Abstract
Objective
Sleep disturbance is a common feature during mood episodes in bipolar disorder. The aim of this study was to investigate the prevalence of such symptoms among euthymic bipolar patients, and their association with risk for mood episode recurrence.
Method
A cohort of bipolar I and II subjects participating in the Systematic Treatment Enhancement Program for Bipolar Disorder who were euthymic for at least eight weeks were included in this analysis. Survival analysis was used to examine the association between sleep disturbance on the Montgomery-Asberg Depression Rating Scale (MADRS) and recurrence risk.
Results
73/483 bipolar I and II subjects reported at least mild sleep disturbance (MADRS sleep item ≥ 2) for the week prior to study entry. The presence of sleep problems was associated with a history of psychosis, number of previous suicide attempts, and anticonvulsant use. Sleep disturbance at study entry was significantly associated with risk for mood episode recurrence.
Conclusions
Sleep disturbance is not uncommon between episodes for individuals with bipolar disorder and may be associated with a more severe course of illness. This suggests that sleep disturbance is an important prodromal symptom of bipolar disorder and should be considered a target for pharmacologic or psychosocial maintenance treatment.
Keywords: Bipolar disorder, sleep disturbance, relapse prevention, treatment
Introduction
Sleep disturbance is common among bipolar patients [Harvey, 2008] and substantially impacts quality of life [Giglio et al., 2009, Gruber et al., 2009] by negatively affecting the course and prognosis of the disorder. Disruptions in sleep often occur before the onset of a mood episode, and are also common symptoms of acute bipolar episodes [Jackson et al., 2003, Harvey et al., 2005, Bauer et al., 2006, Harvey, 2008]. These disruptions are a marker of inadequate recovery from a mood episode [Harvey, 2008] and are often associated with comorbid conditions, such as anxiety [Harvey et al., 2005]. Artificially disrupting sleep (i.e., with behavioral manipulations) in bipolar disorder can cause mania or hypomania (for review, see [Wu et al., 1990], and in depressed bipolar patients, clinically induced sleep deprivation has an antidepressant effect [Wehr et al., 1987, Wu et al., 1990]. Sleep disturbance may also be a defining feature of the disorder in all phases of illness, given that sleep may be problematic even in euthymic bipolar patients [Harvey et al., 2005, Plante et al., 2008]. Individuals with bipolar disorder may have a biological vulnerability to dsyregulated sleep due to having a genetic abnormality in their circadian rhythm system [Wehr et al., 1987, Ehlers et al., 1988, Harvey et al., 2005, Grandin et al., 2006, Murray & Harvey, 2010].
In the current study, we examined euthymic bipolar patients to investigate the prevalence of residual sleep disturbance and its potential association with risk for a subsequent mood episode. Given that comorbid diagnoses, such as anxiety, can exacerbate sleep disturbance in individuals with bipolar disorder and that medications can alter sleep [Harvey et al., 2005], we also examined whether these variables affect the time to mood recurrence after a mood episode. The Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD), a multicenter effectiveness study, afforded an opportunity to study the impact of sleep disturbance in a large and generalizable cohort of well-characterized, clinically-representative bipolar I and II participants.
Methods
Study Overview
STEP-BD was a multicenter study designed to evaluate longitudinal outcomes in individuals with bipolar disorder. All participants received standardized prospective evaluation, including structured and semi-structured assessments and clinical interviews [Sachs et al., 2003]. Subjects were recruited between 1999 and 2005. The human research committees of all 16 participating sites approved the STEP-BD protocol. Participants in the Standard Care Pathway of STEP-BD could receive any clinically-indicated intervention. Study psychiatrists were trained to utilize model practice procedures, including evidence-based pharmacology [Sachs et al., 2003].
Participants
Eligibility in STEP-BD required that participants were at least 15 years of age and met DSM-IV criteria for bipolar I disorder, bipolar II disorder, cyclothymia, bipolar disorder not otherwise specified (NOS), or schizoaffective disorder, bipolar subtype. STEP-BD exclusion criteria included unwillingness or inability to give informed consent or comply with study assessments. Subjects in the present study were a set of 483 individuals with bipolar I or II disorder drawn from the STEP-BD cohort who were euthymic, as defined by Young Mania Rating Scale score ≤ 12, Montgomery-Asberg Depression Rating Scale score ≤ 10, and a clinical status of “in recovery” for at least 8 weeks.
Assessments
Treating psychiatrists in STEP-BD were trained in the empirically-supported guidelines for the treatment of bipolar disorder [Suppes et al., 2005], including use of standardized evaluation tools and rating scales. Methods and assessments used in STEP-BD relevant to the present study are described below; all methods and assessments used in STEP-BD have been described elsewhere [Sachs et al., 2003].
The Affective Disorder Evaluation (ADE) [Sachs et al., 2003] utilizes adaptations of the mood and psychosis modules from the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [Association, 1994] Axis I Disorders, Patient Edition (SCID-P) [First et al., 1996]. It was administered to all participants at study entry, and was the primary means of establishing diagnosis. The ADE also included systematic assessment of lifetime and recent course of illness based on patient report.
The Mini International Neuropsychiatric Interview (MINI Version 4.4) [Sheehan et al., 1998] was used to confirm bipolar diagnosis and establish comorbid axis I diagnoses. The MINI is a brief structured interview designed to identify the major Axis I psychiatric disorders in the fourth edition of the DSM-IV and the tenth revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) [Organization, 1992]. The MINI and ADE were completed by different study clinicians, and a consensus diagnosis of one of the eligible bipolar disorders was required on both the ADE and MINI for study entry.
The Young Mania Rating Scale (YMRS) [Young et al., 1978], an eleven-item, clinician-administered assessment of manic symptoms, and the Montgomery-Åsberg Depression Rating Scale (MADRS) [Montgomery et al., 1979], a ten-item, clinician-administered assessment of depressive symptoms, were administered at each follow-up visit to determine current severity of symptoms [Sachs et al., 2003].
The Clinical Monitoring Form (CMF) [Sachs et al., 2003] was used to collect DSM-IV criteria for depressive, manic, hypomanic, or mixed states and was administered at each follow-up visit to determine clinical status.
Study Outcomes
Independent study assessments were conducted at fixed intervals, and clinical status was assessed at each follow-up visit with the CMF. Remission was defined as two or fewer syndromal features of mania, hypomania, or depression for at least eight weeks. Recurrence was defined as meeting full DSM-IV criteria for a manic, hypomanic, mixed, or depressive episode on any one follow-up visit. Occurrence of subsyndromal mood symptoms during follow-up was not considered recurrence.
MADRS item 4 (‘reduced sleep’) measures the subject’s rating of reduced duration or depth of sleep compared to the subject’s normal sleep pattern, and was used as the primary measure of “sleep disturbance” in the present study. Consistent with previous literature, the definition of “sleep disturbance” is a score of 2 or greater on MADRS item [Williamson et al., 2006]. While the MADRS is not specifically a sleep measure, individual items are considered to be reliable and valid [Montgomery et al., 1979, Welmer et al., 2007].
Statistical Analysis
After confirmation that distribution assumptions were met, parametric univariate tests for association (i.e., Student’s T-tests), or chi square tests, were used to examine the relationship between baseline sociodemographic, clinical features, and sleep disturbance. We used survival analysis to examine time from study entry to first manic, hypomanic, mixed, or major depressive episode. Data was censored after completion of 2 years of follow-up or last recorded visit. Cox regression was utilized after confirmation that the proportional hazards assumption was met by incorporating a variable-by-time term in regression models, and by visual inspection of hazard plots. Baseline sociodemographic and clinical variables (Table 1) were examined as potential confounders by incorporating them individually into a Cox regression model to investigate whether resulting hazard ratios for the sleep disturbance term changed by 10% or more. This strategy is often used in other studies [Tong & Lu, 2000] and is suggested as a means of determining confounding variables [Bliss et al., 2011; Kleinbaum & Klein, 2010;]. All analyses were conducted using Stata 10.0 (College Station, TX).
Table 1.
Demographic and Clinical Variables Association with Sleep Disturbance
| Variable | Sleep Disturbance N (%) |
Odds Ratio | 95% CI |
|---|---|---|---|
| Female | 46 (63.9%) | OR = 0.78 | 0.46–1.27 |
| Age | M = 43.18 (± 12.98) | OR=1.06 | 0.88–1.27 |
| Caucasian | 64 (88.9%) | OR = 0.86 | 0.39–1.93 |
| Married | 33 (45.8%) | OR = 1.39 | 0.84–2.31 |
| Bipolar I Type | 52 (72.2%) | OR = 0.95 | 0.54–1.66 |
| Rapid Cycling | 41 (56.9%) | OR = 1.45 | 0.88–2.40 |
| Depressive Symptoms^ | M= 3.50 (± 2.46) | OR = 0.99 | 0.91–1.08 |
| (Hypo)manic Symptoms^ | M= 3.56 (± 2.89) | OR = 1.14* | 1.05–1.23 |
| Any Anxiety Dx | 13 (18.1%) | OR = 1.24 | 0.64–2.40 |
| Substance Use Dx | 30 (41.7%) | OR = 1.56 | 0.63–4.11 |
| Suicide Attempt | 27 (38.0%) | OR = 1.94* | 1.14–3.30 |
| Psychosis | 24 (33.3%) | OR = .54* | 0.32–0.91 |
Note. Depressive Symptoms is the total score on the MADRS, without the sleep item. (Hypo)manic symptoms is the total score on the YMRS, without the sleep item. Dx= diagnosis.
p < .05
Results
In this cohort, 73/483 (15%) of euthymic participants reported at least mild sleep disturbance. The presence of sleep disturbance was not associated with gender, age, race, marital status, bipolar subtype, rapid cycling in the prior year, or comorbid axis I anxiety or substance abuse disorder (Table 1). Sleep disturbance was less prevalent in individuals with a history of psychosis, but more prevalent in those with a history of suicide attempts and (hypo)manic symptoms (p<.05) (see Table 1).
There was no association of sleep disturbance with pharmacotherapy, defined as use of lithium, valproate, atypical antipsychotics, or lamotrigine (see Table 2). However, there was a significant association with use of other anticonvulsants; individuals taking these medications were significantly more likely to have sleep disturbance (p<.05).
Table 2.
Medications and their Association with Sleep Disturbance
| Medication* | Sleep Disturbance N (%) |
Odds Ratio | 95% CI |
|---|---|---|---|
| Lithium | 33 (45.8%) | OR = 1.33 | 0.80–2.20 |
| Valproate | 25 (34.7%) | OR = 1.05 | 0.62–1.78 |
| Atypical Antipsychotic | 14 (19.4%) | OR = .61 | 0.33–1.13 |
| Any Antipsychotic | 26 (36.1%) | OR = .91 | 0.54–1.52 |
| Lamotrigine | 17 (23.6%) | OR = 1.65 | 0.90–3.01 |
| Other Anticonvulsant** | 16 (22.2%) | OR = 2.73*** | 1.43–5.20 |
Note. Sleep disturbance reported for participants taking each medication type.
Includes the following medications: gabapentin, levetiracetam, tiagabine, oxcarbazepine, and zonisamide.
p < .05
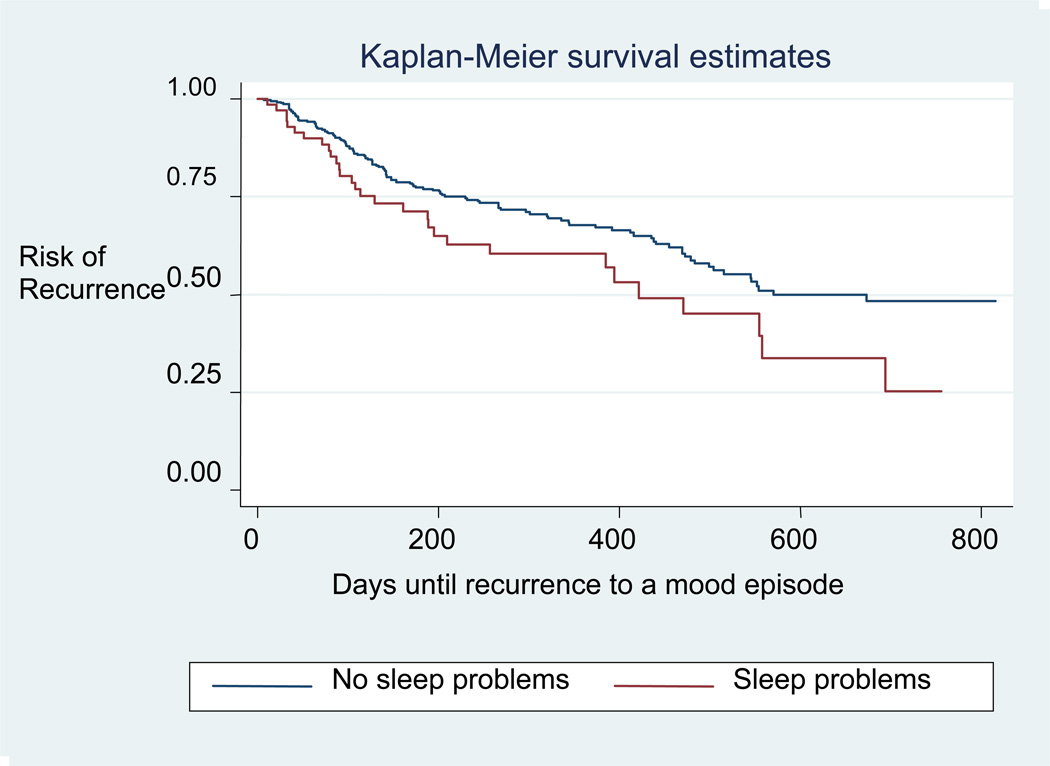
In the survival analysis, we found that the presence of sleep disturbance was significantly associated with greater risk for mood episode recurrence (Kaplan-Meier log rank p<0.05) (see Figure 1). In Cox regression, incorporating terms for sociodemographic and clinical features examined in Table 1 did not change the hazard ratio by 10% or more, suggesting that they do not confound the association between residual sleep disturbance and recurrence risk.
Figure 1.
Risk of Recurrence for Individuals with and without Sleep Problems
For exploratory purposes, we also examined time to recurrence by pole separately for depressive episode and manic/hypomanic/mixed episodes recurrence, with results censored at any recurrence (i.e., if a subject initially relapsed or recurred into mania, subsequent observations for that subject were censored and not further considered in analysis of time-to-depression). We found numerically increased risk for recurrence to both poles, for depression the hazard ratio was 1.45 [95% CI .88–2.40; p=.14] and for mania/hypomania/mixed state, the hazard ratio was 1.79 [95% CI .92–3.4449; p=.09].
Discussion
We found that residual sleep disturbance among euthymic bipolar I and II patients was associated with risk of recurrence of subsequent mood episodes. Sleep disturbance was also associated with a history of psychosis, number of previous suicide attempts, and the use of anticonvulsants, but not with other medications.
This study supports previous findings that bipolar patients experience sleep disturbance even during euthymic periods (e.g., [Plante et al., 2008]. This may explain why only 15% of individuals with bipolar disorder had sleep disturbance. Giglio et al., in a cross-sectional study, found that euthymic bipolar patients with sleep disturbance had worse quality of life and more impairment in social and environmental domains, than those without sleep disturbance [Giglio et al., 2009]. These data could suggest that sleep disturbance may be both a symptom of the disorder, and a significant trigger for acute episodes as evidenced that our exploratory analyses showing a trend for sleep to be more disruptive during bipolar episodes. That sleep disturbance occurs in the absence of a mood episode, and is not associated with most medications, suggests that sleep disturbance and bipolar disorder may share a similar etiology or overlapping pathophysiology [Harvey et al., 2005, Grandin et al., 2006 ]. This possibility is supported by studies that have found sleep disturbance is worse in euthymic bipolar patients than in matched normal controls [Knowles et al., 1986, Millar et al., 2004].
Though the underlying mechanisms have yet to be elucidated, this study demonstrates that residual sleep disturbance is a primary prodromal symptom of bipolar episodes. Given that relapse rates are high in bipolar disorder [Gitlin et al., 1995] and that identification and treatment of prodromal symptoms are crucial for relapse prevention [Otto et al., 2002], sleep maintenance is an important treatment goal. We also found that sleep disturbance is associated with (hypo)manic symptoms which has been well documented in the literature [Wehr et al., 1987, Wehr, 1991], further highlighting the need to assess sleep problems during euthymic periods for individuals with bipolar disorder. Thus, clinicians should be mindful to assess carefully for sleep disturbance, even in the absence of other bipolar symptoms. Interestingly, we did not find that sleep disturbance was associated with depressive symptoms during euthymic periods. There is some evidence that extreme sleep disturbance, or total sleep deprivation, has anti-depressant features [Giedke et al., 2002, Benedetti et al., 2003] but triggers mania [Wehr, 1992]. Yet, the lack of association between sleep disturbance and depressive symptoms is an unusual finding and warrants further research.
Sleep disturbance, among euthymic bipolar individuals, is associated with a history of suicide attempts; often an indicator of severe affect dysregulation. For example, other studies have found that sleep disturbance is associated with increased impulsivity [Dahl et al., 1996, Harvey et al., 2005]. In nonclinical populations, sleep loss has been shown to intensify negative emotions as well as to diminish positive emotions [Zohar et al., 2005]. Thus, managing sleep may improve both affect regulation and impulsivity.
Finally, any discussion of sleep disturbance must consider the use of medications which can alter sleep architecture. In our study, we found that subjects with a history of psychosis were less likely to report sleep disturbance. This may be associated with the use of atypical antipsychotic medications in this population, although sleep disturbance was not more prevalent among patients taking atypical antipsychotics. Sleep disturbance was significantly associated with use of “other” anticonvulsants, although the vast majority of subjects in this category were taking gabapentin, likely reflecting the use of this medication specifically for insomnia.
This study provides prospective data from a large, generalizable outpatient sample of well-characterized euthymic bipolar I and II patients who were treated in a naturalistic setting; however, we lacked a comprehensive characterization of sleep using a validated scale, such as the Pittsburgh Sleep Questionnaire Index [Backhaus et al., 2002]. The MADRS, while a valid scale of depressive symptoms, is not specifically a sleep scale and includes only one sleep item. Given that the MADRS sleep item assesses “reduced sleep,” it does not capture sleep disturbance due to increases in sleep, or over sleeping. This item also does not capture the causality of sleep disturbance, for example, individuals may experience a decreased need for sleep due to hypomania/mania. This is important to note as hypomania symptoms were associated with dysregulated sleep (see Table 1). The MADRS is also based largely on patient self-report, which can be unreliable; however, other studies have used this item to define sleep problems [Lader et al., 2005, Williamson et al., 2006]. In future investigations, the assessments of sleep disturbance with more objective measures, such as polysomnography or actigraphy, would be valuable.
Conclusion
Sleep disturbance is common among euthymic bipolar patients and is an independent risk factor for recurrent mood episodes. Thus, maintenance treatment for bipolar disorder should include careful assessment and management of sleep disturbance.
Acknowledgements
Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the NIMH. Additional detail on STEP-BD can be located at http://www.nimh.nih.gov.proxy.library.emory.edu/health/trials/practical/step-bd/questions-and-answers-for-the-systematic-treatment-enhancement-program-for-bipolar-disorder-step-bd-study-background.shtml.
This study was funded by the National Institute of Mental Health Contract N01MH80001.
Footnotes
Conflict of Interest Statement
Dr. Sylvia is a consultant for United BioSource Corporation.
Dr. Dupuy does not have any support or funding to disclose.
In the past two years, Dr. Ostacher has served on advisory/consulting boards of Pfizer and Schering Plough (now Merck); he has received speaking fees from AstraZeneca, Bristol Myers Squibb, Eli Lilly & Company, and Pfizer.
Ms. Cowperthwait does not have any support or funding to disclose.
Ms. Hay is a consultant for GENOMIND.
Over his lifetime, Dr. Sachs has served as a member of the speakers’ bureaus for Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol Myers Squibb, Eli Lilly & Co, GlaxoSmithKline, Janssen Pharmaceuticals, Memory Pharmaceuticals, Novartis Pharmaceuticals, Pfizer, Sanofi-Aventis, and Wyeth. Dr. Sachs has received research support from Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol Myers Squibb, Eli Lilly & Co, GlaxoSmithKline, Janssen Pharmaceuticals, Memory Pharmaceuticals, the National Institute of Mental Health, Novartis Pharmaceuticals, Pfizer, Repligen, Shire, and Wyeth. In his lifetime, Dr. Sachs has served on advisory/consulting boards of Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol Myers Squibb, Cephalon, CNS Response, Elan Pharmaceuticals, Memory Pharmaceuticals, Merck, Novartis Pharmaceuticals, Organon, Otsuka, Pfizer, Schering Plough, Sepracor, Repligen, Sanofi-Aventis, Shire, Sigma-Tau, Solvay Pharmaceuticals, and Wyeth. Dr. Sachs and his spouse own stock in Concordant Rater Systems. In the past twelve months, Dr. Sachs has received research support from GlaxoSmithKline, the National Institute of Mental Health, and Repligen. In the past twelve months, Dr. Sachs has served on the advisory/consulting boards of AstraZeneca Pharmaceuticals, Bristol Myers Squibb, Cephalon, Concordant Rater Systems, Janssen Pharmaceuticals, Merck, Otsuka, Pfizer, Schering Plough, Sepracor, and Repligen.
Dr. Nierenberg is a full time employee of the Massachusetts General Hospital (MGH). In the past 12 months (as of June 21, 2010), he has served as a consultant to the Appliance Computing Inc. (Mindsite), and Brandeis University. Through the MGH Clinical Trials Network and Institute (CTNI), he has consulted for Brain Cells, Inc, Dianippon Sumitomo/Sepracor, Labopharm, Merck, Methylation Science, Novartis, PGx Health, Shire, Schering-Plough, Targacept, and Takeda/Lundbeck Pharmaceuticals. He received grant/research support through MGH from NIMH, PamLabs, Pfizer Pharmaceuticals, and Shire. He received honoraria from Belvior Publishing, Hillside Hospital, American Society for Clinical Psychopharmacology, Columbia University, IMEDEX, MJ Consulting, New York State, MBL Publishing, Physicians Postgraduate Press, SciMed, SUNY Buffalo, University of Wisconsin, and the University of Pisa. Dr. Nierenberg is a presenter for the Massachusetts General Hospital Psychiatry Academy (MGHPA). The education programs conducted by the MGHPA were supported through Independent Medical Education (IME) grants from the following pharmaceutical companies in 2008: Astra Zeneca, Eli Lilly, and Janssen Pharmaceuticals; in 2009 Astra Zeneca, Eli Lilly, and Bristol-Myers Squibb. No speaker bureaus or boards since 2003. He is on the advisory boards of Appliance Computing, Inc., Brain Cells, Inc., Eli Lilly and Company, and Takeda/Lundbeck and Targacept. Dr. Nierenberg owns stock options in Appliance Computing, Inc. and Brain Cells, Inc. Through MGH, he is named for copyrights to the Clinical Positive Affect Scale and the MGH Structured Clinical Interview for the Montgomery Asberg Depression Scale exclusively licensed to the CTNI.
Dr. Perlis receives speaker's bureau/consulting fees from AstraZeneca, Eli Lilly & Co., Proteus Biomedical, and Concordant Rater Systems. Dr. Perlis receives royalties and has a patent for the Concordant Rater Systems.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-Retest Reliability and Validity of the Pittsburgh Sleep Quality Index in Primary Insomnia. J Psychosom Res. 2002;53:737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- Bauer M, Grof P, Rasgon N, Bschor T, Glenn T, Whybrow PC. Temporal Relation between Sleep and Mood Ni Patients with Bipolar Disorder. Bipolar Disord. 2006;8:160–167. doi: 10.1111/j.1399-5618.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Colombo C, Serretti A, Lorenzi C, Pontiggia A, Barbini B, et al. Antidepressant Effects of Light Therapy Combined with Sleep Deprivation Are Influenced by a Functional Polymorphism within the Promoter of the Serotonin Transporter Gene. Biol Psychiatry. 2003;54:687–692. doi: 10.1016/s0006-3223(02)01894-2. [DOI] [PubMed] [Google Scholar]
- Bliss R, Weinberg J, Vieira V, Webster T. Detecting Confounding And Evaluating The 10% Rule Joint Statistical Meetings. Miami Beach, FL: 2011. [Google Scholar]
- Dahl RE, Ryan ND, Matty MK, Birmaher B, Al-Shabbout M, Williamson DE, et al. Sleep Onset Abnormalities in Depressed Adolescents. Biol Psychiatry. 1996;39:400–410. doi: 10.1016/0006-3223(95)00190-5. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Frank E, Kupfer DJ. Social Zeitgebers and Biological Rhythms. Archives of General Psychiatry. 1988;45:948–952. doi: 10.1001/archpsyc.1988.01800340076012. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer R, Gibbon M. Biometrics Research Department. New York: New York State Psychiatric Institute; 1996. Structured Clinical Interview for Dsm-Iv Axis I Disorders. [Google Scholar]
- Giedke H, Schwärzler F. Therapeutic Use of Sleep Deprivation in Depression. Sleep Med Rev. 2002;6:361–377. [PubMed] [Google Scholar]
- Giglio LM, Andreazza AC, Andersen M, Cereser KM, Sterz L, Kapczinski F. Sleep in Bipolar Patients. Sleep Breath. 2009;13:169–173. doi: 10.1007/s11325-008-0215-5. [DOI] [PubMed] [Google Scholar]
- Gitlin MJ, Swendsen J, Heller TL, Hammen C. Relapse and Impairment in Bipolar Disorder. Am J Psychiatry. 1995;152:1635–1640. doi: 10.1176/ajp.152.11.1635. [DOI] [PubMed] [Google Scholar]
- Grandin LD, Alloy LB, Abramson LY. The Social Zeitgeber Theory, Circadian Rhythms, and Mood Disorders: Review and Evaluation. Clin Psychol Rev. 2006;26:679–694. doi: 10.1016/j.cpr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Gruber J, Harvey AG, Wang PW, Brooks JOR, Thase ME, Sachs GS, et al. Sleep Functioning in Relation to Mood, Function, and Quality of Life at Entry to the Systematic Treatment Enhancement Program for Bipolar Disorder (Step-Bd) J Affec Disord. 2009;114:41–19. doi: 10.1016/j.jad.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG. Sleep and Circadian Rhythms in Bipolar Disorder: Seeking Synchrony, Harmony, and Regulation. Am J Psychiatry. 2008;165:820–829. doi: 10.1176/appi.ajp.2008.08010098. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Schmidt DA, Scarna A, Semler CN, Goodwin GM. Sleep-Related Functioning in Euthymic Patients with Bipolar Disorder, Patients with Insomnia, and Subjects with Sleep Problems. Am J Psychiatry. 2005;162:50–57. doi: 10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- Jackson A, Cavanagh J, Scott J. A Systematic Review of Manic and Depressive Prodromes. J Affec Disord. 2003;74:209–217. doi: 10.1016/s0165-0327(02)00266-5. [DOI] [PubMed] [Google Scholar]
- Kleinbaum DG, Klein M. Logistic Regression: A Self-Learning Text. Third ed. New York: Springer; 2010. [Google Scholar]
- Knowles JB, Cairns J, Maclean AW, Delva N, Prowse A, Waldron J, et al. The Sleep of Remitted Bipolar Depressives: Comparison with Sex and Age-Matched Controls. Can J Psychiatry. 1986;31:295–298. doi: 10.1177/070674378603100402. [DOI] [PubMed] [Google Scholar]
- Lader M, Andersen HF, Baekdal T. The Effect of Escitalopram on Sleep Problems in Depressed Patients. Hum Psychopharmacol. 2005;20:349–354. doi: 10.1002/hup.694. [DOI] [PubMed] [Google Scholar]
- Millar A, Espie CA, Scott J. The Sleep of Remitted Bipolar Outpatients: A Controlled Naturalistic Study Using Actigraphy. J Affect Disord. 2004;80:145–153. doi: 10.1016/S0165-0327(03)00055-7. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Åsberg M. A New Depression Scale Designed to Be Sensitive to Change. Brit J Psychiat. 1979;1979:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Murray G, Harvey AG. Circadian rhythms and bipolar disorder. Bipolar Disorders. 2010;12:459–472. doi: 10.1111/j.1399-5618.2010.00843.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. International Classification of Diseases, Tenth Revision. Worth Health Organization; Geneva, Switzerland: 1992. [Google Scholar]
- Otto MW, Reilly-Harrington N. Cognitive-Behavioral Therapy for the Management of Bipolar Disorder. In: Hofmann SG, Tompson MC, editors. Treating Chronic and Severe Mental Disorders: A Handbook of Empirically Supported Interventions. New York: The Guilford Press, Inc; 2002. [Google Scholar]
- Plante DT, Winkelman JW. Sleep Disturbance in Bipolar Disorder: Therapeutic Implications. Am J Psychiatry. 2008;165:830–843. doi: 10.1176/appi.ajp.2008.08010077. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Thase ME, Otto MW, Baur M, Miklowitz D, Winiewski SR, et al. Rationale, Design, and Methods of the Systematic Treatment Enhancement Program for Bipolar Disorder (Step-Bd) Biol Psychiatry. 2003;53:1028–1042. doi: 10.1016/s0006-3223(03)00165-3. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The Development and Validation of a Structured Diagnostic Psychiatric Interview for Dsm-Iv and Icd-10. J Clin Psychiatry. 1998;59:22–33. quiz 34–57. [PubMed] [Google Scholar]
- Suppes T, Dennehy EB, Hirschfeld RM, Altshuler LL, Bowden CL, Calabrese JR, et al. The Texas Implementation of Medication Algorithms: Update to the Algorithms for Treatment of Bipolar I Disorder. Journal of Clinical Psychiatry. 2005;66:870–886. doi: 10.4088/jcp.v66n0710. [DOI] [PubMed] [Google Scholar]
- Tong S, Lu Y. Identification of Confounders in the Assessment of the Relationship between Lead Exposure and Child Development. Annals of Epidemiology. 2000;11(1):38–45. doi: 10.1016/s1047-2797(00)00176-9. [DOI] [PubMed] [Google Scholar]
- Wehr TA. Sleep-Loss as a Possible Mediator of Diverse Causes of Mania. Br J Psychiatry. 1991;159:576–578. doi: 10.1192/bjp.159.4.576. [DOI] [PubMed] [Google Scholar]
- Wehr TA. Improvement of Depression and Triggering of Mania by Sleep Deprivation. JAMA. 1992;267:548–551. [PubMed] [Google Scholar]
- Wehr TA, Sack DA, Rosenthal NE. Sleep Reduction as a Final Common Pathway in the Genesis of Mania. Am J Psychiatry. 1987;144:201–204. doi: 10.1176/ajp.144.2.201. correction, 144:542. [DOI] [PubMed] [Google Scholar]
- Welmer AK, Von Arbin M, Murray V, Holmqvist LW, Sommerfeld DK. Determinants of Mobility and Self-Care in Older People with Stroke: Importance of Somatosensory and Perceptual Functions. Phys Ther. 2007;87:1633–1641. doi: 10.2522/ptj.20060349. [DOI] [PubMed] [Google Scholar]
- Williamson D, Brown E, Perlis RH, Ahl J, Baker RW, Tohen M. Clinical Relevance of Depressive Symptom Improvement in Bipolar I Depressed Patients. J Affec Disord. 2006;92:261–266. doi: 10.1016/j.jad.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Wu JC, Bunney WE. Sleep Deprivation and Relapse. Am J Psychiatry. 1990;147:14–21. doi: 10.1176/ajp.147.1.14. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A Rating Scale for Mania: Reliability, Validity and Sensitivity. Brit J Psychiat. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zohar D, Tzischinsky O, Epstein R, Lavie P. The Effects of Sleep Loss on Medical Residents' Emotional Reactions to Work Events: A Cognitive-Energy Model. Sleep. 2005;28:47–54. doi: 10.1093/sleep/28.1.47. [DOI] [PubMed] [Google Scholar]