Abstract
Introduction
Dodecafluoropentane emulsion (DDFPe) in 250 nm nanodroplets seems to swell modestly to accept and carry large amounts of oxygen in the body at >29°C. Small particle size allows oxygen delivery even into hypoxic tissue unreachable by erythrocytes. Using permanent cerebral embolic occlusion in rabbits, we assessed DDFPe dose response as a neuroprotectant at 7 and 24 hours post-embolization without lysis of arterial obstructions and investigated blood pharmacokinetics.
Methods
New Zealand White rabbits (N=56) received cerebral angiography and embolic spheres (diameter=700-900 μm) occluded middle and/or anterior cerebral arteries. Intravenous DDFPe dosing (2% w/v emulsion) began at 60 minutes and repeated every 90 minutes until sacrifice at 7 or 24 hours post-embolization. Seven hour groups: 1) Control (embolized without treatment, N=6), and DDFPe treatment: 2) 0.1ml/kg (N=7), 3) 0.3ml/kg (N=9), 4) 0.6ml/kg (N=8). Twenty-four hour groups: 5) Control (N=16), and DDFPe treatment: 6) 0.1ml/kg (N=10). Infarcts as percent of total brain volume were determined using vital stains on brain sections. Other alert normal rabbits (N=8) received IV doses followed by rapid arterial blood sampling and GC-MS analysis.
Results
Percent infarct volume means significantly decreased for all DDFPe treated groups compared with controls, p=<0.004 to <0.03. Blood DDFP (gas) half-life was 1.45±0.17 minutes with R=0.958. Mean blood clearance was 78.5±24.9 ml/min/kg (mean±SE).
Conclusions
Intravenous DDFPe decreases ischemic stroke infarct volumes. Blood half-life values are very short. The much longer therapeutic effect, >90 minutes, suggests multiple compartments. Lowest effective dose and maximum effective therapy duration are not yet defined. Rapid development is warranted.
Keywords: Dodecafluoropentane emulsion, Neuroprotective agent, Stroke model, Rabbit
Introduction
Stroke is the fourth most common cause of death in the USA [1] and ischemic stroke affects 795,000 patients annually, costing $73.7 billion [2]. Few patients receive therapy and current best therapy improves outcomes to the point of independent lives in only 40% of those [3]. The treatment of ischemic stroke is currently focused on prompt revascularization and restoration of oxygenated blood flow. Due to time constraints and diagnostic requirements, therapy reaches fewer than 4% of patients and increases the urgency for the development of new therapies [2]. A neuroprotectant that extends the time window until safe thrombolytic or intra-arterial interventional therapy can be applied would have a profound impact, but no neuroprotectant has yet progressed successfully from animal models into human clinical therapy [4-6]. An effective oxygen transport substance may have therapeutic potential in diverse situations involving blood loss, hypoxia, and ischemic stroke, but this approach using other drugs including various liquid perfluorocarbons and other techniques has not yet proven clinically applicable.
Dodecafluoropentane is a perfluorocarbon (PFC) with a pentane base. In the current formulation it exists as an emulsion (DDFPe) of nanodroplets, 250 to 300 nm in size when below 29°C. Although DDFP has a boiling point of 29°C, due to intravascular pressure it apparently does not shift to microbubble form in the body at 37°C. Rather, the particle size expands only slightly allowing facilitation of respiratory gas dissolution. This mechanism transports high levels of oxygen and other gasses, much higher than other liquid phase PFCs with much higher boiling points [7]. The exceptionally small particle size may allow oxygen delivery into tissues unreachable by erythrocytes. This includes some flow even through occluded major blood vessels by transportation through flaws in clot, through collateral vessels, through microcirculation, and through diffusion gradients into hypoxic tissue. A previous study using a rabbit model of permanent embolic occlusion of the middle cerebral artery showed DDFPe decreased infarct volumes at 4 hours when administration was delayed up to 3 hours post stroke and therapy was successful for seven hours when begun one hour following embolization [8,9]. In this study we assessed DDFPe dose response and efficacy in reducing infarct volume at 7 and 24 hours post-embolization without lysis of arterial obstructions and also investigated basic blood pharmacokinetics.
Methods
All animal procedures were approved by the Institutional Animal Care and Use Committee.
DDFPe effect on infarct volume
These methods were described previously [8,10]. New Zealand White rabbits (N=56; 5.1±0.10 kg) received cerebral angiography from a femoral artery approach. Embolic spheres (700-900 μm) were injected into the internal carotid artery occluding the middle cerebral and/or anterior cerebral arteries. Animals with other occlusions, 10% of cases, were discarded. In all treated groups, intravenous DDFPe (NuvOx Pharma, LLC, Tucson, AZ) dosing over 1-2 minutes with a 2% w/v emulsion began at 1 hour post-embolization via a cannula placed in an ear vein and was repeated every 90 minutes until sacrifice. Rabbits were sacrificed at either 7 or 24 hours post-embolization. At 7 hours the groups were: 1) Control (embolized without treatment, N=6), or treatment with DDFPe: 2) 0.1 ml/kg (N=7), 3) 0.3 ml/kg (N=9), and 4) 0.6 ml/kg (N=8). At 24 hours the groups were: 5) Control (N=16), and treatment with DDFPe: 6) 0.1 ml/kg (N=10). Following euthanasia, the brain was harvested, immediately chilled in saline, and then sliced coronally at 4.0-mm intervals. Brain sections were placed in 1% 2,3,5-triphenyltetrazolium chloride (Sigma-Aldrich; St. Louis, MO) for 45 minutes at 37°C, fixed in 10% formalin, and digitally photographed (Fig. 1). Brain size and areas of infarction were measured using digital analysis (NIH ImageJ) by a technician blinded to treatment groups. Infarct volume was calculated as a percent of total brain volume.
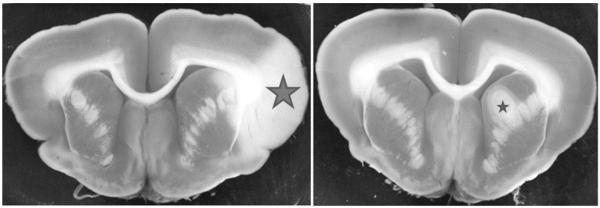
Fig. 1.
Representative brain sections stained with TTC showing infarct areas. Left side: Section from control animal showing the infarct area (large star) of 3.9 % (infarct volume as a percent of total brain volume). Right side: Section from animal having a stroke and treated with DDFPe showing the infarcted area (small star) of 0.8 %.
Pharmacokinetic study
In alert New Zealand White rabbits (5.1±0.5 kg; N=8) cannulae were placed in a vein of one ear and in an artery of the other ear. After intravenous injection of DDFPe (0.6 ml/kg in a 2% w/v emulsion) arterial blood samples were taken over a period of 4 to 7 hours. The dose of DDFPe was repeated every 90 minutes in 4 animals. Samples were stored at −20°C until they were analyzed for DDFP content by a headspace gas chromatograph-mass spectrometer (Varian TSQ).
Statistics
Because infarct volumes were not normally distributed, ranks of infarct volume were analyzed with PROC GLM of SAS software (Kruskal-Wallis equivalent). Blood levels of DDFP were analyzed for exponential curve fit and exponential decay time constant using KaleidaGraph software v4.01 (2005). Values are given as mean±standard error.
Results
Mean percent infarct volumes (%IV) decreased greatly for all DDFPe treated groups compared with controls (Table 1, Fig. 1). For the 7 hour study the %IVs for the DDFPe groups were significantly different from the control group at p values less than 0.009, but were not different from each other. The average %IV for the 0.1, 0.3 and 0.6 ml/kg dose groups was 18.8% of the untreated control group. For the 24 hour study using 0.1 ml/kg of DDFPe the treated group was significantly different from the control group (p=0.03) and had an average %IV of 15.0% of control.
Table 1.
Results of DDFPe treatment on stroke infarct volume at 7 or 24 hours.
| Group No. | DDFPe Dosage | N | Sacrifice Time (h) | Infarct Volume (%) ± SE | P-value (vs. Control) |
|---|---|---|---|---|---|
| 1 | Control | 6 | 7 | 3.88 ± 0.77 | - |
| 2 | 0.1 ml/kg | 7 | 7 | 0.57 ± 0.71 | 0.004 |
| 3 | 0.3 ml/kg | 9 | 7 | 0.59 ± 0.63 | 0.003 |
| 4 | 0.6 ml/kg | 8 | 7 | 1.03 ± 0.67 | 0.009 |
| 5 | Control | 16 | 24 | 3.39 ± 0.76 | - |
| 6 | 0.1 ml/kg | 10 | 24 | 0.51 ± 1.02 | 0.03 |
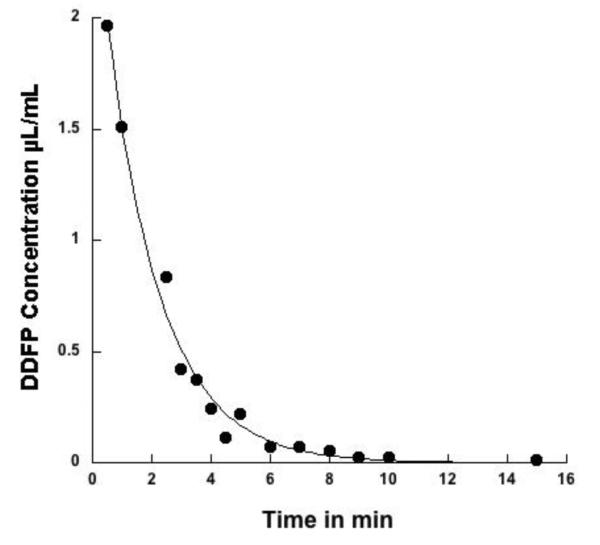
DDFP blood levels were analyzed in animals receiving a single DDFPe dose of 0.6 μl/kg. Examination of the falling phase of blood levels of DDFP yielded peak levels of 1.97 to 3.34 μL/ml with a single exponential decay. The calculated mean half-life was 1.45±0.17 minutes. For curve fit the mean R value for the N=4 animals was 0.958 (Fig. 2). Mean blood clearance was 78.5±24.9 ml/min/kg. Blood levels reached non-detectable levels within 30 minutes. Injections at 90 minute intervals in 4 additional animals showed similar peaks and half-lives. After each dose of 0.6 ml/kg blood levels returned to baseline and no sign of accumulation was seen with up to 5 doses.
Fig. 2.
DDFP clearance from blood as a function of time in a representative rabbit. The DDFPe dose was 0.6 ml/kg of a 2% w/v emulsified preparation. Blood levels of DDFP were determined using a headspace gas chromatograph-mass spectrometer (Varian TSQ). Half-life in blood for this rabbit was 1.68 minutes. R value was 0.994.
Discussion
Several perfluorocarbons have been extensively tested in the search for neuroprotectants and blood substitutes [11,12]. Although animal studies have been very promising, no neuroprotectants have been successful in human trials [4-6]. The initial application of DDFPe as an ultrasound contrast agent was well tolerated in single doses in >2000 humans for that purpose [9] before development stopped for economic reasons [13]. Only recently has the potential therapeutic aspect of this emulsion been investigated [8,14,15]. A preliminary study showed the potential as a neuroprotective agent with complete and permanent occlusion of cerebral arteries in rabbits and up to 3 hours of delay in therapy. Over 80% of infarct volume was protected for up to 7 hours [8] and duration was extended to 24 hours in this study. The minimum required dose seems to be less than 1% of other liquid perfluorocarbons but is not yet clearly defined. In the present study we tested decreasing doses of DDFPe for efficacy and described its basic pharmacokinetics.
Repeated doses show that protection can be maintained for a full 24 hours using the smallest dose (0.1ml/kg) every 90 minutes. The potential clinical applications are extremely broad and may change the basic paradigm for stroke, in both workup and therapy. A dose could be given in the field, provide a period of protection during transportation, be repeated at 90 minutes and provide a continuing period of protection during clinical workup in the hospital. This time advantage, a potential three hour bonus with two injections in a most critical phase, could “pause the clock” on the “Time is Brain” concept at the time of first administration. Since expected good therapeutic results fall quickly with the passage of time, DDFPe as a neuroprotectant could dramatically increase the expected good outcomes of thrombolysis or thrombectomy. Two or three fold improvement in infarct reduction may be possible for the patient who is finally ready to get therapy several hours after receiving DDFPe at 1 hour. Multiple additional doses appear to further expand the “pause” and the limits are not yet defined. Bridging the first few hours following stroke onset may be the critical portion of therapy. If so, we speculate that it can possibly be a complete therapy if collateral flow can be recruited to supply the ischemic areas. Similar applications in cases of blood loss, hypoxia, or trauma may also be efficacious.
The small doses demonstrated here apparently avoid the toxicity of larger doses suggested in an animal study where pulmonary edema was encountered in dogs receiving rapid repeat intravenous injections [16]. Although single doses and double doses separated by 24 hours appeared safe in over 2000 human applications [13,17], toxicity of more frequently administered repeated doses has not yet been defined. In addition, DDFPe can be damaged by uncontrolled fluctuations in storage conditions (i.e. hot/cold cycles) prior to use. This appears to enlarge droplet size. Use of this damaged form may have lead to pulmonary edema and severe toxicity in rabbits (unpublished data). The drug must be maintained at moderate room temperatures in storage but need not be refrigerated.
A mean half-life value of 1.45 ± 0.17 minutes for DDFP in blood after a single 0.6 ml/kg dose agrees with a previous study in humans in which blood data showed a short half-life of 2.2 ± 1.2 minutes [18]. The higher blood clearance rate of 78.5 ± 24.9 ml/min/kg in rabbits compared to 30.1 to 48.6 ml/min/kg in humans may in part be due to the higher heart and respiratory rates and faster circulation time in rabbits. In the human study 99% of DDFP in a single dose was recovered from expired air within 2 hours [18]. This short half-life is not easily reconciled with the supportive effect of DDFPe lasting 90 to 120 minutes when similar doses were given for severe blood loss in swine [15] and rats [14]. Thus, the duration of effect of DDFPe greatly exceeds the duration of measurable blood levels. Analysis of levels of DDFP in rabbit organs is currently being done. One possibility is that DDFPe diffuses into organs and tissues at low levels. Thus, low levels of DDFPe would be available for time periods much longer than 5 half-lives as measured in blood. Another possibility is that molecules of DDFP assemble in series along blood vessels of the penumbra and pass along oxygen in a daisy-chain manner. The latter would require very low levels of DDFP. The effective level of DDFP would need to be rather low because in multiple dose studies in which DDFPe was given at 90 minute intervals, the blood level of DDFP appeared to return to the baseline level less than 30 minutes after each dose. At present the lowest effective dose of DDFPe is unknown but is under investigation.
Limitations
Each dose tested in this study proved effective at reducing infarct volume; therefore, the minimum required dose is not yet defined. Alternative dose schedules or infusion schedules to reach steady state also require better understanding of the pharmacokinetics for appropriate planning. The real limits on duration of effectiveness of a single dose will require additional study, although the previous swine studies showed continued effectiveness of between 90 and 120 minutes when given as an infusion over 30 minutes [14]. The lowest effective dose may be near or below the detection level for the analysis technique used.
Conclusion
Intravenous DDFPe protects brain from ischemic injury and significantly decreases infarct volumes in ischemic stroke. The lowest effective dose and maximum length of effective therapy have not yet been defined. Although DDFPe has a short half-life in blood, 1.45 ± 0.17 minutes, the effect of DDFPe is much longer, >90 minutes, which suggests the possibility of two or more compartments in the model. Rapid development and testing are warranted.
Acknowledgements
Supported by the Hornick Fund for stroke research from UAMS
Footnotes
Conflict of Interest WC Culp and RD Skinner have an application pending to the USPTC regarding the use of DDFPe in numerous medical situations.
References
- 1.Kochanek K, Xu J, Murphy SL, Minino AM, Kung H-C. Deaths: Final data for 2009. Natl Vital Stat Rep. 2011;60(3):1–117. [PubMed] [Google Scholar]
- 2.Roger VL, et al. AHA Statistical Update: Heart Diseases and Stroke Statistics--2012 Update. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. ePub Dec 15, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, Clark W, Budzik R, Zaidat OO, SWIFT Trialists Solitaire flow restoration device versus the Merci Retriever in patients with acute ischemic stroke (SWIFT): a randomized, parallel-group, non-inferiority trial (2012) Lancet. 2012;380(9849):1241–9. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 4.Donnan GA. The 2007 Feinberg Lecture: a new road map for neuroprotection. Stroke. 2009;39:242–248. doi: 10.1161/STROKEAHA.107.493296. [DOI] [PubMed] [Google Scholar]
- 5.Diener HC, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Shuaib A, Ashwood T, Wasiewski W, Alderfer V, Hardemart HG, Rodichok L, SAINT I, II Investigators NXY-059 for the treatment of acute stroke: pooled analysis of the SAINT I and II Trials. Stroke. 2008;39:1751–1758. doi: 10.1161/STROKEAHA.107.503334. [DOI] [PubMed] [Google Scholar]
- 6.Heiss WD, Brainin M, Bornstein NM, Tuomilehto J, Hong Z, Cerebrolysin Acute Stroke Treatment in Asia (CASTA) Investigators Cerebrolysin in patients with acute ischemic stroke in Asia: resulte of a double-blind, placebo-controlled randomized trial. Stroke. 2012;43(3):630–636. doi: 10.1161/STROKEAHA.111.628537. [DOI] [PubMed] [Google Scholar]
- 7.Johnson JLC, Dolezal MC, Kerschen A, Matsunaga TO, Unger EC. In vitro comparison of dodecafluoropentane (DDFP), perfluorodecalin (PFD), and perfluoroctylbromide (PFOB) in the facilitation of oxygen exchange. Artif Cell Blood. 2009;37:156–162. doi: 10.1080/10731190903043192. [DOI] [PubMed] [Google Scholar]
- 8.Culp WC, Woods SD, Skinner RD, Brown AT, Lowery JD, Johnson JLH, Unger EC, Hennings LJ, Borrelli MJ, Roberson PK. Dodecafluoropentane emulsion decreases infarct volume in a rabbit ischemic stroke model. J Vasc Interv Radiol. 2012;23:116–121. doi: 10.1016/j.jvir.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Correas JM, Quay SC. EchoGen emulsion: a new ultrasound contrast agent based on phase shift colloids. Clin Radiol. 1996;51(S1):11–14. [PubMed] [Google Scholar]
- 10.Culp WC, Woods SD, Brown AT, Lowery JD, Hennings LJ, Skinner RD, Borrelli MJ, Roberson PK. Three variations in rabbit angiographic stroke models. J Neurosci Meth. doi: 10.1016/j.jneumeth.2012.10.017. Epub ahead of print Nov 8 2012, 10.1016/j/jneurometh.2012.10.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiss JG. Understanding the fundamentals of perfluorocarbons and perfluorocarbon emulsions relevant to in vivo oxygen delivery. Artif Cells Blood Substit Immobil Biotechnol. 2005;33:47–63. doi: 10.1081/bio-200046659. [DOI] [PubMed] [Google Scholar]
- 12.Remy B, Deby-Dupont G, Lamy M. Red blood cell substitutes: Fluorocarbon emulsions and haemoglobin solutions. Br Med Bull. 1999;55:277–298. doi: 10.1258/0007142991902259. [DOI] [PubMed] [Google Scholar]
- 13.The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit. 1998 Jul 27; CPMP/1342/98. [Google Scholar]
- 14.Lundgren CEG, Bergoe GW, Tyssebotn IM. Intravascular fluorocarbon-stabilized microbubbles protect against fatal anemia in rats. Artif Cell Blood Sub. 2006;34:473–486. doi: 10.1080/10731190600769271. [DOI] [PubMed] [Google Scholar]
- 15.Tyssebotn IM, Lundgren CE, Olszowka AJ, Bergoe GW. Hypoxia due to shunts in pig lung treated with O2 and fluorocarbon-derived intravascular microbubbles. Artif Cells Substit Immobil Biotechnol. 2010;38(2):79–89. doi: 10.3109/10731191003634679. [DOI] [PubMed] [Google Scholar]
- 16.Grayburn PA, Erickson JM, Excobar J, Womack L, Velasco CE. Peripheral intravenous myocardial contrast echocardiography using a 2% dedecafluoropentane emulsion: identification of myocardial risk area and infarct size in the canine model of ischemia. J AM Coll Cardiol. 1995;26(5):1340–7. doi: 10.1016/0735-1097(95)00306-1. [DOI] [PubMed] [Google Scholar]
- 17.Khor SP. Amendment to the pharmacokinetic analysis of clinical data from study SON-3600-1004a: NuvOx Formal Study Report. 2012.
- 18.Correas JM, Meuter AR, Singlas E, Kessler DR, Worah D, Quay SC. Human pharmacokinetics of a perfluorocarbon ultrasound contrast agent evaluated with gas chromatography. Ultrasound Med Biol. 2001;27:565–570. doi: 10.1016/s0301-5629(00)00363-x. [DOI] [PubMed] [Google Scholar]