Abstract
Although it is now clear that cognitive dysfunction is a common accompaniment of cancer chemotherapy, its implications await further research and direction. Most of the clinical research relies on standard neuropsychological tests that were developed to diagnose stable traits. Cognitive dysfunction in patients undergoing treatment varies with time. Its dimensions will vary during the course of treatment, which generally consists of cycles of drug administration followed by recovery periods. To effectively determine the connection between chemotherapy and cognitive function requires neuropsychological tests based on performance, so that they can be administered at specified times during the entire course of treatment and beyond. A number of computerized test batteries, many of which have been developed for environmental neurotoxicology, are now available that fit such criteria. Moreover, cognitive impairment is only one aspect of chemotherapy-induced neurotoxicity. A full appreciation of its scope requires assessment of sensory functions such as vision, audition, and somatosensory properties, and assessment of motor function. A program of research based on animal models is also essential. Only with animal models is it possible to determine dose-response relationships and to couple behavioral with mechanistic indices such as neuroplasticity. Animal behavior models play a vital role in environmental toxicology because, from them, it is possible to derive some index of exposure that limits adverse effects. However, as in human testing, it is critical to choose situations whose properties remain stable over long periods of time so as to trace the time course of neurotoxicity.
Introduction
Oncologists are now aware that cancer chemotherapy can exert subtle as well as blatant neurotoxicity. The latter has been recognized even from the earliest days of chemotherapeutics and certainly in the case of radiation therapy. Gross sensory loss, such as deafness, and evidence of abnormal central nervous system function such as seizures are inarguable. The less obvious outcomes, labeled as chemobrain or chemofog by cancer patients, achieved far less clinical recognition because they came in the form of subjective complaints. The labels describe a syndrome characterized by memory difficulties, episodes of disorientation, inability to concentrate, and other aspects of cognitive impairment. A T-shirt sold in the gift shop at the University of Rochester Medical Center reflects how keenly patients are aware of their difficulties. It is inscribed with one patient’s view: “I have Chemobrain; what’s your excuse?” It reflects a situation that should cause us to ponder the limitations of and constraints imposed upon clinical medicine and cancer chemotherapy.
Within the past decade, but especially quite recently, the application of neuropsychological test methods and their consistent findings has conferred scientific credibility on such patient reports 1, 2,,3, 4. The proportion of treated patients who may suffer neural damage due to chemotherapy is unknown, but longitudinal imaging studies on breast cancer patients treated with chemotherapy have indicated that white matter changes in the Central Nervous System are detectable in up to 70% of patients 5. Other imaging studies also have shown enduring deficits. For example, data based on PET scans 6 showed altered activity in frontal cortex, cerebellum, and basal ganglia in breast cancer survivors 5–10 years after treatment. And inagaki et al7 found, by MRI, diminished volumes of gray and white matter in treated survivors one year after adjuvant chemotherapy for breast cancer.
Oncologists were not surprised to find that patients undergoing the rigors of chemotherapy experienced a multitude of side effects. Given the biological potency of these chemicals, and the awareness that they damaged tissues other than those targeted by therapy, it seemed reasonable that patients would present a variety of complaints, some of which might be correlated with biological indicators such as anemia. Cognitive impairment might be seen as a relatively minor, vague, and reversible component of such effects, as would fatigue and anxiety. How was the clinician expected to weigh such elusive functional deficits against the prospect that chemotherapy may prolong the patient’s life? It was only after the launching of studies based on established neuropsychological tests that the extent and nature of cognitive impairment gained appreciation. These studies also indicated that such adverse effects continued long after therapy ended.
These newer findings are leading oncologists to consider more seriously the full extent of neurotoxic complications stemming from chemotherapy. Schiff and Wen 8 communicated their views in this way: “The CNS is an organ with a unique profile of vulnerability to antineoplastic treatments. In many cases, CNS neurotoxicity is the dose-limiting side effect of treatment for systemic and CNS neoplasms. Novel methods of delivering radiation and chemotherapy agents have led to recognition of new forms of CNS neurotoxicity.”
Moreover, cancer has become a chronic illness, and the number of long-term cancer survivors with neurobehavioral deficits will continue to increase. Cognitive impairment, furthermore, is only one component of chemotherapy-induced neurotoxicity, whose scope also embraces sensory systems (vision, somesthesis, audition, taste and smell), motor function (strength, endurance, coordination), and mood. Often, by the time neurotoxicity is apparent clinically, it has advanced to an irreversible stage. Sensitive tests can detect incipient impairment and forestall more serious conditions, but, especially for new drugs or drug regimens, oncologists do not know what to look for, and may fail to detect the early, emerging indications of neurotoxicity. And, as some commentators have noted, the anxieties and health effects themselves provoked by cancer make it difficult to disentangle them from the neurotoxic effects of chemotherapy. Contrast this with the situation oncologists are familiar with in the case of anthracyclines, which present the risk of cardiac damage. Detection of cardiac damage at the point of imminent heart failure is too late to impede progression of the disease. Therefore, in an attempt to prevent anthracycline-induced cardiomyopathy, a number of surveillance methods have been used to try to detect problems at an earlier stage of chemotherapy. An equivalent rationale should be applied to neurotoxicity. Duffner 9 views this as an urgent need, noting that the mass of evidence indicating brain damage arising from chemotherapy is a “wake-up call to neuro-oncologists.”
One reason for an emphasis on early detection is new information about how certain chemotherapy drugs act on the nervous system. A pioneering paper 10 revealed that the neurotoxic potency of three common chemotherapeutic drugs (carmustine (BCNU)), cisplatin, and cytosine arabinoside (cytarabine) equaled or exceeded their potency as anti-tumor agents. When applied to cultured cells at what were calculated to be clinically relevant exposure levels, they proved more toxic for the progenitor cells of the CNS and for nondividing oligodendrocytes than for the cancer cell lines studied. When administered systemically in mice, these agents were also associated with increased cell death and decreased cell division in the subventricular zone, in the dentate gyrus of the hippocampus, and in the corpus callosum. Some of these effects persisted for weeks after drug administration ended. As they noted, “Our studies have multiple implications for future strategies of cancer treatment … it seems that [doses of] chemotherapeutic agents sufficient to harm cancer cells may also damage many cell populations of the CNS … It is also possible, however, that our results actually understate the extent of damage that occurs in association with chemotherapy.”
These startling results underscore how little we really know about the neurotoxic consequences of cancer chemotherapy, a point emphasized by Noble et al (in press) in their review of chemotherapy-induced neurotoxicity. In fact, they point out that such effects are so widespread, because of the numbers of treated patients that, in essence, they are equivalent in scope to a major neurological disease. In support of their contention, they cite the breadth of data we now possess about the underlying pathological processes.
At this point, the scientific position of chemotherapy-induced neurotoxicity in oncology stands at about where environmental neurotoxicity stood over three decades ago 11. Since then, it has generated a torrent of books, articles, and conferences. It has turned environmental neurotoxicology into a science with multiple dimensions ranging from molecular mechanisms to animal models to epidemiology, all of which are waiting, as it were, to be applied to oncology. Questions about environmental chemicals have also enlisted both clinical neurology and neuroscience in determining the health risks posed by exposures.
Why haven’t more features of this established scientific technology been applied to the neurotoxic risks of cancer chemotherapy? Shouldn’t it be even more important now than in the past to adopt the most effective and precise scientific practices for the evaluation, prediction, and prevention of neurotoxic outcomes? Wefel et al12 have presented a cogent argument for such adoption: “Cancer is becoming a chronic illness, requiring on-going symptom assessment and intervention. The number of long-term cancer survivors will continue to increase as will the number of survivors with neurocognitive and/or neurobehavioural impairment.”
Two Contrasting Views of Neurotoxicity
Environmental Neurotoxicology was propelled by legislation and regulation. Although the Toxic Substances Control Act (TSCA) was finally signed into law in 1976, its roots lay in the growing recognition that we were being exposed to thousands of synthetic chemicals as well as to industrial sources of metals that could threaten public health. The Council on Environmental Quality (CEQ) had issued a statement of concern in 1971: “The environmental effects of most of the substances discussed in this report are not well understood. Testing has largely been confined to their acute effects, and knowledge of the chronic, long-term effects, such as genetic mutation, is inadequate. Although far from complete, available data indicate the potential or actual danger of a number of these substances.” And even earlier, The Federal Insecticide, Fungicide and Rodenticide Act (FIFRA), passed in 1947, had governed the regulation of pesticides in the United States, a responsibiliy enlarged by the 1988 amendments that required pesticide reregistration and that prescribed a Scientific Advisory Panel to oversee the process, particularly from the standpoint of safety. Although both acts require that regulations weigh economic and other benefits against health risks, the latter demanded a process by which those risks could be quantified. Once quantified, exposure standards could then be prescribed that offered a stated degree of risk. Typically, because exposures to environmental chemicals offer no health benefits, the health risks assume priority and exposure standards are sought that offer a robust margin of safety.
Oncologists face a contrasting situation and history. In their universe, the sources of the health risks lie in the cancer itself. Therapy is administered to eliminate or arrest the cancer. Dose is determined by therapeutic effectiveness, and side effects play a secondary role. One constellation of side effects, however, neurotoxicity, has proven to be especially troublesome. The reasons are not difficult to grasp. Subtle cognitive problems, such as memory loss, are often subjective and not easily evaluated in a clinical setting. How is the clinician expected to weigh such elusive functional deficits against the prospect that chemotherapy may prolong the patient’s life? But what if, as noted by Dietrich et al 10 and emphasized by Ahles and Saykin3, the neurotoxic potency of certain treatment options exceeds their anti-tumor potency?
An appraisal of the current literature on chemotherapy-induced neurotoxicity reveals that it is guided primarily by an unstructured, informal clinical approach to some form of neurotoxic risk assessment. The term risk assessment generally denotes an approach that seeks early, or low-dose indices of adverse effects in an effort to to prescribe exposure standards with a high enough margin of safety to escape even minimal effects. In this form, it is not applicable to chemotherapy. Under a less constrained definition, however, it would describe a process in which detection of adverse effects, by sensitive methods, would lead to a re-evaluation of a patient’s regimen.
In practice, clinical oncologists become aware, sometimes because of patient complaints, that certain courses of treatment are inducing some form of neurotoxicity; for example, trouble hearing. Or, investigators pursuing research on chemotherapeutic actions and effectiveness uncover clinically significant neurotoxic effects. They may then ask about the scope and character of such effects, but not in a quantitative sense. Generally, they do not engage in a prolonged or extensive search for the time or dose levels at which adverse effects begin to emerge, nor for how long after the course of treatment they persist. Although these are crucial questions for evaluating patient quality of life and the benefit-risk balance, and can be determined if the proper instruments are applied, they still mostly remain as background issues.
This chapter describes how the kinds of standards, methods, and approaches that have informed progress in environmental neurotoxicology can lead to procedures and techniques that could be applicable, with modification, to the ways in which we evaluate neurotoxic potential and outcomes stemming from chemotherapy. In essence, neurotoxicity assessment can be seen to include three functions. One is simply to insure clinical awareness of the patient’s state. Another is to conduct what in environmental tox would be a risk-benefit analysis. Third is to build a database. Here, we would use advanced assessment techniques, especially for sensory and motor function that lie outside the scope of conventional neuropsychological tests.
In parallel, especially for exploring new therapies, it is crucial that they be evaluated in animal models for neurotoxic potential before they are applied to patients. Although new drugs follow a series of tests for adverse effects before they are administered to humans, the kinds of neurotoxicity of concern to oncologists are not specifically included. A model for such assessments will be described in this chapter.
Dimensions of Neurotoxicity
Cognitive impairment is only one component of neurotoxicity, whose expression also embraces sensory systems, motor function, and mood and personality disorders. Sensory system damage and dysfunction arising from chemotherapy have been noted for vision, somesthesis, audition, and olfaction. Generally, when reported, they have advanced to a clinically detectable stage, and have not been studied to determine at what point function begins to show evidence of impairment. Motor function, except for weakness, has received even less attention. The main lesson we have learned from research on cognitive function is one that neurotoxicolgists learned long ago in their studies of exposed populations such as workers. Namely, that even during the stage of what might be called silent or incipient neurotoxicity, before patients became aware of deficient function, sensitive neurobehavioral tests would have detected impairment and provided clinicians with information that might have forestalled more serious conditions.
Lessons Learned from Studies of Cognitive Dysfunction
Investigations of cognitive dysfunction in chemotherapy were not the product of attempts to set exposure standards, or of the appearance of overt neurotoxic signs such as seizures but, instead, complaints by patients. These complaints drove chemotherapy research in an unaccustomed direction; namely, validation of subjective adverse effects. It is informative to review this history.
Oncologists were not surprised to find that patients undergoing the rigors of chemotherapy experienced a multitude of side effects. Given the biological potency of these chemicals, and the awareness that they damaged tissues other than those targeted by therapy, it seemed reasonable that patients would present a variety of complaints, some of which might be correlated with biological indicators such as anemia. Cognitive impairment might be seen as a relatively minor, vague, and reversible component of such effects, as would fatigue and anxiety. It was only after the launching of studies based on established neuropsychological tests that the extent and nature of cognitive impairment gained appreciation. These studies also indicated that such adverse effects continued long after therapy ended.
Several reviews of these findings have now appeared 1, 2, 3 Although many of the studies reviewed were based on small samples, and although in total they reflect some inconsistencies, the weight of evidence points to effects that in many patients persist for years beyond the termination of treatment. The reviews also agree on the importance of longitudinal prospective studies, on the need for more research on potential mechanisms, on the need for more standardization and perhaps greater breadth of neuropsychological tools and approaches, and the critical role of animal studies to clarify both the scope and mechanisms of impairment.
This literature, although firmly establishing the objective basis of patient reports, is still largely confined to the narrow question of cognitive dysfunction. This chapter maintains that oncologists and cancer researchers should enlarge their view of what constitutes neurotoxicity and how to measure and investigate it. I will adopt, as a means of framing my argument, the approach that would be relied on were chemotherapy viewed as equivalent to an environmental exposure. To do so I will discuss tools and approaches that can be used to trace the status of neurotoxic responses during and after a course of treatment. Optimally, these tools would be employed before chemotherapy begins and would be used to monitor patients on specified occasions during the course of chemotherapy and for some period afterward. Predictive assessments based on animal models will be discussed also.
Cognitive function approaches
Many of the earliest attempts to assess neurotoxicity in humans adopted procedures that had been developed for clinical neuropsychological testing. Such procedures often proved poorly designed for research in neurotoxicology because they evolved as diagnostic instruments, not as tools with which to screen populations or for experimental investigations. They typically were used to provide a functional profile of a patient, often one who had suffered brain damage. For example, they were designed to evaluate stroke patients, or those suffering from disorders such as schizophrenia. They had not been contrived to determine, for example, whether workers exposed to pesticides differed from controls on various psychological dimensions, or to yield a dose-response function for acute exposures relating concentration to performance. They most certainly were not devised to trace the development of adverse effects during a period of exposure to a potentially neurotoxic agent. Nevertheless, lacking more appropriate tools, they were invoked to respond to some pressing questions about exposed populations. The pioneering reports from the Finnish Institute of Occupational Health13 relied heavily on clinical instruments. Neurotoxicology, however, also borrowed techniques from the experimental psychology laboratory. Such techniques lacked the standardization and norms provided by most clinical tests, but offered the virtue of greater specificity, flexibility, and a scientific basis.
The currrent literature on cognitive impairment arising from chemotherapy is almost exclusively based on neuropsychological tests designed to assess a stable and enduring condition. Such tests are not equivalent to the tools required to assess patients repeatedly during treatment to determine whether, and to what degree, they are impaired. The necessary tools, especially for measurement of cognitive function, have different properties. They measure performance.
Performance tests differ from conventional clinical tests in several respects14. Tests devised for clinical applications aim to differentiate between individuals, and to offer or substantiate a diagnosis. Performance tests are designed to differentiate among stressors such as drugs, toxic chemicals, and conditions such as sleep deprivation. Clinical tests should be relatively insensitive to environmental perturbations because they should serve to identify stable traits in the individual, but performance tests are expressly designed to reflect such perturbations. Finally, clinical tests generally are meant to be given only once.
In contrast, performance tests should be capable of repeated administration, as in monitoring changing response patterns over an experimental session, or in overseeing the status of workers in a particular environment where they are exposed chronically to presumed or suspected neurotoxicants and where they undergo repeated assessment. There is now a robust literature describing the kinds of instruments that show promise as assays of nervous system function for monitoring patients undergoing chemotherapy or in following the progression of neurodegenerative disorders such as Alzheimer disease and Parkinson disease. Chemobrain assessments, either for research or for patient evaluations, should be based on performance tests. The primary question in both instances is how function changes over time.
Moreover, the time taken for evaluation may be limited, especially in the workplace, so that a compact but comprehensive test battery is more suitable than the typical, largely paper and pencil tests administered by clinical neuropsychologists. Responses on paper and pencil tasks also have to be scored and transcribed, leading to transcription errors and rescoring.
Faced with the need to assess specified populations exposed to defined hazards, or to evaluate particular stressors experimentally, neurotoxicology turned to the development and adoption of computerized testing. It made the mechanics of testing more efficient; it offered considerably more uniformity in how test stimuli were presented; it made it possible to test several subjects simultaneously; it could use testers who did not require advanced clinical training; it could automate scoring and analyis; it allowed remote testing (as in an exposure chamber); and it proved adaptable for translation of procedures used in the animal laboratory. Perhaps most important of all, it moved human testing from clinical diagnosis to the realm of performance.
Slikker et al15 offer a comprehensive discussion of the properties and usefulness of computerized test batteries and how they reflect and extend traditional approaches. Several current batteries have been used widely enough, and are well-enough established, to be considered as appropriate instruments for neurotoxicology. The CANTAB16 consists of a suite of computerized tests, now numbering 22, that embrace a variety of cognitive functions: visual memory, executive function, working memory, semantic and verbal memory, attention, decision making, and response control (designed to assess behaviours such as impulsivity). Most of the tests are explicity designed to be independent of language and culture. Alternate forms are available for repeated testing. The CANTAB has been used extensively in patients with Alzheimer and Parkinson disease. The BARS (Behavioural Assessment and Research System) battery is specifically designed for the detection of neurotoxicity in populations with limited education or literacy.17 It too can be used for repeated assessments.
One of the newer features of computer-based testing is the incorporation of instructional materials. Particularly because of the variety of populations that undergo assessment for neurobehavioural function, including those unfamiliar with testing procedures and that are often illiterate, more effective means for communicating test instructions have been sought by investigators. The computer itself is a tool that can be adapted for such a purpose. Rohlman et al18 in response to such a need, use computer graphics for the BARS battery to teach subjects how to perform the tests before the test items themselves are presented. The technique relies on a sequence of approximations to the final performance, much like the technique, called shaping, used to train animals on schedule-controlled operant behaviour.
A useful illustration of current technology for neurobehavioral testing is the Cambridge Neuropsychological Test Automated Battery (CANTAB). The CANTAB is a computer administered battery consisting of 14 individual neuropsychological tests (see table). The subtests are designed to measure cognitive abilities reliant on frontal/subcortical circuits and has been used extensively in research on these abilities in non-human primates and in humans with Parkinson and Huntington disease. Included in the CANTAB battery are measures of working memory and cognitive flexibility. Performance on these CANTAB subtests is sensitive to early deficits in un-medicated PD patients. The CANTAB tests have also been used in studies of toxic and metabolic disorders, effects of substance abuse, and evaluation of neurotransmitter modulation in normal controls and disease. The CANTAB has been used extensively in patients with Alzheimer and Parkinson diseases19. The CANTAB is well suited for use in neurotoxicology.16
TABLE 1.
CANTAB Subtests and Abilities Assessed
| CANTAB Subtest | Ability assessed |
|---|---|
| Motor Screening | Visual, movement, and comprehension difficulties |
| Big/Little Circle | Concept formation, learning and reversal |
| Delayed Match to Sample | Immediate and delayed perceptual matching |
| Intra-Extra Dimensional Set Shifting |
Rule acquisition and cognitive flexibility |
| .Matching to Sample Visual Search | Ability to match visual samples and measures reaction and movement time |
| Paired Associates Learning | Episodic memory and learning |
| Pattern Recognition Memory | Recognition memory for patterns |
| Reaction Time | Speed of manual response |
| Rapid Visual Information Processing |
Sustained visual attention |
| Stockings of Cambridge | Spatial planning and motor control |
| Spatial Recognition Memory | Recognition memory for spatial locations |
| Spatial Span | Working memory capacity |
| Spatial Working Memory | Working memory and strategy use |
| Verbal Recognition Memory | Immediate free recall and immediate and delayed recognition memory |
The CANTAB tests have excellent face validity for the constructs measured. Each test was developed from established animal behavior paradigms and validated in patients with damage in specific areas of the brain including frontal and temporal lobe and basal ganglia. In addition, many of the sub-tests (Spatial Span, Spatial Working Memory, IDED Set Shifting; Rapid Visual Information Processing; Paired Associative Learning) have been studied with functional neuroimaging to provide confirmation of the neuro-anatomical substrates supporting each test.
Sensory Function
Vision
Visual system toxicity induced by anticancer chemotherapy is not uncommon and has been recognized from the beginning. A statement by Schmid et al,20 however, points up the discrepancies between what constitutes neurotoxicity by clinical criteria and the criteria that would be used in environmental risk assessment (my italics):
“Many ophthalmic complications have been reported for these new cytotoxic chemotherapeutics, some of which are reversible if detected early enough… At first, many of these ocular toxicities are hardly detected…However, these side effects may turn out to be irreversible by the time the symptoms are recognized.” Among the functional complaints listed by Schmid et al (2006), which could be classified as early indications of potential damage, are blurred vision, decreased color vision, diminished visual acuity, diplopia, night blindness, photopsia, and photophobia. As they note, “The possible reversal of some of these side effects, if discovered in time, emphasizes the need for clinicians to be aware of these ocular reactions and suggests an immediate consultation with an ophthalmologist.”
Is referral for consultation, after a patient complains, an adequate response? Both vision scientists, and neurotoxicologists who employ measures of visual function as an index of adverse effects, would not see it as adequate. Tamoxifen offers an instructive example. Eisner and Incognito 21 undertook a comparison of two groups of middle-aged women 40–69 years of age, as a follow-up to previous work on color vision abnormalities and chemotherapy. One group had been using tamoxifen, both as adjuvant therapy after successful treatment for early-stage breast cancer. They comprised two subgroups, one on medication for over two years, the other treated for less than two years. The controls were not using any hormonally-acting drugs. Relying on a color-naming psychophysical procedure, they fouhd that tamoxifen treatment produced a tendency to label test stimuli of 440 nm, typically called “lavender,” as “white.” This is the kind of subtle functional change that tends to precede clinically evident toxicity.
Although the precise control of wavelength by instrumentation used by Eisner and Incognito21 would be confined to only a few institutions, other means for measuring color discrimination are available. The Farnsworth Munsell 100 Hue Test presents the patient with four trays containing a total of 85 removable color reference caps spanning the visible spectrum in small increments of hue. Color vision abnormalities are assessed by the ability of the patient to arrange the color caps in order of hue. A briefer version, using only 15 color tiles (the FM D-15) is also used, while another brief version, the Lanthony D-15, uses desaturated colors to separate “normal” color perception from the kind of subtle color deficiency that may accompany workplace exposures to substances such as organic solvents 22
In some reports, blurring of vision has been noted as a patient complaint or observation, but not followed up with appropriate tests. The typical Snellen eye chart used to measure visual acuity presents the patient with a high-contrast target, namely, black letters on a white background.
Contrast, however, is an important visual parameter because when we direct our vision to a scene, objects and their surroundings vary in contrast. This kind of pattern vision is explored by vision scientists by displaying what in essence are alternating dark and light bars, or gratings, that are characterized mathematically by their width (or spatial frequency). Charts containing gratings of varying spatial frequency, contrast, and orientation can be used to assess contrast sensitivity and are commercially available. In addition, simpler charts are available that have transformed these parameters into a display of letters on a background. Blurring represents a loss of contrast sensitivity. The tools noted above for assessing contrast sensitivity have shown effects from exposure to chemicals such as methylmercury, acrylamide, and volatile organic solvents. They have also detected visual system impairment in patients with Parkinson disease and multiple sclerosis. They can be used as a relatively quick and simple assay for incipient visual dysfunction of the kind that, unlike conventional visual acuity measures, cannot be corrected with glasses.
Portable charts for this purpose are available. The Pelli-Robson and Mars tests use a single large letter size with contrast varying across groups of letters 23. The Pelli-Robson chart uses letters (6 per line), arranged in groups whose contrast varies from high to low. The Mars test is similar. A more elaborate test, the Functional Acuity Contrast Test uses sine-wave gratings, the standard for vision research, mounted on a chart. It was used by Schreiber et al24
The National Eye Institute (NEI) has devised a questionnaire that can be used for screening. The Visual Functioning Questionnaire – 25 (VFQ-25) can be obtained from the NEI web site. Its core defect is that the more subtle indications of early-stage visual dysfunction escape subjective assessment and detection.
Hearing
The auditory system is vulnerable to many chemical exposures. Drugs such as the aminoglycosides and workplace compounds are examples. Among chemotherapeutic agents, cisplatin is notorious for its ototoxicity. Perhaps as many as 40% of patients report hearing difficulties. As always, because it is so effective a drug against conditions such as testicular cancer, oncologists are reluctant to reduce dosage even when hearing tests indicate that the patient is suffering auditory system damage. Dosage reduction, however, may not be the only alternative. Rademaker-Lakhai et al25 carried out an audiometric study comparing different dosing schedules of cisplatin. They found that hearing impairment was more severe for the schedule administered the every 2 weeks versus every week when the dose levels with the same dose-intensity were compared. If dosing schedule can be altered without reducing the effectiveness of chemotherapy, then patients can benefit if audition is evaluated during the course of therapy and treatment protocols changed to reduce toxicity.
Such flexibility depends on access to audiometric facilities. For those clinical settings wishing to monitor hearing function, it is vital to note that some superficially simple procedures may provide misleading results. A core problem with ototoxicity in chemotherapy is that the National Cancer Institute’s reporting system, Common Terminology Criteria for Adverse Events, or CTCAE does not consider high-frequency hearing loss (above say, 8,000 Hz). Such losses are the first indication of auditory system damage. The frequencies important for vocal communication are significantly lower, so that ordinary patient interviews, such as the Hearing Handicap Inventory in the Elderly, from the Surgeon General. Conventional audiograms will fail to detect the early signs of hearing loss because they typically do not assay frequencies above 4,000 Hz. Tests beyond conventional audiometry such as otoacoustic emissions (OAEs) and evoked potentials (e.g., brainstem auditory evoked responses, or BAERs) make it possible to detect auditory damage at an early stage.
Somatosensory function
Some observers contend that the most disabling form of chemotherapy-induced neurotoxicity is peripheral neuropathy. Cavaletti et al26 noted that it could be the side effect of treatment most likely to elicit a reduction of dose. Postma et al27, relying on a questionnaire survey, believe that the incidence may be as high as 100%. Rating scales, such as the Total Neuropathy Scale (TNS), are useful for assessing symptoms, but their ability to quantify dysfunction is limited. At the same time, the instruments available for quantification have their own limitations. The vibrating probes used by devices such as the Bioesthesiometer deform the skin according to the amount of pressure exerted by the tester, so that crucial variable is essentially uncontrolled. Maurrisen and Weiss28 describe the problems with instruments of that design.
Tactile sensitivity can be addressed by fairly simple devices, however, provided the procedures are conducted according to established psychophysical principles. Examples can be seen in Tremblay et al 29, who directed their study at how age affects tactile sensitivity. The authors used three different tests to measure sensitivity in the right index finger. One was used to determine pressure sensitivity. Skin indentations were produced by applying a set of Semmes–Weinstein nylon monofilaments to the finger. The actual force is scaled approximately logarithmically in mg (but psychophysically it provides a linear scale of perceived intensity). Each filament was applied to the finger in a sequence of increasing perceptual difficulty for one second. However, each trial consissted of a temporal forced-choice decision in which subjects were presented with two time periods, one containing the stimulus (monofilament applied) and one containing no stimulus. Subjects were asked during which period the stimulus was applied. Sensitivity thresholds were calculated by determining which monofilament gave the lowest buckling force at a detection rate of 75%.
Spatial acuity was tested by measuring gap detection. A series of 14 small sqare-shaped blocks made of high-density Styrofoam were precision milled so that one of the sides contained a gap of specific dimensions while the other side was left intact. The subjects were asked to report which side of the block contained the gap when the experimenter pressed the block against the finger. By using a range of gap widths, and a two-alternative forced-choice procedure, the investigtors were able to calculate a gap threshold.The third test, thickness discrimination, consisted of presenting the subject with a set of 12 square Styrofoa plates of differing thickness grasped between the thumb and forefinger. As in the other tests, a standard, 5 mm thickness was compared with a different plate in a two-alternative forced-choice procedure.
For all three procedures, the younger group of subjects (mean age 23 years) were markedly more sensitive than the older group (mean age 70 years). On the basis of these differences, these procedures should prove useful for assessing losses of mechanoreceptor sensitivity due to peripheral neuropathy. Measures of two-point threshold, often determined with calipers, could also prove useful, but the variability introduced by examiner differences in applied pressure can be problematic.
Olfactory Discrimination
Diminished smell acuity is widely recognized as an accompaniment of chemotherapy.30 A simple way to test olfactory function, used in studies of Parkinson disease and Alzheimer disease as well as for workers 31 makes use of the University of Pennsylvania Smell Identification Test (UPSIT). It is a 40-item test and consists of 40 odorants in 4 booklets containing microencapsulated odorants that are released by scratching standardized odor-impregnated test booklets. The score is number of errors. It is the most widely used instrument for assessing smell loss, and has become the standard for such assessments.
Motor function
Most comprehensive neuropsychological test batteries used in environmental neurotoxicology, particularly those based on computer presentation, include some form of motor function assessment. Because cancer patients undergoing chemotherapy frequently report loss of strength, slowing of movement and reactions, and problems with coordination, motor function testing would be an essential component of any test battery aimed at monitoring adverse neurobehavioral effects during and after treatment.
Finger-tapping rate is a common measure. It requires the subject to tap a specific key on the keyboard as rapidly as possible in a 30-second period and has been used in studies of mercury vapor 32 and manganese exposure. The BARS test battery uses a special, simplified keyboard for this purpose.18 The Grooved Pegboard consists of a small board containing a 5×5 set of slotted holes angled in different directions and 25 pegs with a ridge along one side, requiring the peg to be rotated into position for correct insertion. This is a test of fine manipulative dexterity and motor speed. The completion time in seconds is recorded for each hand. It has been used in studies of lead neurotoxicity 33 and mercury vapor 32. More advanced assessment methods are also available; they were designed for situations in which the predominant questions arose from motor effects. For example, Wastensson et al 34 employed a system that measured the speed of rapid alternating pointing movements between two targets and one used to quantify the performance of rapid alternating movements of the forearms.
Animal Models
Purpose of animal models
In their reviews of cognitive dysfunction associated with chemotherapy, Tannock et al 35 and Ahles and Saykin 3, among others, emphasized the need for animal models both to identify the scope of possible adverse responses and to relate them to mechanistic measures. Hardly more than a handful of current publications have attempted to address such questions. Examples include: Lee et al,36 (young and old female rats administered 5-FU or cyclophosphamide and studied with the Morris maze or Stone maze); Seigers et al,37 (rats administered methotrexate and studied with Morris maze and novel object recognition tasks); Foley et al,38 (mice treated with either methotrexate or 5-FU, studied for lever-press acquisition); Konat et al, 39 (combination of adriamycin and cyclophosphamide in rats, and studied with passive avoidance); Mustafa et al,40 (rats administered 5-FU and studied with object location recognition); Winocur et al, 41 (mice administered a combination of methotrexate and 5-FU and tested with different Morris maze tasks). Although such studies have provided much useful data, overall they lack cogency as models for clinical extrapolation for four reasons: first, they tend to rely on methods that typically are applied only once, while chemotherapy regimens generally administer drugs as a series of treatments or cycles. The basic need is for methods capable of monitoring the entire course of treatment as well as the persistence of neurotoxic effects following treatment. Second, most tend to study only a single endpoint while adverse effects in the clinic include multiple endpoints. Third, some typically assess only single drugs, while clinical practice dictates drug combinations. And, If they study combinations, they rarely assay the individual components in depth. Fourth, they offer rather limited dose-response information, tending to choose a single dose or dose combination on the basis of other toxicity information, previous literature, clinical values, etc. Dose-response information provides a basis for mechanistic exploration.
Animal models are needed that are capable of tracing the onset, time course, and persistence of neurotoxicity--the key clinical questions.
Procedures
Appropriate procedures would be built around endpoints that are assessed repeatedly during courses of treatment designed to mimic clinical practice. For example, they might compare a widely-used drug combination with its components. And, following the scheme by which environmental chemical exposure standards are derived, they would explore dose-response functions.
The ultimate aim of animal models would be to lay the foundation for preclinical assessments capable of predicting the neurotoxic profile of various chemotherapy regimens. Such tools, ulimately, would have the potential to be translated into a comprehensive test battery for monitoring patients. The parallel aim would be to provide a test bed, so to speak, for mechanistic research such as that of Dietrich et al10 and Han et al.42
In essence, then, animal models would begin to initiate the development of a suite of preclinical assessments that (1) can be used to predict the neurobehavioral outcomes of individual chemotherapy agents and of multi-drug chemotherapy regimens; (2) can be used in situations requiring reliable, efficient screening for new treatment regimens; (3) can be translated into procedures for monitoring patients; (4) can be used to predict or monitor the usefulness of countermeasures aimed at reducing the neurotoxic effects of chemotherapy. Preclinical assessments would be especially useful in this latter context because cancer treatments are almost never given at the optimal dosages or schedules to kill cancer cells. Instead, treatment choices tend to be governed by the need to limit toxicity, which often takes the form of neurotoxicity.
Choice of doses
Identifying neurotoxicity is not a challenging problem. Even the crudest observational screens are capable of doing so. Useful animal models would include, at some stage treatment protocols congruent with clinical practice. In particular, they would build on the fact that chemotherapy is typically administered for several courses in a series of cycles, with each period of treatment followed by a rest period. Furthermore, because it has been recognized for nearly 30 years that adjuvant polychemotherapy is superior to single-agent strategies (cf., 43, they would assess combinations as well as single agent regimens. For example, a widely-used combination given for adjuvant breast cancer therapy is CMF, or the combination of cyclophosphamide, methotrexate, and 5-fluorouracil. As noted by McArthur and Hudis 43 it is a particularly reasonable option for patients who have lower-risk tumors and It is also an attractive combination for evaluating animal models because it has been shown to produce cognitive impairment in about 50% of treated patients. 44, 45, 46
Only after many environmental neurotoxicants had been studied individually (e.g., lead, methylmercury, PCBs) did investigators begin to consider animal models for the assessment of mixtures. In the current literature on animal models for cancer drugs, when combinations are studied, disentangling the contributions of the individual components to the effects of polychemotherapy regimens is rarely attempted even though it would offer oncologists some basis for decisions about balancing therapeutic effectiveness versus toxicity. A related problem is the lack of dose-response information. Dose-response methodology is critical for setting environmental exposure standards to protect public health. For chemotherapeutic drugs, the aim would be to correlate dose with the incidence and characteristics of adverse effects. Such properties would need to be determined before mixture studies are attempted.
The importance of dose-response information is underlined by the significant proportions of patients who experience effects such as nausea, vomiting, stomatitis, constipation, fatigue and other adverse symptoms. Because these are also the doses associated with impaired cognitive function, one approach to designing a useful animal model would be to use them as the anchors for dose-response calculations. For example, the clinical doses, in the form of conversion to doses for a rat model, might be considered the baseline (100%) doses. Doses equivalent to 50% and 25% of the clinical dose as well as the control vehicle could then be used to choose an appropriate range of doses. Such a strategy might be used to disentangle side effects, such as nausea, from performance effects on neurobehavioral tests and to obtain less confounded, “purer,” measures of neurotoxicity. Equally important, a dose-response function provides a basis for exploring the relationship between mechanistic measures and their expression in behavior.
As noted earlier, one defining feature of chemotherapy is treatment schedule. Treatments are generally given in cycles, with periods of recovery between treatments. Protocols for evaluating neurotoxicity have to take this feature into account when they are being designed. That is, they must be capable of application at least during the periods between treatments as well as for some duration of time after the course of treatment has ended so as to capture the kind of persistent, lingering effects seen in some patients and documented in rodent studies such as that of Han et al. 42
Choice of endpoints
An example of a protocol focused on cognitive performance provides an approach that would prove useful for other kinds of neurotoxicity such as those discussed by Weiss.47 Cognitive complaints by patients were the main incentives for research into the more subtle neurotoxic manifestations of chemotherapy and remain so today.
Schedule-controlled operant behavior
Stable behavioral baselines are required for any scheme aimed at monitoring adverse neurotoxic effects during the course of treatment. Schedule-controlled operant behavior is ideally suited for this role. It is widely used in psychopharmacology because it can be used to compare different drugs and acute doses against a stable criterion that allows repeated testing over extended periods of time (e.g., 48). It is used extensively in environmental neurotoxicology because it can be used to trace changes over time with chronic exposure (e.g., 49 and aging 50).
A typical experimental setting is a standard operant chamber with two levers and a device for delivering food pellets (Figure 1). A prototypical situation is one in which a rat, by depressing one of the levers mounted on the front panel, can trigger the release of a small food pellet. The food pellet is termed a reinforcer and the process is termed reinforcement. The rat's responses produce food delivery according to the contingencies, or schedule, programmed by the experimenter. Typically, rats, say, are maintained at about 80% of free-feeding weight so that they will perform specified behaviors rewarded by pellet deliveries.
Figure 1.
Standard operant chamber containing response levers, feeder (behind panel), and stimulus lights
We use the term operant to refer to learned or acquired behavior that is controlled by its consequences. Most complex human behavior falls into this niche. The term, schedule-controlled, refers to the way in which experimenters define the relationship between a specified response by the organism and the effects of that response. The term schedule describes the relationship between the behavior and its consequences. For example, a fixed-ratio schedule of reinforcement defines a situation in which a specified number of responses, such as lever presses, is required for delivery of a food pellet reward.
Schedule contingencies come in many varieties. Some are based primarily on time. Interval schedules specify relationships between elapsed time and the availability of reinforcement. A fixed-interval schedule might specify that the first response 5 minutes since the last reinforcement will produce the next reinforcement (FI 5). Another way to construct a schedule based on elapsed time is to specify the interval between successive responses; a Differential Reinforcement of Low Rate schedule might require a minimum of 20 secs between responses (DRL 20) for reinforcement. Response number, in the form of ratio schedules, is another widely-used performance criterion. A fixed-ratio schedule might require 100 responses (FR 100) for reinforcement delivery.
The primary virtue of schedule-controlled operant behaviour is its flexibility. It can be used to study rate of responding during steady-state behaviour, or the acquisition of new behaviour against a background of stable behaviour, or the ability to distinguish related visual stimuli, or the speed of responding to a stimulus, or the accuracy and other characteristics of motor control.
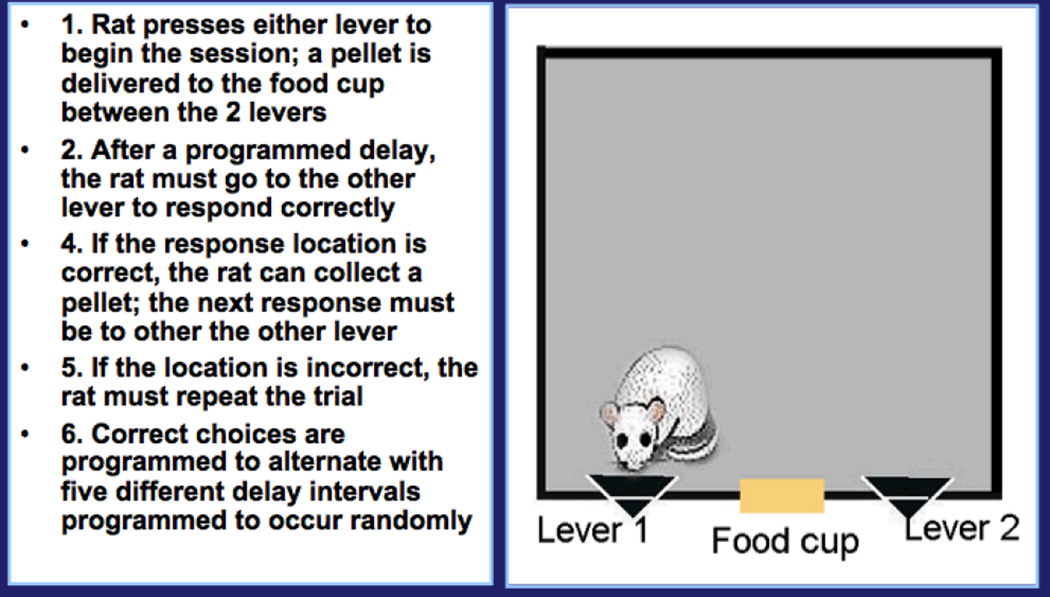
One operant procedure that would serves as a useful example for such a project provides a measure of working memory and is termed Delayed Spatial Alternation. Of all the complaints registered by chemotherapy patients, memory difficulties seem to be among the most frequent and distressing (cf., 51, 47). With this procedure, the rats are tested with a procedure depicted in Figure 2 (e.g., 49, 52, 53). Here, the pellet rewards are delivered for pressing the lever (right or left) opposite the one that previously was designated as the correct one. That is, the correct lever alternates between sides. The memory component is assessed by interposing delays between choices, so that the rat has to remember which was correct on the previous choice. The delays will vary between 0.5 and 12.0 seconds; typically, the longer the delay, the less the accuracy. All delays are sampled during a 45-minute test session. Stable performance is typically achieved by 60 training sessions (12 weeks). With stable performance in place, we can then trace how it varies over the course of treatment; that is, the immediate after-effects of treatment, how much recovery occurs between treatments, and how much impairment (if any) persists beyond that point.
Figure 2.
Schematic for Delayed Spatial Alternation.
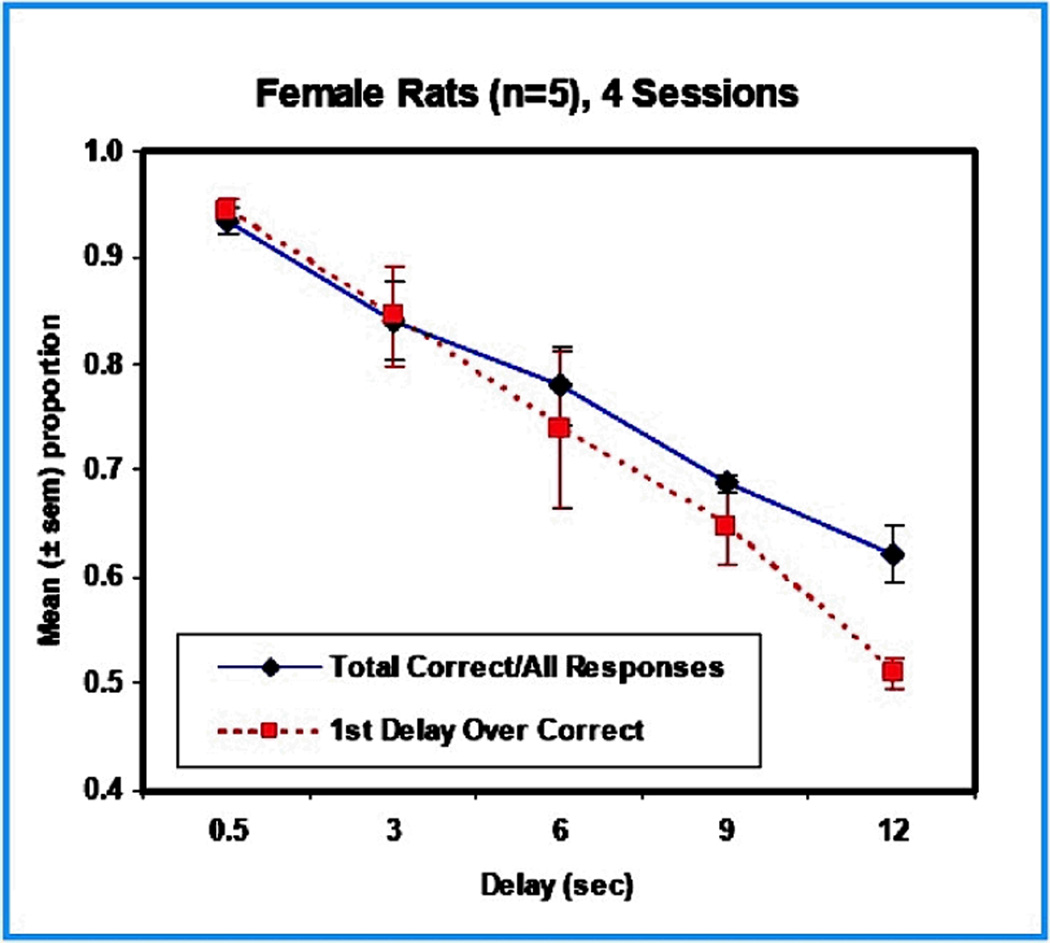
Five different delays are presented within the same session. Generally speaking, the longer the delay, the more difficult it is to remember, with the result being a within session function showing more criterion responding at shorter delays than longer ones. Drugs that interfere with memory will shift the function, but overall responding itself will provide a confirmation of food motivation. Figure 3 presents the results of a study in female rats tested, after preliminary training, with delay values of 0.5, 3, 6, 9, 12-sec. These were presented randomly during a session, each for 40 times, for a total of 200 trials. The chart shows that the number of correct responses varied inversely with delay duration, as would be expected.
Figure 3.
Performance of 5 trained female rats on a Delayed Spatial Alternation task. The delays ranged from 0.5 to 12 seconds. Both measures of performance, total correct, and the first response following the delay, showed the expected decline in accuracy as delay duration increased.
Alternative approaches
Objections are sometimes raised about the resources required for these kinds of studies: that is, the lengthy training periods and the investment in equipment. We find it difficult to conceive of a complex learned behavior, stable over time, that does not require extensive training. Surely the cognitive functions that underlie the difficulties complained of by patients are products of a lifetime of experience, so we can hardly expect to predict such effects by using quick, simple behavioral indices. The equipment issue is easily resolved. If we aim to investigate and compare many different regimens as they become targets for evaluation, we need to be able to study substantial numbers of animals under standard conditions. One laboratory staff member can control and monitor 20 operant chambers per 1-hr session (as in our laboratory), or four 1-hour sessions per day, because of automation, and have the results and even many statistical analyses processed automatically as well. It offers, compared to other approaches, what might be termed a high-throughput solution to testing potential treatment regimens. Procedures that superficially seem less demanding and expensive, such as the Morris maze, can be much more costly. Like similar methods, the water maze requires one staff member to test one animal at a time—a very expensive and time-consuming procedure. In addtion, it is not a procedure that is appropriate for daily testing over a period of months. Further, we have found that staff members differ among themselves in how they handle animals and in their observational skills. This is another source of variability often overlooked.
SUMMARY
This chapter is an attempt to provide a foundation for the evaluation of neurotoxicity evoked by cancer chemotherapy. Its outlook is framed by the experience of how to assess neurotoxic risks posed by environmental chemicals, a situation in which prevention of adverse effects predominates. It has emphasized behavioral testing rather than mechanistic studies because its target is a model for tracing the onset and persistence of neurotoxicity in patients. In accordance with this aim, it also includes an example of how preclinical assessment in animal models might be undertaken. Here, dose-response functions and stable performance baselines are critical, as they have been shown to be in the evaluation of environmental neurotoxicants.
ACKNOWLEDGEMENTS
Preparation supported in part by NIEHS grants ES013247 and ES015509 to B. Weiss and Center grant ES01247.
REFERENCES
- 1.Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: A review of published studies and recommendations for future research. J Clin Oncol. 2007;25(17):2455–2463. doi: 10.1200/JCO.2006.08.1604. [DOI] [PubMed] [Google Scholar]
- 2.Taillibert S, Voillery D, Bernard-Marty C. Chemobrain: Is systemic chemotherapy neurotoxic? Curr Opin Oncol. 2007;19(6):623–627. doi: 10.1097/CCO.0b013e3282f0e224. [DOI] [PubMed] [Google Scholar]
- 3.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7(3):192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hess LM, Insel KC. Chemotherapy-related change in cognitive function: A conceptual model. Oncol Nurs Forum. 2007;34(5):981–994. doi: 10.1188/07.ONF.981-994. [DOI] [PubMed] [Google Scholar]
- 5.Brown MS, Stemmer SM, Simon JH, et al. White matter disease induced by high-dose chemotherapy: Longitudinal study with MR imaging and proton spectroscopy. AJNR Am J Neuroradiol. 1998;19(2):217–221. [PMC free article] [PubMed] [Google Scholar]
- 6.Silverman DH, Dy CJ, Castellon SA, et al. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res Treat. 2007;103(3):303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- 7.Inagaki M, Yoshikawa E, Matsuoka Y, et al. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109(1):146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- 8.Schiff D, Wen P. Central nervous system toxicity from cancer therapies. Hematol Oncol Clin North Am. 2006;20(6):1377–1398. doi: 10.1016/j.hoc.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Duffner PK. The long term effects of chemotherapy on the central nervous system. J Biol. 2006;5(7):21. doi: 10.1186/jbiol51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5(7):22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss B, Laties VG. Behavioral Toxicology. New York: Plenum; 1975. [Google Scholar]
- 12.Wefel JS, Kayl AE, Meyers CA. Neuropsychological dysfunction associated with cancer and cancer therapies: A conceptual review of an emerging target. Br J Cancer. 2004;90(9):1691–1696. doi: 10.1038/sj.bjc.6601772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanninen H. The psychological performance profile in occupational intoxications. Neurotoxicol Teratol. 1988;10(5):485–488. doi: 10.1016/0892-0362(88)90013-x. [DOI] [PubMed] [Google Scholar]
- 14.Wetherell A. Performance tests. Environ Health Perspect. 1996;104(Suppl 2):247–273. doi: 10.1289/ehp.96104s2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slikker W, Beck BD, Cory-Slechta DA, Paule MG, Anger WK, Bellinger D., Jr Cognitive tests: Interpretation for neurotoxicity? (workshop summary) Toxicol Sci. 2000;58(2):222–234. doi: 10.1093/toxsci/58.2.222. [DOI] [PubMed] [Google Scholar]
- 16.Fray PJ, Robbins TW. CANTAB battery: Proposed utility in neurotoxicology. Neurotoxicol Teratol. 1996;18(4):499–504. doi: 10.1016/0892-0362(96)00027-x. [DOI] [PubMed] [Google Scholar]
- 17.Rohlman DS, Gimenes LS, Eckerman DA, Kang SK, Farahat FM, Anger WK. Development of the behavioral assessment and research system (BARS) to detect and characterize neurotoxicity in humans. Neurotoxicology. 2003;24(4–5):523–531. doi: 10.1016/s0161-813x(03)00023-8. [DOI] [PubMed] [Google Scholar]
- 18.Rohlman DS, Lasarev M, Anger WK, Scherer J, Stupfel J, McCauley L. Neurobehavioral performance of adult and adolescent agricultural workers. Neurotoxicology. 2007;28(2):374–380. doi: 10.1016/j.neuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Blackwell AD, Sahakian BJ, Vesey R, Semple JM, Robbins TW, Hodges JR. Detecting dementia: Novel neuropsychological markers of preclinical alzheimer's disease. Dement Geriatr Cogn Disord. 2004;17(1–2):42–48. doi: 10.1159/000074081. [DOI] [PubMed] [Google Scholar]
- 20.Schmid KE, Kornek GV, Scheithauer W, Binder S. Update on ocular complications of systemic cancer chemotherapy. Surv Ophthalmol. 2006;51(1):19–40. doi: 10.1016/j.survophthal.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Eisner A, Incognito LJ. The color appearance of stimuli detected via short-wavelength-sensitive cones for breast cancer survivors using tamoxifen. Vision Res. 2006;46(11):1816–1822. doi: 10.1016/j.visres.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Iregren A, Andersson M, Nylen P. Color vision and occupational chemical exposures: I. an overview of tests and effects. Neurotoxicology. 2002;23(6):719–733. doi: 10.1016/S0161-813X(02)00088-8. [DOI] [PubMed] [Google Scholar]
- 23.Haymes SA, Roberts KF, Cruess AF, et al. The letter contrast sensitivity test: Clinical evaluation of a new design. Invest Ophthalmol Vis Sci. 2006;47(6):2739–2745. doi: 10.1167/iovs.05-1419. [DOI] [PubMed] [Google Scholar]
- 24.Schreiber JS, Hudnell HK, Geller AM, et al. Apartment residents' and day care workers' exposures to tetrachloroethylene and deficits in visual contrast sensitivity. Environ Health Perspect. 2002;110(7):655–664. doi: 10.1289/ehp.02110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rademaker-Lakhai JM, Crul M, Zuur L, et al. Relationship between cisplatin administration and the development of ototoxicity. J Clin Oncol. 2006;24(6):918–924. doi: 10.1200/JCO.2006.10.077. [DOI] [PubMed] [Google Scholar]
- 26.Cavaletti G, Bogliun G, Marzorati L, et al. Grading of chemotherapy-induced peripheral neurotoxicity using the total neuropathy scale. Neurology. 2003;61(9):1297–1300. doi: 10.1212/01.wnl.0000092015.03923.19. [DOI] [PubMed] [Google Scholar]
- 27.Postma TJ, Aaronson NK, Heimans JJ, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The QLQ-CIPN20. Eur J Cancer. 2005;41(8):1135–1139. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Maurissen JPJ, Weiss B. Vibration sensitivity as an index of somato-sensory function in monkeys and humans. In: Spencer PS, Schaumberg HH, editors. Experimental and Clinical Neurotoxicology. New York: Williams and Wilkins; 1980. [Google Scholar]
- 29.Tremblay F, Mireault AC, Dessureault L, Manning H, Sveistrup H. Postural stabilization from fingertip contact II. relationships between age, tactile sensibility and magnitude of contact forces. Exp Brain Res. 2005;164(2):155–164. doi: 10.1007/s00221-005-2238-5. [DOI] [PubMed] [Google Scholar]
- 30.Ravasco P. Aspects of taste and compliance in patients with cancer. Eur J Oncol Nurs. 2005;9(Suppl 2):S84–S91. doi: 10.1016/j.ejon.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Antunes MB, Bowler R, Doty RL. San Francisco/Oakland bay bridge welder study: Olfactory function. Neurology. 2007;69(12):1278–1284. doi: 10.1212/01.wnl.0000276988.50742.5e. [DOI] [PubMed] [Google Scholar]
- 32.Ellingsen DG, Bast-Pettersen R, Efskind J, Thomassen Y. Neuropsychological effects of low mercury vapor exposure in chloralkali workers. Neurotoxicology. 2001;22(2):249–258. doi: 10.1016/s0161-813x(01)00012-2. [DOI] [PubMed] [Google Scholar]
- 33.Bleecker ML, Ford DP, Vaughan CG, Walsh KS, Lindgren KN. The association of lead exposure and motor performance mediated by cerebral white matter change. Neurotoxicology. 2007;28(2):318–323. doi: 10.1016/j.neuro.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Wastensson G, Lamoureux D, Sallsten G, Beuter A, Barregard L. Quantitative assessment of neuromotor function in workers with current low exposure to mercury vapor. Neurotoxicology. 2008;29(4):596–604. doi: 10.1016/j.neuro.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Tannock IF, Ahles TA, Ganz PA, Van Dam FS. Cognitive impairment associated with chemotherapy for cancer: Report of a workshop. J Clin Oncol. 2004;22(11):2233–2239. doi: 10.1200/JCO.2004.08.094. [DOI] [PubMed] [Google Scholar]
- 36.Lee GD, Longo DL, Wang Y, et al. Transient improvement in cognitive function and synaptic plasticity in rats following cancer chemotherapy. Clin Cancer Res. 2006;12(1):198–205. doi: 10.1158/1078-0432.CCR-05-1286. [DOI] [PubMed] [Google Scholar]
- 37.Seigers R, Schagen SB, Beerling W, et al. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behav Brain Res. 2008;186(2):168–175. doi: 10.1016/j.bbr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Foley JJ, Raffa RB, Walker EA. Effects of chemotherapeutic agents 5-fluorouracil and methotrexate alone and combined in a mouse model of learning and memory. Psychopharmacology (Berl) 2008;199(4):527–538. doi: 10.1007/s00213-008-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konat GW, Kraszpulski M, James I, Zhang HT, Abraham J. Cognitive dysfunction induced by chronic administration of common cancer chemotherapeutics in rats. Metab Brain Dis. 2008;23(3):325–333. doi: 10.1007/s11011-008-9100-y. [DOI] [PubMed] [Google Scholar]
- 40.Mustafa S, Walker A, Bennett G, Wigmore PM. 5-fluorouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus. Eur J Neurosci. 2008;28(2):323–330. doi: 10.1111/j.1460-9568.2008.06325.x. [DOI] [PubMed] [Google Scholar]
- 41.Winocur G, Vardy J, Binns MA, Kerr L, Tannock I. The effects of the anti-cancer drugs, methotrexate and 5-fluorouracil, on cognitive function in mice. Pharmacol Biochem Behav. 2006;85(1):66–75. doi: 10.1016/j.pbb.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Han R, Yang YM, Dietrich J, Luebke A, Mayer-Proschel M, Noble M. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J Biol. 2008;7(4):12. doi: 10.1186/jbiol69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McArthur HL, Hudis CA. Advances in adjuvant chemotherapy of early stage breast cancer. Cancer Treat Res. 2008;141:37–53. doi: 10.1007/978-0-387-73161-2_. [DOI] [PubMed] [Google Scholar]
- 44.Kreukels BP, van Dam FS, Ridderinkhof KR, Boogerd W, Schagen SB. Persistent neurocognitive problems after adjuvant chemotherapy for breast cancer. Clin Breast Cancer. 2008;8(1):80–87. doi: 10.3816/CBC.2008.n.006. [DOI] [PubMed] [Google Scholar]
- 45.Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004;26(7):955–969. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- 46.Rugo HS, Ahles T. The impact of adjuvant therapy for breast cancer on cognitive function: Current evidence and directions for research. Semin Oncol. 2003;30(6):749–762. doi: 10.1053/j.seminoncol.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Weiss B. Chemobrain: A translational challenge for neurotoxicology. Neurotoxicology. 2008;29(5):891–898. doi: 10.1016/j.neuro.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winsauer PJ, Quinton MS, Porter JR, et al. Effects of MDMA administration on scopolamine-induced disruptions of learning and performance in rats. Pharmacol Biochem Behav. 2004;79(3):459–472. doi: 10.1016/j.pbb.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 49.Cory-Slechta DA, Pokora MJ, Widzowski DV. Behavioral manifestations of prolonged lead exposure initiated at different stages of the life cycle: IIdelayed spatial alternation. Neurotoxicology. 1991;12(4):761–776. [PubMed] [Google Scholar]
- 50.Newland MC, Reile PA, Langston JL. Gestational exposure to methylmercury retards choice in transition in aging rats. Neurotoxicol Teratol. 2004;26(2):179–194. doi: 10.1016/j.ntt.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Hurria A, Rosen C, Hudis C, et al. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: A pilot prospective longitudinal study. J Am Geriatr Soc. 2006;54(6):925–931. doi: 10.1111/j.1532-5415.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- 52.Markowski VP, Cox C, Preston R, Weiss B. Impaired cued delayed alternation behavior in adult rat offspring following exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin on gestation day 15. Neurotoxicol Teratol. 2002;24(2):209–218. doi: 10.1016/s0892-0362(02)00186-1. [DOI] [PubMed] [Google Scholar]
- 53.Weiss B, Stern S, Cox C, Balys M. Perinatal and lifetime exposure to methylmercury in the mouse: Behavioral effects. Neurotoxicology. 2005;26(4):675–690. doi: 10.1016/j.neuro.2005.05.003. [DOI] [PubMed] [Google Scholar]