Abstract
To date, the surgical anatomy and histopathology of kidneys from patients with stones and small bowel resection have not been studied. We present here materials from 11 cases, 10 Crohn’s disease and one with resection in infancy for unknown cause. Stones are predominantly calcium oxalate (CaOx). Urine stone risks included hyperoxaluria (urine oxalate excretion > 45 mg/day) in half of the cases, and reduced urine volume and pH. As in ileostomy and obesity bypass, inner medullary collecting ducts (IMCD) contain crystal deposits with associated cell injury, interstitial inflammation and papillary deformity. Cortical changes include modest glomerular sclerosis, tubular atrophy, and interstitial fibrosis. Interstitial papillary apatite (Randall’s) plaque is abundant, and CaOx stones grow over deposits as in ileostomy, idiopathic CaOx stone formers, and primary hyperparathyroidism. Abundant plaque is compatible with the low urine volume and pH. IMCD deposits all contain apatite; in 3 cases CaOx is also present. This is similar to findings in obesity bypass but not ileostomy. Mechanisms for CaOx in IMCD appear to include elevated urine, and presumably tubule fluid CaOx SS with a low calcium to oxalate ratio; mechanisms for the universal presence of IMCD apatite are unknown.
INTRODUCTION
Elsewhere [1;2] we have shown that bowel disease can produce a wide range of renal histopathology, and entrain multiple mechanisms for renal stone production and formation of intra-renal crystal deposits. In patients with obesity bypass procedures, calcium oxalate (CaOx) stones are found free in the urinary collecting system. Within the kidney the only deposits are occasional apatite plugs within inner medullary collecting ducts (IMCD), rare microscopic linear deposits of CaOx over apical surfaces of otherwise normal appearing IMCD cells, and a single small IMCD CaOx plug in each of 2 of the 5 cases we have studied [1;2]. Patients with ileostomy produce stones containing both uric acid and CaOx [3]. In their kidneys we have found interstitial apatite (Randall’s) plaque, and IMCD plugs composed of apatite and the sodium and ammonium acid salts of uric acid [3]. Some CaOx stones were found growing over regions of plaque, as is seen in idiopathic CaOx stone formers (ICSF) and stone formers with primary hyperparathyroidism [4–6]. Other stones were found free in the collecting system.
We have little difficulty in explaining the plaque, stones, and urate IMCD deposits we have thus far encountered in bowel patients. Bypass patients produce no excess of plaque above control subjects and their stones never grow attached to renal papillae. The lack of plaque is consistent with our prior findings that plaque is promoted by scanty acidic urine and hypercalciuria; bypass patients have high urine volumes and are not hypercalciuric [1;7]. Their urine oxalate excretion is high, and we can presume with reasonable certainty that hyperoxaluria (urine oxalate excretion > 45 mg/day) raised urine supersaturation (SS) with respect to CaOx and led to crystal formation, perhaps in free solution. By contrast ileostomy patients produce scanty and acidic urine, form abundant plaque, and produce attached CaOx stones over areas of papillary plaque. Their urine has a high uric acid SS that accounts for their uric acid stones. Urine sodium and ammonium urate SS was present in 3 of the 5 ileostomy patients with these salts in their IMCD, meaning that for urate species IMCD and urine were not discordant. Altogether, the types of stones formed by bypass and ileostomy patients we have presented to date, as well as urate IMCD deposits can be reasonably explained by their urine SS, and plaque abundance has seemed to follow urine volume, calcium and pH as in ICSF and normal subjects.
What we cannot easily explain is the discordance between apatite IMCD deposits and both urine SS and compositions of stones formed. Though CaOx makes up the bulk of stones in bypass patients, and they are hyperoxaluric, apatite is the predominant IMCD crystal [2] even though their average urine pH was below 5.5, and correspondingly their SS with respect to calcium phosphate (CaP) was below 1. Discordance is even greater with ileostomy. Although stones were uric acid and CaOx, urine pH was low, and CaP SS well below 1, apatite was the predominant IMCD crystal. In other words, in both diseases, the IMCD environment appears to produce and support apatite that would not be stable or predicted in their urine. Stones reflect the urine conditions, IMCD deposits do not.
Discordance is by no means the rule. Idiopathic CaOx stone formers have very modest CaP SS in urine and never produce any IMCD crystal deposits [2]. Brushite SF have higher urine CaP SS and produce IMCD apatite deposits [8]; the same is true for stone formers with primary hyperparathyroidism [5;9] or distal renal tubular acidosis [10]. So apatite deposits can occur, or not, in accord with urine SS; it is in our two bowel diseases we have encountered a unique disconnect between urine and IMCD whose tubule fluid must closely approximate the final urine under normal circumstances.
One way to advance the problem is to study additional groups of patients with bowel disease, in hope that within the groups we will eventually be able to discern mechanisms for the dissociation of IMCD apatite from final urine conditions and from stones. Here, we present our work on 11 patients who form CaOx stones and have small bowel resection due to mainly Crohn’s disease. For the first time, we can, in some patients, identify sizable IMCD CaOx deposits, which are consistent with both urine SS and with the stones being formed. However, in these deposits we also always find apatite, as unexpected as in ileostomy and bypass patients. The details of these patients, taken along with those from bypass and ileostomy patients, lead to an initial hypothesis that might be of value for future research into the mechanisms for IMCD deposit formation, and round out present knowledge of crystal formation in tissues of stone formers with bowel disease. As well, these patients form interstitial plaque, and their urine findings support our past work indicating that scanty and acidic urine can promote plaque in the absence of overt hypercalciuria.
RESULTS
Clinical Findings
Eleven patients were studied (Table 1), most patients were men and all but one (Case 3) had Crohn’s disease. Bowel surgeries numbered from 1 to 7 procedures; the amount of bowel resected is not known to us. None had ileostomy or colostomy. Stones began before or after the surgery with a wide variation in timing (Table 1). Numbers of stones and procedures were often quite high. Serum creatinine values are not elevated; metabolic acidosis and hypokalemia were not observed. Stones were overwhelmingly CaOx monohydrate.
TABLE 1.
CLINICAL CHARACTERISTICS AND SERUM CHEMISTRIES
| Pt | Sex | Age at First SB Surgery (yr) | Age at First Stone (yr) | Prior Stones (#) | Age at Biopsy (yr) | ESWL | PNL | Total Procedures | Serum Creat (mg/dl) | Serum CO2 (mM/L) | Serum K (mM/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 23 | 47 | 4 | 48 | 1 | 1 | 3 | 1.3 | 32 | 4.1 |
| 2 | F | 31 | 40 | >12 | 52 | 2 | 1 | 4 | 0.9 | 27 | 3.6 |
| 3 | M | 1 | 12 | >30 | 38 | 3 | 1 | 5 | 0.8 | 28 | 4.1 |
| 4 | M | 22 | 20 | >25 | 43 | 2 | 2 | 5 | 1.0 | 25 | 3.6 |
| 5 | M | 34 | 26 | >10 | 49 | 1 | 5 | 8 | 0.9 | 32 | 4.0 |
| 6 | M | 22 | 28 | 3 | 34 | 2 | 1 | 3 | 0.9 | 27 | 4.5 |
| 7 | M | 39 | 39 | 3 | 48 | 2 | 1 | 5 | 1.0 | 26 | 4.3 |
| 8 | M | 24 | 24 | >50 | 60 | 1 | 2 | 7 | 1.1 | 33 | 4.7 |
| 9 | F | 26 | 34 | >12 | 54 | 5 | 6 | 12 | 0.8 | 28 | 4.5 |
| 10 | M | < 40 | < 40 | >10 | 75 | 0 | 5 | 5 | 1.0 | 27 | 4.3 |
| 11 | M | 40 | 47 | 1 | 52 | 3 | 2 | 6 | 0.8 | 28 | 4.7 |
SB, small bowel; ESWL, extracorporeal shock wave lithotripsy; PNL, percutaneous nephrolithotomy; Total procedures: includes cystoscopy, open surgery, ureteroscopy as well as ESWL and PNL.
Surgical Pathology
A majority of papillae contained large deposits of white – interstitial apatite -plaque (Figure 1 a–d; Figure 2a and b). Abundance of white plaque ranged from 1.2 – 14.9% of surface area coverage, with a mean of 5±1 (Table 2). Some stones were found attached to papillae in all but one case (patient 11) (Figure 1c; 2a and b; Table 2); when detached, they were growing over white plaque as is found in ICSF, primary hyperparathyroidism and ileostomy patients [3–5]. All attached stones were CaOx with typical apatite deposits adherent at sites where stones were attached to papillae (Figure 2c–e). In some regions the density of attached stones over plaque was remarkable, and like that found in ICSF (Figure 2b).
Figure 1. Coexistence of attached stones and BD plugging on the same papillae.
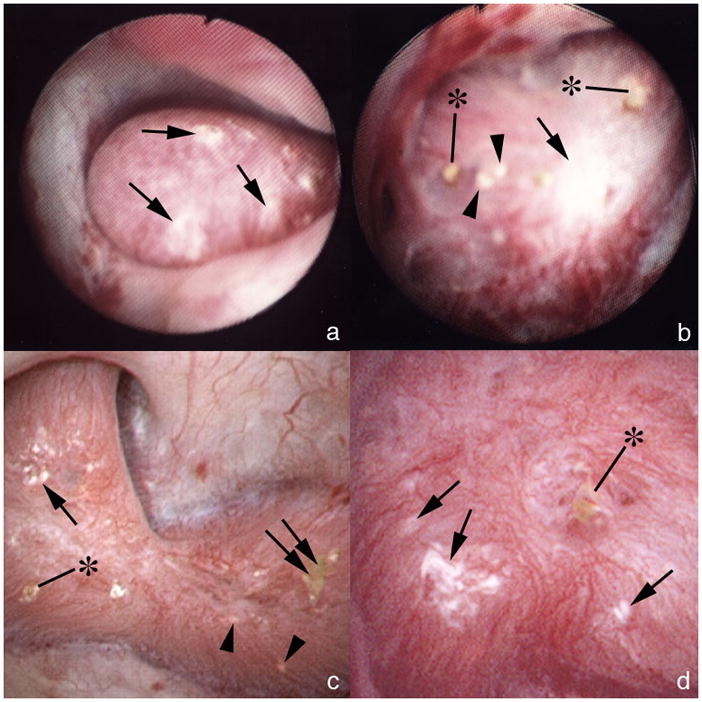
(a) Large area of white interstitial (Randall’s) plaque (single arrows) on one papilla. (b) In another patient a large area of white plaque (single arrow) is seen in intermixed with areas of yellow plaque (IMCD crystal deposits, arrowheads) and crystalline plugs (asterisks) protruding from dilated BD. (c) A papilla from another patient shows a smaller area of white plaque (single arrow) intermixed with yellow plaque (arrowheads), BD plugs (asterisk) and an attached stone (double arrow). (d) At higher magnification, a dilated BD is seen with a protruding crystal plug (asterisk) near several small areas of white plaque (single arrows).
Figure 2. Details of attached stones.
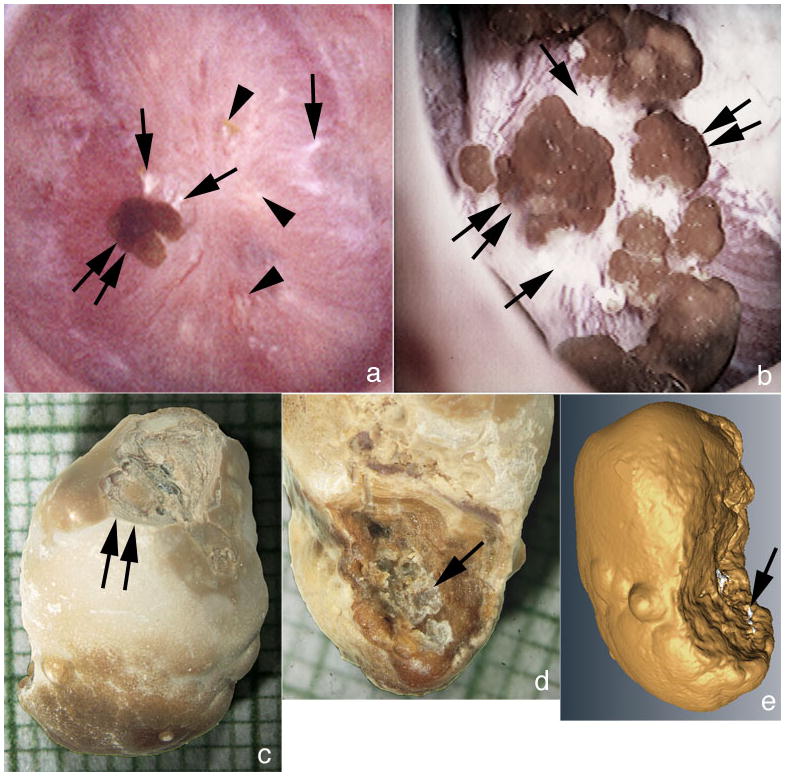
(a) An attached stone (double arrow) is seen resting on a region of white plaque (single arrows) and intermixed with small areas of white (single arrow) and yellow plaque (arrowheads). Patient 8 had numerous attached stones (double arrow) atop an extensive area of white plaque (single arrows) much like that found in some ICSF. Analysis of attached stones by μ-CT revealed these to be composed of primarily of CaOx with small sites of apatite corresponding to a site of attachment to white (Randall’s) plaque. (c & d) g Light microscopic image of an attached revealing the smooth urinary (c) and papillary (d) surface morphology. The papillary surface (d) shows a concave region with crystalline material (single arrow) consistent with an attachment site. The urinary surface (c) shows a damaged region (double arrow) generated during stone removal. (e) Reconstruction of μ-CT images shows regions of CaOx in yellow and areas of apatite in white. The white regions are appear to present the attachment site.
TABLE 2.
SURGICAL AND PATHOLOGICAL FINDINGS AND STONE TYPE
| Pt | Papillary deformity (%) | Plaque Papillary Surface Area (%) | Stones Attached to Plaque | Dilated BD | IMCD BD Deposits | Nature of IMCD Crystals | Glom | Tub At | IF | Stone Type |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 100% | 4.80 | Yes | +++ | 6±2 | HA | - | - | - | COM (2) |
| 2 | 33% | 3.60 | Yes | ++ | 2±1 | HA, CaOx | 19/1/2/4 | 1 | 1 | COM (1) |
| 3 | 10% | 3.03 | Yes | + | 1±1 | HA | 33/1/0/2 | 2 | 2 | COM (1) |
| 4 | 10% | 6.38 | Yes | + | 2±1 | HA, CaOx | - | - | - | COM (2) |
| 5 | 75% | 2.62 | Yes | ++ | 2±1 | HA | 12/0/1/11 | 2 | 2 | CaOx 91%, HA 9% (5) |
| 6 | 10% | 14.90 | Yes | + | 1±1 | HA | 10/2/1/0 | 1 | 1 | COM (1) |
| 7 | 20% | 2.17 | Yes | + | 1±1 | HA | 27/2/3/0 | 1 | 1 | COM (3) |
| 8 | 50% | 6.99 | Yes | ++ | 4±1 | HA | 8/1/1/2 | 2 | 2 | COM (2) |
| 9 | 75% | 1.23 | Yes | +++ | 4±2 | HA | 17/1/2/3 | 2 | 2 | CaOx 95% (4)* |
| 10 | 70% | 7.68 | Yes | ++ | 1±1 | HA, CaOx | 13/1/1/10 | 2 | 2 | COM (12) |
| 11 | 10% | 1.76 | No | + | 1±1 | HA | 5/1/0/2 | 1 | 1 | COM 98%, HA 2% (4) |
% Papillary deformity refers to the fraction of papillae visualized at the time of surgery with deformity; % Plaque papillary surface area, mean percent of papillary surface covered by white plaque; Stones attached to plaque, stones found attached to papilla on plaque at surgery; Dilated BD, degree of dilatation of Bellini ducts; IMCD/BD, inner medullary collecting duct+Bellini duct, mean number of deposits/mm2 of tissue on μCT; IMCD crystals, HA hydroxyapatite, CaOx, calcium oxalate; Glom, # glomeruli/#mild/#moderate/#global sclerosis; Tub At, cortical tubular atrophy, graded 1–3; IF, cortical interstitial fibrosis graded 1–3; COM, calcium oxalate monohydrate;
small amount hydroxyapatite and acid ammonium urate in 1 stone each.
Intermixed with white plaque, we observed ample yellow plaque (Figure 1 b and c), which is the gross reflection of IMCD crystal plugs (Figure 1 b – d). Crystal plugs protrude (Figure 1 b – d) from mouths of dilated Bellini ducts (BD). Dilated BD were most abundant in flattened and deformed papillae (Table 2). The amount of deformity was less than that found in brushite stone formers [8] but greater than that found in ileostomy patients.
Histopathology
Intra-operative papillary micro-biopsies revealed very large amounts of interstitial plaque (Figure 3 a–c). As predicted from the operative appearance, multiple IMCD were plugged with crystal deposits (Figure 3c, d and 4 a–f; Table 2). Patterns of deposit vary from patient to patient. Three patients (Table 2, cases 1, 8 and 9) have higher numbers of deposits than other patients. Two patients who do not have large numbers of deposits (Table 2 cases 4 and 10) nevertheless formed deposits of unusually large size (Figure 4 a, b, e and f); because large, their plugged ducts occupy most of whole regions of papillary tissue in the biopsy sample, to an extent only thus far encountered in renal tubular acidosis and brushite nephropathy [8;10]. Even so, the overall density of plugging, estimated from micro-CT images (Table 2) is less than we have found in ileostomy patients. As usual, we found no inflammation around even extensive interstitial deposits; but around plugged IMCD we found marked interstitial inflammation and fibrosis (Figures 3 d and 4 a–f).
Figure 3. Relative densities of interstitial plaque and IMCD deposits.
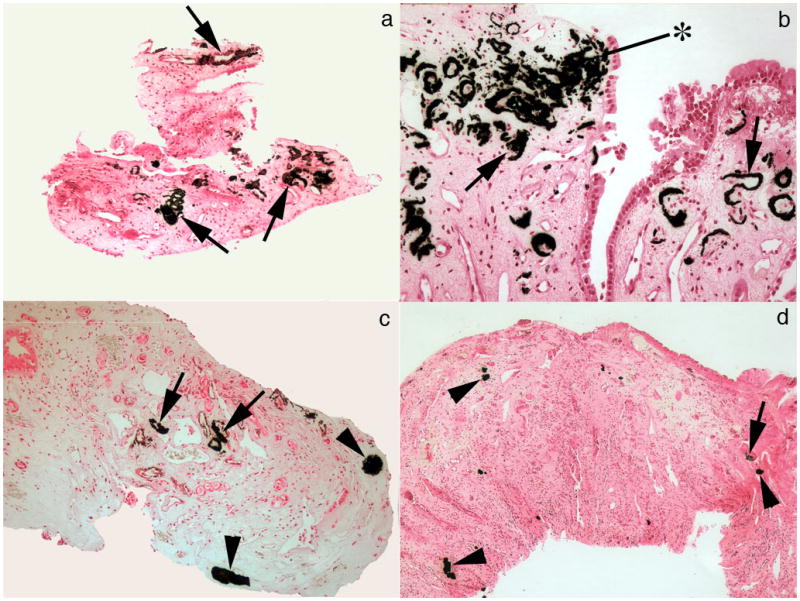
(a,b) Large areas of interstitial plaque (Randall’s) (arrows) are seen surrounding the thin loops of Henle and extending to the base of the urothelial cells (asterisk). (c,d) Areas of interstitial plaque (arrows) and plugged IMCD (arrowheads) are found in the same biopsy sample at varying amounts. None of these IMCD deposits contained birefringent crystals. Interstitial fibrosis was associated with IMCD plugs. Original magnification × 100 (a,b); × 50 (c,d).
Figure 4. IMCD deposits mixture of apatite and CaOx.
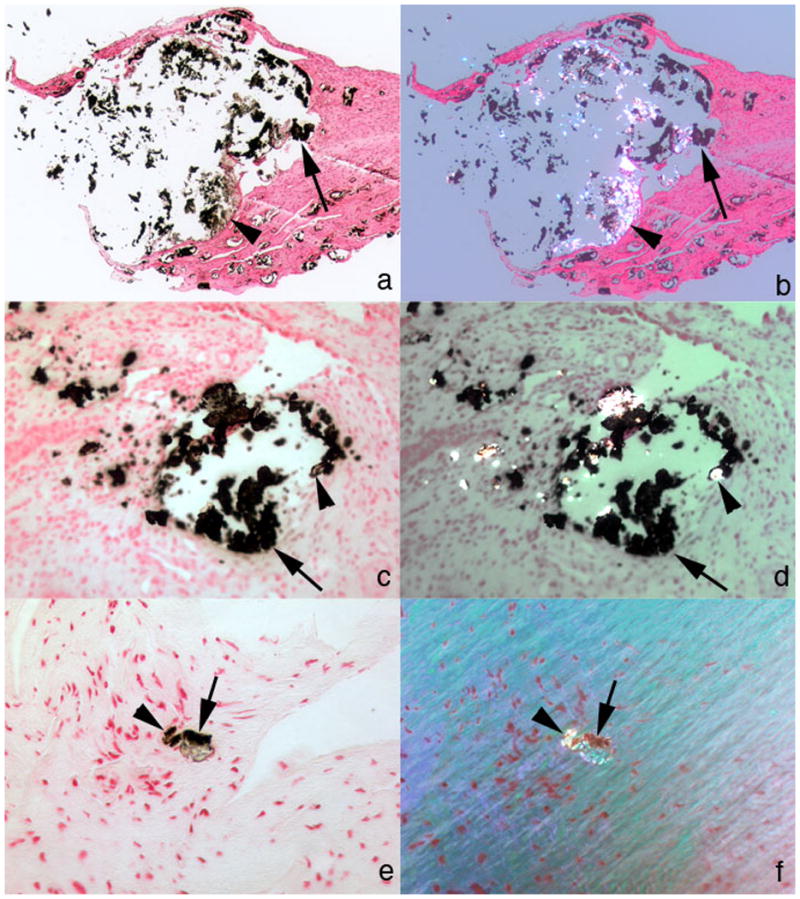
(a–d) Two different large IMCD plugs from separate patients are seen under non-polarizing (a, c) vs polarizing (b, d) optics. These deposits show birefringent (arrowheads) and non-birefringent (single arrows) crystals forming the same deposit yet not admixed. The non-birefringent crystals are probably apatite and the birefringent crystals CaOx. (e, f) An occasional small IMCD plug also possessed both birefringent (see arrowheads in panels e, f) and non-birefringent (see single arrows in panels e, f)) crystals. Original magnification × 25 (a–d); × 100 (e,f).
Cortical changes (Table 2) included moderate glomerular sclerosis in all but 2 cases (patients 5 and 10) who had global sclerosis of a majority of glomeruli. Tubular atrophy and interstitial fibrosis ranged from mild to moderate and did not track closely with glomerular sclerosis (Table 2). Serum creatinine (Table 1) did not reflect cortical changes. No cortical deposits were detected.
Nature of crystal deposits
All patients had some IMCD with non-birefringent crystal deposits, as would be found with apatite (Table 2). Although crystals in most IMCD were exclusively non-birefringent, in three patients (cases 2, 4 and 10; Table 2) we found some IMCD containing both birefringent and non-birefringent crystals. The IMCD plugs with both crystals in fact contained mixtures of CaOx and apatite. Using micro–FTIR, birefringent deposits were always CaOx (Figure 5 illustrates Case 4). Non-birefringent deposits were always apatite (Figure 5 illustrates Case 3). Because we have made these positive determinations by FTIR we have labeled deposits in Table 2 by their crystal structure, not their optical appearance. Though present in the same plugs, CaOx and apatite were never admixed together, but occupied separate regions (Figure 4, a–f).
Figure 5. Micro-Fourier-Transform Infrared spectrometer analysis of IMCD deposits.
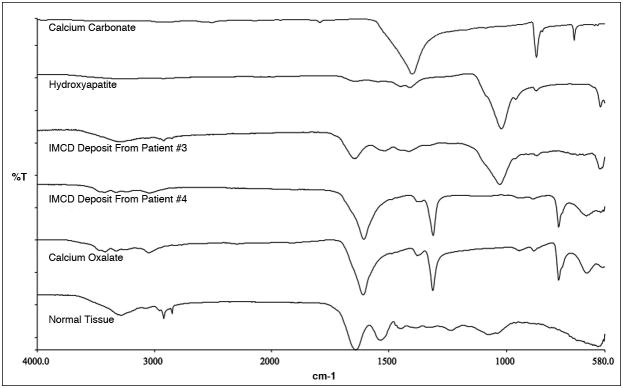
IMCD deposits show a spectral band matching that of the hydroxyapatite and calcite standards in patient #3 and CaOx standard in patient #4. Tissue with embedding medium, a control, displays bands in common with the three standards.
Urine Chemistry Findings
Urine CaOx SS was quite high in most of the patients (Table 3), the exceptions being cases 4, 8 and 9. Uric acid SS was substantial in cases 6, 7 and 10; no stones contained uric acid. One patient (Table 3, case 9) had some ammonium acid urate in one stone. At the time of our study, the high urine volume and unexceptional urine ammonia level did not create a high SS with respect to that salt (not shown). Overall the SS values are reasonably consistent with the predominance of CaOx in stones. Since urine pH is generally below 6 and SS with respect to CaP as brushite is below 1 in almost all patient samples, the presence of abundant apatite in IMCD deposits is unexplained.
TABLE 3.
24 HOUR URINE RESULTS
| Pt | VOL | pH | CIT | CA | OX | NA | UA | NH4 | SUL | SS UA | SS CAOX | SS CAP | Wt (kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.70 | 5.41 | 324 | 117 | 57 | 137 | 430 | 42 | 23 | 1.31 | 9.70 | 0.18 | 75 |
| 2 | 0.66 | 6.05 | 80 | 158 | 43 | 86 | 376 | 64 | 28 | 1.10 | 26.90 | 3.10 | 57 |
| 3 | 2.38 | 5.53 | 248 | 243 | 57 | 272 | 506 | 69 | 37 | 1.00 | 8.81 | 0.36 | 59 |
| 4 | 1.68 | 5.61 | 50 | 69 | 35 | 182 | 460 | 52 | 21 | 1.07 | 4.03 | 0.09 | 57 |
| 5 | 0.75 | 5.67 | 89 | 88 | 21 | 63 | 258 | 31 | 7 | 1.42 | 10.2 | 1.10 | 50 |
| 6 | 1.13 | 5.27 | 260 | 75 | 142 | 239 | 453 | 63 | 27 | 2.37 | 17.03 | 0.18 | 100 |
| 7 | 1.13 | 5.28 | 128 | 117 | 44 | 174 | 507 | 39 | 33 | 2.62 | 10.59 | 0.21 | 91 |
| 8 | 2.47 | 5.70 | 354 | 37 | 104 | 222 | 512 | 36 | 24 | 0.70 | 3.25 | 0.07 | 86 |
| 9 | 2.36 | 5.93 | 434 | 208 | 44 | 178 | 349 | 39 | 23 | 0.36 | 6.36 | 0.84 | 61 |
| 10 | 0.85 | 5.14 | 56 | 33 | 62 | 121 | 280 | 42 | 41 | 2.34 | 11.77 | 0.05 | 58 |
| 11 | 1.96 | 5.61 | 40 | 148 | 106 | 250 | 627 | 53 | 33 | 1.28 | 14.5 | 0.39 | 77 |
Vol, volume l/d; Cit, citrate, mg/d; Ca, calcium, mg/d; Ox, oxalate, mg/d; Na, sodium, mEq/d; UA, uric acid, mg/d; NH4, ammonium, mEq/d; SUL, sulfate, mEq/d; SS, supersaturation; CAOX, calcium oxalate; CAP, calcium phosphate. Results are the mean of two collections for each patient.
DISCUSSION
Perhaps the most informative way to discuss these patients is in relation to our prior findings in patients with ileostomy and bypass for obesity [1;3]. As a group the small bowel, ileostomy, and bypass cases all formed similar numbers of stones per patient year, ~1.1, 1.1, and 0.8, respectively. Surgical procedure rates for stones of all bypass and the present series were about 0.6/pt yr; ileostomy was 1.5/pt yr. Stones in the bypass cases and the present series were essentially only CaOx; ileostomy patients formed uric acid and CaOx stones.
At surgery, bypass patients displayed minimal papillary abnormality which consisted only of an occasional plugged BD. The ileostomy patients displayed less papillary deformity than the present patients, and about the same extent of BD plugging; both markedly exceeded the minimal findings in the bypass patients. Histopathologically, IMCD and BD deposits were far more marked in the ileostomy patients than in the present group (12 vs. 2.2 deposits per mm2, 95% CI of difference 2 – 17.6, p=0.02), and among the bypass patients we found the least of all, only a rare plugged duct. Altogether the three groups form a gradient of pathology, bypass being the least involved, ileostomy the most, and these cases, here, somewhere in between. Only the ileostomy patients formed any uric acid stones.
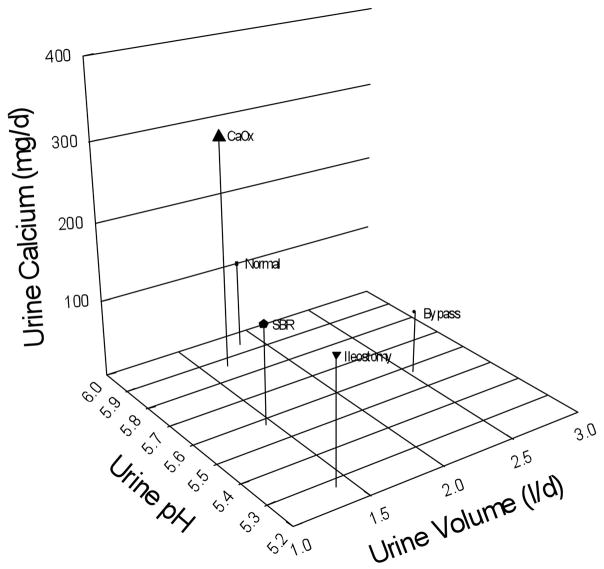
Plaque, in our present series, was abundant, and this is not surprising in that urine pH was low and urine volume often low. Moreover, as noted, urine volume would most likely have been much lower at intervals of worsened diarrhea. Compared with bypass, ileostomy, and routine CaOx stone formers, as well as normal people, the present patients fall between ileostomy and normals in pH and have the low volumes of ileostomy cases (Figure 6). Plaque abundance is reasonable for their position on the 3 axes of this plot. Bypass SF have the urine pH of the present cases, but much higher volume and much less plaque.
Figure 6. Principle determinants of interstitial plaque.
Normal subjects (Normal) and idiopathic CaOx stone formers (CaOx) have urine pH (y-axis) values near 6 as well as similar urine volumes (x-axis), but the stone formers have much higher calcium excretion (z-axis height) and abundant plaque (size of symbol) vs. small amounts for normals. Bypass patients (Bypass) have normal amounts of plaque; low urine calcium and high volume offset the low urine pH. Small bowel resection (SBR) and ileostomy patients (Ileostomy) have low urine pH and volume, and normal calcium excretions, and both form abundant plaque.
The stones are also not problematic. CaOx SS is high and we found CaOx stones growing over regions of apatite plaque. So, unlike the complex problems raised by deposits, the plaque and stones found here are in accord with, and help to confirm the model we have presented elsewhere of plaque formation with a scanty acidic urine [3;7] and subsequent CaOx stone overgrowth driven by urine CaOx supersaturation.
The problem here, as in the other bowel diseases, is to explain apatite IMCD deposits when urine SS would lead one to expect CaOx or uric acid. In bypass cases we found IMCD and BD plugs were almost invariably apatite. CaOx was found only as a microscopic linear deposit over the apical surfaces of a few IMCD cells in 3 of 5 patients, and in a small IMCD plug in 2 of these 3. Deposits in ileostomy patients were apatite, never CaOx; we also found ammonium and sodium salts of uric acid that could be explained by urine SS. Here, again, we find apatite in IMCD of all patients; one IMCD in each of 3 cases had a plug containing CaOx as well as apatite. Two of these plugs were very large, among the largest IMCD plugs we found, and were vastly larger than the CaOx plugs in our bypass patients. Given that stones were CaOx in all three groups, and sometimes uric acid in ileostomy cases, IMCD and BD apatite deposits always have diverged from the stones and urine SS.
How can we account for the divergence, and for the differences between these three groups? One approach is to consider relevant urine chemistry values in relation to the kinds of IMCD deposits encountered. For this purpose, we compared measurements in our 5 published cases of bypass surgery [1;2], 7 patients with ileostomy [3], the present 11 patients, and for reference 15 ICSF [2] who never form any intra-tubular deposits (Table 4). Among these patients, as noted, 6 (3 bypass, 3 of the present cases) have some CaOx in their IMCD (Table 4, column labeled apatite + CaOx). Although SS CaOx does not vary among the 4 groups in Table 4, those with CaOx deposits achieve their supersaturation at a lower molar ratio of calcium to oxalate than do the other 3 groups. CaOx crystallization is known to be more efficient as the molar ratio approaches 1 [11]. Although numbers are too small to establish these comparisons rigorously, these urine data give some preliminary clue to a possible pathogenetic mechanism for CaOx in IMCD plugs. Our much larger report of patients who were not biopsied [12] agrees reasonably well with this table: molar ratios were 2.5, bypass, 5.1, small bowel + colon resection, 5.2, ileostomy, 5.8 small bowel resection alone, and 12.5 for routine stone formers, respectively. Even so, our results do not help explain IMCD apatite.
TABLE 4.
RELATIONSHIP BETWEEN URINE FINDINGS AND IMCD DEPOSITS IN PATIENTS WITH BOWEL DISEASE OR ICSF
| NO IMCD DEPOSITS (15) | APATITE ONLY (11) | APATITE + CAOX (6) | APATITE + UA (5) | |
|---|---|---|---|---|
| SS CAOX | 10±1 | 8±2 | 10±2 | 9±2 |
| OX 24* | 41±7 | 64±8 | 69±11 | 32±12 |
| [OX] | 0.3±0.1 | 0.4±0.1 | 0.5±0.1 | 0.3±0.1 |
| CA 24** | 231±25 | 116±29 | 89±40 | 169±43 |
| [CA]** | 4.2±0.4 | 1.7±0.5 | 1.8±0.7 | 3.8±0.8 |
| [CA]/[OX]*** | 14±1 | 6±2 | 3±2 | 11±2 |
| pH** | 5.84±0.1 | 5.5±0.1 | 5.6±0.1 | 5.3±0.1 |
| SS UA*** | 1.2±0.2 | 1±0.3 | 1±0.3 | 3±0.4 |
| SS CAP | 1±0.2 | 0.3±0.2 | 0.6±0.3 | 0.6±0.3 |
Values are ± SEM.
values differ by ANOVA, p<0.05;
p<0.01;
p<0.001;
ICSF, idiopathic calcium stone formers (n=15); [OX], [Ca] oxalate and calcium molarity (mM/l); SS, supersaturation; CaOx, calcium oxalate; CaP, calcium phosphate; UA, uric acid; Ox24 and Ca24, 24 hour urine oxalate and calcium, (mg/d). Bowel patients include all previously reported patients with bypass surgery for obesity (n=5), ileostomy (n=7), and current patients. One small bowel patient with apatite IMCD deposits missing from table (incomplete data).
For apatite, the urine data are most remarkable in that SS with at least one of the initial apatite precursors, brushite, is always below 1 in the groups with deposits. As in the case of urate species arising in a setting of urine chemistries that favor uric acid, we presume and conjecture that CaOx or uric acid was an initial phase, tubule pH rose secondary to damage, and apatite became predominant. Urine pH for the group with only apatite in deposits averaged 5.5, much below that at which calcium phosphate phases would be stable. We recognize that at this stage all we can offer is conjecture; we expect subsequent experiments by our lab and other labs will eventually clarify the actual mechanisms involved in this very important example of crystal mediated cell injury.
For urate species the same sequence might reasonably apply. Urate was found in 5 cases, all ileostomy. Urine uric acid SS was far higher in these 5 patients than in any other group (Table 4). We propose that uric acid was the initial phase plugging IMCD or BD, and that urate species became the more stable phase with time, perhaps because intra-tubular pH rose secondary to injury to acidification mechanisms from local plugging with obstruction. This speculation requires further experimental confirmation.
We must presume that because of their bowel diseases these patients suffered episodes of dehydration which would have radically altered SS. For ileostomy the effects would predictably be alkali loss and volume loss, with very acid scanty urine favoring uric acid and CaOx. For bypass patients and the present cases, prone to enteric hyperoxaluria, the main prediction would be extreme CaOx SS. The latter could seed IMCD or BD with initial CaOx plugs and entrain the mechanisms we already alluded to.
Although we realize that our sample of patients with bowel disease is relatively small, overall the three groups of patients lead us to a general hypothesis about enteric stone disease that is perhaps most valuable in suggesting new research. Ileostomy is a low volume low pH condition, without enteric hyperoxaluria, which favors uric acid stones and plugs, and CaOx crystals, but at a higher calcium to oxalate ratio than would be produced by enteric hyperoxaluria. Bypass and small bowel disease create enteric hyperoxaluria and would foster CaOx crystals more strongly than ileostomy. We propose a sequence of local tubule injury with reduced IMCD and BD acidification; initial plugging occurs from phases favored in urine, CaOx or uric acid, crystal mediated IMCD cell damage occurs, local IMCD and BD fluid pH rises, along with CaP SS, and apatite forms. This hypothesis is testable and may be fruitful.
MATERIALS AND METHODS
Patients
We studied eleven patients with small bowel resection and kidney stones who required percutaneous nephrolithotomy at this institution (International Kidney Stone Institute, Methodist Clarian Hospital, Indianapolis, IN, USA) during the past 6 years (Table 1) and who consented to participate in the study. Clinical history was obtained along with reviews of old records to obtain stone analyses (Table 2) and the type and number of stone procedures.
Clinical Measurements
Two 24-h urine samples were collected while patients were eating their free choice diet and off medications. In urine we measured volume, pH, calcium, oxalate, citrate, phosphate, uric acid, sodium, potassium, magnesium, sulfate and ammonia using methods detailed elsewhere [13], and calculated supersaturation (SS) with respect to CaOx, brushite, uric acid, sodium acid urate and ammonium acid urate using EQUIL 2 [14]. Routine clinical blood measurements were made on bloods drawn for clinical purposes.
Biopsy protocol and plaque area determination
During PNL all papillae were digitally imaged as described elsewhere [1]. Biopsies were taken from one upper pole, inter-polar and lower pole papillum and from the cortex. Using the intra-operative recordings [7], total surface area of each papilla was measured, and one of us (JEL) outlined areas of white (Randall’s) plaque as well as the entire papilla on one set of prints. The white plaque and total papillary areas were converted to numbers of pixels, giving the ratio of plaque to total papillary pixels, or percent coverage with plaque. No biopsy site inspected intra operatively displayed significant hemorrhage and no post-operative complications related to the biopsy procedures occurred in any patient. The study was approved by the Institutional Review Board Committee for Clarian Health Partners (#98-073).
Tissue analysis
Light microscopy analysis
Thirty-one papillary and nine cortical biopsies were studied using light microscopy. All biopsy specimens were immersed in 5% paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.4), and were dehydrated through a series of graded ethanol concentrations to 100% ethanol prior to embedment in a 50/50 mixture of Paraplast Xtra (Fisher) and Peel-away Micro-Cut (Polysciences). Serial sections were cut at 4μ and stained with the Yasue metal substitution method for calcium histochemistry [15], hematoxalin & eosin for routine histological examination. An additional set of serial sections was cut at 7μ for infrared analysis.
A renal pathologist (CLP) performed a semi-quantitative analysis [8] using the Jones’ silver stained cortical sections from nine of the eleven small bowel patients. Each slide was given a code number in order to remove any patient identification. Tubular atrophy and interstitial fibrosis were independently scored on a scale of 0 to 3 (0 = none, 1 = mild or <34% of sample, 2 = moderate or 34–66%, and 3 = severe or >66%). Glomerulosclerosis was defined as increased mesangial matrix with or without wrinkling, thickening and/or collapse of glomerular basement membranes. Sclerosis of individual glomeruli was scored as segmental (<25% = mild; 25 to 75% = moderate) or global (>75% = severe or total obsolescence). The total number of glomeruli observed and the number of glomeruli in each of the three categories of sclerosis were recorded.
Infrared analysis
Reflectance-absorption (R/A) spectra were collected with a Perkin-Elmer Spotlight 400 infrared imaging microscope interfaced to a Perkin-Elmer Spectrum One FTIR spectrometer [5]. The system employed a 100 × 100 m liquid nitrogen cooled mercury cadmium telluride (HgCdTe) detector. Tissue samples were analyzed using an aperture size appropriate to the size of the sample being studied (e.g. 50 to 20μ in diameter). Each spectrum collected represents the average of 64 or 128 individual scans possessing a spectral resolution of 4 cm−1. A clean area on the low-E slide was employed to collect the background spectrum.
Attenuated total internal reflection (ATR) spectra were collected for all tissue samples with a Perkin-Elmer Spotlight 400 infrared imaging microscope interfaced to a Perkin-Elmer Spectrum One FTIR spectrometer. The system employed a 100 × 100 m liquid nitrogen cooled HgCdTe detector. The standard germanium internal reflection element was employed in conjunction with a 100 × 100μ or a 50 × 50μ aperture. Since the ATR measurement is essentially an immersion measurement, the sampled area using these apertures is 25 × 25 m or 12.5 × 12.5μ, respectively. Each spectrum collected represents the average of 64 or 128 individual scans possessing a spectral resolution of 4 cm−1. A clean potassium chloride surface was employed to collect the background spectrum.
μ-CT analysis
All papillary biopsies underwent μ-CT analysis with the SkyScan-1172 (Vluchtenburgstraat 3, B-2630 Aartselaar, Belgium) high-resolution desk-top μ-CT system allowing nondestructive mapping of the location and size of the crystalline deposits within a biopsy specimen [5]. This μ-CT system can generate a tissue window so that both the mineral deposit and tissue organization are seen at the same time. For this protocol, biopsies are quickly dipped in a 1:10 dilution of Hypaque (50%, Nycomed Inc., Princeton, NJ) / PBS, then coated with a thin layer of paraffin and mounted in the center of a small chuck which is then locked into place in the machine. The sample was positioned in the center of the beam, and the system configuration was set at 35 kV, 209 mA, 180° rotation, with flatfield correction. Images were saved to CD’s and reconstructed with Cone-Reconstruction software by SkyScan. These images were then reconstructed into 3D images with SkyScan’s CTAn + CTVol software. These images allowed us to properly orient each biopsy for future light microscopic analysis. Three separate scans from each patient were used to determine the number of sites of intraluminal deposits per square millimeter.
“Stones from these patients were analyzed using the SkyScan 1172 with reconstruction voxel sizes of 10–20 um. Three-dimenesional reconstructions of stones were examined using ImageJ (http://rsb.info.nih.gov/ij/) and Voxx2 (http://www.nephrology.iupui.edu/imaging/voxx/) to verify the composition. Surface renderings were done using Amira (Visage Imaging, Carlsbad, CA).”
Acknowledgments
Funded by NIH PO1 DK56788
Footnotes
Disclosure:
All the authors declared no competing interests.
Reference List
- 1.Evan AP, Lingeman JE, Coe FL, et al. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest. 2003;111:607–616. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evan AP, Coe FL, Gillen D, et al. Renal intratubular crystals and hyaluronan staining occur in stone formers with bypass surgery but not with idiopathic calcium oxalate stones. Anat Rec (Hoboken) 2008;291:325–334. doi: 10.1002/ar.20656. [DOI] [PubMed] [Google Scholar]
- 3.Evan AP, Lingeman JE, Coe FL, et al. Intra-tubular deposits, urine and stone composition are divergent in patients with ileostomy. Kidney Int. 2009;76:1081–1088. doi: 10.1038/ki.2009.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller NL, Gillen DL, Williams JC, Jr, et al. A formal test of the hypothesis that idiopathic calcium oxalate stones grow on Randall’s plaque. BJU Int. 2009;103:966–971. doi: 10.1111/j.1464-410X.2008.08193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evan AE, Lingeman JE, Coe FL, et al. Histopathology and surgical anatomy of patients with primary hyperparathyroidism and calcium phosphate stones. Kidney Int. 2008;74:223–229. doi: 10.1038/ki.2008.161. [DOI] [PubMed] [Google Scholar]
- 6.Evan AP, Coe FL, Lingeman JE, et al. Mechanism of formation of human calcium oxalate renal stones on Randall’s plaque. Anat Rec (Hoboken) 2007;290:1315–1323. doi: 10.1002/ar.20580. [DOI] [PubMed] [Google Scholar]
- 7.Kuo RL, Lingeman JE, Evan AP, et al. Urine calcium and volume predict coverage of renal papilla by Randall’s plaque. Kidney Int. 2003;64:2150–2154. doi: 10.1046/j.1523-1755.2003.00316.x. [DOI] [PubMed] [Google Scholar]
- 8.Evan AP, Lingeman JE, Coe FL, et al. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int. 2005;67:576–591. doi: 10.1111/j.1523-1755.2005.67114.x. [DOI] [PubMed] [Google Scholar]
- 9.Parks JH, Coe FL, Evan AP, Worcester EM. Clinical and laboratory characteristics of calcium stone-formers with and without primary hyperparathyroidism. BJU Int. 2009;103:670–678. doi: 10.1111/j.1464-410X.2008.08064.x. [DOI] [PubMed] [Google Scholar]
- 10.Evan AP, Lingeman J, Coe F, et al. Renal histopathology of stone-forming patients with distal renal tubular acidosis. Kidney Int. 2007;71:795–801. doi: 10.1038/sj.ki.5002113. [DOI] [PubMed] [Google Scholar]
- 11.Nancollas GH, Singh RP. In vitro system for calcium stone formation: the constant composition model. Contrib Nephrol. 1987;58:49–58. doi: 10.1159/000414487. [DOI] [PubMed] [Google Scholar]
- 12.Parks JH, Worcester EM, O’Connor RC, Coe FL. Urine stone risk factors in nephrolithiasis patients with and without bowel disease. Kidney Int. 2003;63:255–265. doi: 10.1046/j.1523-1755.2003.00725.x. [DOI] [PubMed] [Google Scholar]
- 13.Parks JH, Goldfisher E, Asplin JR, Coe FL. A single 24-hour urine collection is inadequate for the medical evaluation of nephrolithiasis. J Urol. 2002;167:1607–1612. [PubMed] [Google Scholar]
- 14.Werness PG, Brown CM, Smith LH, Finlayson B. Equil 2: a basic computer program for the calculation of urinary saturation. J Urol. 1985;134:1242–1244. doi: 10.1016/s0022-5347(17)47703-2. [DOI] [PubMed] [Google Scholar]
- 15.Yasue T. Histochemical identification of calcium oxalate. Acta Histochem Cytochem. 1969;2:83–95. [Google Scholar]