Abstract
Very little is known about genetic factors that regulate life history transitions during ontogeny. Closely related tiger salamanders (Ambystoma species complex) show extreme variation in metamorphic timing, with some species foregoing metamorphosis altogether, an adaptive trait called paedomorphosis. Previous studies identified a major effect quantitative trait locus (met1) for metamorphic timing and expression of paedomorphosis in hybrid crosses between the biphasic Eastern tiger salamander (Ambystoma tigrinum tigrinum) and the paedomorphic Mexican axolotl (Ambystoma mexicanum). We used existing hybrid mapping panels and a newly created hybrid cross to map the met1 genomic region and determine the effect of met1 on larval growth, metamorphic timing, and gene expression in the brain. We show that met1 maps to the position of a urodele-specific chromosome rearrangement on linkage group 2 that uniquely brought functionally associated genes into linkage. Furthermore, we found that more than 200 genes were differentially expressed during larval development as a function of met1 genotype. This list of differentially expressed genes is enriched for proteins that function in the mitochondria, providing evidence of a link between met1, thyroid hormone signaling, and mitochondrial energetics associated with metamorphosis. Finally, we found that met1 significantly affected metamorphic timing in hybrids, but not early larval growth rate. Collectively, our results show that met1 regulates species and morph-specific patterns of brain transcription and life history variation.
Keywords: amphibian, comparative genomics, linkage mapping, microarray, metamorphic timing, QTL
Introduction
Some closely related species exhibit dramatic phenotypic differences of adaptive significance. Such species provide excellent models to investigate the genetic basis of complex traits and life history evolution. An important and difficult objective in working with natural species is to identify gene and gene functions that are associated with phenotypic differences (Jones 1998; Bradshaw and Schemske 2003; Hoekstra et al. 2006). In some cases, potential candidate genes are predictable given the nature of the phenotypic differences, such as in the case of color polymorphisms (Mundy 2007) and craniofacial variations in birds and fishes (Schneider and Helms 2003; Streelman et al. 2007). However, for most traits, it is difficult to predict genetic and developmental components of the selection process. When it is possible to cross species and segregate molecular and phenotypic variation, quantitative trait locus (QTL) mapping provides an unbiased and efficient method to initially locate genetic factors in the genome. Subsequent comparative and functional genomic approaches can then be used to narrow the search for causative genetic factors and reveal insights about gene functions, development, and evolution.
Here, we consider genetic factors that activate postembryonic developmental programs during ontogeny to enable critical life history transitions. Amphibian and insect larvae undergo a dramatic life history transition known as metamorphosis, during which adult traits develop to enable strategies for predator avoidance, dispersal, resource utilization, and mating (Wilbur 1980). Thyroid hormone (TH) is the master regulator of amphibian metamorphosis, and release of TH by the thyroid is under complex control of the hypothalamic–pituitary–thyroid axis (Shi 2000; Denver et al. 2002). At a molecular level, the effects of TH are mediated by TH receptors, which are nuclear transcription factors that regulate the transcription of genes in a TH-dependent manner (Buchholz et al. 2006). Thus, rising TH levels during larval development bring about metamorphosis by triggering transcriptional programs that activate tissue remodeling, cellular differentiation, and tissue regression—developmental processes that transform larval phenotypes into adult phenotypes (Shi 2000). According to this model, variation in metamorphic timing may trace to variation in the timing of TH-dependent transcriptional events during development.
The timing of metamorphosis is a critical life history trait that evolves in response to aquatic habitat characteristics that determine the rate and plasticity of larval growth and the length of the larval period (Wilbur and Collins 1973; Wilbur 1980; Semlitsch and Wilbur 1989). Species that use relatively permanent habitats for larval development generally have longer larval periods than species that use ephemeral habitats. For example, some anuran tadpoles hatch in rapidly drying desert ponds and metamorphose after only 8 days of development (Newman 1989), whereas salamanders in permanent, high altitude lakes may overwinter as aquatic larvae and transform the following year, or forgo metamorphosis altogether (Petranka 1998). The observation of life history variation between anuran and salamander species is not surprising because these groups diverged from a common ancestor more than 300 Ma. However, there are also substantial differences in developmental timing within both of these orders, of which the pronounced differences in metamorphic timing between closely related tiger salamander lineages (Ambystoma spp.) are an unusually dramatic example. Such species groups provide models for identifying genes for metamorphic timing and investigating the relationships between development, life history variation, and life cycle evolution (Voss et al. 2003, 2012; Voss and Smith 2005).
In most Eastern tiger salamander (A. tigrinum tigrinum) populations, larvae undergo an obligate metamorphosis after a variable period of growth (Petranka 1998). In contrast, Mexican axolotls (A. mexicanum) do not produce a high titer of TH during larval development and thus do not metamorphose (Prahlad 1968; Galton 1992), a process called paedomorphosis (Gould 1977). As a consequence, sexually mature A. mexicanum retain juvenile traits and complete their life cycles in the aquatic habitat. Crosses between A. t. tigrinum and A. mexicanum, followed by backcrosses of F1 hybrids to A. mexicanum, show that a major effect QTL (met1) explains both continuous variation in metamorphic timing and expression of paedomorphosis (Voss and Shaffer 1997, 2000; Voss and Smith 2005; Voss et al. 2012). Heterozygotes that inherit a single A. t. tigrinum met1 allele almost invariably undergo metamorphosis. However, homozygotes that inherit two A. mexicanum met1 alleles metamorphose at a later time than heterozygotes, or fail to undergo metamorphosis altogether and exhibit paedomorphosis. The number of paedomorphs observed from hybrid crosses depends on genetic background. For example, when hybrid crosses are made using A. mexicanum from a laboratory population, the observed number of paedomorphs is statistically consistent with a single gene model (Voss 1995). However, when hybrid crosses are made using wild-caught A. mexicanum, a more complicated genetic basis is implicated and fewer paedomorphs are observed than expected under a single gene model. In these crosses, most of the A. mexicanum met1 homozygotes do not exhibit paedomorphosis. Instead, they metamorphose on average 36 days later than siblings carrying an A. t. tigrinum met1 allele (Voss and Shaffer 1997, 2000; Voss and Smith 2005). Thus, met1 alleles from paedomorphic A. mexicanum delay metamorphosis while met1 alleles from metamorphic A. t. tigrinum decrease the time to metamorphosis, and this consequently decreases the probability of expressing paedomorphosis.
In this study, we used comparative, quantitative, and functional genomic approaches to map the met1 genomic region and investigate the effect of met1 genotype on larval growth, neurodevelopmental gene expression, and metamorphic timing. Our previous work identified met1 using anonymous molecular markers (Voss and Shaffer 1997, 2000). Subsequently, an expressed sequence tag (EST) (ctg325) was identified for met1 that shows nucleotide similarity to human ngfr (Voss and Smith 2005). We show here that ngfr and met1 map to a genomic region that was structured by chromosomal rearrangements after the divergence of salamanders and anurans from a common ancestor. As a result, a unique set of met1 region genes were brought together during evolution, and several of these encode proteins that function in related biological processes. Further, several genes in linkage disequilibrium with the met1 region are differentially expressed in the brain during larval development, indicating that more than one gene from the met1 region may explain phenotypic differences between these species. At a genomic level, more than 200 genes, including genes that code for proteins with mitochondrial functions, were differentially expressed between met1 genotypes that significantly affect metamorphic timing. These changes were observed during a specific interval of time late in the larval period in interspecific hybrids, but at a much earlier time in the parental species. Our results show that met1 regulates temporal changes in brain transcription during larval development and the timing of metamorphosis, but not the rate of larval growth preceding these events.
Materials and Methods
Salamander Care and Genotyping
Second-generation hybrids were generated by backcrossing an A. t. tigrinum–A. mexicanum F1 hybrid male to an A. mexicanum female via in vitro fertilization (Voss 1995). Embryos were transferred to large containers of pond water and aerated. Hatching was synchronized manually and from hatching onward, larvae were reared individually. From hatching until approximately 21 days posthatching (DPH), larvae were fed brine shrimp napuli (Artemia). After this, larvae were fed California blackworms (Lumbriculus). At 19 DPH, tail clippings were collected for genomic DNA extraction using phenol/chloroform. Genotyping was performed to determine whether individuals were carrying A. mexicanum, A. t. tigrinum, or a combination of species-specific alleles at loci from the met1 genomic region. Genotypes for several loci (discussed later) were obtained using AcycloPrime-FP chemistry and a Wallac Victor3 plate reader as described in Smith et al. (2005). The polymerase chain reaction primers and extension probes that were used to type met1 region loci are presented in supplementary table S1, Supplementary Material online.
At 28, 42, 56, 70, 84, 98, and 112 DPH, 18 unique individuals were measured for snout-vent length (SVL) prior to tissue harvesting (i.e., no individual was measured more than once). Whole brains and pituitaries were then removed and immediately flash frozen in liquid nitrogen (six of each met1 genotype per time point). Excluding two recombinants (one at 70 DPH and one at 112 DPH), half of these individuals were homozygous for axolotl alleles across ngfr, rasd1, map2k3, rai1, and shmt1 (i.e., had a met1mex/mex genotype), whereas the other half were heterozygous for these loci (i.e., had a met1mex/Att genotype). An additional 30 animals, including one recombinant (ngfrmex/Att, rasd1mex/mex, map2k3mex/Att, rai1mex/mex, and shmt1mex/mex), were allowed to develop indefinitely, so that we could examine the relationship between met1 genotype and metamorphic timing. All animal care and use procedures were in accordance with protocols approved by the University of Kentucky Institutional Animal Care and Use Committee.
Comparative and QTL Mapping of met1
Previously, we performed A. mexicanum/A. t. tigrinum × A. mexicanum backcrosses and developed markers from ESTs to make linkage maps (Voss 1995; Voss and Shaffer 1997, 2000; Voss and Smith 2005; Voss et al. 2011). In this study, we used the mapping panel and markers from Voss et al. (2011) to perform genome-wide QTL scans for metamorphic timing QTL. Genome-wide marker information is detailed in Voss et al. (2011). Marker orders were determined using MultiPoint 2.2 (MultiQTL Ltd., Hafia, Israel) and the Kosambi mapping function (Kosambi 1944). QTL were identified using R/qtl (Broman et al. 2003), the scanone function, the normal QTL model, and Haley–Knott regression (Haley and Knot 1992). Genome-wide thresholds for evaluating the significance of QTL were determined from 1,000 replicated data sets (Churchill and Doerge 1994). We also used the mapping panel from Voss and Smith (2005) to map additional loci and more finely map the position of met1. Primer sequences, diagnostic polymorphisms, and polymorphism detection assays for met1 region loci are summarized in supplementary table S1, Supplementary Material online.
Statistical Analyses of Growth and Metamorphic Timing in Backcrossed Hybrids
We assessed whether backcrossed hybrids with nonrecombinant heterozygous and homozygous genotypes for ngfr, rasd1, map2k3, rai1, and shmt1 differed in growth trajectory by fitting a general linear model of the following form: SVLtij = βt + Gt + Ti + (GT)ti + T2i + (GT2)ti + εtij, where βt is the intercept for met1mex/mex individuals, Gt is the difference between the intercept for met1mex/mex and met1mex/Att individuals, Ti is the linear regression coefficient for met1mex/mex individuals, (GT)ti is the difference between the linear regression coefficients for met1mex/mex and met1mex/Att individuals, T2i is the quadratic regression coefficient for met1mex/mex individuals, (GT2)ti is the difference between the quadratic regression coefficients for met1mex/mex and met1mex/Att individuals, and εtij is the error term for the jth individual of genotype t (a dummy variable) sampled at time i. This error term is assumed to be normally distributed with mean = 0 and variance = σ2 and describes the deviation of each individual (irrespective of genotype) from its expected value based on the fitted model, which accounts for systemic differences between the genotypes when making predictions. A backward selection procedure was then applied in which parameters with t-statistic P values ≥ 0.05 and deletion test P values ≥ 0.05 were removed from the model (Crawley 2007). When conducting this procedure, the quadratic interaction term between genotype and time (i.e., (GT2)ti) was removed prior to the linear interaction term between genotype and time (i.e., (GT)ti), which was removed prior to the main effect of genotype (i.e., Gt). We also assessed whether biphasic salamanders that were nonrecombinant for ngfr, rasd1, map2k3, rai1, and shmt1 differed in time to completion of metamorphosis by conducting a one-tailed Mann–Whitney test. Salamanders that did not complete metamorphosis within 365 DPH were scored as paedomorphs.
RNA Extraction, Microarray Platform, and Quality Control
Total RNA was extracted individually from the brains of backcrossed hybrids sampled between 42 and 112 DPH. RNA extractions were conducted using TRIzol (Invitrogen) according to the manufacturer’s protocol. Samples were further purified using RNeasy mini columns (Qiagen). Upon extraction, RNA samples were quantified via UV spectrophotometry and qualified via an Agilent Bioanalyzer. Four RNA samples per genotype (i.e., nonrecombinant homozygotes vs. heterozygotes) from each of six time points (42, 56, 72, 84, 98, and 112 DPH) were submitted to the University of Kentucky Microarray Core Facility (UKMCF). The UKMCF generated biotin-labeled cRNA targets for each of the 48 samples and independently hybridized each sample to a custom Ambystoma Affymetrix GeneChip (Page et al. 2007). This microarray platform consists of 4,844 probe sets that were designed using a preferred maximum of 11 probe pairs (match/mismatch) and a possible minimum of 8 probe pairs. Of these 4,844 probe sets, 57 are Affymetrix controls, 4,596 were designed from A. mexicanum sequence, and 191 were designed from A. t. tigrinum sequence. We subjected all microarray data to low-level quality control (QC) as described previously (Page et al. 2010) and used the robust multiarray average (RMA) algorithm (Irizarry et al. 2003) to generate probe set-level expression summaries. We then examined the probe set-level data by computing correlation matrices for replicate GeneChips, conducting principal component analysis, and rendering pair-wise M versus A plots of replicate GeneChips. Upon conducting low-level and probe set-level QC, it was clear that one of the met1mex/mex 42 DPH GeneChips was aberrant. Thus, this GeneChip was removed prior to further processing and analysis. All QC analyses were performed using the R statistical computing environment (www.r-project.org, last accessed July 16, 2013) and the affy and affyPLM packages (Gautier et al. 2004; Bolstad et al. 2005). All sequence data, the raw microarray data (.CEL files), and the microarray annotations are available at Sal-Site (http://www.ambystoma.org/genome-resources, last accessed September 17, 2013).
Identification of Zero Mismatch Probe Sets and Expression Summarization
Despite the shallow phylogenetic distance between A. mexicanum and A. t. tigrinum (Shaffer and McKnight 1996; O’Neill et al. 2013), it is possible that targets from backcrossed hybrids will have variable hybridization efficiencies for probes on the Ambystoma GeneChip due to sequence divergence between the parent species (Bar-Or et al. 2007; Buckley 2007). To address this issue, we used probe sequences as queries in Blast searches of A. mexicanum and A. t. tigrinum EST contig databases. Blast alignments were then used to extrapolate the number of mismatches between each probe’s sequence and A. mexicanum and A. t. tigrinum EST-based contigs, respectively (Page et al. 2010). In total, 1,604 probe sets (∼33%) were designed from contigs with predicted orthologs in A. mexicanum and A. t. tigrinum, and for 1,320 (∼82%) of these, we did not detect any mismatches between the evaluated probe sequences and the contig sequence from the heterologous species. However, for 31 of these probe sets (∼2%), we were only able to assess a subset of the probes, and we therefore refer to these probe sets as equivocal zero mismatch probe sets (EZMMPSs) to distinguish them from the 1,289 zero mismatch probe sets (ZMMPSs) that were fully evaluated. Upon completing this exercise, we used the subset option of the RMA function in the affy package to calculate probe set-level expression summaries based on the 1,320 ZMMPSs and EZMMPSs and used this RMA matrix to identify differentially expressed genes (DEGs; discussed later).
Statistical Analysis of Microarray Data
To arrive at a list of DEGs, we implemented two statistical approaches. First, the limma package (Smyth 2004) was used to compare the alternate met1 genotypes at 42, 56, 70, 84, 98, and 112 DPH. This involved fitting a linear model to each probe set that contained 12 coefficients, one for each time by genotype combination. We then reoriented this model in terms of contrasts between the two genotypes at each of the six time points. We corrected for multiple testing by applying a 0.05 false discovery rate (Benjamini and Hochberg 1995) to the P values associated with the moderated F statistics that resulted from our contrast matrix. In addition, for some downstream analyses, we required that DEGs identified using limma be differentially regulated by ≥1.5-fold as a function of met1 genotype at one or more time points.
To identify genes with modest but temporally consistent expression differences between the met1 genotypes, we also used the maSigPro package to conduct a stepwise quadratic regression analysis (Conesa et al. 2006). We corrected for multiple testing by applying a 0.05 false discovery rate (Benjamini and Hochberg 1995) at the overall model level. We then implemented a backward selection procedure (Conesa et al. 2006) that removed parameters from the full quadratic model with P values > 0.001, a stringent threshold that better enabled us to exclude genes with modestly different temporal trajectories between met1 genotypes, but little to no difference in elevation (i.e., expression levels). We also required that models have R2 ≥ 0.50 and significant genotype, genotype by time, or genotype by time2 terms. Finally, we graphically inspected the 96 genes that met the above criteria and manually selected genes that were robustly different between the alternate met1 genotypes at several time points.
Enrichment Analyses
We used EASE analyses, as implemented by DAVID (Dennis et al. 2003), to identify biological process, cellular component, and molecular function gene ontology terms that were statistically enriched in lists of DEGs. This approach applies a conservative adjustment to the calculation of Fisher’s exact probability from a hypergeometric distribution (Hosack et al. 2003). The ZMMPSs and EZMMPs with predicted orthologies to human proteins were used to generate expected values (i.e., as the background). The count threshold was set to two, and the EASE threshold (i.e., the critical value of the corrected Fisher’s exact P value) was set to 0.05.
Examination of Hybrid DEG Expression in the Parent Species
In a previous study, we used the same microarray platform used here to investigate neurodevelopmental gene expression in A. mexicanum and A. t. tigrinum at 42, 56, 70, and 84 DPH (Page et al. 2010). We used the raw data from this prior experiment (i.e., the .CEL files) to generate a zero mismatch RMA matrix for the parent species that is analogous to the zero mismatch RMA matrix for backcrossed hybrids described earlier. We then obtained log2(RMA) values from this matrix for probe sets that were identified as differentially expressed between met1mex/mex and met1mex/Att hybrids in this study using limma. For each of these probe sets, we used Welch’s t statistic to test for differential expression between A. mexicanum and A. t. tigrinum at each time point that we sampled parental gene expression (Page et al. 2010). In addition, we used Kruskal’s nonmetric multidimensional scaling (NMDS) to examine relationships among hybrid, A. mexicanum, and A. t. tigrinum expression for DEGs that differed between the hybrid met1 genotypes by ≥1.5-fold at one or more time points (Venables and Ripley 2010). NMDS is an ordination technique for dimension reduction that can be used on any measure of dissimilarity (i.e., distance). It uses an iterative regression-based approach to find a numerical solution for representing the distance relations among observations from a high dimensional data set in k dimensions, where k is a small number relative to the dimensionality of the original data set. NMDS is nonmetric in the sense that it only attempts to maintain the rank order of distances in the spatial representation. The stress is a common statistic for assessing the fit of an NMDS solution (Venables and Ripley 2010) that can be interpreted as a measure of the distortion in the distance relationships that results from dimension reduction. When conducting NMDS, we used 1 – r as a measure of dissimilarity where r is Pearson’s correlation coefficient. This measure of dissimilarity ensures that all distances fall between zero and two and that the correlation across genes between samples determines the magnitude of dissimilarity, with positively correlated samples treated as similar and negatively correlated samples treated as dissimilar. We allowed a maximum of 150 iterations and conducted NMDS for k = 1–10 dimensions to examine how the stress changed as a function of the number of dimensions used for ordination.
Results
Effect of met1 Genotype on Larval Growth and Metamorphic Timing in Backcrossed Hybrids
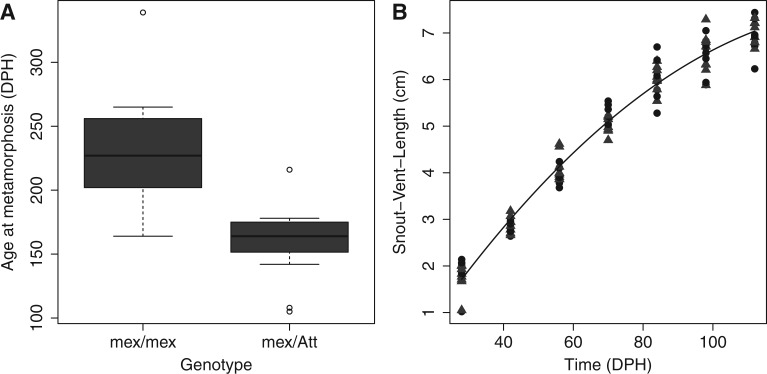
Of the 30 individuals allowed to develop indefinitely, only two met1mex/mex individuals failed to metamorphose within 365 days and were thus scored as paedomorphs. The single recombinant for the met1 region (ngfrmex/Att, rasd1mex/mex, map2k3mex/Att, rai1mex/mex, and shmt1mex/mex) completed metamorphosis at a relatively early time (164 DPH), consistent with met1 associating more closely with map2k3 and ngfr than the other loci (discussed later). Twenty-seven individuals that were nonrecombinant for the met1 region metamorphosed. Among these, a highly statistically significant difference in metamorphic timing was observed between met1mex/mex and met1mex/Att individuals (fig. 1A). Despite this pronounced difference in metamorphic timing, there was no difference between the met1 genotypes in larval growth trajectory throughout the time period that tissues were collected (table 1 and fig. 1B). These results show that met1 affects the timing of metamorphosis and the probability of expressing paedomorphosis without affecting early larval growth rate.
Fig. 1.—
(A) Box plots comparing the ages at which spontaneous metamorphosis was completed by biphasic met1mex/mex (n = 8) and met1mex/Att (n = 19) hybrids. The difference in metamorphic timing between the alternate met1 genotypes is highly statistically significant (one-sided Mann–Whitney test, W = 142.5, P = 0.0002). (B) Scatter plot comparing the growth trajectories of met1mex/mex hybrids (black circles) and met1mex/Att hybrids (gray triangles). The six parameter dummy variable regression model described in Materials and Methods and table 1 was fit to the data. Stepwise deletion tests revealed that retaining a separate quadratic regression coefficient (F1,118 = 0.051, P = 0.822), a separate linear regression coefficient (F1,119 = 0.858, P = 0.356), and a separate intercept (F1,120 = 0.083, P = 0.774) for met1mex/Att hybrids was not necessary. Thus, the curve shown corresponds to the selected model (F2,121 = 2,181, P << 0.0001) and implies that there is no difference between the growth trajectories of the alternate met1 genotypes.
Table 1.
Parameter Estimates, t Statistics, t Statistic P Values, and Adjusted R2 Values for the Models Fit During the Selection Procedure Used to Compare the Growth Trajectories of met1mex/mex and met1mex/Att Hybrids
| Estimate | Full Model | Step 1 | Step 2 | Step 3 |
|---|---|---|---|---|
| Βt ± SE | −1.34 ± 2.57 × 10−1 | −1.30 ± 1.96 × 10−1 | −1.36 ± 1.83 × 10−1 | −1.36 ± 1.81 × 10−1 |
| Βt t value | −5.19 | −6.63 | −7.46 | −7.52 |
| P(Βt) = 0 | 8.73 × 10−7 | 1.03 × 10−9 | 1.48 × 10−11 | 1.05 × 10−11 |
| Gt ± SE | −3.56 × 10−2 ± 3.64 × 10−1 | −1.11 × 10−1 ± 1.47 × 10−1 | 1.57 × 10−2 ± 5.47 × 10−2 | Removed |
| Gt t value | −0.10 | −0.75 | 0.29 | Removed |
| P(Gt) = 0 | 0.92 | 0.45 | 0.77 | Removed |
| Ti ± SE | 1.21 × 10−1 ± 8.15 × 10−3 | 1.20 × 10−1 ± 5.84 x10−3 | 1.21 × 10−1 ± 5.74 × 10−3 | 1.21 × 10−1 ± 5.72 × 10−3 |
| Ti t value | 14.88 | 20.55 | 21.09 | 21.18 |
| P(Ti) = 0 | <2.00 × 10−16 | <2.00 × 10−16 | <2.00 × 10−16 | <2.00 × 10−16 |
| T2i ± SE | −4.20 × 10−4 ± 5.74 × 10−5 | −4.11 × 10−4 ± 4.06 × 10−5 | −4.12 × 10−4 ± 4.05 × 10−5 | −4.12 × 10−4 ± 4.04 × 10−5 |
| T2i t value | −7.32 | −10.13 | −10.16 | −10.21 |
| P(T2i) = 0 | 3.28 × 10−11 | <2.00 × 10−16 | <2.00 × 10−16 | <2.00 × 10−16 |
| (GT)ti ± SE | −7.44 × 10−4 ± 1.15 × 10−2 | 1.81 × 10−3 ± 1.96 × 10−3 | Removed | Removed |
| (GT)ti t value | −0.07 | 0.93 | Removed | Removed |
| P[(GT)ti] = 0 | 0.95 | 0.36 | Removed | Removed |
| (GT2)ti ± SE | 1.83 × 10−5 ± 8.14 × 10−5 | Removed | Removed | Removed |
| (GT2)ti t value | 0.23 | Removed | Removed | Removed |
| P[(GT2)ti] = 0 | 0.82 | Removed | Removed | Removed |
| Adjusted R2 | 0.9721 | 0.9723 | 0.9724 | 0.9726 |
Note.—To further emphasize the rationale used during the model selection process, additional decimal places are shown for adjusted R2 relative to the other reported values.
Comparative and QTL Mapping of met1
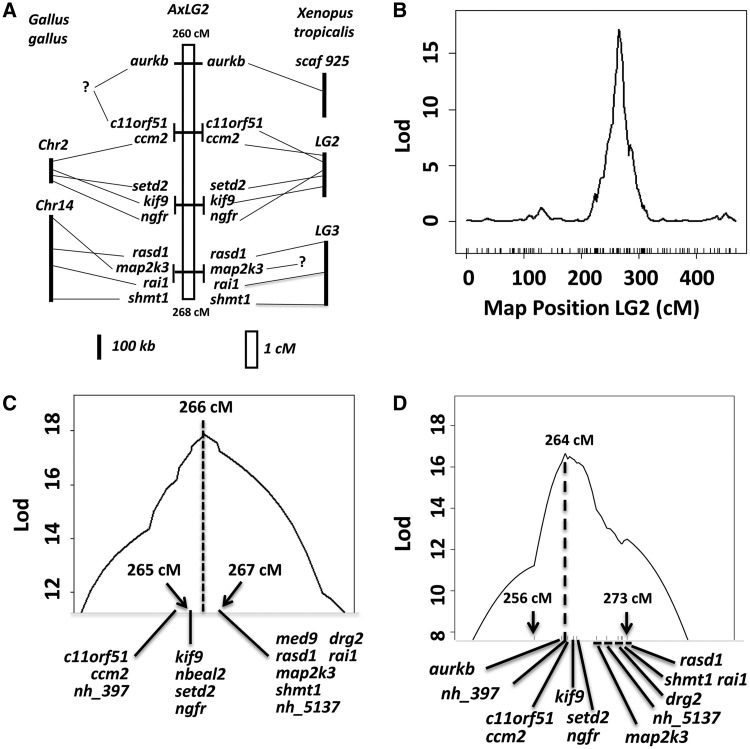
Previous studies identified ngfr as the closest protein-coding marker to met1 in the Ambystoma genetic linkage map (Smith et al. 2005; Voss and Smith 2005). To obtain linked markers for ngfr, we scanned genomic regions flanking ngfr in the Homo sapiens and Gallus gallus genomes and then selected loci that were good candidates for met1. This strategy was not very successful because the ngfr chromosome in H. sapiens (Hsa17) has undergone considerable rearrangement during evolution and the ngfr chromosome in G. gallus (Gga28) contains a ngfr-like gene sequence that does not show high sequence identity to the Ambystoma ortholog. We eventually mapped map2k3 to within 5 cM of ngfr, an unexpected result because map2k3 is approximately 2.6 × 107 bases from ngfr in the human genome and these loci occur on different chromosomes in the chicken genome (fig. 2A). Linkage of map2k3-ngfr implicated a new set of candidate genes for met1 and, in particular, genes from the Smith–Magenis syndrome (SMS) genomic region (Hsa17p11.2). Four additional loci (rasd1, rai1, shmt1, and drg2) were subsequently mapped to establish linkage of SMS loci to map2k3. Several genes were then mapped within 5 cM on the opposite flank of ngfr, including setd2, c11orf51, ccm2, kif9, and aurkb. All of these genes except aurkb are syntenic within the same genomic scaffold in Xenopus tropicalis, and setd2 and ccm2 are linked on chromosome 2 in G. gallus. However, the ngfr-setd2-c11orf51-ccm2-kif9 conserved synteny group does not locate to the SMS conserved synteny group in the X. tropicalis genome, nor are these genes found together in fish or amniote genomes. These results show that ngfr and map2k3 flank the position of an ancestral chromosomal breakpoint that is not observed in representative anuran (Xenopus) and reptile (Gallus) genomes and therefore may be unique to Ambystoma and possibly other salamanders (fig. 2A).
Fig. 2.—
(A) Comparative map showing marker positions on Ambystoma linkage group 2 (AxLG2; center) and the positions of their orthologs in Gallus gallus (left) and Xenopus tropicalis (right). (B) Results of a genome-wide scan for life history pathway QTL (metamorphosis vs. paedomorphosis) using the LAB mapping panel. A single significant LOD peak corresponding to met1 was identified on Ambystoma LG2. (C) Local map of the met1 genomic region on LG2 based on the LAB mapping panel. (D) Result of a genome-wide scan for metamorphic timing QTL based on the WILD2 mapping panel. A single significant LOD peak corresponding to met1 was identified on LG2.
The most likely position of met1 was determined by QTL analysis using two different mapping panels: LAB (N = 90) and WILD2 (N = 496). In LAB, the segregation of discrete metamorphic and paedomorphic phenotypes was observed to be largely consistent with a single gene model of inheritance (Voss 1995). Accordingly, binary QTL mapping was used to map met1 in LAB. A genome-wide scan for QTL using approximately 900 markers identified only one locus on linkage group 2 (LG2) with LOD scores exceeding an empirically determined significance threshold (LOD > 3.35, P = 0.05; fig. 2B). The maximum LOD score obtained by interval mapping placed met1 between conserved syntenies defined by ngfr-setd2-kif9 and map2k3-rasd1-rai1-shmt1 (fig. 2C). However, this position is based on a single recombinant genotype, and the LOD is equivalent when estimated at the position of any of these flanking markers. To more accurately map met1, we turned to the larger WILD2 panel. In WILD2, we previously reported that ∼90% of individuals showed continuous variation in metamorphic timing, and this variation was largely explained by met1 (Voss and Smith 2005). Thus, we typed genes from the met1 genomic region and performed QTL analysis of metamorphic timing to identify the most likely position for met1 in WILD2. Gene orders were resolved to a finer degree using the WILD2 mapping panel (fig. 2D). The maximum LOD peak coincided with the position of an EST contig (nh_397) that is presumptively unique to A. tigrinum spp. because it does not show sequence identity to any NCBI nucleotide or protein-coding sequence. However, the LOD was approximately equivalent for a 3-cM interval defined by flanking genes aurkb and setd2/ngfr. The LOD score dropped approximately 2 LOD between ngfr and map2k3 and declined further for other SMS loci, although a minor peak was observed at the position of rasd1. Under the assumption that met1 corresponds to a single gene, the most likely position for this gene is between aurkb and map2k3.
Effect of met1 Genotype on Gene Expression in Backcrossed Hybrids
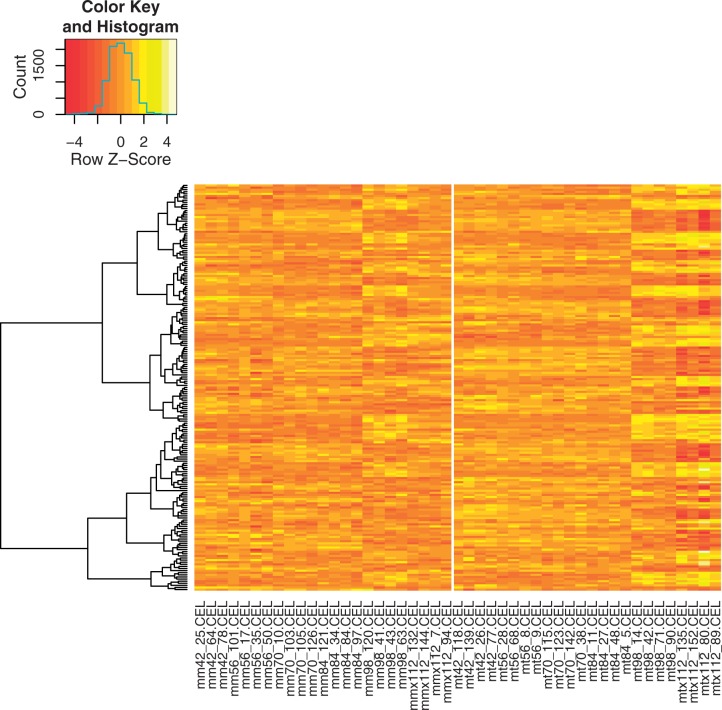
We identified 187 ZMMPSs and 6 EZMMPSs that measured differential transcript abundance between the alternate met1 genotypes at one or more time points using limma (supplementary table S2, Supplementary Material online). Thus, there is no statistical evidence that this list of DEGs is enriched with EZMMPSs (odds ratio = 1.41, lower 95% confidence limit = 0.47, upper 95% confidence limit = 3.59, P[odds ratio = 1] = 0.44). A few of these genes were identified as differentially expressed at the first five time points (42, 56, 70, 84, and 98 DPH), but most were differentially expressed at the last time point (112 DPH). A substantial proportion of these DEGs exhibited temporal changes in both genotypes between 84 and 98 DPH (fig. 3). However, between 98 and 112 DPH, most of these genes returned to expression levels similar to those observed between 42 and 84 DPH in met1mex/mex individuals (fig. 3). Conversely, the expression changes initiated between 84 and 98 DPH continued or intensified in met1mex/Att individuals (fig. 3). The dummy variable quadratic regression approach identified 93 ZMMPSs and 3 EZMMPSs that measured different temporal patterns of expression between the alternate met1 genotypes (supplementary table S3, Supplementary Material online). Hence, there is also no statistical support for the idea that the DEGs identified via maSigPro are statistically enriched with EZMMPSs (odds ratio = 1.38, lower 95% confidence limit = 0.26, upper 95% confidence limit = 4.59, P[odds ratio = 1] = 0.49). Visual inspection of these 96 profiles identified 10 genes with robust expression differences between the alternate met1 genotypes (table 2; supplementary fig. S1, Supplementary Material online). Two of the genes from this list map to LG2 (table 2), and 6 of the 13 DEGs identified from both statistical approaches that map to LG2, located within 35 cM of met1. These patterns indicate that met1 genotype is associated with cis and trans transcriptional changes that commence late in the larval period, before morphological metamorphosis.
Fig. 3.—
Heat map showing the row scaled data from the 193 genes identified using limma. The columns correspond to individual GeneChips with the prefixes mm and mt corresponding to met1mex/mex and met1mex/Att hybrids, respectively. The time points in DPH are denoted by the numbers immediately following the lettered prefixes. Met1mex/mex and met1mex/Att GeneChips are separated by a vertical white line. The dendrogram to the left was obtained via hierarchical clustering of a Euclidean distance matrix.
Table 2.
The 10 Genes Identified via Dummy Variable Regression and Graphical Inspection with Temporally Consistent Differences in mRNA Abundance between Backcrossed Hybrids with Alternate met1 Genotypes
| Gene | Probe Set ID | Sal-Site Version 3 ID |
|---|---|---|
| Atp6v0c* | SRV_01135_a_at | Contig18638 |
| Dhcr7 | SRV_00879_s_at | Contig40972 |
| Eif4a1 | SRV_00923_a_at | Mex_NM_001416_Contig_9 |
| Gdi2 | SRV_00964_a_at | Mex_NM_001494_Contig_1 |
| H2afy2 | SRV_13464_a_at | Tig_NM_018649_Contig_1 |
| Hdac2 | SRV_02393_a_at | Contig40786 |
| Hsp90b1 | SRV_01812_a_at | Mex_NM_003299_Contig_6 |
| NHO | SRV_09160_a_at | Contig77873 |
| Rac1* | SRV_03102_a_at | Contig20873 |
| Tomm70a | SRV_03611_a_at | Contig02244 |
Note.—Sal-Site identifiers correspond to assembly version 3. Profiles of these genes are shown in supplementary figure S1, Supplementary Material online. NHO, no established human ortholog. Asterisk indicates genes that map to Ambystoma LG2.
Met1 Is Associated with the Expression of Genes with Mitochondrial and Neurodevelopmental Functions
Amphibian metamorphosis depends on transcriptional activation of numerous biological processes that transform larval phenotypes into adult phenotypes (Shi 2000). Because our analyses suggested that the vast majority of met1’s transcriptional effects are exerted late in the larval period, we used genes that were differentially expressed between met1mex/mex and met1mex/Att hybrids at 112 DPH to identify biological processes associated with met1. As can be seen in table 3, DEGs upregulated in met1mex/Att hybrids at 112 DPH were highly enriched for genes that function in the mitochondria, with particular enrichment for genes associated with electron transport (supplementary table S4, Supplementary Material online). The 24 DEGs that mapped to the mitochondrion ontology term are associated with a variety of functions including protein synthesis (mrpl13, mrpl30, and mrps7), steroid metabolism (hint2), and apoptosis (cycs). In addition, six genes (ndufa4, ndufa7, ndufab1, ndufc1, ucrc, and uqcrh) associated with mitochondrial ATP synthesis coupled to electron transport were also upregulated in met1mex/Att hybrids at 112 DPH, indicating a change in mitochondrial energetics in individuals that metamorphosed (on average) at significantly earlier times. The DEGs that were upregulated in met1mex/mex individuals (i.e., the genes downregulated in met1mex/Att individuals) at 112 DPH were enriched with genes that mapped to a number of biological processes associated with the endomembrane system, translation, and neurodevelopment (table 3; supplementary table S5, Supplementary Material online). Of particular interest are several genes (actb, mtpn, rhoa, rac1, rtn4, and ubc) associated with neurogenesis in mammals that were upregulated in met1mex/mex hybrids relative to met1mex/Att hybrids. Collectively, these results suggest divergent patterns of brain development and function between met1mex/mex and met1mex/Att hybrids. In particular, met1mex/Att hybrids increase transcription of genes associated with mitochondrial bioenergetics at a time that precedes metamorphosis.
Table 3.
Gene Ontology Terms that Were Significantly Enriched in Genes that Were Differentially Up and Downregulated in met1mex/Att Hybrids Relative to met1mex/mex Hybrids
| GO ID | GO Term | N | P | Total | Enrichment |
|---|---|---|---|---|---|
| GO terms enriched in the DEGs upregulated in met1mex/Att hybrids at 112 DPH | |||||
| 0005743 | Mitochondrial inner membrane | 17 | 2.32 × 10−6 | 58 | 3.70 |
| 0022900 | Electron transport chain | 9 | 2.39 × 10−5 | 18 | 6.24 |
| 0005739 | Mitochondrion | 24 | 2.17 × 10−4 | 143 | 2.12 |
| 0006119 | Oxidative phosphorylation | 9 | 2.72 × 10−4 | 24 | 4.68 |
| 0015078 | Hydrogen ion transmembrane transporter activity | 8 | 4.92 × 10−4 | 21 | 5.01 |
| 0003735 | Structural constituent of ribosome | 13 | 9.44 × 10−4 | 60 | 2.85 |
| 0042775 | Mitochondrial ATP synthesis coupled electron transport | 6 | 3.06 × 10−3 | 14 | 5.35 |
| 0016491 | Oxidoreductase activity | 13 | 0.01 | 79 | 2.16 |
| 0015980 | Energy derivation by oxidation of organic compounds | 7 | 0.02 | 29 | 3.01 |
| 0006414 | Translational elongation | 10 | 0.03 | 57 | 2.19 |
| 0051234 | Establishment of localization | 25 | 0.03 | 213 | 1.46 |
| 0006120 | Mitochondrial electron transport, NADH to ubiquinone | 4 | 0.04 | 10 | 4.99 |
| GO terms enriched in the DEGs downregulated in met1mex/Att hybrids at 112 DPH | |||||
| 0012505 | Endomembrane system | 14 | 2.13 × 10−4 | 69 | 3.10 |
| 0008135 | Translation factor activity, nucleic acid binding | 6 | 0.01 | 22 | 4.11 |
| 0003924 | GTPase activity | 7 | 0.01 | 31 | 3.41 |
| 0022008 | Neurogenesis | 6 | 0.02 | 25 | 3.59 |
| 0006417 | Regulation of translation | 5 | 0.03 | 19 | 3.94 |
Note.—In total, 70 gene ontology terms were enriched in the list of DEGs that were upregulated in met1mex/Att hybrids and 32 gene ontology terms were enriched in the list of DEGs that were downregulated in met1mex/Att hybrids. The data in the table correspond to a subset of these results, which are shown in their entirety in supplementary tables S4 and S5, Supplementary Material online. P = modified Fisher’s exact P value. N = the number of DEGs that annotate to a given ontology term. Total = the number of genes on the array that annotate to a given ontology term. Enrichment = the increase in DEGs annotating to a given ontology term relative to the number of genes expected given a random draw from the array of equal size.
Expression of Hybrid DEGs in the Parent Species
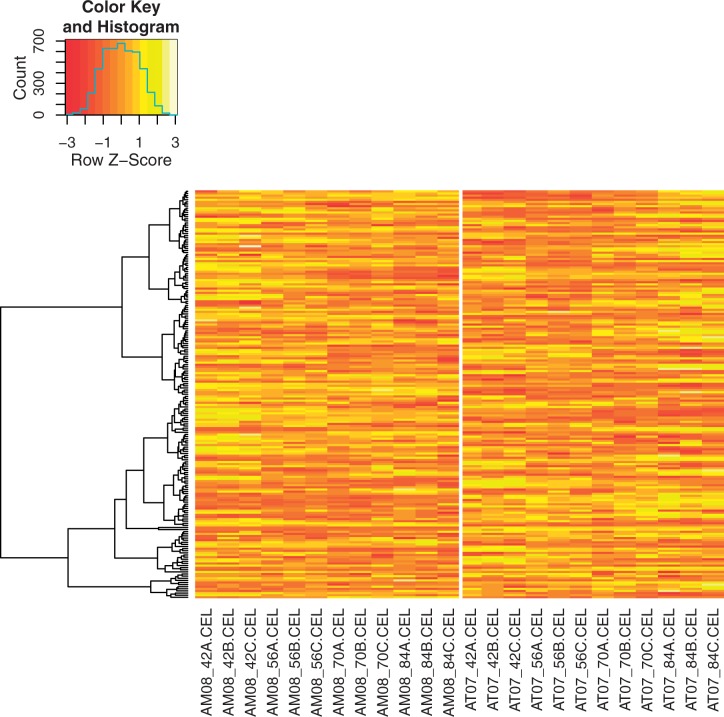
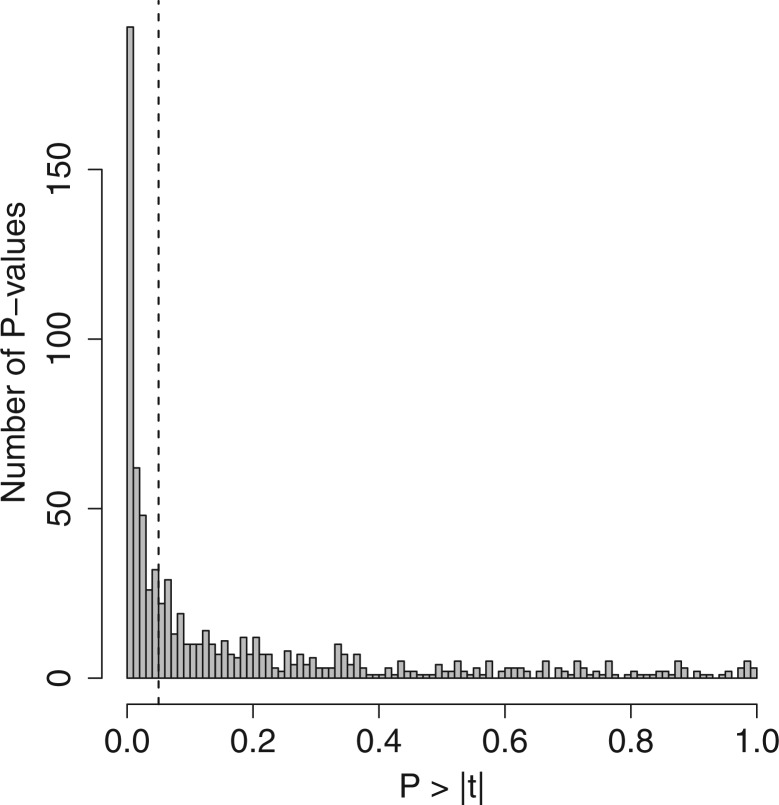
In a previous study, we characterized growth rates and neurodevelopmental gene expression in A. mexicanum and A. t. tigrinum larvae from 42 to 84 DPH (Page et al. 2010). In that study, A. t. tigrinum larvae grew at a faster rate, and all A. t. tigrinum showed definitive signs of morphological metamorphosis by 84 DPH. Metamorphosis-associated genes were identified from A. t. tigrinum that changed in abundance as larval development proceeded, and these changes were particularly pronounced at the later time points. In addition, 419 probe sets with zero nucleotide mismatches between A. mexicanum and A. t. tigrinum measured consistently different magnitudes of expression between species, with expression generally higher in A. t. tigrinum. Reexamination of how the 193 DEGs identified from hybrids in this study using limma were expressed in the parent species revealed a robust pattern of gene expression divergence (fig. 4). The distribution of P values for the 772 t statistics we computed from the parental data differs greatly from the uniform distribution that would be expected if there were no expression differences between the species (fig. 5). This general pattern also holds for each time point when the P values are not pooled (not shown). Approximately 65% of these genes were differentially expressed (α = 0.05) between A. mexicanum and A. t. tigrinum at one or both of the two earliest time points (42 or 56 DPH). Moreover, approximately 78% of these genes were differentially expressed between the parent species (α = 0.05) at one or more of the four time points that we sampled parental gene expression. These results show that many of the genes that are differentially expressed as a function of met1 genotype at 112 DPH in backcrossed hybrids are also differentially expressed between A. mexicanum and A. t. tigrinum, and that many of these differences are detectable in the parent species early during the larval period.
Fig. 4.—
Heat map showing row scaled data from the parent species for the 193 genes identified from backcrossed hybrids using limma. The columns correspond to individual GeneChips with the prefixes AM and AT corresponding to A. mexicanum and A. t. tigrinum samples, respectively. The time points in DPH are denoted by the numbers immediately following the underscore. GeneChips from different species are separated by a vertical white line. The dendrogram to the left was obtained via hierarchical clustering of a Euclidean distance matrix.
Fig. 5.—
Histogram showing the distribution of the 772 t statistic P values that were calculated from previously generated data on A. mexicanum and A. t. tigrinum. The dashed vertical line denotes P > |t| = 0.05.
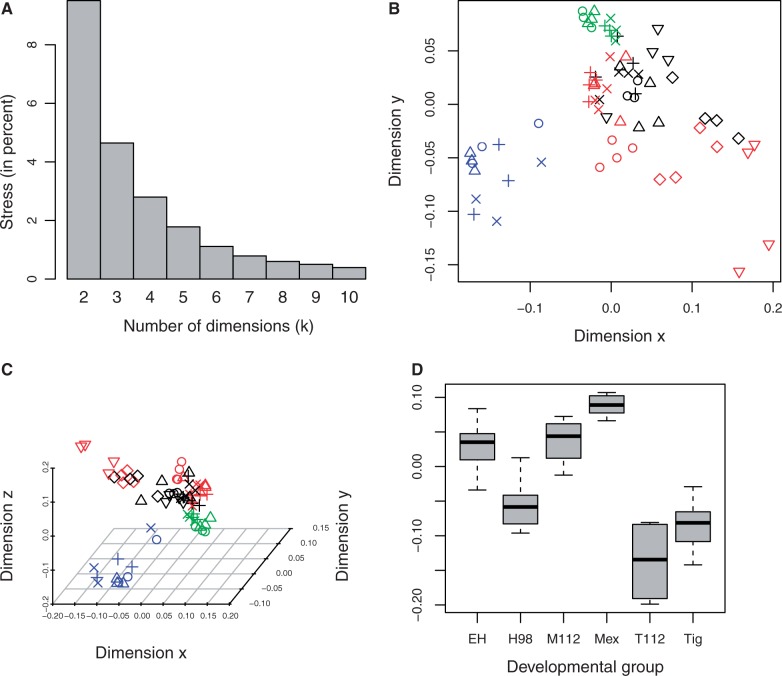
To visualize the degree of similarity in expression between hybrid met1 genotypes and the parent species, we conducted NMDS on the 24 DEGs that were differentially expressed between met1mex/mex and met1mex/Att hybrids by ≥1.5-fold at one or more time points. The algorithm converged within 150 iterations for solutions in k = 2–10 dimensions. As can be seen in figure 6A, the stress did not level off until k was beyond a number of dimensions that could be easily visualized. Nevertheless, there are several features of the NMDS solutions where k = 2 (fig. 6B) and k = 3 (fig. 6C) that suggest there is: 1) hybrid intermediacy in gene expression, 2) a change in both hybrid met1 genotypes at 98 DPH that causes their expression profiles to more closely resemble A. t. tigrinum expression along at least one dimension, and 3) a reversal of the temporal trajectory initiated between 84 and 98 DPH in met1mex/mex hybrids that is not observed in met1mex/Att hybrids (fig. 3). When k = 3, these patterns are readily apparent in the values obtained for the horizontal axis depicted in figure 6C. The distributions of the coordinates for this dimension are shown for several developmentally relevant groups in figure 6D. Collectively, our reexamination of hybrid DEGs in the parental species suggests that many differences between A. mexicanum and A. t. tigrinum in neurodevelopmental gene expression trace to genetic factors from the met1 genomic region.
Fig. 6.—
Results of the NMDS analysis: (A) scree plot showing the stress (in percent) for NMDS solutions based on different numbers of dimensions, (B) scatter plot of the coordinates obtained when k = 2, (C) scatter plot of the coordinates obtained when k = 3, and (D) box plots of various developmental groups’ coordinates for the horizontal dimension shown in (C). In (B) and (C), green indicates A. mexicanum; blue, A. t. tigrinum; black, met1mex/mex hybrids; red, met1mex/Att hybrids. Circles = 42 DPH; triangles = 56 DPH; + = 70 DPH; × = 84 DPH; diamonds = 98 DPH; and inverted triangles = 112 DPH. In (D), EH = 42–84 DPH hybrids irrespective of genotype, H98 = 98 DPH hybrids irrespective of genotype, Mex = A. mexicanum irrespective of sampling time, M112 = 112 DPH met1mex/mex hybrids, T112 = 112 DPH met1mex/Att hybrids, and Tig = A. t. tigrinum irrespective of sampling time.
Discussion
Evolutionary History of the met1 Genomic Region
An important objective in evolutionary genetics is to identify genes for adaptive traits (Feder and Mitchell-Olds 2003). This objective has been pursued most often by testing a priori selected candidate genes (Voss et al. 2000, 2003), with fewer studies using unbiased approaches to identify candidate genes for QTL. A logical first step in searching for candidate genes is to cross-reference a QTL genomic region to a reference genome or genetic map. In our case, some of the met1-associated genes that we initially mapped were not found to be syntenic in any vertebrate genome. Through trial and error mapping of candidate loci and application of a functional genomics approach, we efficiently identified genes linked to met1. In the process, we obtained a sufficient number of loci to reconstruct the dynamic and lineage-specific history of chromosomal rearrangements that structured LG2 and the met1 genomic region. LG2 primarily contains three ancestral blocks of loci that correspond to ancestral vertebrate chromosomes (Voss et al. 2011). In the chicken genome, these blocks correspond to Gga12, Gga14, and a portion of chicken chromosome 2 (Gga2). Genes from these three blocks are not syntenic in Xenopus and thus were fused after the divergence of anurans and salamanders from a common ancestor. During salamander phylogenesis, intrachromosomal rearrangements occurred between segments corresponding to Gga2 and Gga14, interleaving conserved syntenic groups of loci and creating unique gene orders. This is uncharacteristic of the Ambystoma genome, which has undergone a lower overall chromosomal rearrangement rate than other vertebrates and especially mammals (Smith and Voss 2006). In fact, the chromosome associated with LG2 contains more lineage-specific, intrachromosomal rearrangements than are predicted from any other Ambystoma linkage group (Voss et al. 2011). Additional comparative mapping studies are needed to determine whether this chromosomal evolutionary history is unique to A. tigrinum spp. or shared among all extant salamanders. Of particular relevance to this study is the fact that these chromosomal rearrangements brought several genes with associated functions into linkage during evolution.
Gene Expression and the met1 Genomic Region
Our functional genomics approach accomplished two important objectives. First, by comparing the abundances of RNAs between individuals that inherited different met1 genotypes, we efficiently identified expression differences for loci linked to met1. Second, by comparing transcription within developing larval brains, we identified expression differences that are functionally associated with species-specific differences, including life history variation. Although it remains possible that met1 corresponds to a single gene, the observation that several genes in linkage disequilibrium with met1 were differentially expressed raises the possibility that more than one gene underlies the effect of met1 on metamorphic timing and gene expression. Moreover, when considering genes mapped in this study, and other genes that are predicted by synteny to map to the reconstructed met1 region, we find that several sets of functionally associated genes were brought into linkage during evolution. These include genes associated with brain development (ngfr, ccm2, setd2, cspg5, and rasd1), transcriptional regulation (setd2, smarcc1, spop, and med9), lipid biosynthesis (srebf1, scap, and pemt), and mitochondrial function (tmem11, atpaf2, and nt5m). Notably, srebf1 and scap encode proteins that physically interact to regulate transcription of genes associated with cellular lipid homeostasis (Brown and Goldstein 1999). Linkage of functionally associated genes suggests that met1 presents characteristics of a supergene. A supergene is a group of linked loci that cosegregate alleles for specifying suites of coadapted traits (Joron et al. 2006). In the case of met1, genes that were brought into linkage may specify multiple brain phenotypic differences between metamorphic A. t. tigrinum and paedomorphic A. mexicanum. It will be interesting to test the idea that met1 is a supergene in natural populations where tiger salamanders express metamorphosis or paedomorphosis as a polymorphism to exploit ephemeral and permanent ponds, respectively.
Although it is unknown which mechanism functions in A. mexicanum to yield a paedomorphic developmental outcome, a recent study showed that met1 is a TH-responsive QTL (Voss et al. 2012). We extend this finding to hypothesize that met1 affects the brain’s response to TH during development, and as a result, this causes variation in transcription and timing of metamorphosis. We observed a large number of genes that were expressed differently between the hybrid met1 genotypes by 112 DPH, and many of these DEGs were also differentially expressed between A. mexicanum and A. t. tigrinum as early as 42 DPH and extending to 84 DPH. It is well established that TH activates new patterns of gene expression during anuran metamorphosis and vertebrate brain development (Shi 2000), including changes in cellular metabolism not unlike the highly enriched mitochondrial biological processes that we observed in our study. In particular, genes that function in electron transport and ATP synthesis were enriched in our study, suggesting a critical role for oxidative phosphorylation in meeting the energetic demands associated with neuronal differentiation and developmental processes that occur during metamorphosis. Overall, our results show that high and low brain bioenergetics profiles are established very early in the larval period between A. t. tigrinum and A. mexicanum, but later in the case of hybrids. This may explain why these species show different larval growth rates and life history outcomes.
Growth, Differentiation, and Metamorphic Timing
The timing of metamorphosis is an ecologically and evolutionarily important trait because it is influenced by temporal aspects of environmental factors that determine whether and when the adult habitat will become more suitable for growth and reproduction than the larval habitat. Consequently, the earliest and most influential ecological model of amphibian metamorphosis proposed that an organism’s growth history is a critical determinant of metamorphic timing (Wilbur and Collins 1973). According to this model, there is a range of body sizes in which metamorphosis occurs, and within this range, metamorphosis will not proceed until the growth rate drops below a threshold value. Although there is strong empirical support for the idea that a threshold model is the appropriate framework for conceptualizing metamorphic timing (Morey and Reznick 2000), there is debate over whether growth rate or differentiation rate, two traits that are often highly correlated, is the crucial determinant (Smith-Gill and Berven 1979). At 112 DPH, we observed large-scale differences in transcription between met1 genotypes that were not preceded by differences in growth rate. Moreover, many of these differences in gene expression appear to be associated with metamorphic differentiation events that are eminent in met1mex/Att hybrids, but not met1mex/mex hybrids. Although the relevance of our findings remains to be established for natural populations, our results show that differences in metamorphic timing are not necessarily coupled to differences in larval growth history.
Conclusion
We showed in this study that met1 locates to a region of the Ambystoma linkage map that has been structured by chromosomal rearrangements, creating a gene order that has not been observed in any other vertebrates. Some of the loci in the met1 region are associated with bioenergetics and brain development and function. Several of these genes, and many other genes that are not linked to met1, are differentially expressed between hybrids with alternate met1 genotypes. In addition, we showed that many of the genes that were differentially expressed late in the larval period between hybrids with alternate met1 genotypes were also differentially expressed between A. mexicanum and A. t. tigrinum at an earlier time of development. These results support the idea that differences between A. mexicanum and A. t. tigrinum in metamorphic timing and neurodevelopmental gene expression are associated with genetic factors in the met1 genomic region. Finally, we have shown that despite its profound effects on developmental timing and gene expression, met1 does not influence the larval growth trajectories of backcrossed hybrids prior to its effects on transcription in the brain. Overall, our study underscores the importance of using an integrative genomics approach to resolve genetic aspects of life history variation.
Supplementary Material
Supplementary tables S1–S5 and figure S1 are available at Genome Biology and Evolution online (http://www.gbe.oxfordjournals.org/).
Acknowledgments
This work was supported by the Office of Research Infrastructure Programs at the National Institutes of Health (grant number 2R24OD010435 to S.R.V)., National Science Foundation (grant numbers IBN-9982719, IBN-0242833, IBN-0080112, and DBI-0951484 to S.R.V). R.B.P. was supported by the Wallace Endowment to the Department of Biology at the University of Louisville.
Literature Cited
- Bar-Or C, Czosnek H, Koltai H. Cross-species microarray hybridizations: a developing tool for studying species diversity. Trends Genet. 2007;23:200–207. doi: 10.1016/j.tig.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Methodol. 1995;57:289–300. [Google Scholar]
- Bolstad BM, et al. Quality assessment of affymetrix GeneChip data. In: Gentleman R, Carey VJ, Huber W, Irizarry RA, Dudoit S, editors. Bioinformatics and computational biology solutions using R and bioconductor. New York: Springer; 2005. pp. 33–47. [Google Scholar]
- Bradshaw HD, Schemske DW. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature. 2003;426:176–178. doi: 10.1038/nature02106. [DOI] [PubMed] [Google Scholar]
- Broman KW, Sen WH, Churchill GA. R/qtl: QTL mapping in experimental crosses. Bioinformatics. 2003;19:889–890. doi: 10.1093/bioinformatics/btg112. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci U S A. 1999;96:11041–11048. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DR, Paul BD, Fu L, Shi YB. Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen Comp Endocrinol. 2006;145:1–19. doi: 10.1016/j.ygcen.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Buckley BA. Comparative environmental genomics in non-model species: using heterologous hybridization to DNA-based microarrays. J Exp Biol. 2007;209:1602–1606. doi: 10.1242/jeb.002402. [DOI] [PubMed] [Google Scholar]
- Churchill GA, Doerge RW. Empirical threshold values for quantitative trait mapping. Genetics. 1994;138:963–971. doi: 10.1093/genetics/138.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conesa A, Nueda MJ, Ferrer A, Talon M. maSigPro: a method to identify significantly differential expression profiles in time-course microarray experiments. Bioinformatics. 2006;22:1096–1102. doi: 10.1093/bioinformatics/btl056. [DOI] [PubMed] [Google Scholar]
- Crawley MJ. The R Book. West Sussex (UK): Wiley; 2007. [Google Scholar]
- Dennis G, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:R60. [PubMed] [Google Scholar]
- Denver RJ, Glennemeier KA, Boorse GC. Endocrinology of complex life cycles: amphibians. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Rubin R, editors. Hormones, brains, and behavior. San Diego (CA): Academic Press; 2002. pp. 469–513. [Google Scholar]
- Feder ME, Mitchell-Olds T. Evolutionary and ecological functional genomics. Nat Rev Genet. 2003;4:651–657. doi: 10.1038/nrg1128. [DOI] [PubMed] [Google Scholar]
- Galton VA. Thyroid hormone receptors and iodothyronine deiodinases in the developing Mexican axolotl, Ambystoma mexicanum. Gen Comp Endocrinol. 1992;85:62–70. doi: 10.1016/0016-6480(92)90172-g. [DOI] [PubMed] [Google Scholar]
- Gautier L, Cope L, Bolstad BM, Irizarry RA. Affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20:307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- Gould SJ. Ontogeny and phylogeny. Cambridge (MA): Harvard University Press; 1977. [Google Scholar]
- Haley CS, Knott SA. A simple regression method for mapping quantitative trait loci in line crosses using flanking markers. Heredity. 1992;69:315–324. doi: 10.1038/hdy.1992.131. [DOI] [PubMed] [Google Scholar]
- Hoekstra HE, Hirschmann RJ, Bundey RA, Insel PA, Crossland JP. A single amino acid mutation contributes to adaptive beach mouse color pattern. Science. 2006;313:101–104. doi: 10.1126/science.1126121. [DOI] [PubMed] [Google Scholar]
- Hosack DA, Dennis G, Jr, Sherman BT, Lane HC, Lempicki RA. Identifying biological themes within lists of genes with EASE. Genome Biol. 2003;4:R70. doi: 10.1186/gb-2003-4-10-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Jones CD. The genetic basis of Drosophila sechellia’s resistance to a host plant toxin. Genetics. 1998;149:1899–1908. doi: 10.1093/genetics/149.4.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joron M, et al. A conserved supergene locus controls colour pattern diversity in Heliconius butterflies. PLoS Biol. 2006;4:e303. doi: 10.1371/journal.pbio.0040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosambi D. The estimation of map distances from recombination values. Ann Eugen. 1944;12:172–175. [Google Scholar]
- Morey S, Reznick D. A comparative analysis of plasticity in larval development in three species of spadefoot toads. Ecology. 2000;81:1736–1749. [Google Scholar]
- Mundy NI. Coloration and the genetics of adaptation. PLoS Biol. 2007;5:e250. doi: 10.1371/journal.pbio.0050250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman RA. Developmental plasticity of Scaphiopus couchii tadpoles in an unpredictable environment. Ecology. 1989;70:1775–1787. [Google Scholar]
- O’Neill EM, et al. Parallel tagged amplicon sequencing reveals major lineages and phylogenetic structure in the North American tiger salamander (Ambystoma tigrinum) species complex. Mol Ecol. 2013;22:111–129. doi: 10.1111/mec.12049. [DOI] [PubMed] [Google Scholar]
- Page RB, Boley MA, Smith JJ, Putta S, Voss SR. Microarray analysis of a salamander hopeful monster reveals transcriptional signatures of paedomorphic brain development. BMC Evol Biol. 2010;10:199. doi: 10.1186/1471-2148-10-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RB, et al. Microarray analysis identifies keratin loci as sensitive biomarkers for thyroid hormone disruption in the salamander Ambystoma mexicanum. Comp Biochem Physiol C Pharmacol Toxicol. 2007;145:15–27. doi: 10.1016/j.cbpc.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Petranka JW. Salamanders of the United States and Canada. Washington (DC): Smithsonian Institution Press; 1998. [Google Scholar]
- Prahlad KV. Induced metamorphosis: rectification of a genetic disability by thyroid hormone in the Mexican axolotl Siredon mexicanum. Gen Comp Endocrinol. 1968;11:21–30. doi: 10.1016/0016-6480(68)90103-2. [DOI] [PubMed] [Google Scholar]
- Schneider RA, Helms JA. The cellular and molecular origins of beak morphology. Science. 2003;299:565–568. doi: 10.1126/science.1077827. [DOI] [PubMed] [Google Scholar]
- Semlitsch RD, Wilbur HM. Artificial selection for paedomorphosis in the salamander Ambystoma talpoideum. Evolution. 1989;43:105–112. doi: 10.1111/j.1558-5646.1989.tb04210.x. [DOI] [PubMed] [Google Scholar]
- Shaffer HB, McKnight ML. The polytypic species revisited: genetic differentiation and molecular phylogenetics of the tiger salamander Ambystoma tigrinum (amphibian: caudate) complex. Evolution. 1996;50:417–433. doi: 10.1111/j.1558-5646.1996.tb04503.x. [DOI] [PubMed] [Google Scholar]
- Shi YB. 2000. Amphibian metamorphosis: from morphology to molecular biology. New York: Wiley-Liss.
- Smith JJ, Kump DK, Walker JA, Parichy DM, Voss SR. A comprehensive expressed sequence tag linkage map for tiger salamanders and the Mexican axolotl: enabling gene mapping and comparative genomics in Ambystoma. Genetics. 2005;171:1161–1171. doi: 10.1534/genetics.105.046433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JJ, Voss SR. Gene order data from a model amphibian: new perspectives on vertebrate genome structure and evolution. BMC Genomics. 2006;7:219. doi: 10.1186/1471-2164-7-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Gill SJ, Berven KA. Predicting amphibian metamorphosis. Am Nat. 1979;113:563–585. [Google Scholar]
- Smyth GK. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- Streelman JT, Peichel CL, Parichy DM. Developmental genetics of adaptation in fishes: the case for novelty. Annu Rev Ecol Evol Syst. 2007;38:655–681. [Google Scholar]
- Venables WN, Ripley BD. Modern applied statistics with S. 4th ed. New York: Springer; 2010. [Google Scholar]
- Voss SR. Genetic basis of paedomorphosis in the axolotl, Ambystoma mexicanum: a test of the single gene hypothesis. J Hered. 1995;86:441–447. [Google Scholar]
- Voss SR, Kump DK, Walker JA, Shaffer HB, Voss GJ. Thyroid hormone responsive QTL and the evolution of paedomorphic salamanders. Heredity. 2012;109:293–298. doi: 10.1038/hdy.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss SR, Prudic KL, Oliver JC, Shaffer HB. Candidate gene analysis of metamorphic timing in ambystomatid salamanders. Mol Ecol. 2003;12:1217–1223. doi: 10.1046/j.1365-294x.2003.01806.x. [DOI] [PubMed] [Google Scholar]
- Voss SR, Shaffer HB. Adaptive evolution via a major gene effect: paedomorphosis in the Mexican axolotl. Proc Natl Acad Sci U S A. 1997;94:14185–14189. doi: 10.1073/pnas.94.25.14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss SR, Shaffer HB. Evolutionary genetics of metamorphic failure using wild-caught vs. laboratory axolotls (Ambystoma mexicanum) Mol Ecol. 2000;9:1401–1407. doi: 10.1046/j.1365-294x.2000.01025.x. [DOI] [PubMed] [Google Scholar]
- Voss SR, Shaffer HB, Taylor J, Safi R, Laudet V. Candidate gene analysis of thyroid hormone receptors in metamorphosing vs. nonmetamorphosing salamanders. Heredity. 2000;85:107–114. doi: 10.1046/j.1365-2540.2000.00714.x. [DOI] [PubMed] [Google Scholar]
- Voss SR, Smith JJ. Evolution of salamander life cycles: a major-effect quantitative trait locus contributes to discrete and continuous variation for metamorphic timing. Genetics. 2005;170:275–281. doi: 10.1534/genetics.104.038273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss SR, et al. Origin of avian and amphibian chromosomes by fusion, fission, and retention of ancestral chromosomes. Genome Res. 2011;8:1306–1312. doi: 10.1101/gr.116491.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur HM. Complex life cycles. Annu Rev Ecol Syst. 1980;11:67–93. [Google Scholar]
- Wilbur HM, Collins JP. Ecological aspects of amphibian metamorphosis. Science. 1973;28:1305–1314. doi: 10.1126/science.182.4119.1305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.