Abstract
Many insects rely on bacterial symbionts with tiny genomes specialized for provisioning nutrients lacking in host diets. Xylem sap and phloem sap are both deficient as insect diets, but differ dramatically in nutrient content, potentially affecting symbiont genome evolution. For sap-feeding insects, sequenced symbiont genomes are available only for phloem-feeding examples from the suborder Sternorrhyncha and xylem-feeding examples from the suborder Auchenorrhyncha, confounding comparisons. We sequenced genomes of the obligate symbionts, Sulcia muelleri and Nasuia deltocephalinicola, of the phloem-feeding pest insect, Macrosteles quadrilineatus (Auchenorrhyncha: Cicadellidae). Our results reveal that Nasuia-ALF has the smallest bacterial genome yet sequenced (112 kb), and that the Sulcia-ALF genome (190 kb) is smaller than that of Sulcia in other insect lineages. Together, these symbionts retain the capability to synthesize the 10 essential amino acids, as observed for several symbiont pairs from xylem-feeding Auchenorrhyncha. Nasuia retains genes enabling synthesis of two amino acids, DNA replication, transcription, and translation. Both symbionts have lost genes underlying ATP synthesis through oxidative phosphorylation, possibly as a consequence of the enriched sugar content of phloem. Shared genomic features, including reassignment of the UGA codon from Stop to tryptophan, and phylogenetic results suggest that Nasuia-ALF is most closely related to Zinderia, the betaproteobacterial symbiont of spittlebugs. Thus, Nasuia/Zinderia and Sulcia likely represent ancient associates that have co-resided in hosts since the divergence of leafhoppers and spittlebugs >200 Ma, and possibly since the origin of the Auchenorrhyncha, >260 Ma.
Keywords: gene loss, genome evolution, leafhopper, nutritional symbioses, Nasuia deltocephalinicola, Sulcia muelleri
Introduction
Obligate symbioses with bacteria have enabled insects to exploit novel niches, previously out of adaptive reach (Moran 2007). Prominent examples include plant sap-feeding insects in the order Hemiptera that have evolved partnerships with a diverse consortium of bacterial symbionts (Buchner 1965). By providing essential nutrients lacking in xylem sap or phloem sap, these relationships have permitted insect hosts to tap into these specialized diets (Buchner 1965; Baumann 2005; Moran et al. 2008). Symbiont acquisition has thereby enabled the Hemiptera to diversify into a species-rich and geographically ubiquitous insect order.
Specialization on xylem or phloem in sap-feeding Hemiptera is a conserved trait, corresponding to ancient clades (Grimaldi and Engel 2005; Cryan and Urban 2012). Both diet types are deficient in most essential amino acids and many cofactors, but otherwise they have vastly different nutritive qualities (Mattson 1980; Andersen et al. 1989; Sandström and Petterson 1994; Sandström and Moran 1999). In general, phloem is more nutritious, with 10-fold greater levels of available organic nitrogen and a 1000-fold greater level of carbohydrates, mostly sugars (Mattson 1980; Andersen et al. 1989). However, the influence of phloem versus xylem sap diets on the evolution of symbioses remains unknown. Because phloem-feeding systems have been studied only in the hemipteran suborder, Sternorrhyncha (e.g., Buchnera-aphid symbiosis; Shigenobu and Wilson 2011), and xylem-feeding examples only in the Cicadomorpha (McCutcheon and Moran 2010), which is one of the two clades comprising the hemipteran suborder Auchenorrhyncha, diet type and phylogenetic relationships are confounded in comparisons.
Species in the Auchenorrhyncha rely on two obligate symbionts: Sulcia muelleri (Bacteroidetes), which has been present within Auchenorrhyncha since it emerged 260–280 Ma (Moran et al. 2005), and an array of co-primary symbionts from different bacterial divisions (fig. 1). In several groups of xylem feeders, genomes of Sulcia and its partner symbiont have been sequenced; these include cicadas (Sulcia plus Hodgkinia cicadicola [Alphaproteobacteria]; McCutcheon et al. 2009b), spittlebugs (Sulcia plus Zinderia insecticola [Betaproteobacteria]; McCutcheon and Moran 2010; Koga et al. 2013), and sharpshooter leafhoppers (Sulcia plus Baumannia cicadellinicola [Gammaproteobacteria]; Wu et al. 2006). In each case, Sulcia can produce most of the 10 essential amino acids, but is unable to produce histidine or methionine. And, in each case, these missing pathways are perfectly complemented by those encoded in the co-primary symbionts (reviewed by McCutcheon and Moran 2010). This is in contrast to the aphid-Buchnera model system in which the provisioning of all 10 essential amino acids is accomplished by a single obligate symbiont (Shigenobu et al. 2000, reviewed by Baumann 2005). Like aphids, most groups of Auchenorrhyncha are phloem feeders, including the treehoppers and most leafhopper groups (Membracoidea: Membracidae, most Cicadellidae), and most planthopper groups (e.g., Fulgoroidea: Cixiidae, Delphacidae, Flatidae, Fulgoridae) (Dietrich 2003). Most of these are also host to dual bacterial symbionts (fig. 1), based on microscopy and 16S rRNA screening (Buchner 1965; Noda et al. 2012; Urban and Cryan 2012; Koga et al. 2013), but to date none has been studied using genomic approaches.
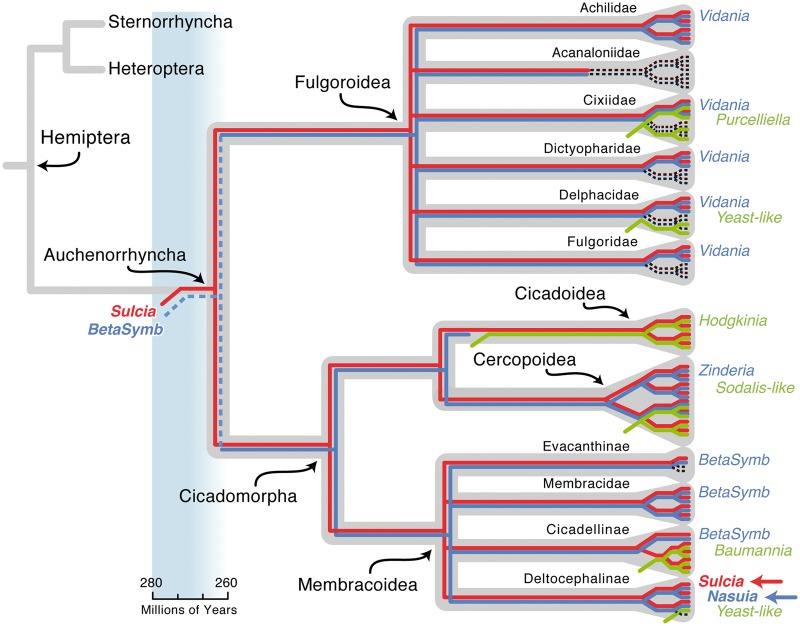
Fig. 1.—
Schematic summary of the evolution of symbiotic associations in major lineages of Auchenorrhyncha, with emphasis on the Cicadomorpha (cicadas, spittlebugs, leafhoppers, and treehoppers). The focal symbionts, Nasuia and Sulcia from Macrosteles quadrilineatus (Cicadellidae: Deltocephalinae), are demarcated with a blue and red arrow, respectively. Phylogenies show evolutionary events in symbioses based on molecular phylogenetic studies (Moran et al. 2003, 2005; Takiya et al. 2006; Urban and Cryan 2012; Noda et al. 2012; Bressan and Mulligan 2013; Hou et al. 2013; Koga et al. 2013). Events are color-coded as follows: Host insect phylogeny (from Cryan and Urban 2012) gray background; Sulcia, red; betaproteobacterial symbiont lineage (BetaSymb = Zinderia + Nasuia + Vidania), blue; symbiont loss, black dashed line; and symbiont replacement, green. Identified symbiont names are given at the tips. This schematic is not a full account of host relationships and symbiont associations, as many species and genera remain to be explored, and some inferred losses may reflect incomplete knowledge. Sulcia is hypothesized to have been associated with the Auchenorrhyncha since its emergence 260–280 Ma (see time scale and corresponding blue box; Moran et al. 2005). Although further evidence is required to test the hypothesis that the Auchenorrhyncha share a common betaproteobacterial symbiont (BetaSymb) that was acquired early in its diversification, this potential relationship is shown here with a blue dashed line. The hypothesis that the Cicadomorpha do share the closely related BetaSymb (Zinderia + Nasuia), as evidenced by our genomic and phylogenetic results, is shown in a solid blue line (as is Vidania found throughout the Fulgoroidea based on Urban and Cryan 2012).
A central aspect of symbiont evolution is extreme genomic reduction resulting from maternal transmission and the internment of symbionts within specialized host cells (bacteriocytes) for millions of years. Repeatedly, symbionts lose pathways and genes involved in every functional category, and particularly in functions related to cellular regulation, DNA repair, cell envelope biosynthesis, and secondary metabolism (Shigenobu et al. 2000; Tamames et al. 2007; Moran et al. 2008; McCutcheon and Moran 2010, 2012). Indeed, auchenorrhynchan symbionts possess the smallest known cellular genomes. In some cases, these genomes appear insufficient to support cellular life (e.g., McCutcheon and von Dohlen 2011; McCutcheon and Moran 2012), yet they retain a subset of genes for synthesizing essential amino acids required by hosts (McCutcheon and Moran 2012).
The similarity in amino acid biosynthetic abilities of different Sulcia genomes suggests that Sulcia lost pathways for biosynthesis of methionine and histidine early during the diversification of the Auchenorrhyncha. Sulcia has continuously maintained associations with symbionts providing these functions, even as these partners were sometimes replaced over evolutionary time in particular lineages (fig. 1; Buchner 1965; Moran et al. 2005; McCutcheon and Moran 2010). It remains unclear whether the original partner of Sulcia persists in any modern Auchenorrhyncha. However, Zinderia of spittlebugs, Nasuia deltocephalinicola of deltocephaline leafhoppers (Noda et al. 2012), and Vidania fulgoroideae of fulgorid planthoppers (Gonella et al. 2011) form a clade within Betaproteobacteria in 16S rDNA-based phylogenies. This BetaSymb lineage has been proposed to represent the ancestral partner of Sulcia that has been replaced in several descendant lineages of the Auchenorrhyncha, perhaps in response to dietary or ecological shifts by hosts (see fig. 1; Koga et al. 2013). Conclusions from sequence-based phylogenies are hampered by extreme acceleration in sequence evolution and extreme nucleotide base compositional biases of symbionts. Shared genomic features across Zinderia, Nasuia, and Vidania could provide more definitive support for BetaSymb as a clade and as an ancestral symbiont of Auchenorrhyncha. But, to date, only a single Zinderia genome has been available.
To address the questions of 1) the pattern of genome evolution in dual symbionts of a phloem-feeding host, and 2) the origin for the BetaSymb lineage, we have sequenced the complete genomes of symbionts of the Aster Leafhopper (ALF), Macrosteles quadrilineatus (Cicadellidae: Deltocephalinae). This species is a widespread pest of North American agriculture (Frost et al. 2011). As found in other members of Deltocephalinae, it harbors Sulcia and the betaproteobacterial co-primary symbiont, Nasuia (Noda et al. 2012; Koga et al. 2013).
Materials and Methods
Material Acquisition and Sequencing
Samples of M. quadrilineatus were field-collected from Yale West Campus, West Haven, CT, USA, on Symphyotrichum lanceolatum (Asteraceae). Specimens were identified according to Kwon (1988) and with the barcoding mitochondrial locus Cytochrome Oxidase I (following Le Roux and Rubinoff 2009). In order to confirm the bacteriome association of the targeted bacterial lineages, whole-specimen Fluorescent in situ Hybridization was performed following Koga et al. (2009). Fluorescing probes were designed as follows: Bet940 (5′-TTAATCCACATCATCCACCG-3′) for the Betaproteobacteria labeled with 5′ AlexaFlour-555 probe modification, and Sulc664R (5′-CCMCACATTCCAGMTACTCC-3′) for Sulcia, labeled with a 5′ AlexaFlour-647 modification (Invitrogen). Briefly, specimens were dissected in 70% EtOH, fixed in Carnoy’s solution, and incubated in alcoholic hydrogen peroxide for 2 weeks. Material was then rinsed and stored in 100% ethanol, rehydrated in PBSTx, and rinsed with a hybridization buffer. Specimens were incubated in a hybridization-probe solution, containing the oligo-probes and DAPI counterstain. Specimens were imaged with a Nikon Eclipse TE2000-U epifluorescence microscope (fig. 2).
Fig. 2.—
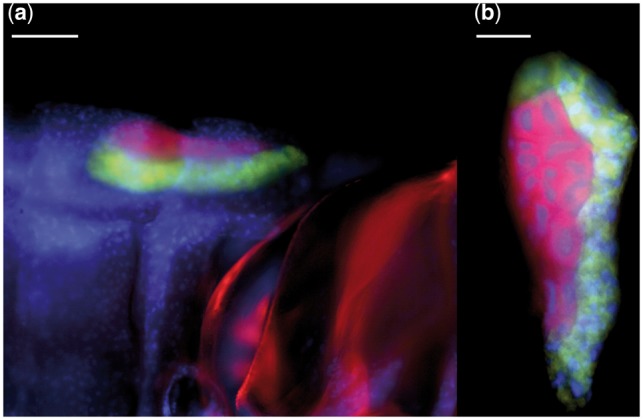
Fluorescence in situ hybridization of the bacteriome of Macrosteles quadrilineatus (Cicadellidae: Deltocephalinae), housing Sulcia muelleri (green; Bacteroidetes), and Nasuia deltocephalinicola (red; Betaproteobacteria). Blue fluorescence shows host nuclear DNA. (a) Localization of the bacteriome along the anterolateral abdominal segments. (b) The dissected bacteriome comprising two distinct bacteriocyte types that house Sulcia-ALF and Nasuia-ALF. White scale bars equal 100 μm (a) and 50 μm. (b) Note.—Red autofluorescence of the host thoracic plates.
Ten individual insects were pooled for genomic DNA (gDNA) sequencing. Total gDNA was extracted using Qiagen DNeasy extraction kit (Qiagen Corp.) in a 50 μl volume. Genomic sequencing was done using Illumina’s MiSeq platform. gDNA was sheared into library insert sizes of 500 bp, and library amplification (eight polymerase chain reaction [PCR] cycles) was performed with high-fidelity polymerase (KAPA Bio HiFi) to reduce known problems of biased amplification in AT-rich symbiont genomes (Aird et al. 2011; Sloan and Moran 2012a). The prepared library was sequenced using paired-end orientation on a 2 × 250 bp lane. Library preparation and sequencing were done at the Yale Center for Genome Analysis.
Assembly
Because sequenced samples include a complex metagenomic mixture of host and bacterial symbionts, we implemented a multi-tiered approach to produce preliminary genome contigs, collect associated bacterial reads, and reassemble and improve scaffolds (Sloan et al. 2013). A first pass assembly was done on 1/16th of the read data (1,937,157 reads) using Velvet v1.2.08 with a kmer length of 63 (Zerbino and Birney 2008). Whole genome assembly returned nearly 12,000 contigs greater than 500 bp, the majority of which had relatively low kmer coverage. Velvet yielded 10 large contigs of over 10 kb in length, which were identified using BlastN searches against the NCBI nr database. The three largest contigs, one at 190 kb and two at ∼56 kb, were unambiguously assigned to Sulcia and to Betaproteobacteria, respectively (supplementary fig S1, Supplementary Material online). We hereafter refer to these symbiont lineages as Sulcia-ALF (of the Aster Leafhopper) and Nasuia-ALF. The remaining large contigs represent insect nuclear DNA and the complete mitochondrial genome.
To remove symbiont-derived reads from the total read pool, data were mapped to the Velvet contigs using SOAP2 v2.21 (settings: -m 10 -×1000 -g 3 -r 2; Li et al. 2009). Isolated symbiont reads were then reassembled using MIRA (Chevreux et al. 1999) with paired-end insert size set to 100–600 bp and normal quality settings. MIRA confirmed the 190 kb scaffold of Sulcia-ALF, but broke Nasuia-ALF sequences into three large contigs: 12, 42, and ∼54 kb. MIRA also produced a 5 kb contig including a 1.1 kb tandem repeat that assembled with the ends of the two largest contigs, overlapping each end by over 450 bp. The Velvet and Mira contigs were assembled into a consensus scaffold that filled most ambiguous sections introduced by Velvet (e.g., strings of Ns). This merged the two largest Nasuia-ALF contigs, leaving two large scaffolds of ∼102 kb and ∼12 kb in length. These were assembled using PCR and Sanger sequencing (primers: 98836F-ACGCCGCCGAGAATAAGTG and 100182R-ATACACAACGCGCCCTTCG). The remaining ends of the Nasuia-ALF scaffold contained a 400 bp overlap that was further supported by paired-end read data that bridged the two ends.
To assess the quality of the merged scaffolds, to improve genome assemblies, and to determine overall coverage for the symbiont genomes, total read data were mapped to the symbiont scaffolds using SOAP2. Read mapping was run iteratively until it converged on a consensus genome map, using custom Perl scripts (Sloan and Moran 2012a). This corrected indels and base pair substitutions within initial assemblies. To verify complete coverage of the circular bacterial genomes, scaffolds were broken, reoriented, and reads were remapped to confirm closure. Sulcia-ALF read coverage was relatively uniform, whereas Nasuia-ALF mapping revealed a coverage spike of roughly 3-fold, starting at the nucleotide position 55,964, and supporting the potential repeat region assembled by Mira. The 1.1 kb repeat is too large to verify with the 250 bp reads, so PCR amplification and Sanger sequencing of the region was used to confirm the boundaries where the assembly initially broke (primers: 55201F-ATTCGCCTCATCCCCTCTCG + 56233R-AGCCATAACCTTTTGCGTCGT) and to confirm the existence of the internal repeat with inverse primers (primers: 56954F-AAATTAAGAGAGCTAGG + 56087R-TTTAACATCTATCGCTTG). The copy number of genomic repeats is difficult to verify and likely varies within insect hosts and within the symbionts that are polyploid (see Woyke et al. 2010), as has been shown in Portiera, the endosymbiont of whiteflies (Sloan and Moran 2013). Thus, the reported genome includes a single copy of the repeating motif flagged with a repeat annotation.
To determine whether other symbionts are present in the samples, and to search for potentially unintegrated Sulcia-ALF and Nasuia-ALF sequences, Velvet contigs longer than 500 bp were identified using translated Blastx searches against the NCBI nr database and taxonomically binned with MEGAN4 (Huson et al. 2011). A vast majority of the reads were placed in insect groups or were unassignable; these likely represent high copy insect genomic regions. Twenty-four contigs (500–700 bp in length) were close matches to regions of the genome of Arsenophonus nasoniae (Gammaproteobacteria). Arsenophonus is a cluster of bacterial symbionts present in many insect groups and in some plants (Nováková et al. 2009). Given the extremely fragmented genomic contigs for Arsenophonus and its low relative coverage, it is unlikely to represent an obligate primary symbiont of Macrosteles.
Genome Annotation
First pass annotations were done with RAST and the Joint Genome Institute IMG ER pipelines (Aziz et al. 2008; Markowitz et al. 2009), using the general bacterial translation table. These pipelines produced a relatively complete annotated Sulcia-ALF genome sequence, but yielded many unidentifiable short open reading frames (ORFs) for Nasuia-ALF. Previous research indicated that the small genomes of Hodgkinia and Zinderia use an alternative genetic code that has reassigned the UGA stop codon to tryptophan (McCutcheon et al. 2009b; McCutcheon and Moran 2010). To see if this is the case for Nasuia, Glimmer3 was used to predict coding regions with the modified translation table 4 (Delcher et al. 2007). Use of the alternative table extended many previously truncated protein-coding genes to their expected lengths, providing strong evidence that Nasuia uses a UGA Stop → Trp recoding. Many genes utilize UGA-Trp codons at UGG-Trp conserved in other species with the standard bacterial code (e.g., hisI, rpoB, dnaN, etc.; see also McCutcheon and Moran 2010). All inferred genes for both symbionts were manually checked and curated using phmmer and hmmerscan searches with available databases (NCBI nr, TIGRfam, SwissProt, and Pfam) in HMMER3 (Finn et al. 2011). The EcoCyc and KEGG databases were used further to determine gene pathways and function (Moriya et al. 2007; Keseler et al. 2011). RNA coding regions were identified using RNAammer, tRNAscan-SE, and searches against the Rfam database (Schattner et al. 2005; Lagesen et al. 2007; Burge et al. 2012). Genomes were manually annotated with Geneious Pro v5.5.8 (Drummond et al. 2010).
Phylogenomics
To infer phylogenetic relationships of Nasuia-ALF, we used Phyla_Amphora2, which identifies and aligns conserved genomic markers from a set of phylum-specific genes (Wang and Wu 2013). Conserved orthologs were determined using Amphora’s marker scan with HMMER3 (E-value cutoff of 0.01), which generated a 42 gene amino acid alignment (approx. 10k columns) for 102 Betaproteobacterial taxa (supplementary table S1, Supplementary Material online). The Betaproteobacterial symbiont of the mealybug Planococcus citri, Tremblaya princeps (placed in Betaproteobacteria: Burkholderiaceae; see Alves et al. 2013), was not included in the analysis as many of its genes were missing and our match threshold excluded nearly half of the available genes. Four outgroups were selected from Gammaproteobacteria and Alphaproteobacteria, and homologous genes were extracted using Blastp searches. Individual gene sets were aligned using MAFFT v7.017 with the L-INS-i model (Katoh and Standley 2013), and manually curated to remove large gaps and ambiguously aligned regions. Amino acid likelihood substitution models were determined for each gene matrix with ProtTest3 (Darriba et al. 2011). Phylogenetic trees were inferred using maximum likelihood criteria with RAxML v7.4.4 (Stamatakis 2006). A concatenated matrix was partitioned by loci and assigned gene-specific GTR-based amino acid model. Tree searches were conducted for 1000 bootstraps with a final maximum likelihood search.
Results
Genome Assembly
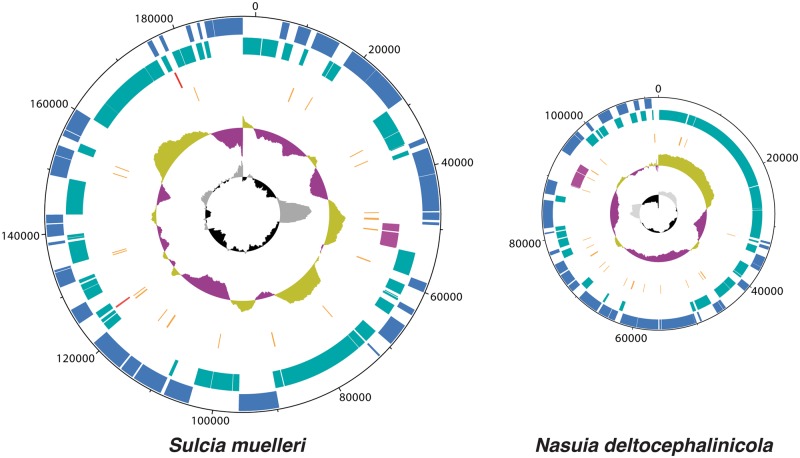
The Illumina MiSeq run yielded 30,994,516 quality-filtered reads. Sequence assembly and PCR verification produced complete genomes for Sulcia-ALF and Nasuia-ALF, with each genome comprising roughly 1.3% of total sequencing data. Coverage mapping of the merged assemblies for Sulcia-ALF and the Nasuia-ALF produced unbroken scaffolds with high mean sequencing depths of 500× and 850×, respectively. Genome sizes for Sulcia-ALF and Nasuia-ALF are 190,733 and 112,091 bp (counting only a single copy of the 1.1 kb tandem repeat; see Materials and Methods), respectively. Both are the smallest in their phyla, and Nasuia-ALF represents the smallest bacterial genome reported to date (fig. 3; McCutcheon and Moran 2012). The GC content of both symbionts is low: Sulcia-ALF at 24.0% and Nasuia-ALF at 17.1%.
Fig. 3.—
Genome map of the co-primary symbionts of Macrosteles quadrilineatus: Sulcia-ALF (left) and Nasuia-ALF (right). Genomes are scaled relative to nucleotide count. The outermost track (blue) shows genes coded on the plus strand, whereas the adjacent tracks (teal) show genes coded on the minus strand. The other tracks show RNA genes, including the rRNA operons (purple), ncRNA (red), and tRNA (orange). The two graphs show GC skew (outermost: green = values above genome average, purple = below genome average) and GC content (innermost: gray = values above genome average, black = below genome average). Plots were created with DNAPlotter (Carver et al. 2009).
Sulcia: Gene Loss in a Stable Symbiont
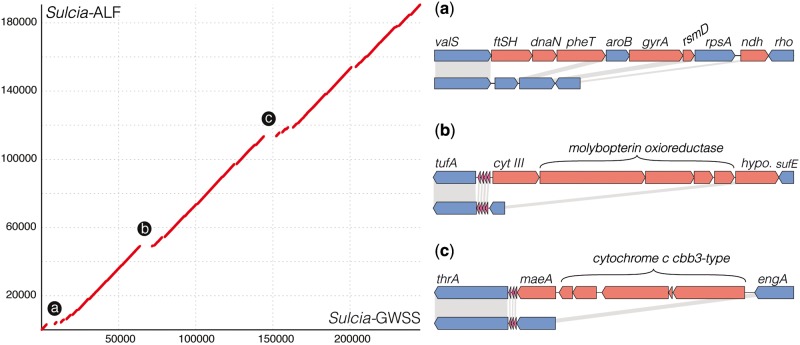
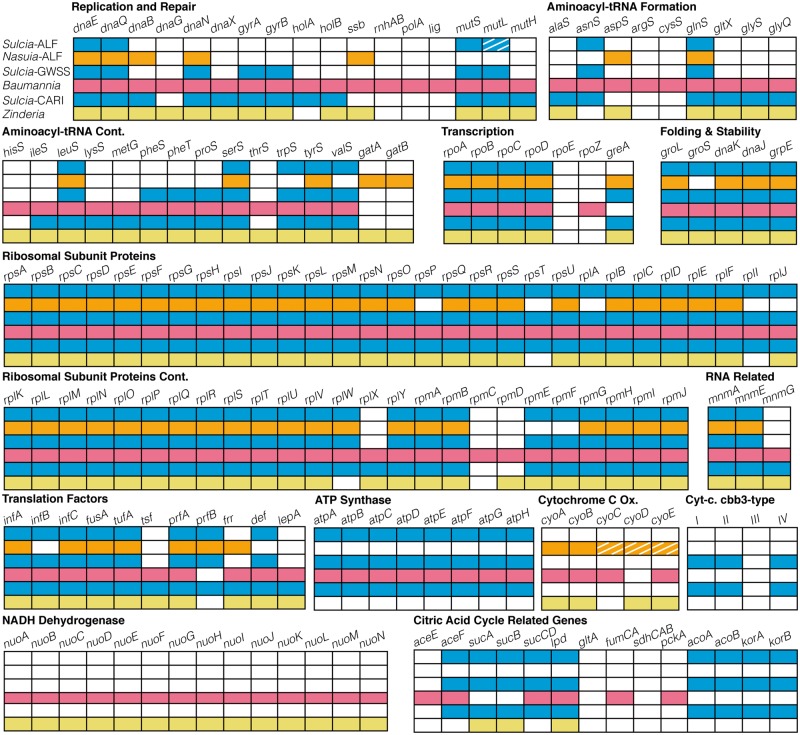
Sulcia-ALF contains a genome of only 190 kb, whereas previous Sulcia genomes, from several xylem-feeding auchenorrhynchan species, are 245–280 kb in size (fig. 4; McCutcheon and Moran 2007, 2010; McCutcheon et al. 2009a). The genome is perfectly syntenic with those of other Sulcia lineages; however, protein-coding content is dramatically reduced (figs. 4 and 5). Sulcia-ALF encodes 190 predicted protein-coding genes (five hypothetical and two pseudogenes), a single ribosomal RNA operon, noncoding RNA (rnpB), ssra RNA, and 30 tRNAs capable of recognizing all codons (fig. 5 and supplementary table S2, Supplementary Material online). In contrast, Sulcia of the sharpshooter leafhoppers and cicadas contain 226–228 and 246 genes, respectively (figs. 4 and 5; McCutcheon and Moran 2007; McCutcheon et al. 2009a; Woyke et al. 2010). Compared with previously sequenced Sulcia genomes, the Sulcia-ALF genome retains the same set of orthologs for the synthesis of eight essential amino acids: leucine, isoleucine, threonine, lysine, arginine, tryptophan, phenylalanine, and valine (fig. 5) (McCutcheon and Moran 2007, 2010; McCutcheon et al. 2009a; Woyke et al. 2010).
Fig. 4.—
Synteny plot showing gene order conservation and gene loss between Sulcia-ALF (Macrosteles quadrilineatus) and Sulcia-GWSS (Homalodisca coagulata). Axes are in the number of nucleotides. Lettered breaks correspond to gene maps on the right hand side. Plots a–c show specific gene loss between Sulcia-GWSS (top) and Sulcia-ALF (bottom). Conserved orthologs are colored blue connected with gray shading, whereas genes that are lost in Sulcia-ALF are shown in red.
Fig. 5.—
Genomic content of the co-primary symbionts from the leafhoppers, Macrosteles quadrilineatus (Sulcia-ALF and Nasuia-ALF) and Homalodisca coagulata (Sulcia-GWSS and Baumannia); and, the spittlebug, Clastoptera arizonana (Sulcia-CARI & Zinderia). The major pathways include DNA replication, DNA repair, translation and processing, protein stability, and energy metabolism. Colored boxes indicate genes that are present, and white boxes indicate gene absence. Striped boxes indicate genes that are present, but possibly pseudogenized, or with uncertain function.
Sulcia-ALF has lost many genes underlying oxidative phosphorylation (OXPHOS) and DNA replication and repair (figs. 4 and 5). It lacks nearly all OXPHOS-related genes, including those encoding the complete cytochrome C oxidase complex, NADH dehydrogenase, menaquinone and ubiquinone biosynthesis proteins, and the molybdopterin oxioreductase complex that is involved in the terminal electron acceptor, DMSO reductase (Kisker et al. 1999). Sulcia-ALF does, however, retain genes encoding F-Type ATP synthase machinery that could function to synthesize ATP from the built-up electrochemical gradient generated by missing enzymes. In the absence of most protein complexes in the OXPHOS pathway, its function remains unclear.
Further gene losses involve DNA replication and repair (figs. 4 and 5), including genes encoding the DNA polymerase III beta (DnaN) subunit and DNA gyrase (GyrAB), which are responsible for the efficient replication of DNA by clamping the polymerase and relaxing coiling ahead of the DNA helicase (DnaB), respectively (Johanson and McHenry 1980; Sugino et al. 1980). Based on studies in model systems, these losses are expected to reduce replication efficiency (Burgers and Kornberg 1982; Smelkova and Marians 2001). Additionally, Sulcia-ALF retains only a single gene (mutS) encoding a subunit of the methyl-directed DNA mismatch repair complex, which is responsible for correcting mismatches and frame-shift associated insertions and deletions in Escherchia coli (Schofield and Hsieh 2003; Polosina et al. 2009). The gene mutL is pseudogenized and is identifiable in Sulcia-ALF as a 135 bp segment, whereas homologs in other Sulcia lineages are 1.1 kb in length (McCutcheon et al. 2009a).
Nasuia: The Functions of the Smallest Symbiont Genome
The Nasuia-ALF genome is 112 kb in size, 27 kb smaller than the next smallest known bacterial genome, that of Tremblaya princeps, a symbiont of the mealybug P. citri (139 kb; fig. 3; McCutcheon and von Dohlen 2011), and 30 kb smaller than the genome of Hodgkinia cicadicola, the symbiont that co-resides with Sulcia in cicadas (143 kb; McCutcheon et al. 2009b). The Nasuia-ALF genome encodes 137 predicted protein-coding genes, a single ribosomal operon, and 29 tRNAs predicted to be capable of recognizing all necessary codons (fig. 5 and supplementary table S3, Supplementary Material online). Fifteen predicted ORFs are unassignable to class or function. Nasuia-ALF contains a 1.1 kb tandem repeat that encodes part of MetF, a hypothetical protein, and a cold shock protein (CspA). Similar large genomic repeats (ranging from 150 bp to 6.1 kb) and major structural inversions have been found in other reduced symbiont genomes including those of Carsonella (psyllids), Portiera (whiteflies), and Tremblaya (McCutcheon and von Dohlen 2011; Sloan and Moran 2012b, 2013).
Nasuia-ALF uses an alternative genetic code in which UGA is reassigned from Stop to Trp. Roughly 51% of all protein-coding genes contain at least one UGA codon. This reassignment also has been found in two other bacterial symbionts, Zinderia and Hodgkinia (McCutcheon et al. 2009b; McCutcheon and Moran 2010). Nasuia-ALF is further missing prfB encoding the peptide release RF2 that is responsible for recognizing the UGA stop codon (Capecchi 1967), as also observed in Zinderia and Hodgkinia.
Nasuia-ALF retains the pathways for synthesizing both histidine and methionine, as found in other symbionts that co-reside with Sulcia (McCutcheon and Moran 2010). Nasuia-ALF’s histidine pathway is complete. For methionine biosynthesis, Nasuia-ALF uses a cobalamin-independent direct sulfhydrylation pathway as previously found for Zinderia (McCutcheon and Moran 2010). Both retain CysHI enzymes for the sulfhydrylation pathway and methionine synthase (MetE; Hacham et al. 2003; McCutcheon and Moran 2010). Although both symbionts retain genes encoding homoserine acetyltransferase (MetX), they appear to use two different sulfhydrylases: Nasuia-ALF encodes succinylhomoserine sulfhydrylase ortholog (MetB), whereas Zinderia uses acetyl-homoserine ortholog (MetY; McCutcheon and Moran 2010). Nasuia-ALF appears able to initiate acetyl-driven methionine biosynthesis initiated by MetX, but not the succinyl alternative. MetB is a versatile enzyme shown to utilize multiple homoserine-esterified intermediates, including both succinylhomoserine and acetylhomoserine (Hacham et al. 2003), and it may function to catabolize the latter. The plant-sap diet seems unlikely to furnish the missing metabolites, as plants use a phosphorylhomoserine pathway (Macnicol et al. 1981), and it has been suggested that the requisite homoserine is obtained from Sulcia’s threonine synthesis pathways (McCutcheon and Moran 2007).
Nasuia-ALF retains few other large pathways, but does retain genes underlying basic processes of replication, transcription, and translation, including genes encoding DNA and RNA polymerase subunits, RNA modification enzymes, ribosomal proteins, and some tRNA synthetases (fig. 5). It appears able to perform a limited part of the OXPHOS pathway. It encodes the machinery to synthesize and assemble a cytochrome C oxidase, including both precursors (e.g., CcsB) and assembly proteins (e.g., SCO1-like proteins), although many of these proteins have incurred AT-biased substitutions and are difficult to identify specifically, or may be pseudogenes. Finally, Nasuia-ALF retains genes encoding several enzymes that function in the metabolism of carbohydrate and sugars, including flavodoxin reductase (Fpr) and phosphoglycerate mutase (GpmA); however, these pathways are greatly reduced and appear incomplete. Other gene losses have left Nasuia without a complete set of aminoacyl tRNA synthetases (Nasuia-ALF has lost 14 additional genes for tRNA synthetases relative to Zinderia), most TCA cycle-related genes (e.g., sucAB and lpd), and genes for several components of the DNA replication machinery (dnaG, dnaX, and holB).
Phylogenomics
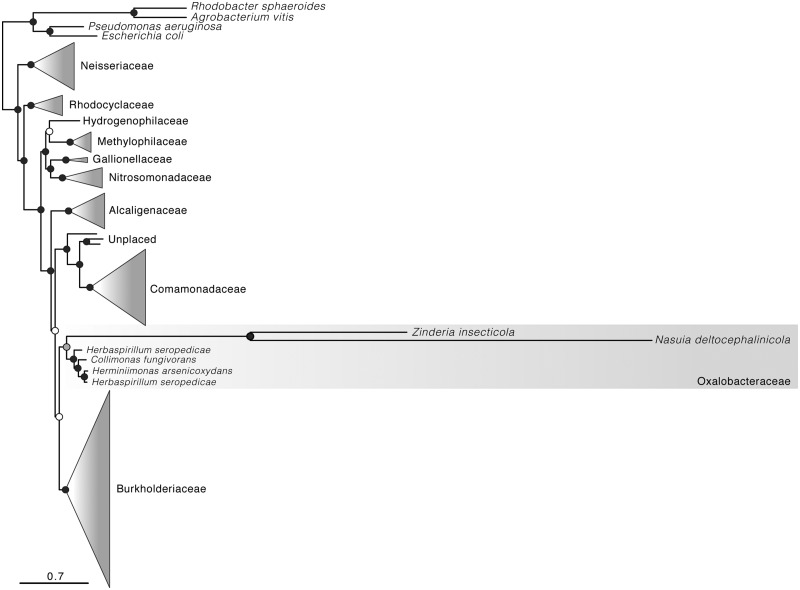
Phylogenetic analyses based on amino acid sequences of 42 proteins place Zinderia and Nasuia as sister lineages with high bootstrap support (BS = 100; fig. 6). Both lineages exhibit high levels of sequence divergence relative to other species of Betaproteobacteria. Both are placed within the family Oxalobacteriaceae (BS = 75). This placement was supported in previous phylogenetic results for Betaproteobacteria, including analyses based on 16S rRNA including Zinderia and Nasuia representatives (Noda et al. 2012; Koga et al. 2013) and analyses based on proteins from genome sequences including only Zinderia (McCutcheon and Moran 2010; Alves et al. 2013).
Fig. 6.—
Relationships between Nasuia deltocephalinicola and other Betaproteobacteria. Phylogenetic tree was inferred with maximum likelihood criteria for 107 taxa for 42 proteins (approx. 10,000 amino acid columns). Circles at nodes indicate bootstrap support values: black = >75, gray = 55–75, and white = <55. See supplementary table S1, Supplementary Material online, for complete taxon sampling, and supplementary figure S2, Supplementary Material online, for full phylogeny.
Discussion
The Origins of the Auchenorrhyncha Symbionts
Macrosteles quadrilineatus is associated with two obligate symbionts, Sulcia-ALF and Nasuia-ALF, as previously observed for some other members of the Deltocephalinae (Noda et al. 2012; Ishii et al. 2013). Most groups of Auchenorrhyncha are associated with Sulcia; however, symbionts that co-reside with Sulcia vary, implying multiple evolutionary transitions in major auchenorrhynchan clades (see fig. 1; Moran et al. 2003; McCutcheon et al. 2009b; Koga et al. 2013). Phylogenomic results and other biological characteristics support a close relationship of Nasuia with Zinderia, which co-resides with Sulcia in most cercopoid spittlebugs (Koga et al. 2013). Sequence-based phylogenetic analyses should be interpreted cautiously in the inference of bacterial symbiont relationships, as apparent relationships might reflect from a combination of convergence and long branch attraction due to the extreme AT nucleotide bias, rapid sequence evolution, and ancient divergence of symbionts relative to other bacteria (see McCutcheon and Moran 2012). Despite this, our genomic results provide additional biological support for a close relationship between Nasuia and Zinderia. First, Nasuia and Zinderia share cobalamin-independent, direct methionine synthesis pathways not found in the other co-primary symbionts of the Cicadomorpha (McCutcheon and Moran 2010). Second, and more distinctively, the reassignment of UGA observed in Nasuia and Zinderia has occurred only a few times in the history of life, including the small AT-rich genomes of mycoplasmas, several mitochondrial lineages, and Hodgkinia (Yamao et al. 1985; Knight et al. 2001; McCutcheon et al. 2009b; McCutcheon and Moran 2010). The reassignment is absent from most other reduced, often AT-rich genomes, such as those of Blattabacterium, Blochmannia, Buchnera, Carsonella, Portiera, Uzinura, and Tremblaya (Shigenobu et al. 2000; Clark et al. 2001; Gil et al. 2003; McCutcheon et al. 2009b; Sabree et al. 2009, 2013; McCutcheon and von Dohlen 2011; Sloan and Moran 2012b).
Several hypotheses exist to explain the evolution of codon reassignment, including the complete extinction of the UGA stop codon and its reemergence as a Trp codon, along with UGG (Osawa and Jukes 1988). However, even small and AT-biased genomes contain many UGA Stop codons. An alternative hypothesis, proposed for Hodgkinia, is that the reassignment is triggered by an inactivating mutation in RF2 (prfB), which causes some peptides to be extended, but is not lethal (McCutcheon et al. 2009b). Such a mutation would resemble other deleterious mutations and gene inactivations that are fixed through genetic drift in reduced symbiont genomes. Selection would then favor replacing UGA stop codons with fully functional stop codons (UAA or UAG), freeing up UGA to bind with the tRNA-Trp anticodon. The tRNA-Trp may evolve to enhance the weak binding already present between its anticodon and UGA. Because all cases of this recoding occur in genomes with extensive gene loss (Nasuia, Zinderia, Hodgkinia, Mycoplasma, some mitochondrial genomes), the initial loss of prfB as a triggering event is a potential commonality.
Our results bolster support for a symbiont clade containing Nasuia and Zinderia, and suggest that this BetaSymb clade represents an ancient associate of Sulcia, with these two symbionts co-residing in hosts at least since the origin of the Cicadomorpha (figs. 1 and 6). Preliminary phylogenetic evidence further suggests that the fulgorid symbiont, Vidania, also may be derived from a common ancestor shared with Nasuia and Zinderia (Urban and Cryan 2012; Koga et al. 2013). Both Vidania and Zinderia have co-diversified with their respective host groups for at least 200 million years (McCutcheon and Moran 2010; Urban and Cryan 2012). We hypothesize that Vidania and Zinderia represent subclades of BetaSymb, and share an ancestor that was present in the shared ancestor of the fulgoroid and cicadomorph lineages prior to their split 260 Ma (see fig. 1; Scherbakov 2002). A genomic sequence of Vidania, not yet available, could provide further evidence for or against this hypothesis.
In earlier work on Auchenorrhyncha symbionts, based largely on light microscopy, Buchner (1965) and his student Müller (1940, 1962) hypothesized the existence of the symbiont later characterized with molecular data and named Sulcia (Moran et al. 2005). They further hypothesized companion symbionts for different auchenorrhynchan groups, but, lacking molecular evidence, did not recognize common ancestry of betaproteobacterial symbionts present in several auchenorrhynchan groups.
The hypothesis that Sulcia and BetaSymb were co-resident in the shared ancestor of Cicadomorpha implies that replacements of BetaSymb with alternative symbionts have occurred multiple times and at different evolutionary depths (summarized in fig. 1). These transitions include switches to Hodgkinia (Alphaproteobacteria) in cicadas and to the Sodalis-like symbiont (Gammaproteobacteria) in philaenine spittlebugs (Koga et al. 2013). Within the leafhoppers, the replacement of Nasuia with Baumannia appears to be correlated with the origin and diversification of the monophyletic xylem-feeding sharpshooters, 25–40 Ma (Moran et al. 2003; Takiya et al. 2006). Baumannia retains one of the largest obligate symbiont genomes to date, encoding nearly complete pathways for energy metabolism and vitamin biosynthesis (Wu et al. 2006), and contrasting with the reduced genome of Nasuia-ALF in other leafhoppers. Xylem feeding creates serious challenges, requiring physical and metabolic compensations to overcome negative tension and low energy content (Brodbeck et al. 1993; Zimmermann et al. 1994; Novotny and Wilson 1997). By providing new gene repertoires, the acquisition of a novel bacterial symbiont may have enabled hosts to overcome these challenges, allowing the diversification of xylem-specialized insects (Takiya et al. 2006). However, symbiont replacements and losses have sometimes also occurred in phloem-feeding lineages. For example, Scaphoideus titanus, another phloem feeder classified in the Deltocephalinae, lacks both Sulcia and Nasuia and instead possesses transovarially transmitted Cardinium (Bacteroidetes) symbionts plus a yeast-like symbiont related to those found in certain lineages of planthoppers and aphids (Sacchi et al. 2008). Some other leafhopper groups, such as the subfamily Typhlocybinae that feeds on more nutritious parenchyma plant tissues, appear to have lost their symbionts entirely (Buchner 1965; Moran et al. 2005). Nutritional aspects of these reported cases of loss or replacements have not been investigated.
Extreme Conservation of Essential Amino Acid Synthesis
A main role of these bacterial symbionts is to provision host insects with the essential amino acids that their hosts can neither synthesize, nor obtain in sufficient quantities from their plant sap diets. In general, plant sap offers low concentrations of the essential amino acids relative to the nonessential amino acids (Brodbeck et al. 1993; Sandström and Moran 1999). Essential amino acid synthesis by the symbionts of M. quadrilineatus is achieved by the conserved complementation pattern previously observed in dual symbiont systems: Sulcia-ALF synthesizes 8 of the 10 required by the host, whereas Nasuia-ALF synthesizes the remaining 2 (reviewed by McCutcheon and Moran 2010). The only exception to this pattern reported so far is in spittlebugs, where Sulcia-CARI has additionally lost the tryptophan pathway, which is retained by Zinderia along with histidine and methionine synthesis pathways (McCutcheon and Moran 2010). All previous examples of this complementation in the Auchenorrhyncha were from xylem feeders (McCutcheon and Moran 2010). Our study shows that a similar pattern occurs in phloem feeders. Although phloem is far more nutritious than is xylem, having 10- to 100-fold higher concentration of free amino acids, the amino acid profile is usually unbalanced relative to animal nutritional needs, and it varies with plant species and growth stage (Sandström and Moran 1999). A high relative concentration of single amino acids can render sap noxious or unusable to insects (Brodbeck and Strong 1987), and insects have been shown to perform better on sap in which amino acids’ profiles resemble experimentally determined nutritional requirements for insects (Dadd 1985).
The amino acid pathways in the symbionts of M. quadrilineatus, and also in the other Auchenorrhyncha, are relatively complete and do not appear to require the host or companion symbionts to contribute intermediate steps. The only proposed exception is the transfer of homoserine produced by Sulcia to its co-primary symbionts for biosynthesis of methionine (McCutcheon and Moran 2012). In contrast, in symbiont systems in the sister suborder, Sternorrhyncha (e.g., psyllids, whiteflies, aphids, and scale insects), some pathways are missing intermediate and terminal catabolic steps. For example, many primary symbionts in the suborder are missing the aspartate amino transferase (AspC) required for the final step in phenylalanine synthesis, and, with the exception of Carsonella in psyllids, they have also lost the branched-chain aminotransferase (IlvE) required to complete the synthesis of isoleucine, leucine, and valine (Shigenobu et al. 2000; Nakabachi et al. 2006; McCutcheon and von Dohlen 2011; Sloan and Moran 2012b). For the few systems for which genome sequences are available for insect hosts, host enzymes capable of completing these pathways are encoded (Wilson et al. 2010; McCutcheon and von Dohlen 2011), and these genes are upregulated in aphid bacteriocytes (Hansen and Moran 2011; Poliakov et al. 2011). In addition, the synthesis of tryptophan and/or arginine is accomplished by pathway integration between co-occurring symbionts in psyllids (Sloan and Moran 2013), the aphid Cinara cedri (Gosalbes et al. 2008), and mealybugs (McCutcheon and von Dohlen 2011). The fact that all sequenced symbionts of the Auchenorrhyncha, despite millions of years of divergence and several replacements, encode relatively autonomous pathways suggests that the host and companion symbionts cannot contribute interspersed steps in the synthesis of essential amino acids. The complementary, but separate, synthesis of amino acids in the Auchenorrhyncha may reflect the ancient segregation of symbionts into different bacteriocytes (Buchner 1965; Moran et al. 2005; Koga et al. 2013).
Finally, the difference in the methionine synthesis pathways between Zinderia and Nasuia remains difficult to interpret, as only these two genomes are available for betaproteobacterial symbionts of Auchenorrhyncha and amino acid sequence identity for both loci to available sequences is low. (Zinderia’s MetY is 55% identical to that of Herbaspirillum and Nasuia’s MetB is 35% identical to that of Burkholderia.) Given the hypothesized antiquity of Zinderia and Nasuia, it is possible that their shared ancestor had both copies and that one or the other has been lost in the different lineages. Several available genomes for Betaproteobacteria indicate that multiple copies of sulfhydrylase can be present in members of Oxalobacteraceae (family in which Zinderia and Nasuia are placed) (Pedrosa et al. 2011) and the related Burkholderiaceae (Holden et al. 2004). An alternative hypothesis, that one of the sulfhydrase genes was acquired through horizontal transfer, seems less likely, as reduced symbiont genomes generally do not acquire foreign genes and the gene sequences display no signs of foreign origin.
The Shared Loss of Energy Metabolism Genes
The most striking loss of genes from Sulcia-ALF and Nasuia-ALF involve the pathways underlying energy metabolism and particularly the OXPHOS pathway. Both symbionts retain only a single OXPHOS gene complex, and neither retain genes required to synthesize NADH dehydrogenase, which is a unique loss in M. quadrilineatus’ symbionts compared with symbionts of previously studied auchenorrhynchan species. These losses indicate that both symbionts have a reduced capacity to synthesize ATP. Although ATP demands may be reduced, as basic cellular functions have been stripped from both symbionts, ATP is required for numerous pathways in both symbionts, raising the question of how they generate energy.
Speculatively, the loss of these functions, relative to other auchenorrhynchan symbiont systems, is related to the enriched sugars and energy-rich nature of phloem. Carbohydrate concentrations in phloem are several orders of magnitude higher (∼1000×) than those in xylem (Mattson 1980). Evidence from the excreta of the xylem- and phloem-feeding insects indicates that less than 1% of xylem feeders’ waste is organic carbon, but that it may be as high as 70% in the waste of phloem feeders (van Hook et al. 1980; Andersen et al. 1989). This excess of sugar in phloem sap potentially reduces the need for OXPHOS-generated ATP in symbionts, leading to the gross reduction of these pathways in the Sulcia-ALF and Nasuia-ALF genomes. As expected under this hypothesis, the genomes of the other xylem-feeding Auchenorrhyncha do retain more complete OXPHOS pathways, including the missing NADH dehydrogenase (Wu et al. 2006; McCutcheon and Moran 2007, 2010).
In the Sternorrhyncha, OXPHOS genes are variably retained in symbiont genomes. Buchnera of aphids and Portiera of whiteflies have near-complete sets of OXPHOS-related genes, but several have lost the ATP synthase complex (e.g., Shigenobu et al. 2000; Perez-Brocal et al. 2006). Similar losses are observed in the Tremblaya–Moranella dual symbionts of the mealybug P. citri (McCutcheon and von Dohlen 2011). Sequenced genomes of Carsonella from several psyllid species reveal that they have completely lost NADH and other genes from OXPHOS protein complexes (Sloan and Moran 2012b). Sets of genes lost show some overlap between the symbiont systems of phloem-feeding Auchenorrhyncha and Sternorrhyncha, although differences are evident. Perhaps much of energy metabolism is a nonessential symbiont trait that the host is able to supplant when pathways are lost.
In order to metabolize phloem’s carbohydrate-rich diet, symbionts would need to retain the genetic mechanisms to operate the glycolytic, pyruvate metabolic, and citric acid pathways. Both Sulcia-ALF and Nasuia-ALF do possess some genes for metabolizing carbohydrates; and, for Sulcia-CARI of cicadas, several of these (e.g., gapA, lpd, sucAB) are among the most highly expressed genes based on proteome analyses (McCutcheon et al. 2009a). However, these genetic repertoires in Macrosteles’ symbionts are incomplete, calling into question whether or how well each symbiont can metabolize sugars. Possibly, the host exchanges intermediate metabolites required for carbohydrate metabolism directly with the bacteriocytes. Insects can metabolize sugars independently of their symbionts, and they maintain carbohydrate and fatty acid stores in nearby fat tissues (Kunieda et al. 2006; Arrese and Soulages 2010). In the obligate intracellular symbionts of the grain weevil, Sitophilus zeamis (Curculionidae), host-encoded sugar transport proteins are indeed expressed in bacteriocyte tissues (Heddi et al. 2005). It is hypothesized that these transporters allow sugars to enter bacteriocytes along a chemiosmotic gradient where they are metabolized in response to the carbohydrate-rich nature of the host insect’s diet (e.g., wheat; Heddi et al. 2005).
An alternative explanation is that the host may supply its symbionts with ATP directly, or that the required missing OXPHOS genes are provided by one of the symbiotic partners. Sulcia-ALF retains F-type ATP synthase, which requires an electrochemical gradient to drive the synthesis of ATP (Kasimoglu et al. 1996). However, in the absence of all other members of the OXPHOS chain, it is not clear how the electrochemical gradient is achieved by Sulcia-ALF. Possibly, the existing OXPHOS genes are shared between co-primary symbionts because Sulcia-ALF and Nasuia-ALF encode complementary components, although how these would be integrated is not clear. Proteins or protein complexes may be shared between the host and symbiont partners through the endoplasmic reticulum (ER), which is closely associated with host-derived membranes that encase intracellular symbionts (reviewed by Houk and Griffiths 1980). The loss and retention of single, yet complementary, genes in the OXPHOS pathway by Sulcia-ALF and Nasuia-ALF is a mystery, requiring further work to understand symbiont and host contributions to the metabolism of energy in this symbiosis.
Supplementary Material
Supplementary figure S1 and tables S1–S3 are available at Genome Biology and Evolution online (http://www.gbe.oxfordjournals.org/).
Acknowledgments
The authors thank John Overton at the YCGA for assistance with sequencing and Ryuichi Koga, Dan Sloan, and Philipp Engel for discussion and comments. This work was supported by the National Science Foundation (NSF) 1062363 and Yale University.
Literature Cited
- Aird D, et al. Analyzing and minimizing PCR amplification bias in Illumina sequencing libraries. Genome Biol. 2011;2:R18. doi: 10.1186/gb-2011-12-2-r18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves JMP, et al. Genome evolution and phylogenomic analysis of Candidatus Kinetoplastibacterium, the betaproteobacterial endosymbiont of Strigomonas and Angomonas. Genome Biol Evol. 2013;5:138–350. doi: 10.1093/gbe/evt012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen PC, Brodbeck BV, Mizell RF. Metabolism of amino acids, organic acids and sugars extracted from the xylem fluid of four host plants by adult Homalodisca coagulata. Entomol Exp Appl. 1989;50:149–159. [Google Scholar]
- Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol. 2010;55:207–225. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz RK, et al. The RAST server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu Rev Microbiol. 2005;59:155–189. doi: 10.1146/annurev.micro.59.030804.121041. [DOI] [PubMed] [Google Scholar]
- Bressan A, Mulligan KL. Localization and morphological variation of three bacteriome-inhabiting symbionts within a planthopper of the genus Oliarus (Hemiptera: Cixiidae) Environ Microbiol Rep. 2013;5:499–505. doi: 10.1111/1758-2229.12051. [DOI] [PubMed] [Google Scholar]
- Brodbeck B, Strong S. Amino acid nutrition of herbivorous insects and stress to host plants. In: Barbosa P, Schultz JC, editors. Insect outbreaks. New York: Academic Press; 1987. pp. 347–364. [Google Scholar]
- Brodbeck VB, Mizell RF, Andersen PC. Physiological and behavioral adaptations of three species of leafhoppers in response to the dilute nutrient content of xylem fluid. J Insect Physiol. 1993;39:73–81. [Google Scholar]
- Buchner P. Endosymbiosis of animals with plant microorganisms. New York: Wiley Interscience; 1965. [Google Scholar]
- Burge SW, et al. Rfam 11.0: 10 years of RNA families. Nucleic Acids Res. 2012;41:D226–D232. doi: 10.1093/nar/gks1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgers PM, Kornberg A. ATP activation of DNA polymerase III holoenzyme of Escherichia coli. I. ATP-dependent formation of an initiation complex with a primed template. J Biol Chem. 1982;10:11468–11473. [PubMed] [Google Scholar]
- Capecchi MR. Polypeptide chain termination in vitro: isolation of a release factor. Biochemistry. 1967;58:1144–1151. doi: 10.1073/pnas.58.3.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver T, Thomson N, Bleasby A, Berriman M, Parkhill J. DNAPlotter: circular and linear interactive genome visualization. Bioinformatics. 2009;25:119–120. doi: 10.1093/bioinformatics/btn578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevreux B, Wetter T, Suhai S. Genome sequence assembly using trace signals and additional sequence information. Computer Science and Biology: Proceedings of the German Conference on Bioinformatics (GCB) 1999;99:45–56. [Google Scholar]
- Clark MA, Baumann L, Thao ML, Moran NA, Baumann P. Degenerative minimalism in the genome of a psyllid endosymbiont. J Bacteriol. 2001;183:1853–1861. doi: 10.1128/JB.183.6.1853-1861.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JR, Urban JM. Higher-level phylogeny of the insect order Hemiptera: is Auchenorrhyncha really paraphyletic? Syst Entomol. 2012;37:7–21. [Google Scholar]
- Dadd RH. Nutrition: organisms. In: Kerkut GA, Gilbert LT, editors. Comprehensive insect physiology, biochemistry and pharmacology. New York: Pergamon Press; 1985. pp. 313–390. [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics. 2011;27:1164–1165. doi: 10.1093/bioinformatics/btr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcher AL, Bratke KA, Powers EC, Salzberg SL. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics. 2007;23:673–679. doi: 10.1093/bioinformatics/btm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich CH. Auchenorrhyncha (Cicadas, spittlebugs, leafhoppers, trehoppers and planthoppers) In: Resh VH, Cardé RT, editors. Encyclopedia of insects. New York: Academic Press; 2003. pp. 66–74. [Google Scholar]
- Douglas A. Nutritional interactions in insect-microbial symbioses: Aphids and their symbiotic bacteria Buchnera. Annu Rev Entomol. 1998;43:17–37. doi: 10.1146/annurev.ento.43.1.17. [DOI] [PubMed] [Google Scholar]
- Drummond AJ, et al. Geneious v5.1. 2010 [cited 2013 Aug 21]. Available from: http://www.geneious.com. [Google Scholar]
- Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 2011;39:W29–W37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost KE, Willis DK, Groves RL. Detection and variability of aster yellows phytoplasma titer in its insect vector, Macrosteles quadrilineatus (Hemiptera: Cicadellidae) J Econ Entomol. 2011;104:1800–1815. doi: 10.1603/ec11183. [DOI] [PubMed] [Google Scholar]
- Gil R, et al. The genome sequence of Blochmannia floridanus: comparative analysis of reduced genomes. Proc Natl Acad Sci U S A. 2003;100:9388–9393. doi: 10.1073/pnas.1533499100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonella E, et al. Bacterial endosymbiont localization in Hyalesthes obsoletus, the insect vector of Bois noir in Vitis vinifera. Appl Environ Microbiol. 2011;77:1423–1435. doi: 10.1128/AEM.02121-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosalbes MJ, Lamelas A, Moya A, Latorre A. The striking case of tryptophan provision in the cedar aphid Cinara cedri. J Bacteriol. 2008;190:6026–6029. doi: 10.1128/JB.00525-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi D, Engel MS. Evolution of the insects. New York: Cambridge University Press; 2005. [Google Scholar]
- Hacham Y, Gophna U, Amir R. In vivo analysis of various substrates utilized by cystathionine c-synthase and O-acetylhomoserine sulfhydrylase in methionine biosynthesis. Mol Biol Evol. 2003;20:1513–1520. doi: 10.1093/molbev/msg169. [DOI] [PubMed] [Google Scholar]
- Hansen AK, Moran NA. Aphid genome expression reveals host-symbiont cooperation in the production of amino acids. Proc Natl Acad Sci U S A. 2011;108:2849–2854. doi: 10.1073/pnas.1013465108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heddi A, et al. Molecular and cellular profiles of insect bacteriocytes: mutualism and harm at the initial evolutionary step of symbiogenesis. Cell Microbiol. 2005;7:293–305. doi: 10.1111/j.1462-5822.2004.00461.x. [DOI] [PubMed] [Google Scholar]
- Holden MT, et al. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc Natl Acad Sci U S A. 2004;101:14240–14245. doi: 10.1073/pnas.0403302101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Ma Z, Dong S, Chen Y, Yu X. Analysis of yeast-like symbiote diversity in the brown planthopper (BPH), Nilaparvata lugens (Stal), using a novel nested PCR-DGGE protocol. Curr Microbiol. 2013;67:263–270. doi: 10.1007/s00284-013-0356-z. [DOI] [PubMed] [Google Scholar]
- Houk EJ, Griffiths GW. Intracellular symbiotes of the Homoptera. Ann Rev Entomol. 1980;25:161–187. [Google Scholar]
- Huson DH, Mitra S, Ruscheweyh H, Weber N, Schuster SC. Integrative analysis of environmental sequences using MEGAN4. Genome Res. 2011;21:1552–1560. doi: 10.1101/gr.120618.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y, Matsuura Y, Kakizawa S, Nikoh N, Fukatsu T. Diversity of bacterial endosymbionts associated with Macrosteles leafhoppers vectoring phytopathogenic phytoplasmas. Appl Environ Microbiol. 2013;79:5013–5022. doi: 10.1128/AEM.01527-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson KO, McHenry CS. Purification and characterization of the beta-subunit of the DNA polymerase III holoenzyme of Escherichia coli. J Biol Chem. 1980;22:10984–10990. [PubMed] [Google Scholar]
- Kasimoglu E, Park SJ, Malek J, Tseng CP, Gunsalus RP. Transcriptional regulation of the proton-translocating atpase (atpIBEFHAGDC) operon of Escherichia coli: control by cell growth rate. J Bacteriol. 1996;178:5563–5567. doi: 10.1128/jb.178.19.5563-5567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keseler IM, et al. EcoCyc: a comprehensive database of Escherichia coli biology. Nucleic Acids Res. 2011;39:D583–D590. doi: 10.1093/nar/gkq1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisker C, et al. A structural comparison of molybdenum cofactor-containing enzymes. FEMS Microbiol Rev. 1999;22:503–521. doi: 10.1111/j.1574-6976.1998.tb00384.x. [DOI] [PubMed] [Google Scholar]
- Knight RD, Freeland SJ, Landweber LF. Rewiring the keyboard: evolvability of the genetic code. Nat Rev Genet. 2001;2:49–58. doi: 10.1038/35047500. [DOI] [PubMed] [Google Scholar]
- Koga R, Bennett GM, Cryan JR, Moran NA. Evolutionary replacement of obligate symbionts in an ancient and diverse insect lineage. Environ Microbiol. 2013;15:2073–2081. doi: 10.1111/1462-2920.12121. [DOI] [PubMed] [Google Scholar]
- Koga R, Tsuchida T, Fukatsu T. Quenching autofluorescence of insect tissues for in situ detection of endosymbionts. Appl Entomol Zool. 2009;44:281–291. [Google Scholar]
- Kunieda T, et al. Carbohydrate metabolism genes and pathways in insects: insights from the honeybee genome. Insect Mol Biol. 2006;15:563–576. doi: 10.1111/j.1365-2583.2006.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YJ. Taxonomic revision of the leafhopper genus Macrosteles Fieber of the world [PhD thesis] Cardiff (United Kingdom): University of Wales; 1988. [Google Scholar]
- Lagesen K, et al. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35:3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux JJ, Rubinoff D. Molecular data reveals California as the potential source of an invasive leafhopper species, Macrosteles sp. nr. severini transmitting the aster yellows phytoplasma in Hawaii. Ann Appl Biol. 2009;154:429–439. [Google Scholar]
- Li R, et al. SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics. 2009;25:1966–1967. doi: 10.1093/bioinformatics/btp336. [DOI] [PubMed] [Google Scholar]
- Macnicol PK, Datko AH, Giovanelli J, Mudd SH. Homocysteine biosynthesis in green plants: physiological importance of the transsulfuration pathway in Lemna paucicostata. Plant Physiol. 1981;68:619–625. doi: 10.1104/pp.68.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz VM, et al. IMG ER: a system for microbial genome annotation, expert review and curation. Bioinformatics. 2009;25:2271–2278. doi: 10.1093/bioinformatics/btp393. [DOI] [PubMed] [Google Scholar]
- Mattson WJ. Herbivory in relation to plant nitrogen content. Annu Rev Ecol Syst. 1980;11:119–161. [Google Scholar]
- McCutcheon JP, McDonald BR, Moran NA. Convergent evolution of metabolic roles in bacterial co-symbionts of insects. Proc Natl Acad Sci U S A. 2009a;106:15394–15399. doi: 10.1073/pnas.0906424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JP, McDonald BR, Moran NA. Origin of an alternative genetic code in the extremely small and GC-rich genome of a bacterial symbiont. PLoS Genet. 2009b;5:e1000565. doi: 10.1371/journal.pgen.1000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JP, Moran NA. Parallel genomic evolution and metabolic interdependence in an ancient symbiosis. Proc Natl Acad Sci U S A. 2007;104:19392–19397. doi: 10.1073/pnas.0708855104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JP, Moran NA. Functional convergence in reduced genomes of bacterial symbionts spanning 200 My of evolution. Genome Biol Evol. 2010;2:708–718. doi: 10.1093/gbe/evq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JP, Moran NA. Extreme genome reduction in symbiotic bacteria. Nat Rev Microbiol. 2012;10:13–26. doi: 10.1038/nrmicro2670. [DOI] [PubMed] [Google Scholar]
- McCutcheon JP, von Dohlen CD. An interdependent metabolic patchwork in the nested symbiosis of mealybugs. Curr Biol. 2011;21:1366–1372. doi: 10.1016/j.cub.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran NA. Symbiosis as an adaptive process and source of phenotypic complexity. Proc Natl Acad Sci U S A. 2007;104:8627–8633. doi: 10.1073/pnas.0611659104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran NA, Dale C, Dunbar H, Smith WA, Ochman H. Intracellular symbionts of sharpshooters (Insecta: Hemiptera: Cicadellinae) form a distinct clade with a small genome. Environ Microbiol. 2003;5:116–126. doi: 10.1046/j.1462-2920.2003.00391.x. [DOI] [PubMed] [Google Scholar]
- Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet. 2008;42:165–190. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- Moran NA, Tran P, Gerardo NM. Symbiosis and insect diversification: an ancient symbiont of sap-feeding insects from the bacterial phylum Bacteroidetes. Appl Environ Microbiol. 2005;71:8802–8810. doi: 10.1128/AEM.71.12.8802-8810.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya Y, Itoh M, Okuda S, Yoshizawa AC, Kanehisa M. KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007;35:W182–W185. doi: 10.1093/nar/gkm321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller HJ. Die symbiose der Fulgoroiden (Homoptera: Cicadina) Zoologica. 1940;98:111–220. [Google Scholar]
- Müller HJ. Neuere vorstellungen über verbreitung and phylogenie der endosymbiosen der zikaden. Zeitschrift für Morphologie und Ökologie der Tiere. 1962;51:190–210. [Google Scholar]
- Nakabachi A, et al. The 160-kilobase genome of the bacterial endosymbiont Carsonella. Science. 2006;314:267. doi: 10.1126/science.1134196. [DOI] [PubMed] [Google Scholar]
- Noda H, et al. Bacteriome-associated endosymbionts of the green rice leafhopper Nephotettix cincticeps (Hemiptera: Cicadellidae) Appl Entomol Zool. 2012;47:217–225. [Google Scholar]
- Nováková E, Hypsa V, Moran NA. Arsenophonus, an emerging clade of intracellular symbionts with a broad host distribution. BMC Microbiol. 2009;9:143. doi: 10.1186/1471-2180-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny V, Wilson MR. Why are there no small species among xylem-sucking insects? Evol Ecol. 1997;11:419–437. [Google Scholar]
- Osawa S, Jukes TH. Evolution of the genetic code as affected by anticodon content. Trends Genet. 1988;4:191–198. doi: 10.1016/0168-9525(88)90075-3. [DOI] [PubMed] [Google Scholar]
- Pedrosa F, et al. Genome of Herbaspirillum seropedicae strain SmR1, a specialized diazotrophic endophyte of tropical grasses. PLoS Genet. 2011;7:e1002064. doi: 10.1371/journal.pgen.1002064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Brocal V, et al. A small microbial genome: the end of a long symbiotic relationship? Science. 2006;314:312–313. doi: 10.1126/science.1130441. [DOI] [PubMed] [Google Scholar]
- Poliakov A, et al. Large-scale label-free quantitative proteomics of the pea aphid-Buchnera symbiosis. Mol Cell Proteomics. 2011;10:110007039. doi: 10.1074/mcp.M110.007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosina YY, Mui J, Pitsikas P, Cupples CG. The Escherichia coli mismatch repair protein MutL recruits the Vsr and MutH endonucleases in response to DNA damage. J Bacteriol. 2009;191:4041–4043. doi: 10.1128/JB.00066-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabree ZL, Huan CY, Okusu A, Moran NA, Normark BB. The nutrient supplying capabilities of Uzinura, an endosymbiont of armored scale insects. Environ Microbiol. 2013;15:1988–1999. doi: 10.1111/1462-2920.12058. [DOI] [PubMed] [Google Scholar]
- Sabree ZL, Kambhampati S, Moran NA. Nitrogen recycling and nutritional provisioning in Blattabacterium, the cockroach endosymbiont. Proc Natl Acad Sci U S A. 2009;106:19521–19526. doi: 10.1073/pnas.0907504106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi L, et al. Multiple symbiosis in the leafhopper Scaphoideus titanus (Hemiptera: Cicadellidae): details of transovariol transmission of Cardinium and yeast-like symbionts. Tissue Cell. 2008;40:231–242. doi: 10.1016/j.tice.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Sandström J, Moran NA. How nutritionally imbalanced is phloem sap for aphids? Entomol Exp Appl. 1999;91:203–210. [Google Scholar]
- Sandström J, Pettersson J. Amino acid composition of phloem sap and the relation to intraspecific variation in pea aphid (Acyrthosiphon pisum) performance. J Insect Physiol. 1994;40:947–955. [Google Scholar]
- Schattner P, Brooks A, Lowe TM. The tRNAsca-se, snoscan, snoGPS web servers for detection of tRNAs ad snoRNAs. Bioinformatics. 2005;33:w686–w689. doi: 10.1093/nar/gki366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherbakov D. The 270 million year history of Auchenorrhyncha. Denisia. 2002;4:29–36. [Google Scholar]
- Schofield MJ, Hsieh P. DNA mismatch repair: molecular mechanisms and biological function. Annu Rev Microbiol. 2003;57:579–608. doi: 10.1146/annurev.micro.57.030502.090847. [DOI] [PubMed] [Google Scholar]
- Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa H. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature. 2000;407:81–86. doi: 10.1038/35024074. [DOI] [PubMed] [Google Scholar]
- Shigenobu S, Wilson ACC. Genomic rebelations of mutualism: the pea aphid and its obligate bacterial symbiont. Cell Mol Life Sci. 2011;68:1297–1309. doi: 10.1007/s00018-011-0645-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DB, Bennett GB, Engel P, Williams D, Ochman H. Disentangling associated genomes. Method Enzymol. 2013 doi: 10.1016/B978-0-12-407863-5.00020-4. (In press) [DOI] [PubMed] [Google Scholar]
- Sloan DB, Moran NA. Endosymbiotic bacteria as a source of carotenoids in whiteflies. Biol Lett. 2012a;8:986–989. doi: 10.1098/rsbl.2012.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DB, Moran NA. Genome reduction and co-evolution between the primary and secondary bacterial symbionts of psyllids. Mol Biol Evol. 2012b;29:3781–3792. doi: 10.1093/molbev/mss180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DB, Moran NA. The evolution of genomic instability in the obligate endosymbionts of whiteflies. Gen Biol Evol. 2013;5:783–793. doi: 10.1093/gbe/evt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smelkova N, Marians JJ. Timely release of both replication forks from oriC requires modulation of origin topology. J Biol Chem. 2001;276:39186–3919. doi: 10.1074/jbc.M104411200. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Sugino A, Higgins NP, Cozzarelli NR. DNA gyrase subunit stoichiometry and the covalent attachment of subunit A to DNA during DNA cleavage. Nucleic Acid Res. 1980;8:3865–3874. doi: 10.1093/nar/8.17.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiya DM, Tran PL, Dietrich CH, Moran NA. Co-cladogenesis spanning three phyla: leafhoppers (Insecta: Hemiptera: Cicadellidae) and their dual bacterial symbionts. Mol Ecol. 2006;15:4175–4191. doi: 10.1111/j.1365-294X.2006.03071.x. [DOI] [PubMed] [Google Scholar]
- Tamames J, et al. The frontier between cell and organelle: genome analysis of Candidatus Carsonella ruddii. BMC Evol Biol. 2007;7:181. doi: 10.1186/1471-2148-7-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JM, Cryan JR. Two ancient bacterial endosymbiont have coevolved with the planthoppers (Insecta: Hemiptera: Fulgoroidea) BMC Evol Biol. 2012;12:87. doi: 10.1186/1471-2148-12-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hook RI, Nielsen MG, Shugart HH. Energy and nitrogen relations for a Macrosiphum liriodendri (Homoptera: Aphididae) population in an east Tennessee Liriodendron tulipifera stand. Ecology. 1980;61:960–975. [Google Scholar]
- Wang Z, Wu M. A phylum-level bacterial phylogenetic marker database. Mol Biol Evol. 2013;30:1258–1262. doi: 10.1093/molbev/mst059. [DOI] [PubMed] [Google Scholar]
- Wilson AC, et al. Genomic insight into the amino acid relations of the pea aphid, Acyrthosiphon pisum, with its symbiotic bacterium Buchnera aphidicola. Insect Mol Biol. 2010;19:249–258. doi: 10.1111/j.1365-2583.2009.00942.x. [DOI] [PubMed] [Google Scholar]
- Woyke T, et al. One bacterial cell, one complete genome. PLoS One. 2010;5:e10314. doi: 10.1371/journal.pone.0010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, et al. Metabolic complementarity and genomics of the dual bacterial symbiosis of sharpshooters. PLoS Biol. 2006;4:e188. doi: 10.1371/journal.pbio.0040188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamao F, et al. UGA is read as tryptophan in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1985;82:2306–2309. doi: 10.1073/pnas.82.8.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18:821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann U, et al. Xylem water transport: is the available evidence consistent with the cohesion theory? Plant Cell Environ. 1994;17:1169–1181. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.