Abstract
Background
United Kingdom (U.K.) and United States (U.S.) guidelines for risk stratification after polypectomy differ, as do recommended surveillance intervals.
Objective
To compare risk of advanced colorectal neoplasia at one-year colonoscopy among patients cross-classified by U.S. and U.K. surveillance guidelines.
Design
Pooled analysis of four prospective studies, 1984-1998.
Setting
Academic and private clinics in the U.S.
Patients
3226 post-polypectomy patients with 6-18 month follow-up colonoscopy.
Measurements
Rates of advanced neoplasia (adenoma ≥ 1cm, high-grade dysplasia, >25% villous histology, or invasive cancer) at one year, compared across U.S. and U.K. risk categories.
Results
Advanced neoplasia (95% CI) was detected one year post-polypectomy in 3.8% (2.7%-4.9%) of lower-risk and 11.2% (9.8%-12.6%) of higher-risk patients, by U.S. criteria. Using U.K. criteria, 4.4% (3.3%-5.4%), 9.9% (8.3%-11.5%), and 18.7% (14.8%-22.5%) of low-, intermediate-, and high-risk patients, respectively, presented with advanced neoplasia; U.K. high-risk patients comprised 12% of all patients. All U.S. lower-risk patients were low-risk by U.K. criteria; however, since the U.K. guidelines do not consider histological features, more patients are classified as low-risk. U.S. higher-risk patients distributed across the three U.K. categories. Considering all patients with advanced neoplasia, 26.3% were reclassified by the U.K criteria to a higher and 7.0% to a lower risk category, with a net 19% benefiting from two-year earlier detection. Overall, substitution of U.K. for U.S. guidelines resulted in an estimated 0.03 additional colonoscopies per five years per patient.
Limitations
Patients were enrolled 15-20 years ago; colonoscopy quality measures were unavailable. Patients lacking follow-up colonoscopy or with surveillance colonoscopy after 6-18 months, and those with cancer or insufficient baseline adenoma characteristics were excluded (2076/5302).
Conclusions
Application of the U.K. guidelines in the U.S. could identify a subset of patients whose high risk may warrant a one-year clearing colonoscopy, without substantially increasing colonoscopy rates.
Keywords: adenoma, colonoscopy, colorectal cancer, polypectomy, surveillance guidelines
Introduction
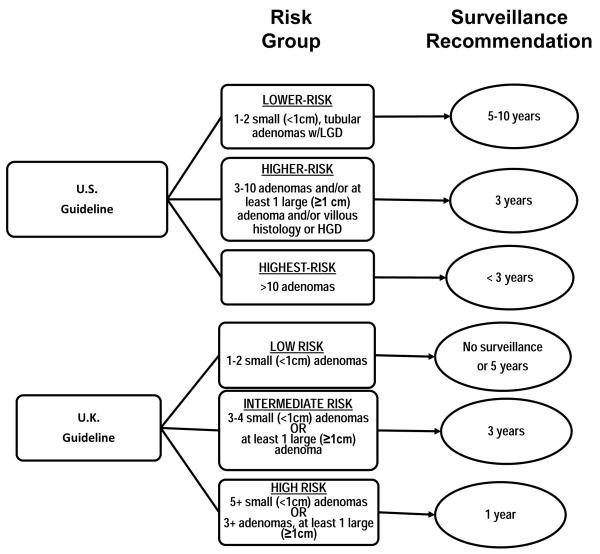
Colonoscopic polypectomy likely accounts for much of the recent decline in colorectal cancer incidence in the U.S. (1) and elsewhere in the Western world (2). On colonoscopic screening, 20-50% of patients are found to harbor colorectal adenomas, which can progress to colorectal cancer if not removed (3-5), and patients with resected adenomas are advised to have follow-up surveillance colonoscopy to reduce their risk of cancer (6-9). Guidelines for colonoscopy surveillance intervals in the U.S. and U.K. are based on results from controlled trials and observational studies (6-9). However, the U.S. and U.K. guidelines differ in how they define risk strata as well as in the recommended surveillance intervals (Figure 1).
Figure 1.
U.S. and U.K. Colonoscopy Surveillance Guidelines according to Risk Group. Participants in the pooled studies required one or more adenoma(s) to be eligible. LGD, low-grade dysplasia; HGD, high grade dysplasia.
In 2006, the U.S. Multi-Society Task Force on Colorectal Cancer and the American Cancer Society jointly developed guidelines for colonoscopy surveillance intervals after polypectomy (6) that are based on the number, size, and histology (villous architecture and degree of dysplasia) of polyps removed, and an update of these guidelines in 2012 (9) reaffirmed the recommended surveillance intervals. Guidelines of the British Society of Gastroenterology and the Association of Coloproctology for Great Britain and Ireland (7, 8) stratify patients into risk groups based on the size and number of adenomas, without considering histological features. One key difference between the two sets of guidelines is that the U.K. criteria recommend a single clearing examination at one year for patients classified as high-risk (i.e., those with ≥ 5 small adenomas or ≥ 3 adenomas at least one of which is ≥ 1cm). In contrast, the U.S. guidelines recommend follow-up surveillance in 3 years for this type of patient, except that a shorter interval (< 3 years) is recommended for those with more than 10 adenomas. Patients with 1-2 small adenomas or histological features of high grade dysplasia (HGD) or villous histology are recommended for a 3-year follow-up interval under the U.S. guidelines and either no surveillance or a 5-year interval under the U.K. guidelines. The clinical and public health consequences of using one of these guidelines rather than the other have not been explored.
We therefore compared the performance of the U.S. and U.K. surveillance guidelines, with regard to their identification of patients with advanced colorectal neoplasia (adenoma ≥1cm, presence of high-grade dysplasia (HGD), >25% villous histology, or invasive cancer) as detected at a one-year colonoscopy.
Methods
Patient and Data Sources
As part of a previously-reported pooled analysis of colorectal neoplasia risk following colonoscopic polypectomy (10), we assembled patient-level data from eight studies in North America that met the following criteria: 800 or more study participants; study protocol requiring complete baseline colonoscopy with removal of one or more adenomas and all visualized lesions; specified schedule of surveillance follow-up colonoscopies; availability of end point data regarding the adenoma features and colorectal cancers detected at follow-up; and study findings reported by June 2005. The current analysis uses data from patients in the four prevention trials whose protocol called for a colonoscopy one year after the initial examination: the Antioxidant Polyp Prevention Study (APPS) (11), the Calcium Polyp Prevention Study (CPPS) (12), the Polyp Prevention Trial (PPT) (13), and the Wheat Bran Fiber (WBF) study (14). The studies all used self-administered questionnaires to ascertain age, sex, history of colorectal cancer in first-degree relatives, and history of colorectal polyps preceding the baseline colonoscopy. Data on the number, size, location and histology of baseline adenomas were abstracted from endoscopy and pathology reports (10); however, the pathology reports for some patients did not specify whether villous features were present, and the CPPS did not record degree of dysplasia for baseline adenomas. The coordinating centers for each study obtained approval from their respective institutional review board for the current protocol.
Risk Categories
The U.S. and U.K. surveillance guidelines are described in detail elsewhere (6-9). The 2012 U.S. guidelines group patients into risk categories based on the following findings at baseline colonoscopy: small (<10 mm) hyperplastic polyps in the rectum of sigmoid; one or two small (< 10 mm) tubular adenomas; three to ten tubular adenomas; more than ten adenomas; one or more tubular adenomas >10 mm; one or more villous adenomas; adenomas with HGD; serrated lesions; serrated polyposis syndrome. The protocols for the four trials included in our current analyses required that patients have at least one adenoma and a complete baseline colonoscopy with removal of all visible polyps shortly before enrollment and no data on serrated lesions were routinely collected; thus, our studies only included patients who would be categorized into three groups based on their surveillance intervals, which for convenience we have denoted as “lower-risk” (one or two small (< 10 mm) tubular adenomas without HGD), “higher-risk” (any adenoma >10 mm or with villous histology or HGD, or three to ten adenomas), and “highest-risk” (more than ten adenomas). The U.K. guidelines stratify patients as: “low-risk”, with only 1-2 small (<1cm) adenomas; “intermediate-risk”, with 3-4 small adenomas or at least one adenoma ≥1cm (but not both); and “high-risk”, with ≥ 5 small adenomas or ≥3 adenomas at least one of which is ≥1cm. Figure 1 depicts the relevant U.S. and U.K. guideline risk groups and the corresponding surveillance follow-up recommendations.
Outcomes
Advanced colorectal neoplasia detected between six and 18 months post-polypectomy was the primary endpoint, defined as any adenoma of size ≥1cm diameter, >25% villous histology (tubulovillous or villous histology), presence of HGD, and/or invasive colorectal cancer. Neoplasms reported from examinations conducted in the first six months were not considered as outcomes, as these procedures were likely performed because of concerns about the completeness of the baseline colonoscopy; only 41 (1.3%) of the total 3226 patients had a procedure within six months of baseline.
Statistical Analyses
We used characteristics of the adenoma(s) identified at the baseline colonoscopy to cross-classify patients into risk groups defined according to the U.S. and U.K. surveillance guidelines. Absolute risk and exact 95% confidence intervals (CI) of advanced neoplasia and of HGD or invasive cancer at one year post-polypectomy were computed for each risk group. We compared the net effect of applying the U.K. guidelines to the U.S. using a net reclassification improvement approach (15, 16), comparing the proportion and direction of reclassification for those with and without advanced neoplasia.
Our data were from four clinical trials with different interventions intended to prevent metachronous (recurrent) adenomas, and we evaluated potential heterogeneity by graphically displaying absolute risk and 95% confidence intervals by treatment arm within each risk stratum (Supplemental Figures 1a and 1b). As a sensitivity analysis, we computed risk estimates including the 471 CPPS patients who lacked atypia data, by assuming they were lower-risk by the U.S. criteria. Analyses were conducted using SAS version 9.2, SAS Institute Inc. (Cary, NC).
Results
Of the 5302 patients enrolled in the four trials, we excluded three with invasive cancer at baseline, 221 with no follow-up colonoscopy, and 1062 whose first surveillance colonoscopy was performed more than 18 months after randomization, leaving 4016 patients (Table 1). We further excluded patients that could not be classified by both sets of guidelines: 680 with 1-2 small adenomas but incomplete information regarding degree of dysplasia and/or villous architecture, and 110 without adenoma size recorded. HGD information was not routinely collected in the CPPS trial, and 471 of the 790 excluded patients were CPPS participants with 1-2 small tubular adenomas of unknown dysplasia status; however, we did include the 44 CPPS participants who had 1-2 small villous adenomas.
Table 1.
Description of studies and available sample size for analysis
| APPS | CPPS | PPT | WBF | Total | |
|---|---|---|---|---|---|
|
|
|||||
| Study Design (number of centers) |
4-arm trial (6) | 2-arm trial (7) | 2-arm trial (8) | 2-arm trial (1) | |
| Recruitment period | 1984-1988 | 1991-1998 | 1991-1998 | 1990-1998 | |
| Follow-up colonoscopy schedule | yrs 1 and 4 | yrs 1 and 4 | yrs 1 and 4 | yrs 1 and 3 | |
|
| |||||
| Number enrolled in the trial | 864 | 930 | 2079 | 1429 | 5302 |
| Colorectal cancer at baseline | −1 | 0 | 0 | −2 | −3 |
| No follow-up colonoscopy | −26 | −17 | −55 | −123 | −221 |
| No follow-up colonoscopy <18 months | −61 | −31 | −673 * | −297 † | −1062 |
|
|
|||||
| No. with 6-18 month colonoscopy, no CRC at baseline | 776 | 882 | 1351 | 1007 | 4016 |
| Not classifiable by U.S. and U.K.‡ | −2 | −2 | −81 | 0 | −85 |
| Not classifiable by U.K. only§ | −1 | −1 | −23 | 0 | −25 |
| Not classifiable by U.S. only∥ | −15 | −471 | −67 | −127 | −680 |
|
|
|||||
| Participants available for this analysis | 758 | 408 | 1180 | 880 | 3226 |
Abbreviation: CRC, colorectal cancer; APPS, Antioxidant Polyp Prevention Study; CPPS, Calcium Polyp Prevention Study; PPT, Polyp Prevention Trial; WBF, Wheat Bran Fiber trial.
Participants were intended to undergo a one-year colonoscopy, which was defined as at least 180 days after randomization but less than 2 years afterward. For the current analysis, we have only considered colonoscopies 6-18 months post-polypectomy, hence the high number of exclusions.
Participants were intended to undergo a colonoscopy one year after randomization, however the national recommendations regarding the frequency of colonoscopic surveillance of patients with a history of colorectal adenomas changed during the study from one and three years after the initial resection to three years after resection; thus many participants did not obtain a one year colonoscopy.
Missing data for adenoma size for all 85 participants; missing data for number of adenomas for 13 participants. These participants cannot be classified by U.S. or U.K. criteria.
Missing data for adenoma size for all 25 participants; these participants could have been classified by the US based on number and histology.
Missing data for villous component for APPS, PPT, and WBF participants, as well as 9 of the CPPS participants. No data were collected for dysplasia at baseline in the CPPS trial. These participants could have been classified by the UK based on number and size.
Baseline Characteristics
Participants who were excluded were somewhat younger, had fewer and smaller adenomas, and were more likely to be missing histology data than those included in the analysis (Supplemental Table 1). The 3226 patients with sufficient data to be included in our analyses were predominantly male (70%), with median age (interquartile range) of 64 (57-70) years. The U.S. guidelines assigned 1194 (37%) to the lower-risk group, 2028 (62.9%) to the higher-risk group, and four (<1%) to the highest-risk group. The U.K. guidelines assigned 1460 (45.3%) to the low-risk group, 1375 (42.6%) to the intermediate-risk group, and 391 (12.1%) to the high-risk group. Table 2 shows the distribution of patients across the two sets of risk groups, according to baseline characteristics.
Table 2.
Baseline patient characteristics by U.S. and U.K. risk group categories (n=3226)
| U.S. Risk Groups* |
U.K. Risk Groups† |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower (n=1194) |
Higher (n=2028) |
Highest (n=4) |
Low (n=1460) |
Intermediate (n=1375) |
High (n=391) |
|||||||
| Characteristic | No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) |
|
|
|
|||||||||||
| Age, years | ||||||||||||
| <50 | 153 | (47.1) | 172 | (52.9) | 0 | (0.0) | 173 | (53.2) | 134 | (41.2) | 18 | (5.5) |
| 50-59 | 280 | (36.6) | 483 | (63.1) | 2 | (0.3) | 330 | (43.1) | 345 | (45.1) | 90 | (11.8) |
| 60-69 | 470 | (35.7) | 847 | (64.3) | 0 | (0.0) | 583 | (44.3) | 561 | (42.6) | 173 | (13.1) |
| 70+ | 291 | (35.5) | 526 | (64.2) | 2 | (0.2) | 374 | (45.7) | 335 | (40.9) | 110 | (13.8) |
| Sex | ||||||||||||
| Female | 385 | (39.5) | 589 | (60.3) | 0 | (0.0) | 472 | (48.5) | 422 | (43.3) | 80 | (8.2) |
| Male | 809 | (35.9) | 1439 | (63.9) | 4 | (0.2) | 988 | (43.9) | 953 | (42.3) | 311 | (13.8) |
| Family history of CRC | ||||||||||||
| No | 875 | (37.1) | 1481 | (62.8) | 1 | (0.0) | 1075 | (45.6) | 1003 | (42.6) | 279 | (11.8) |
| Yes | 264 | (38.5) | 419 | (61.1) | 3 | (0.4) | 317 | (46.2) | 284 | (41.4) | 85 | (12.4) |
| Unknown | 55 | (30.1) | 128 | (69.9) | 0 | (0.0) | 68 | (37.2) | 88 | (48.1) | 27 | (14.8) |
| Previous polyp | ||||||||||||
| No | 808 | (33.9) | 1572 | (66.0) | 2 | (0.1) | 996 | (41.8) | 1084 | (45.5) | 302 | (12.7) |
| Yes | 335 | (47.1) | 374 | (52.6) | 2 | (0.3) | 402 | (56.5) | 233 | (32.8) | 76 | (10.7) |
| Unknown | 51 | (38.3) | 82 | (61.7) | 0 | (0.0) | 62 | (46.6) | 58 | (43.6) | 13 | (9.8) |
| Number of adenomas | ||||||||||||
| 1 | 919 | (50.0) | 920 | (50.0) | 0 | (0.0) | 1109 | (60.3) | 730 | (39.7) | 0 | (0.0) |
| 2 | 275 | (39.7) | 418 | (60.3) | 0 | (0.0) | 351 | (50.6) | 342 | (49.4) | 0 | (0.0) |
| 3+ | 0 | (0.0) | 690 | (99.4) | 4 | (0.6) | 0 | (0.0) | 303 | (43.7) | 391 | (56.3) |
| Adenoma location | ||||||||||||
| Distal/Rectal only | 638 | (38.2) | 1031 | (61.8) | 0 | (0.0) | 797 | (47.8) | 810 | (48.5) | 62 | (3.7) |
| Proximal only | 434 | (54.5) | 362 | (45.5) | 0 | (0.0) | 514 | (64.6) | 242 | (30.4) | 40 | (5.0) |
| Proximal and Distal | 94 | (13.5) | 600 | (86.0) | 4 | (0.6) | 117 | (16.8) | 293 | (42.0) | 288 | (41.3) |
| Unknown | 28 | (44.4) | 35 | (55.6) | 0 | (0.0) | 32 | (50.8) | 30 | (47.6) | 1 | (1.6) |
| Size of largest adenoma | ||||||||||||
| <10mm | 1194 | (65.2) | 634 | (34.6) | 2 | (0.1) | 1460 | (79.8) | 303 | (16.6) | 67 | (3.7) |
| 10 to <20mm | 0 | (0.0) | 1096 | (99.8) | 2 | (0.2) | 0 | (0.0) | 852 | (77.6) | 246 | (22.4) |
| 20+ mm | 0 | (0.0) | 296 | (100.0) | 0 | (0.0) | 0 | (0.0) | 220 | (74.3) | 76 | (25.7) |
| Unknown | 0 | (0.0) | 2 | (100.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 2 | (100.0) |
| Adenoma histology | ||||||||||||
| Tubular | 1194 | (57.6) | 876 | (42.3) | 3 | (0.1) | 1229 | (59.3) | 675 | (32.6) | 169 | (8.2) |
| TV/Villous | 0 | (0.0) | 989 | (99.8) | 1 | (0.1) | 217 | (21.9) | 572 | (57.8) | 201 | (20.3) |
| Other‡ | 0 | (0.0) | 163 | (100.0) | 0 | (0.0) | 14 | (8.6) | 128 | (78.5) | 21 | (12.9) |
| High grade dysplasia | ||||||||||||
| No | 1194 | (48.2) | 1279 | (51.7) | 3 | (0.1) | 1348 | (54.4) | 913 | (36.9) | 215 | (8.7) |
| Yes | 0 | (0.0) | 342 | (100.0) | 0 | (0.0) | 68 | (19.9) | 200 | (58.5) | 74 | (21.6) |
| Not Reported | 0 | (0.0) | 407 | (99.8) | 1 | (0.2) | 44 | (10.8) | 262 | (64.2) | 102 | (25.0) |
| Study§ | ||||||||||||
| APPS | 268 | (35.4) | 489 | (64.5) | 1 | (0.1) | 351 | (46.3) | 299 | (39.4) | 108 | (14.2) |
| CPPS | 0 | (0.0) | 407 | (99.8) | 1 | (0.2) | 44 | (10.8) | 262 | (64.2) | 102 | (25.0) |
| PPT | 584 | (49.5) | 594 | (50.3) | 2 | (0.2) | 644 | (54.6) | 432 | (36.6) | 104 | (8.8) |
| WBF | 342 | (38.9) | 538 | (61.1) | 0 | (0.0) | 421 | (47.8) | 382 | (43.4) | 77 | (8.8) |
Abbreviations: CRC, colorectal cancer; TV, tubulovillous
Lower-risk corresponds to U.S. Group 2, higher-risk to U.S. Group 3, and highest-risk to U.S. Group 4, as defined in reference (6).
Low-, Intermediate- and high-risk groups, as defined in reference (8).
Other histology includes flat, serrated, undecided, and unknown.
APPS, Antioxidant Polyp Prevention Study; CPPS, Calcium Polyp Prevention Study; PPT, Polyp Prevention Trial; WBF, Wheat Bran Fiber trial.
Risk of Advanced Neoplasia
Follow-up colonoscopy was performed at a median of 12.8 months (interquartile range: 12.0-14.1) after baseline. Advanced neoplasms were detected one year post-polypectomy in 273 patients, an estimated absolute risk of 8.5% (95% CI, 7.5%-9.4%); of these, 26, or 0.8% (95% CI, 0.5%-1.1%), had HGD (n=16) or cancer (n=10). Risk of advanced neoplasia increased substantially with increasing risk stratum with both the U.S. or U.K guidelines (Table 3). The highest risk (18.7%; 95% CI, 14.8%-22.5%) was observed in the U.K. high-risk group; there were too few patients in the U.S. highest-risk group to be informative. Risks for the lowest-risk groups were similar for the two sets of guidelines (U.S.: 3.8%; 95% CI, 2.7%-4.9%; U.K.: 4.4%; 95% CI, 3.3-5.4%). Estimated risk was also similar between the U.S. higher-risk group (11.2%; 95 % CI, 9.8%-12.6%) and the U.K. intermediate-risk group (9.9%; 95% CI, 8.3%-11.5%). For the outcome of HGD/colorectal cancer, a similar trend of increasing risk was seen for both sets of guidelines. Lastly, including the 471 CPPS patients who lacked HGD data in the U.S. lowest-risk group resulted in absolute risks of 3.6% (95 % CI, 2.7-4.5) for advanced neoplasia and 0.2% (95% CI, 0.0-0.5) for HGD or colorectal cancer.
Table 3.
Absolute risk of advanced neoplasia and of high-grade dysplasia/colorectal cancer (HGD/CRC) one year post-polypectomy, by baseline risk category as calculated by U.S. or U.K. guidelines
| Baseline Classification |
Advanced Neoplasia at One Year |
HGD/CRC at One Year |
|||
|---|---|---|---|---|---|
|
|
|||||
| Risk Group | No. (%) | No. Events/ No. at risk* |
Absolute Risk (95% CI)† |
No. of Events/ No. at risk‡ |
Absolute Risk (95% CI)† |
|
|
|||||
| U.S. Classification | |||||
| Lower-risk | 1194 (37.0) | 45/1194 | 3.8 (2.7-4.9) | 3/1194 | 0.3 (0.0-0.5) |
| Higher-risk | 2028 (62.9) | 227/2028 | 11.2 (9.8-12.6) | 23/2028 | 1.1 (0.7-1.6) |
| Highest-risk | 4 (0.1) | 1/4 | § | § | |
| Total | 3226 (100) | 273/3226 | 8.5 (7.5-9.4) | 26/3226 | 0.8 (0.5-1.1) |
| U.K. Classification | |||||
| Low-risk | 1460 (45.3) | 64/1460 | 4.4 (3.3-5.4) | 5/1460 | 0.3 (0.0-0.6) |
| Intermediate-risk | 1375 (42.6) | 136/1375 | 9.9 (8.3-11.5) | 13/1375 | 0.9 (0.4-1.5) |
| High-risk | 391 (12.1) | 73/391 | 18.7 (14.8-22.5) | 8/391 | 2.0 (0.6-3.5) |
| Total | 3226 (100) | 273/3226 | 8.5 (7.5-9.4) | 26/3226 | 0.8 (0.5-1.1) |
Number of patients with advanced neoplasia, defined as one or more of the following: large adenoma (≥1cm), presence of high-grade dysplasia, adenoma with tubulovillous or villous histology, or colorectal cancer.
Percent (95% confidence interval).
Number of patients with high-grade dysplasia (HGD) or colorectal cancer (CRC).
Not estimated due to small numbers
Heterogeneity across Studies
We plotted arm-specific absolute risks of advanced neoplasia for the U.S. and U.K risk groups by study date (Supplemental Figures 1a and 1b). Risks ranged from 2.2% to 8.5% and 7.4% to 15.2%for the U.S. lower- and higher-risk groups, respectively. The pattern was similar for U.K. risk categories. There was no consistent pattern of risk by study date.
Cross-classification of Patients by U.S. and U.K. Risk Strata
All 1194 patients who were lower-risk by the U.S. guidelines were also low-risk by the U.K. criteria (Table 4, row 1). However, 266 of the 1460 (18%) U.K. low-risk patients (Table 4 column 1; Supplementary Figure 2) had HGD or villous histology and thus were classified by the U.S. criteria as higher-risk; their absolute risk of advanced neoplasia was higher (7.1%; 95% CI, 4.1-10.2) than that of the 1194 who remained in the U.S. lower-risk category (3.8%; 95% CI, 2.7-4.9).
Table 4.
One-year risk of advanced colorectal neoplasia, cross-classified by U.S. and U.K. baseline risk category
| U.K. Guidelines |
||||
|---|---|---|---|---|
| U.S. Guidelines | Low | Intermediate | High | Total |
| Lower | 45/1194* 3.8% (95% CI, 2.7-4.9) |
--/0 | --/0 | 45/1194 3.8% (95% CI, 2.7-4.9) |
|
| ||||
| Higher | 19/266 7.1% (95% CI, 4.1-10.2) |
136/1375 9.9% (95% CI, 8.3-11.5) |
72/387 18.6% (95% CI, 14.7-22.5) |
227/2028 11.2% (95% CI, 9.8-12.6) |
|
| ||||
| Highest | --/0 | --/0 | 1/4 NA** |
1/4 NA** |
|
| ||||
| Total | 64/1460 4.4% (95% CI, 3.3-5.4) |
136/1375 9.9% (95%CI, 8.3-11.5) |
73/391 18.7% (95% CI, 14.8-22.5) |
273/3226 8.5% (95% CI, 7.5-9.4) |
Number of events at one year/number in baseline risk category; percent; 95% confidence interval
NA,number in baseline risk category too small to report risk.
The 2028 patients in the U.S. higher-risk group were distributed across the three U.K. risk categories: 266 (13%) were U.K. low-risk, 1375 (68%) U.K. intermediate-risk, and 387 (19%) U.K. high-risk (Table 4, row 2; Supplementary Figure 2). Patients reclassified upwards into the U.K. high-risk group had a higher risk of advanced neoplasia (18.6%; 95% CI, 14.7-22.5) compared to those who remained in the U.K. intermediate risk group (9.9%; 95% CI, 8.3-11.5). The four patients in the U.S. highest-risk group were all high-risk by the U.K. criteria, and were too few to provide meaningful risk estimates.
Potential Net Cost and Benefit of Substituting U.K. for U.S Guidelines
Of the 273 patients with advanced neoplasia detected at one year, 72 (26.4%) were correctly reclassified upwards to a higher U.K. risk category, and would have surveillance recommendation changed from three- to one-year follow-up, with consequent earlier detection of their lesion. However, 19 (7.0%) of these patients were incorrectly reclassified downwards to a lower U.K. risk category, with surveillance recommendation changed from a three- to five-year colonoscopy, delaying detection of their lesion. Thus, if the U.K. criteria rather than the U.S. criteria were applied to a population similar to our study, a net 19.4% (53/273) of patients with advanced neoplasia at one year would have their lesion detected earlier, and this is 1.6% (53/3226) of all patients (Table 4). Of 2953 patients in our study without advanced neoplasia at one year, 315 (10.7%) were incorrectly reclassified upwards to a U.K. higher-risk category, with surveillance recommendation changed from three- to one-year colonoscopy, resulting in no benefit as no advanced lesions are detected at one year in these patients. However, 247 (8.4%) of these patients were reclassified downwards to a U.K. lower-risk category, with surveillance recommendation changed from three- to five-year follow-up. The net effect of applying the U.K. rather than U.S. criteria in a similar population would be an increase in surveillance colonoscopies for 2.3% (68/2953) of patients without advanced neoplasia at one year, which is 2.1% (68/3226) of all patients.
To estimate the net change in surveillance colonoscopies associated with following the U.K. rather than U.S. guidelines, we assumed that following the U.S. guidelines for the higher-risk group (follow-up colonoscopy every three years) entails 1/3 colonoscopy per year or 1⅔ colonoscopies over 5 years. For the U.K. high-risk group, an additional clearing colonoscopy is recommended at one year, for an estimated 2 1/3 colonoscopies over a five-year period (1 colonoscopy in years 1 and 4, and 1/3 in year 5). The recommended colonoscopy schedule for the U.K. intermediate-risk and the U.S. higher-risk groups is three years; whereas for the lower-risk groups, it is five to ten years for the U.S. and five years or no surveillance for the U.K.
Applying these numbers to the proportion of patients observed in each risk group in our study yields an estimated five-year average of 1.42 colonoscopies per patient using the U.S. criteria and 1.45 using the U.K. criteria. In practice, no follow-up is the default recommendation for low-risk patients in the U.K., as per the guideline recommendations (8, 17); although those in the eligible age range are offered average risk screening by fecal occult blood testing, which is offered to the whole population , and five-year follow-up is reserved for special circumstances. Forgoing colonoscopy follow-up in these U.K. low-risk patients would result in an average of 0.99 colonoscopies per patient per five years.
Discussion
Using data pooled from four prospective studies with one-year post-polypectomy follow-up, we found that the estimated risk of advanced colorectal neoplasia, as well as of HGD/colorectal cancer, increased across risk categories for both the U.S. and U.K. surveillance guidelines. Risk estimates in the lowest risk groups were similar between the two sets of guidelines; however, the U.K. guidelines do not consider histological features and thus classify substantially more patients into a low-risk category that entails a five-year or no surveillance colonoscopy. Patients classified as high-risk by the U.K. criteria (≥ 5 small adenomas or ≥3 adenomas with at least one ≥1cm) comprised 12.1% of the total population and had an 18.7% absolute risk of advanced neoplasia at one year, substantially higher than that of the U.S. higher-risk group; the U.K. criteria recommend a single one-year clearing colonoscopy for these patients rather than waiting for three years. We estimated that the net result of applying the U.K. criteria rather than the U.S. criteria to a population comparable to the one we studied would confer clinical benefit (two-year earlier detection of advanced adenomas) for 19% of patients that harbor these lesions at one year at a net cost of 0.03 additional colonoscopies per five years for all followed patients.
The U.S. surveillance guidelines have been informed by controlled trials of surveillance intervals. In particular, the NPS reported that the yield of advanced neoplasia in patients with three-year surveillance colonoscopy only did not differ from patients with both one and three-year colonoscopy (18). NPS investigators concluded that the first follow-up colonoscopy can safely be delayed in most patients for up to three years. However, the NPS also identified a subgroup of patients at substantially increased risk at one year (those with ≥ 3 adenomas at baseline), and also suggested that there is a low-risk group for which a much longer interval might suffice. Our study results generally agree with these observations.
An optimal colonoscopy surveillance program would prevent colorectal cancer mortality using the most cost-effective follow-up protocol. Substantial evidence indicates that many U.S. physicians frequently advise patients to have surveillance exams at shorter intervals than proposed by the guidelines (19-24). These reports illustrate the need for evidenced-based risk criteria to better inform physicians and improve adherence to guidelines (9). Data that reliably identify subsets of patients at high- and low-risk for clinically important interval lesions may provide needed assurance to the endoscopists about the appropriateness of guidelines. Risk of advanced neoplasia at one year was low for both the U.K. and U.S. low-risk groups (4.4% and 3.8%, respectively), both of which allow at most two small adenomas. However, the U.S. lower-risk group excludes patients with villous adenomas or HGD who would be classified as U.K. low-risk. The recently-adopted European Guidelines for Quality Assurance in Colorectal Cancer Screening and Diagnosis (25) suggest that programs may wish to consider adenoma histology in the risk classification of the low/intermediate risk groups. However, imperfect agreement within and between pathologists in classifying villous histology and degree of dysplasia (26, 27) may limit the value of this approach. We found that patients whose adenomas had villous histology or HGD and were reclassified into the U.S. higher-risk group from the U.K. low-risk group had a greater risk of advanced neoplasia than those who remained in the U.K. low-risk group. These results suggest information on histology and HGD provide additional, though modest discrimination between lower- and higher-risk patients. A better understanding of this discrimination by use of quantitative biological markers that capture the underlying biology is warranted for further refining risk stratification.
While we cannot know with certainty why advanced neoplasms were detected at the one-year surveillance colonoscopies in our study patients, most of these lesions were plausibly missed or below the limits of detection at the previous exam. If this assumption is correct, the quality of the prior colonoscopy may be the principal determinate of one-year advanced colorectal neoplasia, and high-risk patients, because of large or numerous polyps, may especially benefit from improved colonoscopy quality. In fact, the 2012 U.S. surveillance guidelines place emphasis on a high-quality baseline colonoscopy, as this minimizes the risk of missed lesions (9); this is an important consideration since lack of removal of all polyps at prior colonoscopy is associated with higher risk of developing colorectal cancer (28). Wide variation in adenoma detection rates among endoscopists has also been shown to be associated with risk of interval cancers, especially when the detection rates fall below 20% (29). Improvements in our understanding of the biology and histological heterogeneity of colorectal polyps (30, 31), advances in endoscopy techniques (32), and improvements in colonoscopy quality are likely to lead to fewer missed lesions and higher adenoma detection rates (33-36).
Strengths of our study include its prospective design, the large population of patients with scheduled one-year colonoscopies, pooling of patient-level data, and direct comparison of two sets of surveillance guidelines on the same patients. However, the relatively small number of patients with HGD or invasive colorectal cancer at one year limited our ability to compare risk estimates for these rare endpoints. Data were not collected on colonoscopy quality measures such as withdrawal time, caecal intubation, and endoscopists’ adenoma-detection rates; thus, we could not determine the extent to which variation in colonoscopy quality accounted for the findings of advanced neoplasms at the one-year colonoscopy. The baseline colonoscopies for this study were performed between 1984 and 1998; arguably colonoscopic technique and quality have changed since that time. Nevertheless, rates of detection of advanced neoplasia at one year did not appear to vary by study date. Thus, in spite of recent improvement in endoscopy quality, relative differences between U.S. and U.K. criteria are likely to persist. Our study population represents patients enrolled in prevention trials, and their risk distribution may not be representative of the general population. However, variation in risk among trials was modest, and less than the variation between risk groups as defined by both the U.K and the U.S. criteria.
In conclusion, our results suggest that the U.K. guidelines identify a subset of high-risk patients who would benefit from a one-year clearing colonoscopy without substantially increasing the overall rate of surveillance colonoscopies. Further study of the effectiveness of including histology in the U.K. criteria is warranted, particularly for patients with low-risk adenomas. Advanced neoplasia is an important target of colorectal screening and surveillance, and these risk estimates may help to inform the decision process, for both the individual patient and the public health community.
Supplementary Material
Supplemental Figure 1. Advanced neoplasia at a one-year colonoscopy according to U.S. (1a) and U.K. (1b) risk groups, stratified by study trial arms. APPS, Antioxidant Polyp Prevention Study; CPPS, Calcium Polyp Prevention Study; PPT, Polyp Prevention Trial; WBF, Wheat Bran Fiber trial; BC, beta carotene; Vit C, vitamin C.
Supplemental Figure 2. Patients classified by U.S. Colonoscopy Surveillance Risk Groups (6, 9)reclassified according to U.K. Colonoscopy Surveillance Risk Groups (7, 8), with corresponding surveillance intervals and absolute risk of advanced neoplasms and high-grade dysplasia/colorectal cancer (HGD/CRC) found at colonoscopy one year post-polypectomy (N=3226). Advanced neoplasia is defined as one or more of the following: large adenoma (≥1cm), presence of high-grade dysplasia, adenoma with tubulovillous or villous histology, or colorectal cancer. *Percent (95% confidence intervals).
Supplemental Table 1. Comparison of participants included to those excluded by primary reason for exclusion*
Acknowledgments
Primary Funding Source: Work was supported by the European Union Public Health Programme (Development of European Guidelines for Quality Assurance of Colorectal Cancer Screening (CRC), grant agreement no. 2005317)., by U.S. Public Health Service grants CA-41108, CA-23074, CA95060, CA37287, CA104869, CA23108, CA59005, and CA26852 from the National Cancer Institute.
References
- 1.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59(6):366–78. doi: 10.3322/caac.20038. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343(3):162–8. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 4.Schoenfeld P, Cash B, Flood A, Dobhan R, Eastone J, Coyle W, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med. 2005;352(20):2061–8. doi: 10.1056/NEJMoa042990. [DOI] [PubMed] [Google Scholar]
- 5.Kahi CJ, Anderson JC, Waxman I, Kessler WR, Imperiale TF, Li X, et al. High-definition chromocolonoscopy vs. high-definition white light colonoscopy for average-risk colorectal cancer screening. Am J Gastroenterology. 2010;105:1301–07. doi: 10.1038/ajg.2010.51. [DOI] [PubMed] [Google Scholar]
- 6.Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O’Brien MJ, Levin B, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin. 2006;56(3):143–59. doi: 10.3322/canjclin.56.3.143. [DOI] [PubMed] [Google Scholar]
- 7.Atkin WS, Saunders BP. Surveillance guidelines after removal of colorectal adenomatous polyps. Gut. 2002;51(Suppl V):6–9. doi: 10.1136/gut.51.suppl_5.v6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups. Gut. 2010;59(5):666–89. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonsocopy surveillance afer screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroentereolgy. 2012 Jul 2; doi: 10.1053/j.gastro.2012.06.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Martínez ME, Baron JA, Lieberman DA, Schatzkin A, Lanza E, Winawer SJ, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroentereolgy. 2009;136(3):832–41. doi: 10.1053/j.gastro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg ER, Baron JA, Tosteson TD, Freeman DH, Beck GJ, Bond JH, et al. A clinical trial of antioxidant vitamins to prevent colorectal adenoma. N Engl J Med. 1994;331(3):141–7. doi: 10.1056/NEJM199407213310301. [DOI] [PubMed] [Google Scholar]
- 12.Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler MD, et al. Calcium supplements for the prevention of colorectal adenomas. N Engl J Med. 1999;340(2):101–7. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 13.Schatzkin A, Lanza E, Corle D, Lance P, Iber F, Caan B, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp Prevention Trial Study Group. N Engl J Med. 2000;342(16):1149–55. doi: 10.1056/NEJM200004203421601. [DOI] [PubMed] [Google Scholar]
- 14.Alberts DS, Martinez ME, Roe DJ, Guillen-Rodriguez JM, Marshall JR, van Leeuwen JB, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians’ Network. N Engl J Med. 2000;342(16):1156–62. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- 15.Cook NR, Ridker PM. Advances in measuring he effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Int Med. 2009;150(11):795–802. doi: 10.7326/0003-4819-150-11-200906020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pencina MJ, D’Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NHS Cancer Screening Programmes Adenoma Surveillance BCSP Guidance Note No 1 Version 1. 2009.
- 18.Winawer S, Zauber A, O’Brien M, Ho M, Gottlieb L, Sternberg S, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. N Eng J Med. 1993;328(13):901–6. doi: 10.1056/NEJM199304013281301. [DOI] [PubMed] [Google Scholar]
- 19.Klabunde CN, Frame PS, Meadow A, Jones E, Nadel M, Vernon SW. A national survey of primary care physicians’ colorectal cancer screening recommendations and practices. Prev Med. 2003;36(3):352–62. doi: 10.1016/s0091-7435(02)00066-x. [DOI] [PubMed] [Google Scholar]
- 20.Yabroff KR, Klabunde CN, Yuan G, McNeel TS, Brown ML, Casciotti D, et al. Are physicians’ recommendations for colorectal cancer screening guideline-consistent? J Gen Intern Med. 2010;26(2):177–84. doi: 10.1007/s11606-010-1516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ransohoff DF, Yankaskas B, Gizlice Z, Gangarosa L. Recommendations for post-polypectomy surveillance in community practice. Dig Dis Sci. 2011;56(9):2623–30. doi: 10.1007/s10620-011-1791-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nodora JN, Martz WD, Ashbeck EL, Jacobs ET, Thompson PA, Martinez ME. Primary care physician compliance with colorectal cancer screening guidelines. Cancer Causes Control. 2011;22(9):1277–87. doi: 10.1007/s10552-011-9801-0. [DOI] [PubMed] [Google Scholar]
- 23.Boolchand V, Olds G, Singh J, Singh P, Chak A, Cooper GS. Colorectal screening after polypectomy: a national survey study of primary care physicians. Ann Intern Med. 2006;145(9):654–9. doi: 10.7326/0003-4819-145-9-200611070-00007. [DOI] [PubMed] [Google Scholar]
- 24.Mysliwiec PA, Brown ML, Klabunde CN, Ransohoff DF. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med. 2004;141(4):264–71. doi: 10.7326/0003-4819-141-4-200408170-00006. [DOI] [PubMed] [Google Scholar]
- 25.Atkin WVR, Kuipers EJ, Hoff G, Senore C, Segnan N, Jover R, Schmiegel W, Lambert R, Pox C. Colonoscopic surveillance following adenoma removal. In: Segnan NPJ, von Karsa L, editors. European Guidelines for Quality Assurance in Colorectal Cancer Screening and Diagnosis. First Edition. European Union; Luxembourg: 2010. [DOI] [PubMed] [Google Scholar]
- 26.Terry MB, Neugut AI, Bostick RM, Potter JD, Haile RW, Fenoglio-Preiser CM. Reliability in the classification of advanced colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2002;11:660–3. [PubMed] [Google Scholar]
- 27.Costantini M, Sciallero S, Giannini A, Gatteschi B, Rinaldi P, Bonelli L, et al. Interobserver agreement in the histologic diagnosis of colorectal polyps: the experience of the multicenter adenoma colorectal study (SMAC) J Clin Epidemiol. 2003;56(3):209–14. doi: 10.1016/s0895-4356(02)00587-5. [DOI] [PubMed] [Google Scholar]
- 28.Brenner H, Chang-Claude J, Jansen L, Seiler CM, Hoffmeister M. Role of colonoscopy and polyp characteristics in colorectal cancer after colonoscopic polyp detection. Ann Int Med. 2012;157:225–32. doi: 10.7326/0003-4819-157-4-201208210-00002. [DOI] [PubMed] [Google Scholar]
- 29.Kaminski M, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Eng J Med. 2010;362(19):1795–803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 30.Ahnen DJ. The American College of Gastroenterology Emily Couric Lecture--the adenoma-carcinoma sequence revisited: has the era of genetic tailoring finally arrived? Am J Gastroenterol. 2011;106(2):190–98. doi: 10.1038/ajg.2010.423. [DOI] [PubMed] [Google Scholar]
- 31.Rex DK. Colorectal cancer prevention with colonoscopy: recent research and debate. Gastroenterol Hepatol. 2010;6(7):428–30. [PMC free article] [PubMed] [Google Scholar]
- 32.Rex DK, Fennerty MB, Sharma P, Kaltenbach T, Soetikno R. Bringing new endoscopic imaging technology into everyday practice: what is the role of professional GI societies? Polyp imaging as a template for moving endoscopic innovation forward to answer key clinical questions. Gastrointest Endosc. 2010;71(1):142–6. doi: 10.1016/j.gie.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Kahi CJ, Hewett DG, Norton DL, Eckert GJ, Rex DK. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 2011;9(1):42–6. doi: 10.1016/j.cgh.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355(24):2533–41. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 35.Rex DK. Maximizing detection of adenomas and cancers during colonoscopy. Am J Gastroenterol. 2006;101(12):2866–77. doi: 10.1111/j.1572-0241.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- 36.Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol. 2007;102(4):856–61. doi: 10.1111/j.1572-0241.2006.01054.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Advanced neoplasia at a one-year colonoscopy according to U.S. (1a) and U.K. (1b) risk groups, stratified by study trial arms. APPS, Antioxidant Polyp Prevention Study; CPPS, Calcium Polyp Prevention Study; PPT, Polyp Prevention Trial; WBF, Wheat Bran Fiber trial; BC, beta carotene; Vit C, vitamin C.
Supplemental Figure 2. Patients classified by U.S. Colonoscopy Surveillance Risk Groups (6, 9)reclassified according to U.K. Colonoscopy Surveillance Risk Groups (7, 8), with corresponding surveillance intervals and absolute risk of advanced neoplasms and high-grade dysplasia/colorectal cancer (HGD/CRC) found at colonoscopy one year post-polypectomy (N=3226). Advanced neoplasia is defined as one or more of the following: large adenoma (≥1cm), presence of high-grade dysplasia, adenoma with tubulovillous or villous histology, or colorectal cancer. *Percent (95% confidence intervals).
Supplemental Table 1. Comparison of participants included to those excluded by primary reason for exclusion*