excretion in the anterior stroma of corneas with significant injury interfere with keratocyte contribution of critical components to the BM (collagen type VII, for example) that results in defective epithelial BM regeneration. Only when the epithelial BM is finally regenerated, which may take years in some corneas with haze, and epithelium-derived TGF-β1 levels fall, do myofibroblasts undergo apoptosis and keratocytes reabsorb disorganized extracellular matrix and thereby restore transparency.117,119,120 Thus, the epithelial BM likely functions as a corneal regulatory structure that limits the fibrotic response in the stroma by modulating the availability of epithelium-derived TGF-β1, PDGF, and perhaps other growth factors and extracellular matrix components, to stromal cells, including myofibroblast precursors. It may also regulate levels of stromal cell–produced epithelial modulators of motility, proliferation, and differentiation like keratinocyte growth factor (KGF) that transition through the BM in the opposite direction.121,122 Thus, corneal epithelial BM may modulate epithelial-to-stroma and stroma-to-epithelial interactions by regulating cytokines and growth factor movement from one cell layer to the other.
Latvala et al.123 observed that α6 and β4 integrins have changes in distribution adjacent to the BM during epithelial wound healing after epithelial abrasion in the rabbit cornea. Stepp et al.124 have demonstrated that the re-epithelialization of small wounds is accompanied by increased α6β4 integrin. Epithelial cell migration is also affected by the distribution of laminin and collagen IV during corneal wound healing and BM regeneration.19 Thus, α3(IV) and α4(IV) collagen chains may be important for the healthy corneal epithelium. Upon injury, the BM is remodeled to include α1(IV) and α2(IV) collagen, recapitulating corneal epithelial expression during development.
Pathologic Alterations Associated With the Corneal Epithelial Basement Membrane
Many ocular abnormalities and diseases have been described that relate to the corneal epithelial BM. Basement membrane abnormalities are associated with recurrent corneal erosions,125 lattice corneal dystrophy,126 Alport syndrome,82,127 and thin BM disease.128 Electron microscopic ultrastructural analysis confirmed that irregularities of BM formation and BM composition are likely central factors in epithelial BM dystrophy and recurrent corneal erosion syndrome.129 In this condition, redundant layers of BM prevent deeper epithelial cells from moving anterior through the epithelial tissue to be discharged from the apical epithelial surface, as they are in the normal epithelial maturation process. These desquamating cells became entrapped beneath the sheets of aberrant BM and formed cysts, which contained cellular and nuclear debris.125 Thus, the epithelium is not firmly anchored to the underlying stroma as it is in normal corneas. Immunohistochemical and electron microscopic evidence of structural alterations in the BM were also noted in lattice corneal dystrophy compared to normal corneas.126 Abnormalities in epithelial cell–matrix adhesion molecules and BM components were noted in many types of diseased corneas, especially in those with subepithelial amyloid deposits.126 In Alport syndrome and thin BM disease, the α3 (IV), α4 (IV), and/or α5 (IV) collagens chains are defective, which leads to many associated ocular abnormalities, such as corneal arcus, corneal dystrophies (including posterior polymorphous corneal dystrophy), and corneal epithelial opacities.127,128
Kenney et al.130 reported abnormalities in the human corneal epithelial BM and in the extracellular matrix in keratoconus corneas stained for epithelial BM components such as nidogen, fibronectin, α3-α5 chains of type IV collagen, chains of laminin-332, perlecan, and type VII collagen. Tuori et al.131 also suggested that the defects in the BM and changes in the BM composition have a role in the pathogenesis of keratoconus. These authors speculated that the characteristic breaks in Bowman's layer in many corneas with keratoconus are formed due to scarring or activation of proteolytic enzymes. They hypothesized that the breaks in the BM initiated a wound healing process in cornea in which the basal epithelial cells attempt to maintain BM integrity by upregulating the secretion of BM structural proteins and increasing expression of collagen α1/2 (IV) chains, type VII collagen, laminin-111, and laminin-332. They further hypothesized that the degradation process outstrips the restoration process, leading to overall degradation of BM proteins. They reported that this is manifest in discontinuities in the expression of laminin-111, laminin-332, collagen α5/6 (IV) chains, type VII collagen, and integrin β4. They surmised that this process ends up producing the subepithelial scarring that is noted in some corneas with keratoconus.
Keratoconus corneas also frequently have epithelial hyperplasia and/or epithelial thickening.132 Sykakis and coworkers132 found a positive correlation between breaks in Bowman's layer, and presumably the BM, and epithelial thickness in corneas with keratoconus. Disorders of BM barrier function controlling stromal cell–derived growth factors such as KGF could be an important factor in the pathophysiology leading to corneal epithelial hyperplasia and epithelial thickening in this disease (Fig. 4).
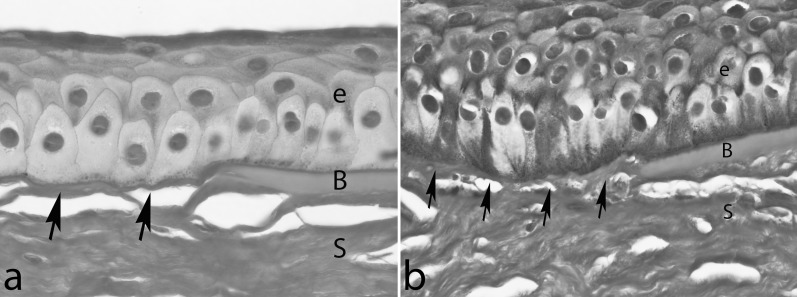
Figure 4. .
Histopathologic analysis of corneal sections of two patients (a, b) with keratoconus stained with periodic acid Schiff (PAS). Note the breaks or attenuations (arrows) in Bowman's layer (B). Ultrastructural transmission electron microscopy studies have demonstrated that the epithelial basement membrane is abnormally thin or missing in areas of the central cornea in most keratoconus corneas removed at the time of corneal transplantation.150 Epithelial (e) thickness tends to be highly variable in keratoconus, often within one cornea of a single patient. In the area shown in (b), there is hyperplasia and hypertrophy of epithelial cells (e), while in (a), the epithelium (e) is relatively normal in thickness. In other areas (not shown), the epithelium can be very thin. S, stroma. Magnification: ×630.
Acquired abnormalities of the BM also can be associated with other diseases. Patients with diabetes mellitus are at increased risk for developing corneal epithelial defects, recurrent epithelial erosions, corneal ulcers, and corneal edema.133,134 Ljubimov et al.135 reported reductions in the expression of nidogens, laminin-111, and laminin-511 and their binding to α3β1 integrin in diabetic corneas. Azar et al.136 reported a decreased formation of HD by corneal epithelial cells on denuded BM when either the stroma or the epithelium was derived from diabetic animals. These alterations may be attributable to decreased BM and integrin synthesis, and may be associated with abnormal growth factor expression in diabetic eyes. Alternatively, some of these growth factors may become elevated in diabetic corneas by diffusion from the diseased vitreous and thereby affect corneal epithelial BM integrin production.135 Another possibility is that BM components and/or integrins may be altered because of their increased degradation by the proteinases elevated in these corneas.135
Bullous keratopathy is a corneal disorder that commonly occurs as a result of intraocular surgical damage to the corneal endothelial cells or the effects of intraocular lenses or glaucoma shunts on these cells. Clinical features include loss of endothelial cells, subendothelial fibrosis, chronic corneal edema, formation of epithelial bullae (blisters), subepithelial fibrosis, and opacification of the stroma.137 While the initiating factor in bullous keratopathy is known to be endothelial dysfunction and loss of endothelial cells leading to stromal edema, a lack of adhesive extracellular matrix proteins such as fibronectin, laminin, and type IV collagen in the epithelial BM has been implicated in enhancing bullae formation.138,139 Tenancin-C (TN-C) is a large glycoprotein that is important in wound healing and tissue remodeling, repair, and development. In the normal adult cornea it is usually restricted to the limbus. Previous studies have associated the antiadhesive effect of TN-C with the development of bullae.137,138,140 Other characteristics of bullous keratopathy include the presence of matrix metalloproteinase-2 at the site of fibrosis141 and the accumulation of inflammatory cells, but not myofibroblasts, within the stroma.142
Basement membrane may also act as a physical barrier against the penetration of viruses and bacteria into the corneal stroma. Alarcon et al.143 suggested that BM provided a barrier to the penetration of Pseudomonas aeruginosa bacteria. These authors suggested that the bacteria penetrated into the corneal stroma from the overlying corneal epithelium only in regions where the BM was discontinuous. That study was consistent with previous studies reporting that epithelial BM of other tissues, such as columnar genital epithelium and epidermal layers of the skin or the lining of the gut, can act as barriers to herpes simples virus and Rift Valley fever virus.144–146 While the mechanism for protection was found to involve direct physical trapping within the BM—likely due to a filtering effect of its small pores—there may also be associated effects on epithelial permeability and epithelial antimicrobial activities.143
Final Considerations
Many studies indicate that the corneal epithelial BM is more than a thin acellular layer separating epithelial cells from the adjacent anterior stroma. This critical structure participates in early developmental stages and undergoes significant compositional changes between infancy and the adult stage, and may continue to undergo alterations during the lifetime of the individual, especially in people with genetic abnormalities affecting the BM. In normal corneas, the epithelial BM plays an important role in corneal homeostasis and downregulation of the wound healing cascades. In mutant animals, such as perlecan knockouts or laminin-deficient mice, it has been noted that specific BM components have important roles in modulating corneal epithelial cell growth, proliferation, and differentiation.
The corneal wound healing response is an excellent example of how the epithelial BM regulates corneal homeostasis. Studies have shown that corneal injuries lead to structural and/or functional defects in the epithelial BM that allow cytokines such as epithelium-derived TGF-β1 and PDGF to gain access to the stroma at sufficient concentration to trigger the differentiation of myofibroblast precursor cells. After repair of the epithelial BM there is a resulting fall in TGF-β1 and PDGF levels in the corneal stroma that leads to apoptosis of stromal myofibroblasts dependent on TGF-β1 for survival.118,147–149 Thus, BM functions both by regulating stromal and epithelial levels of key cytokines and growth factors and through direct interaction of BM components with epithelial, and perhaps stromal, cell surface receptors.
Acknowledgments
Supported in part by US Public Health Service Grants EY10056 and EY015638 from the National Eye Institute, National Institutes of Health, Bethesda, Maryland, and Research to Prevent Blindness, New York, New York. The authors alone are responsible for the content and writing of the paper.
Disclosure: A.A.M. Torricelli, None; V. Singh, None; M.R. Santhiago, None; S.E. Wilson, None
References
- 1. Kruegel J, Miosge N. Basement membrane components are key players in specialized extracellular matrices. Cell Mol Life Sci. 2010; 67: 2879–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Timpl R, Brown JC. Supramolecular assembly of basement membranes. Bioessays. 1996; 18: 123–132 [DOI] [PubMed] [Google Scholar]
- 3. Merker HJ. Morphology of the basement membrane. Microsc Res Tech. 1994; 28: 95–124 [DOI] [PubMed] [Google Scholar]
- 4. Tuori A, Uusitalo H, Burgeson RE, Terttunen J, Virtanen I. The immunohistochemical composition of the human corneal basement membrane. Cornea. 1996; 15: 286–294 [DOI] [PubMed] [Google Scholar]
- 5. Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009; 326: 1216–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schlotzer-Schrehardt U, Dietrich T, Saito K, et al. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp Eye Res. 2007; 85: 845–860 [DOI] [PubMed] [Google Scholar]
- 7. Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004; 265: 23–32 [DOI] [PubMed] [Google Scholar]
- 8. Russell AJ, Fincher EF, Millman L, et al. Alpha 6 beta 4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of alpha 3 beta 1 integrin. J Cell Sci. 2003; 116: 3543–3556 [DOI] [PubMed] [Google Scholar]
- 9. Hamelers IH, Olivo C, Mertens AE, et al. The Rac activator Tiam1 is required for (alpha)3(beta)1-mediated laminin-5 deposition, cell spreading, and cell migration. J Cell Biol. 2005; 171: 871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamill KJ, Kligys K, Hopkinson SB, Jones JC. Laminin deposition in the extracellular matrix: a complex picture emerges. J Cell Sci. 2009; 122: 4409–4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sehgal BU, DeBiase PJ, Matzno S, et al. Integrin beta4 regulates migratory behavior of keratinocytes by determining laminin-332 organization. J Biol Chem. 2006; 281: 35487–35498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wiradjaja F, DiTommaso T, Smyth I. Basement membranes in development and disease. Birth Defects Res C Embryo Today. 2010; 90: 8–31 [DOI] [PubMed] [Google Scholar]
- 13. Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011; 3: 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tisdale AS, Spurr-Michaud SJ, Rodrigues M, Hackett J, Krachmer J, Gipson IK. Development of the anchoring structures of the epithelium in rabbit and human fetal corneas. Invest Ophthalmol Vis Sci. 1988; 29: 727–736 [PubMed] [Google Scholar]
- 15. Sevel D, Isaacs R. A re-evaluation of corneal development. Trans Am Ophthalmol Soc. 1988; 86: 178–207 [PMC free article] [PubMed] [Google Scholar]
- 16. Gipson IK, Spurr-Michaud S, Tisdale A, Keough M. Reassembly of the anchoring structures of the corneal epithelium during wound repair in the rabbit. Invest Ophthalmol Vis Sci. 1989; 30: 425–434 [PubMed] [Google Scholar]
- 17. Thomas T, Dziadek M. Genes coding for basement membrane glycoproteins laminin, nidogen, and collagen IV are differentially expressed in the nervous system and by epithelial, endothelial, and mesenchymal cells of the mouse embryo. Exp Cell Res. 1993; 208: 54–67 [DOI] [PubMed] [Google Scholar]
- 18. Weiser MM, Sykes DE, Killen PD. Rat intestinal basement membrane synthesis. Epithelial versus nonepithelial contributions. Lab Invest. 1990; 62: 325–330 [PubMed] [Google Scholar]
- 19. Fujikawa LS, Foster CS, Gipson IK, Colvin RB. Basement membrane components in healing rabbit corneal epithelial wounds: immunofluorescence and ultrastructural studies. J Cell Biol. 1984; 98: 128–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sta Iglesia DD, Stepp MA. Disruption of the basement membrane after corneal debridement. Invest Ophthalmol Vis Sci. 2000; 41: 1045–1053 [PubMed] [Google Scholar]
- 21. Torricelli AA, Singh V, Agrawal V, Santhiago MR, Wilson SE. Transmission electron microscopy analysis of epithelial basement membrane repair in rabbit corneas with haze. Invest Ophthalmol Vis Sci. 2013; 54: 4026–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kabosova A, Azar DT, Bannikov GA, et al. Compositional differences between infant and adult human corneal basement membranes. Invest Ophthalmol Vis Sci. 2007; 48: 4989–4999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kolega J, Manabe M, Sun TT. Basement membrane heterogeneity and variation in corneal epithelial differentiation. Differentiation. 1989; 42: 54–63 [DOI] [PubMed] [Google Scholar]
- 24. Marshall GE, Konstas AG, Lee WR. Immunogold fine structural localization of extracellular matrix components in aged human cornea. II. Collagen types V and VI. Graefes Arch Clin Exp Ophthalmol. 1991; 229: 164–171 [DOI] [PubMed] [Google Scholar]
- 25. Odermatt BF, Lang AB, Ruttner JR, Winterhalter KH, Trueb B. Monoclonal antibodies to human type IV collagen: useful reagents to demonstrate the heterotrimeric nature of the molecule. Proc Natl Acad Sci U S A. 1984; 81: 7343–7347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scheinman JI, Tsai C. Monoclonal antibody to type IV collagen with selective basement membrane localization. Lab Invest. 1984; 50: 101–112 [PubMed] [Google Scholar]
- 27. Konomi H, Hayashi T, Nakayasu K, Arima M. Localization of type V collagen and type IV collagen in human cornea, lung, and skin. Immunohistochemical evidence by anti-collagen antibodies characterized by immunoelectroblotting. Am J Pathol. 1984; 116: 417–426 [PMC free article] [PubMed] [Google Scholar]
- 28. Marshall GE, Konstas AG, Lee WR. Collagens in ocular tissues. Br J Ophthalmol. 1993; 77: 515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakayasu K, Tanaka M, Konomi H, Hayashi T. Distribution of types I, II, III, IV and V collagen in normal and keratoconus corneas. Ophthalmic Res. 1986; 18: 1–10 [DOI] [PubMed] [Google Scholar]
- 30. Tsuchiya S, Tanaka M, Konomi H, Hayashi T. Distribution of specific collagen types and fibronectin in normal and keratoconus corneas. Jpn J Ophthalmol. 1986; 30: 14–31 [PubMed] [Google Scholar]
- 31. Cleutjens JP, Havenith MG, Kasper M, Vallinga M, Bosman FT. Absence of type IV collagen in the centre of the corneal epithelial basement membrane. Histochem J. 1990; 22: 688–694 [DOI] [PubMed] [Google Scholar]
- 32. Ljubimov AV, Burgeson RE, Butkowski RJ, Michael AF, Sun TT, Kenney MC. Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest. 1995; 72: 461–473 [PubMed] [Google Scholar]
- 33. Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008; 71: 357–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hudson BG. The molecular basis of Goodpasture and Alport syndromes: beacons for the discovery of the collagen IV family. J Am Soc Nephrol. 2004; 15: 2514–2527 [DOI] [PubMed] [Google Scholar]
- 35. Sakai LY, Keene DR, Morris NP, Burgeson RE. Type VII collagen is a major structural component of anchoring fibrils. J Cell Biol. 1986; 103: 1577–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maatta M, Heljasvaara R, Sormunen R, Pihlajaniemi T, Autio-Harmainen H, Tervo T. Differential expression of collagen types XVIII/endostatin and XV in normal, keratoconus, and scarred human corneas. Cornea. 2006; 25: 341–349 [DOI] [PubMed] [Google Scholar]
- 37. Kato T, Chang JH, Azar DT. Expression of type XVIII collagen during healing of corneal incisions and keratectomy wounds. Invest Ophthalmol Vis Sci. 2003; 44: 78–85 [DOI] [PubMed] [Google Scholar]
- 38. Saika S, Okada Y, Miyamoto T, et al. Protein expression pattern of collagen type XV in mouse cornea. Graefes Arch Clin Exp Ophthalmol. 2004; 242: 432–436 [DOI] [PubMed] [Google Scholar]
- 39. Sasaki T, Fukai N, Mann K, Gohring W, Olsen BR, Timpl R. Structure, function and tissue forms of the C-terminal globular domain of collagen XVIII containing the angiogenesis inhibitor endostatin. EMBO J. 1998; 17: 4249–4256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sasaki T, Larsson H, Tisi D, Claesson-Welsh L, Hohenester E, Timpl R. Endostatins derived from collagens XV and XVIII differ in structural and binding properties, tissue distribution and anti-angiogenic activity. J Mol Biol. 2000; 301: 1179–1190 [DOI] [PubMed] [Google Scholar]
- 41. Gordon MK, Fitch JM, Foley JW, et al. Type XVII collagen (BP 180) in the developing avian cornea. Invest Ophthalmol Vis Sci. 1997; 38: 153–166 [PubMed] [Google Scholar]
- 42. Hirako Y, Usukura J, Uematsu J, Hashimoto T, Kitajima Y, Owaribe K. Cleavage of BP180, a 180-kDa bullous pemphigoid antigen, yields a 120-kDa collagenous extracellular polypeptide. J Biol Chem. 1998; 273: 9711–9717 [DOI] [PubMed] [Google Scholar]
- 43. Wessel H, Anderson S, Fite D, Halvas E, Hempel J, SundarRaj N. Type XII collagen contributes to diversities in human corneal and limbal extracellular matrices. Invest Ophthalmol Vis Sci. 1997; 38: 2408–2422 [PubMed] [Google Scholar]
- 44. Akimoto Y, Yamakawa N, Furukawa K, Kimata K, Kawakami H, Hirano H. Changes in distribution of the long form of type XII collagen during chicken corneal development. J Histochem Cytochem. 2002; 50: 851–862 [DOI] [PubMed] [Google Scholar]
- 45. Massoudi D, Malecaze F, Soler V, et al. NC1 long and NC3 short splice variants of type XII collagen are overexpressed during corneal scarring. Invest Ophthalmol Vis Sci. 2012; 53: 7246–7256 [DOI] [PubMed] [Google Scholar]
- 46. Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004; 20: 255–284 [DOI] [PubMed] [Google Scholar]
- 47. Burgeson RE, Chiquet M, Deutzmann R, et al. A new nomenclature for the laminins. Matrix Biol. 1994; 14: 209–211 [DOI] [PubMed] [Google Scholar]
- 48. Aumailley M, Bruckner-Tuderman L, Carter WG, et al. A simplified laminin nomenclature. Matrix Biol. 2005; 24: 326–332 [DOI] [PubMed] [Google Scholar]
- 49. Bystrom B, Virtanen I, Rousselle P, Miyazaki K, Linden C. Pedrosa Domellof F. Laminins in normal, keratoconus, bullous keratopathy and scarred human corneas. Histochem Cell Biol. 2007; 127: 657–667 [DOI] [PubMed] [Google Scholar]
- 50. Ebihara N, Watanabe Y, Nakayasu K, Kanai A. The expression of laminin-5 and ultrastructure of the interface between basal cells and underlying stroma in the keratoconus cornea. Jpn J Ophthalmol. 2001; 45: 209–215 [DOI] [PubMed] [Google Scholar]
- 51. Gilmour TK, Meyer PA, Rytina E, Todd PM. Antiepiligrin (laminin 5) cicatricial pemphigoid complicated and exacerbated by herpes simplex virus type 2 infection. Australas J Dermatol. 2001; 42: 271–274 [DOI] [PubMed] [Google Scholar]
- 52. Zenker M, Aigner T, Wendler O, et al. Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004; 13: 2625–2632 [DOI] [PubMed] [Google Scholar]
- 53. Yurchenco PD, Cheng YS, Colognato H. Laminin forms an independent network in basement membranes. J Cell Biol. 1992; 117: 1119–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Miner JH. Laminins and their roles in mammals. Microsc Res Tech. 2008; 71: 349–356 [DOI] [PubMed] [Google Scholar]
- 55. Smyth N, Vatansever HS, Murray P, et al. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999; 144: 151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aviezer D, Hecht D, Safran M, Eisinger M, David G, Yayon A. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell. 1994; 79: 1005–1013 [DOI] [PubMed] [Google Scholar]
- 57. Mongiat M, Taylor K, Otto J, et al. The protein core of the proteoglycan perlecan binds specifically to fibroblast growth factor-7. J Biol Chem. 2000; 275: 7095–7100 [DOI] [PubMed] [Google Scholar]
- 58. Boonen KJ, Post MJ. The muscle stem cell niche: regulation of satellite cells during regeneration. Tissue Eng Part B Rev. 2008; 14: 419–431 [DOI] [PubMed] [Google Scholar]
- 59. Fuchs E. Finding one's niche in the skin. Cell Stem Cell. 2009; 4: 499–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Iozzo RV. Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol. 2005; 6: 646–656 [DOI] [PubMed] [Google Scholar]
- 61. Whitelock JM, Melrose J, Iozzo RV. Diverse cell signaling events modulated by perlecan. Biochemistry. 2008; 47: 11174–11183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nikolova G, Strilic B, Lammert E. The vascular niche and its basement membrane. Trends Cell Biol. 2007; 17: 19–25 [DOI] [PubMed] [Google Scholar]
- 63. Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nat Genet. 1999; 23: 354–358 [DOI] [PubMed] [Google Scholar]
- 64. Handler M, Yurchenco PD, Iozzo RV. Developmental expression of perlecan during murine embryogenesis. Dev Dyn. 1997; 210: 130–145 [DOI] [PubMed] [Google Scholar]
- 65. Knox S, Melrose J, Whitelock J. Electrophoretic, biosensor, and bioactivity analyses of perlecans of different cellular origins. Proteomics. 2001; 1: 1534–1541 [DOI] [PubMed] [Google Scholar]
- 66. Zhou Z, Wang J, Cao R, et al. Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer Res. 2004; 64: 4699–4702 [DOI] [PubMed] [Google Scholar]
- 67. Sher I, Zisman-Rozen S, Eliahu L, et al. Targeting perlecan in human keratinocytes reveals novel roles for perlecan in epidermal formation. J Biol Chem. 2006; 281: 5178–5187 [DOI] [PubMed] [Google Scholar]
- 68. Vittitow J, Borras T. Genes expressed in the human trabecular meshwork during pressure-induced homeostatic response. J Cell Physiol. 2004; 201: 126–137 [DOI] [PubMed] [Google Scholar]
- 69. Inomata T, Ebihara N, Funaki T, et al. Perlecan-deficient mutation impairs corneal epithelial structure. Invest Ophthalmol Vis Sci. 2012; 53: 1277–1284 [DOI] [PubMed] [Google Scholar]
- 70. Fox JW, Mayer U, Nischt R, et al. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO J. 1991; 10: 3137–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Timpl R, Dziadek M, Fujiwara S, Nowack H, Wick G. Nidogen: a new, self-aggregating basement membrane protein. Eur J Biochem. 1983; 137: 455–465 [DOI] [PubMed] [Google Scholar]
- 72. Ho MS, Bose K, Mokkapati S, Nischt R, Smyth N. Nidogens-extracellular matrix linker molecules. Microsc Res Tech. 2008; 71: 387–395 [DOI] [PubMed] [Google Scholar]
- 73. Dong L, Chen Y, Lewis M, et al. Neurologic defects and selective disruption of basement membranes in mice lacking entactin-1/nidogen-1. Lab Invest. 2002; 82: 1617–1630 [DOI] [PubMed] [Google Scholar]
- 74. Murshed M, Smyth N, Miosge N, et al. The absence of nidogen 1 does not affect murine basement membrane formation. Mol Cell Biol. 2000; 20: 7007–7012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Schymeinsky J, Nedbal S, Miosge N, et al. Gene structure and functional analysis of the mouse nidogen-2 gene: nidogen-2 is not essential for basement membrane formation in mice. Mol Cell Biol. 2002; 22: 6820–6830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Miosge N, Sasaki T, Timpl R. Evidence of nidogen-2 compensation for nidogen-1 deficiency in transgenic mice. Matrix Biol. 2002; 21: 611–621 [DOI] [PubMed] [Google Scholar]
- 77. Maguen E, Rabinowitz YS, Regev L, Saghizadeh M, Sasaki T, Ljubimov AV. Alterations of extracellular matrix components and proteinases in human corneal buttons with INTACS for post-laser in situ keratomileusis keratectasia and keratoconus. Cornea. 2008; 27: 565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bader BL, Smyth N, Nedbal S, et al. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol Cell Biol. 2005; 25: 6846–6856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bose K, Nischt R, Page A, Bader BL, Paulsson M, Smyth N. Loss of nidogen-1 and -2 results in syndactyly and changes in limb development. J Biol Chem. 2006; 281: 39620–39629 [DOI] [PubMed] [Google Scholar]
- 80. Nan H, Xu M, Zhang J, et al. Genome-wide association study identifies nidogen 1 (NID1) as a susceptibility locus to cutaneous nevi and melanoma risk. Hum Mol Genet. 2011; 20: 2673–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Parikh DH, Leibl M, Tam PK, Edgar D. Abnormal expression and distribution of nidogen in Hirschsprung's disease. J Pediatr Surg. 1995; 30: 1687–1693 [DOI] [PubMed] [Google Scholar]
- 82. Xu JM, Zhang SS, Zhang Q, et al. Ocular manifestations of Alport syndrome. Int J Ophthalmol. 2010; 3: 149–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gipson IK, Spurr-Michaud SJ, Tisdale AS. Anchoring fibrils form a complex network in human and rabbit cornea. Invest Ophthalmol Vis Sci. 1987; 28: 212–220 [PubMed] [Google Scholar]
- 84. Zhang H, Labouesse M. The making of hemidesmosome structures in vivo. Dev Dyn. 2010; 239: 1465–1476 [DOI] [PubMed] [Google Scholar]
- 85. Chan LS. Human skin basement membrane in health and in autoimmune diseases. Front Biosci. 1997; 2: d343–d352 [DOI] [PubMed] [Google Scholar]
- 86. Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol. 1999; 112: 411–418 [DOI] [PubMed] [Google Scholar]
- 87. Stanley JR, Tanaka T, Mueller S, Klaus-Kovtun V, Roop D. Isolation of complementary DNA for bullous pemphigoid antigen by use of patients' autoantibodies. J Clin Invest. 1988; 82: 1864–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sawamura D, Li K, Chu ML, Uitto J. Human bullous pemphigoid antigen (BPAG1). Amino acid sequences deduced from cloned cDNAs predict biologically important peptide segments and protein domains. J Biol Chem. 1991; 266: 17784–17790 [PubMed] [Google Scholar]
- 89. Wiche G, Becker B, Luber K, et al. Cloning and sequencing of rat plectin indicates a 466-kD polypeptide chain with a three-domain structure based on a central alpha-helical coiled coil. J Cell Biol. 1991; 114: 83–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. McLean WH, Pulkkinen L, Smith FJ, et al. Loss of plectin causes epidermolysis bullosa with muscular dystrophy: cDNA cloning and genomic organization. Genes Dev. 1996; 10: 1724–1735 [DOI] [PubMed] [Google Scholar]
- 91. Tanaka T, Parry DA, Klaus-Kovtun V, Steinert PM, Stanley JR. Comparison of molecularly cloned bullous pemphigoid antigen to desmoplakin I confirms that they define a new family of cell adhesion junction plaque proteins. J Biol Chem. 1991; 266: 12555–12559 [PubMed] [Google Scholar]
- 92. Ruhrberg C, Watt FM. The plakin family: versatile organizers of cytoskeletal architecture. Curr Opin Genet Dev. 1997; 7: 392–397 [DOI] [PubMed] [Google Scholar]
- 93. Stanley JR, Hawley-Nelson P, Yuspa SH, Shevach EM, Katz SI. Characterization of bullous pemphigoid antigen: a unique basement membrane protein of stratified squamous epithelia. Cell. 1981; 24: 897–903 [DOI] [PubMed] [Google Scholar]
- 94. Stepp MA, Spurr-Michaud S, Tisdale A, Elwell J, Gipson IK. Alpha 6 beta 4 integrin heterodimer is a component of hemidesmosomes. Proc Natl Acad Sci U S A. 1990; 87: 8970–8974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kurpakus MA, Stock EL, Jones JC. Analysis of wound healing in an in vitro model: early appearance of laminin and a 125 × 10(3) Mr polypeptide during adhesion complex formation. J Cell Sci. 1990; 96 (pt 4): 651–660 [DOI] [PubMed] [Google Scholar]
- 96. Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996; 13: 370–373 [DOI] [PubMed] [Google Scholar]
- 97. Dowling J, Yu QC, Fuchs E. Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol. 1996; 134: 559–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Burgeson RE, Christiano AM. The dermal-epidermal junction. Curr Opin Cell Biol. 1997; 9: 651–658 [DOI] [PubMed] [Google Scholar]
- 99. Adachi E, Hopkinson I, Hayashi T. Basement-membrane stromal relationships: interactions between collagen fibrils and the lamina densa. Int Rev Cytol. 1997; 173: 73–156 [DOI] [PubMed] [Google Scholar]
- 100. Shimizu H, Ishiko A, Masunaga T, et al. Most anchoring fibrils in human skin originate and terminate in the lamina densa. Lab Invest. 1997; 76: 753–763 [PubMed] [Google Scholar]
- 101. Uitto J, Pulkkinen L. Molecular complexity of the cutaneous basement membrane zone. Mol Biol Rep. 1996; 23: 35–46 [DOI] [PubMed] [Google Scholar]
- 102. Brittingham R, Uitto J, Fertala A. High-affinity binding of the NC1 domain of collagen VII to laminin 5 and collagen IV. Biochem Biophys Res Commun. 2006; 343: 692–699 [DOI] [PubMed] [Google Scholar]
- 103. Villone D, Fritsch A, Koch M, Bruckner-Tuderman L, Hansen U, Bruckner P. Supramolecular interactions in the dermo-epidermal junction zone: anchoring fibril-collagen VII tightly binds to banded collagen fibrils. J Biol Chem. 2008; 283: 24506–24513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Uitto J, Christiano AM. Dystrophic forms of epidermolysis bullosa. Semin Dermatol. 1993; 12: 191–201 [PubMed] [Google Scholar]
- 105. Uitto J, Pulkkinen L. Molecular genetics of heritable blistering disorders. Arch Dermatol. 2001; 137: 1458–1461 [DOI] [PubMed] [Google Scholar]
- 106. Brittingham R, Colombo M, Ito H, et al. Single amino acid substitutions in procollagen VII affect early stages of assembly of anchoring fibrils. J Biol Chem. 2005; 280: 191–198 [DOI] [PubMed] [Google Scholar]
- 107. Fine JD, Johnson LB, Weiner M, et al. Eye involvement in inherited epidermolysis bullosa: experience of the National Epidermolysis Bullosa Registry. Am J Ophthalmol. 2004; 138: 254–262 [DOI] [PubMed] [Google Scholar]
- 108. Aclimandos WA. Corneal perforation as a complication of epidermolysis bullosa acquisita. Eye (Lond). 1995; 9 (pt 5): 633–636 [DOI] [PubMed] [Google Scholar]
- 109. Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004; 78: 433–446 [DOI] [PubMed] [Google Scholar]
- 110. Rodrigues M, Ben-Zvi A, Krachmer J, Schermer A, Sun TT. Suprabasal expression of a 64-kilodalton keratin (no. 3) in developing human corneal epithelium. Differentiation. 1987; 34: 60–67 [DOI] [PubMed] [Google Scholar]
- 111. Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986; 103: 49–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Chi C, Trinkaus-Randall V. New insights in wound response and repair of epithelium. J Cell Physiol. 2013; 228: 925–929 [DOI] [PubMed] [Google Scholar]
- 113. Pal-Ghosh S, Pajoohesh-Ganji A, Tadvalkar G, Stepp MA. Removal of the basement membrane enhances corneal wound healing. Exp Eye Res. 2011; 93: 927–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Jester JV, Huang J, Barry-Lane PA, Kao WW, Petroll WM, Cavanagh HD. Transforming growth factor(beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest Ophthalmol Vis Sci. 1999; 40: 1959–1967 [PubMed] [Google Scholar]
- 115. Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci U S A. 1996; 93: 4219–4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wilson SE, Liu JJ, Mohan RR. Stromal-epithelial interactions in the cornea. Prog Retin Eye Res. 1999; 18: 293–309 [DOI] [PubMed] [Google Scholar]
- 117. Singh V, Agrawal V, Santhiago MR, Wilson SE. Stromal fibroblast-bone marrow-derived cell interactions: implications for myofibroblast development in the cornea. Exp Eye Res. 2012; 98: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Singh V, Santhiago MR, Barbosa FL, et al. Effect of TGFbeta and PDGF-B blockade on corneal myofibroblast development in mice. Exp Eye Res. 2011; 93: 810–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Fini ME, Stramer BM. How the cornea heals: cornea-specific repair mechanisms affecting surgical outcomes. Cornea. 2005; 24: S2–S11 [DOI] [PubMed] [Google Scholar]
- 120. Stramer BM, Zieske JD, Jung JC, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003; 44: 4237–4246 [DOI] [PubMed] [Google Scholar]
- 121. Wilson SE, Walker JW, Chwang EL, He YG. Hepatocyte growth factor, keratinocyte growth factor, their receptors, fibroblast growth factor receptor-2, and the cells of the cornea. Invest Ophthalmol Vis Sci. 1993; 34: 2544–2561 [PubMed] [Google Scholar]
- 122. Wilson SE, He YG, Weng J, Zieske JD, Jester JV, Schultz GS. Effect of epidermal growth factor, hepatocyte growth factor, and keratinocyte growth factor, on proliferation, motility and differentiation of human corneal epithelial cells. Exp Eye Res. 1994; 59: 665–678 [DOI] [PubMed] [Google Scholar]
- 123. Latvala T, Paallysaho T, Tervo K, Tervo T. Distribution of alpha 6 and beta 4 integrins following epithelial abrasion in the rabbit cornea. Acta Ophthalmol Scand. 1996; 74: 21–25 [DOI] [PubMed] [Google Scholar]
- 124. Stepp MA, Zhu L, Cranfill R. Changes in beta 4 integrin expression and localization in vivo in response to corneal epithelial injury. Invest Ophthalmol Vis Sci. 1996; 37: 1593–1601 [PubMed] [Google Scholar]
- 125. Laibson PR. Recurrent corneal erosions and epithelial basement membrane dystrophy. Eye Contact Lens. 2010; 36: 315–317 [DOI] [PubMed] [Google Scholar]
- 126. Resch MD, Schlotzer-Schrehardt U, Hofmann-Rummelt C, Kruse FE, Seitz B. Alterations of epithelial adhesion molecules and basement membrane components in lattice corneal dystrophy (LCD). Graefes Arch Clin Exp Ophthalmol. 2009; 247: 1081–1088 [DOI] [PubMed] [Google Scholar]
- 127. Colville DJ, Savige J. Alport syndrome. A review of the ocular manifestations. Ophthalmic Genet. 1997; 18: 161–173 [DOI] [PubMed] [Google Scholar]
- 128. Colville D, Savige J, Branley P, Wilson D. Ocular abnormalities in thin basement membrane disease. Br J Ophthalmol. 1997; 81: 373–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Payant JA, Eggenberger LR, Wood TO. Electron microscopic findings in corneal epithelial basement membrane degeneration. Cornea. 1991; 10: 390–394 [DOI] [PubMed] [Google Scholar]
- 130. Kenney MC, Nesburn AB, Burgeson RE, Butkowski RJ, Ljubimov AV. Abnormalities of the extracellular matrix in keratoconus corneas. Cornea. 1997; 16: 345–351 [PubMed] [Google Scholar]
- 131. Tuori AJ, Virtanen I, Aine E, Kalluri R, Miner JH, Uusitalo HM. The immunohistochemical composition of corneal basement membrane in keratoconus. Curr Eye Res. 1997; 16: 792–801 [DOI] [PubMed] [Google Scholar]
- 132. Sykakis E, Carley F, Irion L, Denton J, Hillarby MC. An in depth analysis of histopathological characteristics found in keratoconus. Pathology. 2012; 44: 234–239 [DOI] [PubMed] [Google Scholar]
- 133. Hatchell DL, Magolan JJ, Jr,, Besson MJ, Goldman AI, Pederson HJ, Schultz KJ. Damage to the epithelial basement membrane in the corneas of diabetic rabbits. Arch Ophthalmol. 1983; 101: 469–471 [DOI] [PubMed] [Google Scholar]
- 134. Herse PR. A review of manifestations of diabetes mellitus in the anterior eye and cornea. Am J Optom Physiol Opt. 1988; 65: 224–230 [DOI] [PubMed] [Google Scholar]
- 135. Ljubimov AV, Huang ZS, Huang GH, et al. Human corneal epithelial basement membrane and integrin alterations in diabetes and diabetic retinopathy. J Histochem Cytochem. 1998; 46: 1033–1041 [DOI] [PubMed] [Google Scholar]
- 136. Azar DT, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Altered epithelial-basement membrane interactions in diabetic corneas. Arch Ophthalmol. 1992; 110: 537–540 [DOI] [PubMed] [Google Scholar]
- 137. Kenney MC, Zorapapel N, Atilano S, Chwa M, Ljubimov A, Brown D. Insulin-like growth factor-I (IGF-I) and transforming growth factor-beta (TGF-beta) modulate tenascin-C and fibrillin-1 in bullous keratopathy stromal cells in vitro. Exp Eye Res. 2003; 77: 537–546 [DOI] [PubMed] [Google Scholar]
- 138. Maseruka H, Ataullah SM, Zardi L, Tullo AB, Ridgway AE, Bonshek RE. Tenascin-cytotactin (TN-C) variants in pseudophakic/aphakic bullous keratopathy corneas. Eye (Lond). 1998; 12 (pt 4): 729–734 [DOI] [PubMed] [Google Scholar]
- 139. Quantock AJ, Meek KM, Brittain P, Ridgway AE, Thonar EJ. Alteration of the stromal architecture and depletion of keratan sulphate proteoglycans in oedematous human corneas: histological, immunochemical and X-ray diffraction evidence. Tissue Cell. 1991; 23: 593–606 [DOI] [PubMed] [Google Scholar]
- 140. Akhtar S, Bron AJ, Hawksworth NR, Bonshek RE, Meek KM. Ultrastructural morphology and expression of proteoglycans, betaig-h3, tenascin-C, fibrillin-1, and fibronectin in bullous keratopathy. Br J Ophthalmol. 2001; 85: 720–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kenney MC, Chwa M, Alba A, Saghizadeh M, Huang ZS, Brown DJ. Localization of TIMP-1, TIMP-2, TIMP-3, gelatinase A and gelatinase B in pathological human corneas. Curr Eye Res. 1998; 17: 238–246 [DOI] [PubMed] [Google Scholar]
- 142. Kenney MC, Chwa M, Lin B, Huang GH, Ljubimov AV, Brown DJ. Identification of cell types in human diseased corneas. Cornea. 2001; 20: 309–316 [DOI] [PubMed] [Google Scholar]
- 143. Alarcon I, Kwan L, Yu C, Evans DJ, Fleiszig SM. Role of the corneal epithelial basement membrane in ocular defense against Pseudomonas aeruginosa. Infect Immun. 2009; 77: 3264–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Huard J, Feero WG, Watkins SC, Hoffman EP, Rosenblatt DJ, Glorioso JC. The basal lamina is a physical barrier to herpes simplex virus-mediated gene delivery to mature muscle fibers. J Virol. 1996; 70: 8117–8123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Romoser WS, Turell MJ, Lerdthusnee K, et al. Pathogenesis of Rift Valley fever virus in mosquitoes--tracheal conduits & the basal lamina as an extra-cellular barrier. Arch Virol Suppl. 2005; 1: 89–100 [DOI] [PubMed] [Google Scholar]
- 146. Weeks BS, Ramchandran RS, Hopkins JJ, Friedman HM. Herpes simplex virus type-1 and -2 pathogenesis is restricted by the epidermal basement membrane. Arch Virol. 2000; 145: 385–396 [DOI] [PubMed] [Google Scholar]
- 147. Barbosa FL, Chaurasia SS, Kaur H, de Medeiros FW, Agrawal V, Wilson SE. Stromal interleukin-1 expression in the cornea after haze-associated injury. Exp Eye Res. 2010; 91: 456–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kaur H, Chaurasia SS, Agrawal V, Suto C, Wilson SE. Corneal myofibroblast viability: opposing effects of IL-1 and TGF beta1. Exp Eye Res. 2009; 89: 152–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res. 2006; 82: 788–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Mathew JH, Goosey JD, Bergmanson JP. Quantified histopathology of the keratoconic cornea. Optom Vis Sci. 2011; 88: 988–997 [DOI] [PMC free article] [PubMed] [Google Scholar]