Abstract
Essential tremor is a common disorder that lacks molecular targets for therapeutic development. T-type calcium channel activation has been postulated to underlie rhythmicity in the olivo-cerebellar system that is implicated in essential tremor. We therefore tested whether compounds that antagonize T-type calcium channel currents suppress tremor in two mouse models that possess an essential tremor-like pharmacological response profile. Tremor was measured using digitized spectral motion power analysis with harmaline-induced tremor and in the GABAA receptor α1 subunit-null model. Mice were given ethosuximide, zonisamide, the neuroactive steroid (3β,5α,17β)-17-hydroxyestrane-3-carbonitrile (ECN), the 3,4-dihydroquinazoline derivative KYS05064, the mibefradil derivative NNC 55-0396, or vehicle. In non-sedating doses, each compound reduced harmaline-induced tremor by at least 50% (range of maximal suppression: 53–81%), and in the GABAA α1-null model by at least 70% (range 70–93%). Because the T-type calcium channel Cav3.1 is the dominant subtype expressed in the inferior olive, we assessed the tremor response of Cav3.1-deficient mice to harmaline, and found that null and heterozygote mice exhibit as much tremor as wild-type mice. In addition, ECN and NNC 55-0396 suppressed harmaline tremor as well in Cav3.1-null mice as in wild-type mice. The finding that five T-type calcium antagonists suppress tremor in two animal tremor models suggests that T-type calcium channels may be an appropriate target for essential tremor therapy development. It is uncertain whether medications developed to block only the Cav3.1 subtype would exhibit efficacy.
Keywords: Essential tremor, Harmaline, Ethosuximide, Zonisamide, Calcium channel, Cerebellum, Inferior olive
1. Introduction
In 1982, Llinás and Jahnsen described the T-type calcium channel as responsible for post-inhibitory rebound in thalamocortical neurons, whereby a vigorous depolarization with an action potential burst occurs after hyperpolarization (Llinás and Jahnsen, 1982). The T-type calcium channel became recognized as critical to the thalamic oscillation, in which excitatory thalamocortical collaterals to the reticular thalamic nucleus evoke inhibitory outflow back to thalamic projection neurons, triggering a post-inhibitory rebound, with repetition of the cycle. It became appreciated that various processes affect this oscillatory dynamic and synchrony, so that absence seizures may occur (Huguenard and McCormick, 2007). Deletion of the T-type channel gene subtype Cav3.1 eliminates absence seizures in animal models (Kim et al., 2001); and ethosuximide, which reduces T-type calcium currents (Gomora et al., 2001), suppresses absence seizures clinically and in animal models (Aizawa et al., 1997).
At the same time Llinás’ group studied the thalamic T-type calcium conductance, they described this current in inferior olive neurons as part of a voltage oscillation that depends on low- and high-threshold calcium conductances and on potassium currents (Llinás and Yarom, 1981).
On the basis of physiological, neuropathological, and other studies (Köster et al., 2002; Axelrad et al., 2008), essential tremor (ET) is recognized as a cerebellar disorder. An important influence on cerebellar functioning is exerted by the inferior olive. In animals, harmaline hyperpolarizes olivary neurons and induces burst-firing that is transmitted through the cerebellum, manifested behaviorally as action tremor (Bernard et al., 1984). Harmaline tremor shares several similarities with ET, including suppression by anti-ET drugs (Martin et al., 2005). Llinás’ group demonstrated that 1-octanol, a low-threshold calcium channel antagonist, suppresses harmaline-induced tremor (Sinton et al., 1989), and predicted that T-type current antagonists would be effective for ET. However 1-octanol has other actions at concentrations below that of half-maximal T-type calcium channel blockade, 122 μM (Todorovic and Lingle, 1998), offering alternative explanations for its tremor suppression, such as GABA current potentiation at 50 μM (Dildy-Mayfield et al., 1996).
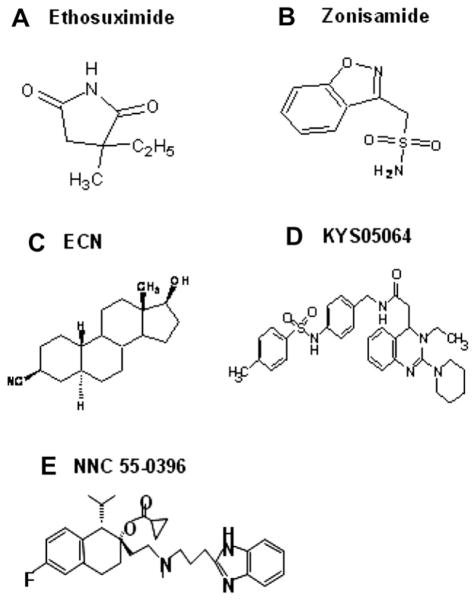
In contrast to the growth in understanding the role of T-type channels in absence seizures and other thalamocortical rhythms, their potential role in ET has not been adequately studied. In this study we re-visited the prediction made 20 years ago that T-type calcium antagonists may effectively suppress tremor (Sinton et al., 1989). We tested whether five compounds with T-type calcium channel antagonist activity can suppress tremor in the harmaline and the GABAA receptor α1 subunit knockout mouse models. The latter model is genetic and also responds to anti-ET drugs (Kralic et al., 2005). We tested two drugs with clinical anti-absence activity, ethosuximide and zonisamide (Wilfong and Schultz, 2005), as well as the neuroactive steroid ECN, the 3,4-dihydroquinazoline derivative KYS05064, and the mibefradil derivative NNC 55-0396, three compounds with in vitro activity against T-type calcium channels (Todorovic et al., 1998; Park et al., 2006; Huang et al., 2004). Despite the structural dissimilarities among these five compounds (Fig. 1), all demonstrated anti-tremor efficacy in both models, suggesting that T-type calcium channels represent a credible therapeutic target for ET.
Fig. 1.
Structures of T-type calcium channel antagonists tested for anti-tremor efficacy.
2. Materials and methods
2.1. Animals
Male ICR mice (20–24 g, Harlan, Indianapolis, Indiana, USA) were used in harmaline experiments. Cav3.1-null mice on a C57BL/6 background, created by K. Sakimura by deleting exon 5 of the cacna1g gene as previously described (Petrenko et al., 2007), were obtained from Riken as embryos, and bred with C57BL/6 wild-type mice and genotyped at Jackson Labs before delivery to the Veterans Affairs animal facility where they were used in harmaline experiments. Genotyping was repeated at our facility on animals utilized in this paper to confirm the genotype. GABAA receptor α1 heterozygous mice (+/−) of the F10+ generation on a mixed genetic background (~25% C57BL/6J, ~25% strain 129/Sv/SvJ, and ~50% FVB/N) were generated in Pittsburgh as previously described (Vicini et al., 2001). Heterozygotes were shipped to the VA Greater Los Angeles and subsequently interbred to produce knockout littermates for the current studies. Mice were housed in groups with free access to rodent diet and water. Genotyping was performed using previously described primers and methods (Ortinski et al., 2004). Experiments were approved by the Institutional Animal Care and Use Committee, in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health. All efforts were made to reduce animal suffering, and the number used was kept to a minimum.
2.2. Drugs
Harmaline, pentylenetetrazole, ethosuximide, zonisamide, NNC 55-0396 [(1S,2S)-2-(2-(N-[(3-benzimidazol-2-yl)propyl]-N-methylamino)ethyl)-6-fluoro-1,2, 3,4-tetrahydro-1-isopropyl-2-naphtyl cyclopropanecarboxylate dihydrochloride], and (2-hydroxypropyl)-β-cyclodextrin were purchased from Sigma–Aldrich (St Louis, Missouri, USA). Harmaline, pentylenetetrazole, ethosuximide, zonisamide, and NNC 55-0396 were dissolved in sterile physiological saline. KYS05064 (3-Ethyl-2-(piperidin-1-yl)-4-[N-4-(p-toluenesulfonamido)benzylacetamido]-3,4-dihydroquinazoline) was synthesized by author J. Y. Lee and dissolved in 10% DMSO/saline. ECN ((3β,5α,17β)-17-hydroxyestrane-3-carbonitrile) was synthesized by authors K. Krishnan and D. F. Covey and dissolved in (2-hydroxypropyl)-β-cyclodextrin 45% w/v in water. Harmaline was injected s.c., other drugs i.p., in a volume of 4 ml/kg. When available, the literature guided the choice of dose. In addition we utilized the “horizontal wire test” to assess for potential sedation or ataxia (Vanoveret al.,1999). In this test, mice are held by the base of the tail, their forepaws brought in contact with a 2-mm diameter, 25-cm long horizontal wire, then released. In order to be scored a pass, mice had to bring at least one hindpaw in contact with the wire and not to fall within 10 s. With each compound the dose was determined at which all 6/6 ICR mice passed on all tests at 10-min intervals over 1.5 h, and the dose at which 3/6 failed the test. In each model, pilot experiments were performed to find the dosages capable of suppressing tremor by approximately 50% and 80%. Some α1-null mice were used in more than one experiment; at least 3 days separated experiments.
2.3. Tremor measurement in the harmaline model
Motion activity was measured with a Convuls-1 Replacement Sensing Platform model 1335-1A (Columbus Instruments, Columbus, Ohio, USA), a metal platform with a load sensor beneath it, connected to a Grass model P511 AC amplifier (Grass Instruments, West Warwick, Rhode Island, USA) with 1 and 70 Hz filter settings. Digitally recorded motion power was analyzed using Spike2 software (Cambridge Electronic Design, United Kingdom) to perform Fourier transformation of the data into frequency spectra. Data were sampled at 128 Hz. The motion power percentage (MPP) is the tremor bandwidth divided by overall motion power: (10–16 Hz power)/ (0–34 Hz power) × 100. Baseline motion data were collected for 20 min, then harmaline, 20 mg/kg, administered, followed by test drug or vehicle 15 min later (time 0), when tremor had fully developed. Motion power was recorded for five successive 20-min epochs.
2.4. Tremor measurement in the GABAA receptor subunit α1-null model
Tremor was measured using the above apparatus while the mouse was suspended by the base of the tail with a padded clip from the ceiling of a plexiglass box resting on the motion detector platform. Tremor-associated motion power data (usually 22–27 Hz) were sampled at 454 Hz for 30 s, then the mouse returned to its cage. Each mouse had tremor sampled four times with 1-min rest periods in between during baseline, and again four times at a specified time after drug treatment. The mean 22–27 Hz motion power from each quartet of measures was used for data analysis.
2.5. Pentylenetetrazole seizures
Mice received ethosuximide, 100 mg/kg, ECN, 50 mg/kg, NNC 55-0396, 40 mg/kg, or vehicle, followed 30 min later by pentylenetetrazole, 70 mg/kg i.p. Each mouse was then placed in a plexiglass chamber and videotaped for 600 s. Each videotape was later viewed by an examiner blinded to treatments. Analyzed measures were latency to first myoclonic jerk, latency to first seizure, maximum seizure severity attained. If no myoclonic jerk or seizure occurred, the mouse was assigned the score 600 s. Seizure severity was rated (Löscher et al., 1991) as follows: 0, no seizure activity; 1, myoclonic jerks; 2, generalized clonic seizure without loss of righting reflex; 3, generalized clonic seizure with loss of righting reflex; 4, loss of righting reflex with forelimb tonus; 5, loss of righting reflex with hindlimb tonus.
2.6. Statistical analysis
A repeated measures ANOVA model was applied to MPP values in each harmaline experiment and dose effects tested for. In addition, post-hoc Student t-tests compared drug-treated groups with the vehicle-treated group under the model using the Tukey–Fisher significance criterion, employing JMP (SAS Inc., Cary, North Carolina, USA).
We used the formula 100 × (1 − [(DT − DB)/(mean VT − mean VB)]), to calculate the mean percentage by which tremor was suppressed compared to the vehicle-treated group, where DT is MPP during a drug treatment epoch, DB is MPP during the mouse’s baseline, mean VT is the vehicle group mean MPP for the same epoch as the drug treatment, and mean VB is mean MPP for the vehicle group during baseline.
For GABAA receptor α1-null mice the measure of analysis was the percent change in the mean motion power for the tremor bandwidth during drug treatment compared to the mean power during baseline. Student’s t-test was employed to determine whether the percent change in tremor was greater during drug treatment than for vehicle treatment. In the pentylenetetrazol seizure model, measures were compared between vehicle and drug-treated groups using the Wilcoxon rank sum test. P values less than 0.05 were considered significant.
3. Results
3.1. Comparison of the two tremor models
Harmaline rapidly induces whole body postural and kinetic tremor that in mice lasts over 1.5 h. Visible tremor corresponds to the generation of a digitized spectral motion peak at 10–16 Hz; drugs such as propranolol that suppress visible tremor reduce this peak as we have previously described (Martin et al., 2005). The percentage of digitized 10–16 Hz motion power to background 0–34 Hz motion power (motion power percentage, MPP) as the tremor measure of analysis serves to reduce variability due to fluctuating activity levels. In untreated mice, this is approximately 30%, representing the normal non-tremor motor activity falling within 10–16 Hz. Values in excess of this correspond to visible tremor (Martin et al., 2005).
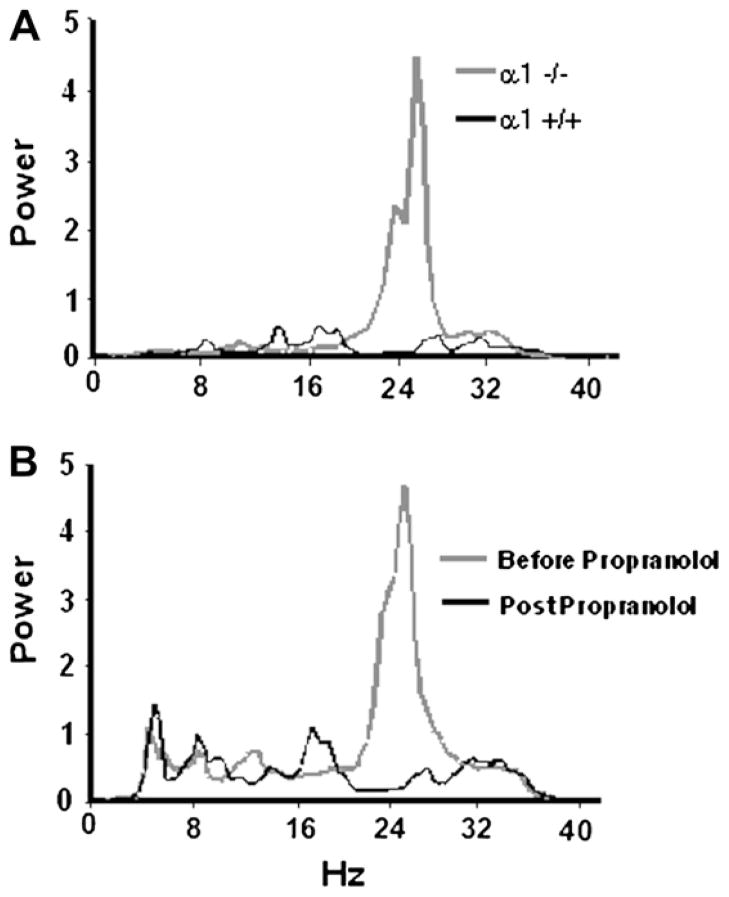
In the GABAA α1-null model, tremor is evident after weaning and remains throughout adulthood. Confirming previous observations by Kralic et al. (2005), we found that tail suspension reliably elicits tremor lasting at least 30 s. In contrast to the harmaline model, the normal erect position on all 4 paws does not reliably elicit tremor. Tremor in α1-null mice is associated with a motion power peak not occurring in heterozygote or wild-type mice (Fig. 2A), most commonly at 22–27 Hz. Similar to clinical ET, this tremor-associated peak is propranolol-sensitive (Fig. 2B), as previously demonstrated by Kralic et al. (2005). The tremor frequency peak varies slightly between animals so that the 5-Hz tremor band falls within an 18–29 Hz range. Pilot trials identified the 5-Hz frequency band to be utilized for each mouse in subsequent experiments.
Fig. 2.
Spectral motion power in GABAA receptor α1 subunit model. (A) During a 30-s tail suspension, an α1-null mouse displays a tremor-associated motion power peak at 22–27 Hz. By comparison, a wild-type mouse does not have tremor or display this peak. (B) Motion power spectra of an α1-null mouse before and after administration of the anti-ET drug propranolol, 20 mg/kg i.p. Corresponding to tremor suppression, the 22–27 Hz motion power peak is eliminated by propranolol.
3.2. Ethosuximide suppresses tremor in the harmaline and GABAA α1-null models
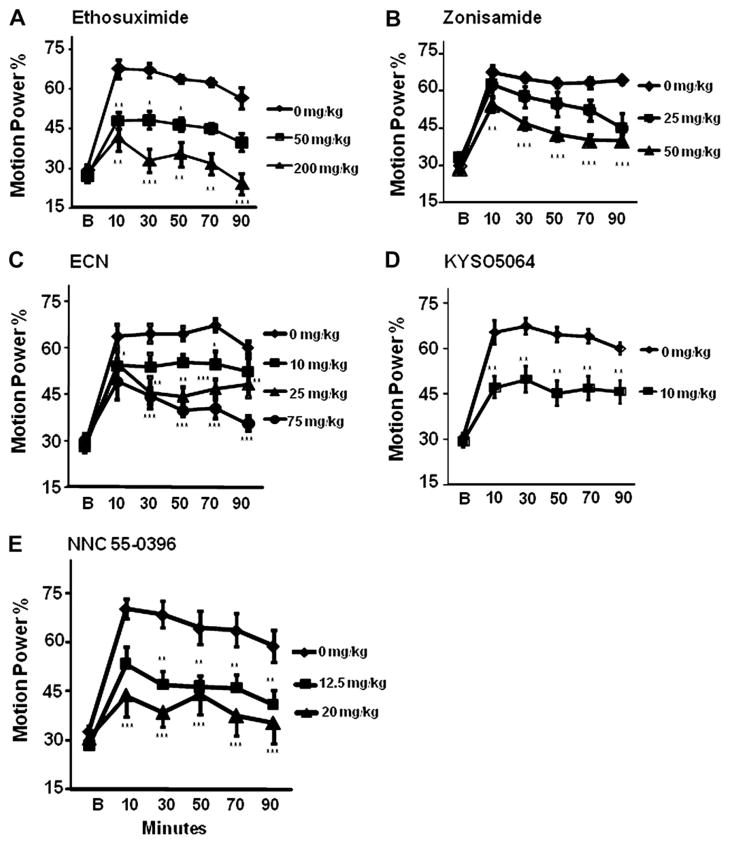
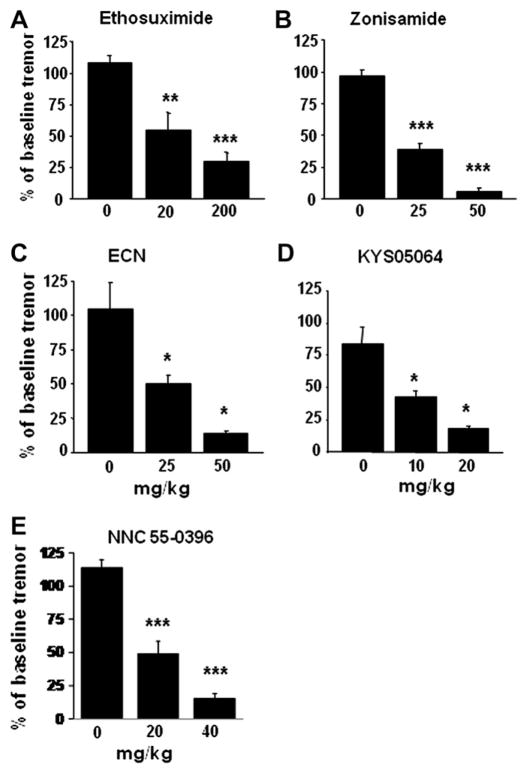
The succinimide derivative ethosuximide is a clinical anti-absence seizure medication. At 400 mg/kg, 3/6 mice failed the horizontal wire test, whereas 6/6 passed at 300 mg/kg. At 200 mg/ kg, a dose that suppresses absence seizures in mice (Aizawa et al., 1997), it reduced harmaline-induced tremor by 80.8% at 20–40 min and by 66.4% at 40–60 min after administration, and in a dose of 50 mg/kg by 43.5% and 43.2% compared to harmaline-vehicle mice at these times (Fig. 3A). In the GABAA α1-null model, tremor was reduced at 1 h after ethosuximide, 200 mg/kg, to a degree comparable to that in the harmaline model, while 20 mg/ kg was sufficient to reduce tremor by approximately 50% (Fig. 4A).
Fig. 3.
Effect of T-type calcium antagonists on tremor in harmaline mouse model. Motion data were collected in a 20-min Baseline epoch (B), then harmaline, 20 mg/kg s.c. administered, followed by compounds or vehicle i.p. 15 min later (at time 0). The panels display motion power in the tremor bandwidth (10–16 Hz) as a percent of overall motion power (0–34 Hz) for Baseline and post-drug 20-min epochs (mid-epoch times shown). Doses of ethosuximide and other drugs employed in these experiments were those that had been determined not to cause sedation or motor impairment as assessed in the horizontal wire test in separate ICR mice not administered harmaline. (A) Ethosuximide, (B) zonisamide, (C) ECN, (D) KYS05064, (E) NNC 55-0396 each significantly suppressed harmaline tremor. n = 6 per group. Error bars = SEM. *p < 0.05 **p < 0.005 ***p < 0.001.
Fig. 4.
Response of tremor in GABAA receptor α1-null mice to treatments during re-test compared to pre-drug baseline tremor. Tremor was assessed at 30 min (zonisamide, KYS05064, ECN, NNC 55-0396, or vehicle) or at 60 min post-injection (etho-suximide or vehicle). Each compound suppressed tremor compared to the vehicle treatment. n = 5–8 animals. Error bars = SEM. +p < 0.01, **p < 0.001, ***p < 0.0001.
Ethosuximide has maximal T-type calcium inhibitory activity that is only partial. Although this action has been reported to be operative at pharmacologically relevant concentrations (Gomora et al., 2001), whether ethosuximide’s actions on sodium and potassium channels may contribute to its clinical effect has been a point of discussion. Thus the finding that ethosuximide suppresses tremor in both models is suggestive for T-type channel involvement in tremor expression but requires corroboration.
3.3. Zonisamide suppresses tremor in the harmaline and GABAA α1-null models
The benzisoxazole derivative zonisamide, also clinically effective for absence seizures, similarly exerts partial maximal inhibition of T-type calcium channels (Kito et al., 1996). At 225 mg/kg, 3/6 mice failed the horizontal wire test, whereas 6/6 passed at 100 mg/kg. A dose of 25 mg/kg suppressed tremor by 30.8% at 20–40 min and 35.1% at 40–60 min, with a progressive decline so that tremor was suppressed by 60.8% at 80–100 min. Zonisamide, 50 mg/kg, reduced tremor by 48.6% and 58.9% at 20–40 min and 40–60 min, with inhibition by 67.8% at 80–100 min (Fig. 3B). Our finding of harmaline tremor suppression in mice extends a similar observation in rats (Miwa et al., 2008). In the α1-null model, zonisamide appeared to be more potent, with 50 mg/kg suppressing tremor by 93.8%, and 25 mg/kg by 61.6% at 30 min post administration (Fig. 4B).
Like ethosuximide, zonisamide does not enhance GABA currents (Rock et al., 1989). In addition to blocking T-type channels, zonisamide antagonizes sodium channels and carbonic anhydrase (Baulac, 2006). The latter properties are not likely to account for zonisamide’s anti-tremor effect, as drugs with these properties such as carbamazepine, phenytoin, and acetazolamide generally lack efficacy for ET (Gunal et al., 2000). Given the off-target actions of zonisamide and ethosuximide, we sought to extend these observations by testing other T-type calcium channel antagonists. The novel compounds ECN, KYS05064, and NNC 55-0396 have been reported to block T-type calcium channels potently in vitro. Of these, systemic administration of only ECN has been reported (Latham et al., 2009).
3.4. The neuroactive steroid ECN suppresses harmaline and genetic tremor
ECN, a 5α-reduced steroid with a carbonitrile side chain but no C-19 methyl group, was found during structure–activity studies at Washington University to demonstrate high selectivity for T-type calcium current antagonism. It is a partial antagonist, with maximal inhibition of 41.6% in dorsal root ganglion cells, but a low IC50 at 0.3 μM. It is much less potent against high voltage calcium channels in these cells (IC50’s: 9–16 μM). ECN does not potentiate the activity of GABA receptors, or exert more than minimal effects on sodium or potassium channels at 10 μM (Todorovic et al., 1998; Nakashima et al., 1999).
We found that at 100 mg/kg 3/6 mice fail the horizontal wire test, whereas 6/6 passed at 75 mg/kg. ECN, 10 mg/kg, suppressed harmaline tremor by 30.8% and 26.7% at 20–40 and 40–60 min after injection, and at 25 mg/kg by 51.7% and 54.9% at these times, and at 75 mg/kg by 60.7% and 73.4% at these times, respectively (Fig. 3C). In the GABAA α1-null model, ECN at 25 mg/kg reduced tremor by 49.8% at 30 min, comparable to that seen with harmaline tremor. The 50 mg/kg dose was sufficient to reduce tremor by 85.8% (Fig. 4C).
3.5. KYS05064 suppresses harmaline and genetic tremor
A group led by author J.Y. Lee at Kyung Hee University, Seoul, found that the 3,4-dihydroquinazoline KYS05064, with piperidine and ethyl side chains (Fig. 1) has an IC50 of 1.0 μM against T-type calcium currents, comparable to that of mibefradil. It has less activity against hERG (IC50: 4.2 μM), and none against N-type channels (Parket al., 2006). We found that at a dose of 75 mg/kg, KYS05064 caused 3/ 6 mice to fail the horizontal wire test, whereas 6/6 passed at 40 mg/ kg. KYS05064, 10 mg/kg, suppressed harmaline tremor by 44.7% and 53.3% at 20–40 and 40–60 min after injection respectively. Tremor suppression was apparent within 20 min, suggesting that it readily enters the brain, and was stable throughout the 100-min experiment (Fig. 3D). The dose 30 mg/kg, although well tolerated in the horizontal wire test as sole therapy, was associated with sedation when combined with harmaline and thus could not be tested. This may have been due to a pharmacokinetic interaction with harmaline. In the genetic model, KYS05064,10 mg/kg, suppressed tremor by 58.0% 30 min post-injection, and 20 mg/kg by 82.2% (Fig. 4D).
3.6. NNC 55-0396 suppresses harmaline and genetic tremor
Mibefradil has high potency for T-type calcium channels, but an ester side chain is cleaved intracellularly, forming an L-type calcium channel antagonist, so that mibefradil loses specificity. A group led by M. Li substituted cyclopropane for the methoxy moiety on the side chain, creating the mibefradil derivative NNC 55-0396 (Fig. 1) that resists cleavage (Huang et al., 2004). NNC 55-0396 exerts no effect against high voltage calcium channels at 100 μM, but inhibits Cav3.1 T-type channels as potently as mibefradil (IC50 6.8 versus 10.1 μM). We found that at a dose of 300 mg/kg, NNC 55-0396 caused 3/6 mice to fail the horizontal wire test, whereas 6/6 passed at 200 mg/kg. NNC 55-0396, 12.5 mg/kg, suppressed harmaline tremor by 48.1% and 43.6% at 20–40 and 40–60 min after injection respectively, while 20 mg/kg suppressed tremor by 78.0% and 57.1% at these times respectively (Fig. 3E). In the α1-null genetic model, NNC 55-0396, 20 and 40 mg/kg, suppressed tremor by 54.2% and 88.3% respectively (Fig. 4E).
3.7. Effect of Cav3.1 deletion on harmaline-induced tremor
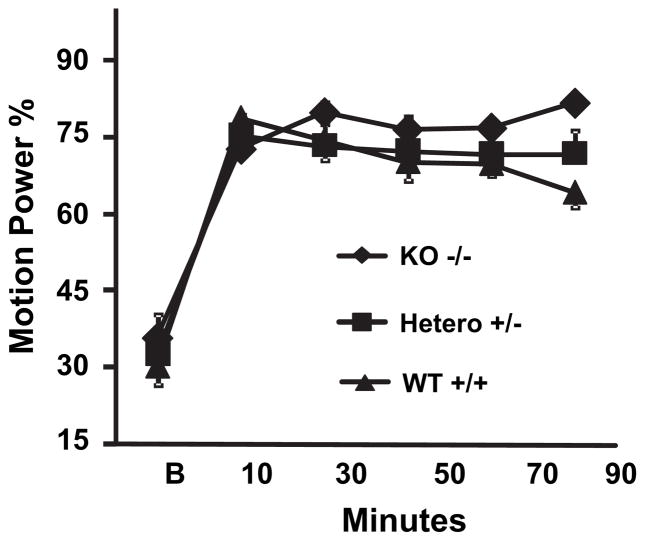
Because the Cav3.1 subtype of T-type calcium channels is dominant in the inferior olive, it was anticipated that Cav3.1 KO mice may show a reduced tremor response to harmaline. As shown in Fig. 5, Cav3.1-null and -heterozygous mice displayed as much tremor response during a 100-min exposure to harmaline as did wild-type controls (p = 0.10, 0.79 respectively).
Fig. 5.
Effect of Cav3.1 genotype on tremor response to harmaline. Cav3.1+/+, Cav3.1+/−, and Cav−/− mice were injected will harmaline, 20 mg/kg s.c. and motion power accessed in successive 20-min epochs starting at 15 min post-injection, as described in Fig. 3. Heterozygote and null Cav3.1-deficient mice displayed as much tremor as wild-type controls. n = 6 per group. Error bars = SEM.
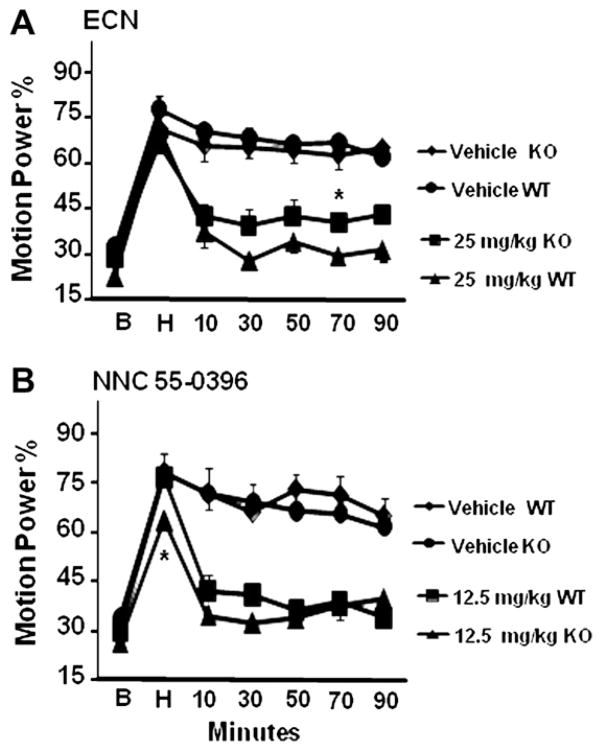
We next asked whether the preserved tremor response to harmaline in KO mice might be mediated by T-type calcium channels or was T-type channel-independent. Accordingly, following a harmaline tremor epoch, ECN, 25 mg/kg or vehicle, was administered to Cav3.1 KO and WT mice. Prior to drug treatment, harmaline induced as much tremor in KO mice as in WT mice, and in vehicle-treated mice the tremor was as sustained (Fig. 6A). ECN suppressed tremor in KO mice to a degree comparable to that seen in WT mice, with a difference seen only at 60–80 min post-injection (p < 0.01; Fig. 6A). Similarly, NNC 55-0396, 12.5 mg/kg, suppressed tremor to a comparable degree in Cav3.1 KO and WT mice, with no statistical difference at any time point post-drug administration (Fig. 6B).
Fig. 6.
Effect of Cav3.1 genotype on harmaline tremor suppression by ECN and NNC 55-0396. Cav3.1 wild-type (WT) or knockout (KO) mice were administered harmaline and motion power measured as described in Fig. 3, but tremor was also measured during a 20-min post-harmaline-injection (H) epoch prior to treatments with drugs or vehicle. (A) Harmaline-induced tremor was comparable in KO and WT mice before treatments and after vehicle treatment. ECN, 25 mg/kg, potently suppressed tremor in both WT and KO groups to a comparable degree. (B) NNC 55-0396, 12.5 mg/kg, potently and similarly suppressed tremor in WT and KO mice. n = 6 per group. Error bars = SEM. *p < 0.05.
3.8. Effect of NNC 55-0396 and ECN on pentylenetetrazole seizures
The anti-absence drug ethosuximide prolongs latency to clonic seizures in the pentylenetetrazole model, as does zonisamide. If the novel T-type calcium antagonists tested above for tremor share common mechanisms with ethosuximide and zonisamide, they should exhibit anti-seizure efficacy. We tested NNC 55-0396, 40 mg/kg, and ECN, 50 mg/kg, and compared their effects in this model with those of ethosuximide, 100 mg/kg. Ethosuximide robustly prevented the occurrence of myoclonic jerks or prolonged the latency to the first jerk (p = 0.009, Fig. 7A), and suppressed clonic seizures as indicated by the latency measure (p < 0.001, Fig. 7B) or maximum seizure severity attained, which on average was only myoclonic jerking (p < 0.001, Fig. 7C). NNC 55-0396 similarly prolonged latency to first myoclonic jerk (p < 0.001), and reduced maximal seizure severity (p = 0.003), but did not significantly prolong latency to first seizure (p = 0.12). ECN prolonged latency to first myoclonic jerk (p < 0.001), latency to first seizure (p = 0.003), and reduced maximal seizure severity (p = 0.008). These findings suggest that NNC 55-0396 and ECN, like ethosuximide, exert anti-epileptic effects in doses that do not cause impairment in the horizontal wire test, and likely enter the brain to do so.
Fig. 7.
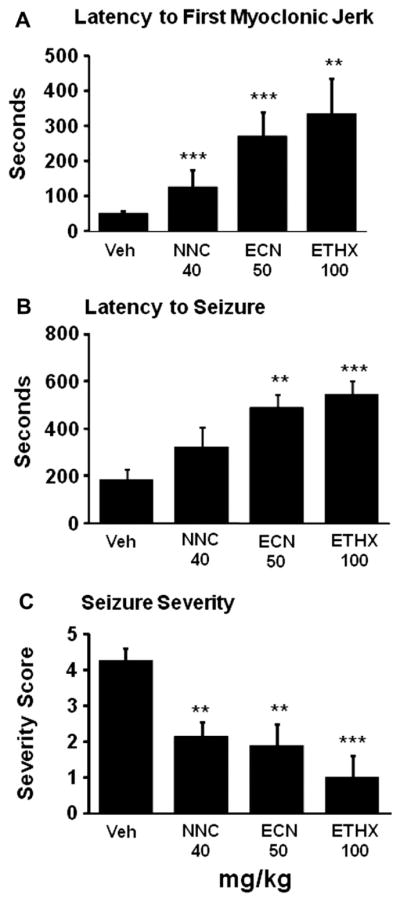
Effect of T-type calcium channel antagonists on epileptiform activity measures in the pentylenetetrazole seizure model. Vehicle (VEH), NNC 55-0396 (NNC), 40 mg/kg, ECN, 50 mg/kg, ethosuximide (ETHX), 100 mg/kg, were administered 30 min prior to pentylenetetrazole, 70 mg/kg, and behavior recorded for 600 s. n = 8–11 per group. Means and SEMs are shown. **p < 0.01, ***p < 0.001, Wilcoxon rank sum.
4. Discussion
ET is a common movement disorder, affecting 4.0–4.6% of persons above age 65, with half seeking medical treatment, yet specific molecular targets for therapeutic development have been lacking. The utilization of two animal models offers a strategy to achieve progress towards this goal. Harmaline induces a postural/ kinetic tremor that, like ET, is associated with cerebellar metabolic hyperactivation (Bernard et al., 1984; Bucher et al., 1997), and responds to ethanol, propranolol, primidone, 1-octanol, and benzodiazepines reviewed by Martin et al., 2005; Stöhr et al., 2008. Harmaline-induced tremor is the most-studied ET model and has received increasing attention as a pre-clinical screening tool. Tremor suppression by 1-octanol in the harmaline model successfully predicted clinical efficacy (Sinton et al., 1989; Bushara et al., 2004). Tremor in the GABAA receptor α1-null mouse genetic model is also postural/kinetic, but is most reliably seen with tail suspension. Tremor responds to the anti-ET agents ethanol, propranolol, 1-octanol, primidone, and gabapentin (Kralic et al., 2005). Tremor in this model is not drug-driven but genetic so that anti-tremor efficacy may potentially reflect clinical efficacy better, and pharmacokinetic interactions with harmaline are avoided. The α1-null mouse has thus also been advocated as a valuable ET model. This report is the first to utilize both models to assess anti-tremor efficacy of novel compounds.
We employed five T-type calcium channel antagonists, reasoning that, given their different structures (Fig. 1), it would be unlikely that a shared off-target action would explain a similar action on tremor in the two models. The dose at which half the mice failed the horizontal wire test exceeded the dose required to suppress half the tremor in the two models by 4- to 15-fold, and dosages used in the efficacy studies were not associated with horizontal wire test failures, thus sedation, ataxia or loss of muscle tone appear unlikely to explain these results. Although it is possible that effects on sodium (or potassium) channels could underlie tremor suppression by ethosuximide and zonisamide, this would not apply to ECN, which has negligible effects on these channels. Unlike 1-octanol, ECN also does not enhance GABA currents (Todorovic et al.,1998). NNC 55-0396 is derived from the classic and highly specific T-type calcium channel antagonist mibefradil, but is even more specific in that it is not metabolized to an L-type calcium channel antagonist (Huang et al., 2004).
These results are reinforced by recent findings with two potent and selective T-type calcium antagonists, fluoropiperidine derivatives, reported by Merck workers to suppress tremor in the harmaline model (Shipe et al., 2008; Yang et al., 2008). The compound in the Yang et al. report suppressed harmaline tremor markedly and inhibited experimental absence seizures in rat after oral 1 mg/kg administration, with a plasma level of 33 nM, well below the nearest off-target binding site affinity,1.4 μM, uncovered in a screen of 170 receptors, channels and enzymes.
These combined findings that seven compounds with highly different structures, but sharing the property of T-type calcium channel antagonism, suppress experimental essential tremor strongly suggests that this channel plays an important role in tremor expression, and represents a credible therapeutic target for ET. Interestingly, L-type calcium channel antagonists with significant T-type antagonist activity, nimodipine and nicardipine, have been reported effective for ET in small clinical trials (Biary et al., 1995; Jiménez-Jiménez et al., 1994), whereas nifedipine, with poor T-type channel antagonism, does not suppress ET tremor (Topaktas et al., 1987).
An attractive hypothesis is that just as absence seizures reflect excessive synchrony in the T-type calcium channel-dependent thalamocortical oscillatory circuit, ET similarly results from excessive synchrony in T-type calcium channel-dependent olivocerebellar pathways. Given the critical role of inferior olive burst-firing for tremor expression in the harmaline model (Bernard et al., 1984), it was logical to conjecture that olivary Cav3.1 channels are a critical link in the expression of tremor. However we found that Cav3.1-null mice exhibit just as much tremor in response to harmaline as wild-type mice controls. The ability of Cav3.1 KO mice to exhibit tremor does not appear due to developmental compensations resulting in a non-T-type channel-dependent tremor mechanism, as these animals still show suppression of tremor by the specific T-type antagonists ECN and NNC 55-0396. We hypothesize that, in addition to enhancing Cav3.1 calcium channel currents in the inferior olive, harmaline may also activate rebound bursting via Cav3.2 or Cav3.3 channels to induce tremor, although the location of this action within the cerebellum is not clear. Cav3.2 and 3.3 channels are found on some deep cerebellar neurons and on stellate and basket interneurons (Molineux et al., 2006). NNC 55-0396 has been shown in whole cell experiments to block rebound firing in deep cerebellar neurons (Alviňa et al., 2009). Mibefradil, the parent molecule of NNC 55-0396, is a potent Cav3.2 antagonist (Perez-Reyes et al., 2009), thus NNC 55-0396 likely blocks Cav3.2 as well. ECN blocks Cav3.2 channels; mice deficient for these channels fail to show the expected analgesic response to systemically administered ECN (Latham et al., 2009).
Our findings in a pilot evaluator-blinded trial of zonisamide for ET suggest efficacy (Handforth et al., 2009). Zonisamide’s drawbacks include limited maximal T-type current inhibition, and off-target actions that may be responsible for limiting tolerability. With the recent upsurge of interest in developing selective and potent T-type calcium channel antagonists, we anticipate that promising candidates will emerge for evaluation in ET clinical trials.
Acknowledgments
Cav3.1 mice embryos were provided by the Riken BRC through the National Bio-Resource Project of the MEXT, Japan. This work was supported by the International Essential Tremor Foundation (AQ), The Ralph M. Parsons Foundation, Veterans Affairs (AH), NIH AA10422 (GEH), NIH GM47969 (DFC), and Vision 21 Program, Korea Institute of Science and Technology (JYL). We are grateful for the technical assistance of Brandon Bunker, Ana Artero, and Christopher Shin.
References
- Aizawa M, Ito Y, Fukuda H. Pharmacological profiles of generalized absence seizures in lethargic, stargazer and γ-hydroxybutyrate-treated model mice. Neuroscience Research. 1997;29:17–25. doi: 10.1016/s0168-0102(97)00066-7. [DOI] [PubMed] [Google Scholar]
- Alviňa K, Ellis-Davies G, Khodakhah K. T-type calcium channels mediate rebound firing in intact deep cerebellar neurons. Neuroscience. 2009;158:635–641. doi: 10.1016/j.neuroscience.2008.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrad JE, Louis ED, Honig LS, Flores I, Ross GW, Pahwa R, Lyons KE, Faust PL, Vonsattel JP. Reduced Purkinje cell number in essential tremor: a postmortem study. Archives of Neurology. 2008;65:101–107. doi: 10.1001/archneurol.2007.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulac M. Introduction to zonisamide. Epilepsy Research. 2006;68 (Suppl 2):S3–S9. doi: 10.1016/j.eplepsyres.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Buisseret-Delmas C, Compoint C, Laplante S. Harmaline induced tremor. III. A combined simple units, horseradish peroxidase, and 2-deoxyglucose study of the olivocerebellar system in the rat. Experimental Brain Research. 1984;57:128–137. doi: 10.1007/BF00231139. [DOI] [PubMed] [Google Scholar]
- Biary N, Bahou Y, Soff MA, Thomas W, al Deeb SM. The effect of nimodipine on essential tremor. Neurology. 1995;45:1523–1525. doi: 10.1212/wnl.45.8.1523. [DOI] [PubMed] [Google Scholar]
- Bucher SF, Seelos KC, Dodel RC, Reiser M, Oertel WH. Activation mapping in essential tremor with functional magnetic resonance imaging. Annals of Neurology. 1997;41:32–40. doi: 10.1002/ana.410410108. [DOI] [PubMed] [Google Scholar]
- Bushara KO, Goldstein SR, Grimes GJ, Jr, Burstein AH, Hallett M. Pilot trial of 1-octanol in essential tremor. Neurology. 2004;62:122–124. doi: 10.1212/01.wnl.0000101722.95137.19. [DOI] [PubMed] [Google Scholar]
- Dildy-Mayfield JE, Mihic SJ, Liu Y, Deitrich RA, Harris RA. Actions of long chain alcohols on GABAA and glutamate receptors: relation to in vivo effects. British Journal of Pharmacology. 1996;118:378–384. doi: 10.1111/j.1476-5381.1996.tb15413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomora JC, Daud AN, Weiergräber M, Perez-Reyes E. Block of cloned human T-type calcium channels by succinimide antiepileptic drugs. Molecular Pharmacology. 2001;60:1121–1132. [PubMed] [Google Scholar]
- Gunal DI, Afşar N, Bekiroglu N, Aktan S. New alternative agents in essential tremor therapy: double-blind placebo-controlled study of alprazolam and acetazolamide. Neurological Sciences. 2000;21:315–317. doi: 10.1007/s100720070069. [DOI] [PubMed] [Google Scholar]
- Handforth A, Martin FC, Kang GA, Vanek Z. Zonisamide for essential tremor: an evaluator-blinded study. Movement Disorders. 2009;24:437–440. doi: 10.1002/mds.22418. [DOI] [PubMed] [Google Scholar]
- Huang L, Keyser BM, Tagmose TM, Hansen JB, Taylor JT, Zhuang H, Zhang M, Ragsdale DS, Li M. NNC 55-0396 [(1 S,2 S)-2-(2-(N-[(3-benzimidazol-2-yl)propyl]-N-methylamino)ethyl)-6-fluoro-1,2, 3,4-tetrahydro-1-isopropyl-2-naphtyl cyclopropanecarboxylate dihydrochloride]: a new selective inhibitor of T-type calcium channels. Journal of Pharmacology and Experimental Therapeutics. 2004;309:193–199. doi: 10.1124/jpet.103.060814. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, McCormick DA. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends in Neuroscience. 2007;30:350–356. doi: 10.1016/j.tins.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Jiménez-Jiménez FJ, Garcia-Ruiz PJ, Cabrera-Valdivia F. Nicardipine versus propranolol in essential tumor. Acta Neurologica (Napoli) 1994;16:184–188. [PubMed] [Google Scholar]
- Kim D, Song I, Keum S, Lee T, Jeong MJ, Kim SS, McEnery MW, Shin HS. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking α1G T-type Ca2+ channels. Neuron. 2001;31:35–45. doi: 10.1016/s0896-6273(01)00343-9. [DOI] [PubMed] [Google Scholar]
- Kito M, Maehara M, Watanabe K. Mechanisms of T-type calcium channel blockade by zonisamide. Seizure. 1996;5:115–119. doi: 10.1016/s1059-1311(96)80104-x. [DOI] [PubMed] [Google Scholar]
- Köster B, Deuschl G, Lauk M, Timmer J, Guschlbauer B, Lücking CH. Essential tremor and cerebellar dysfunction: abnormal ballistic movements. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;73:400–405. doi: 10.1136/jnnp.73.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralic JE, Criswell HE, Osterman JL, O’Buckley TK, Wilkie ME, Matthews DB, Hamre K, Breese GR, Homanics GE, Morrow AL. Genetic essential tremor in γ-aminobutyric acidA receptor α1 subunit knockout mice. Journal of Clinical Investigation. 2005;115:774–779. doi: 10.1172/JCI23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham JR, Pathirathna S, Jagodic MM, Joo Choe W, Levin ME, Nelson MT, Yong Lee W, Krishnan K, Covey DF, Todorovic SM, Jevtovic-Todorovic V. Selective T-type calcium channel blockade alleviates hyperalgesia in ob/ob mice. Diabetes. 2009;58:2656–2665. doi: 10.2337/db08-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982;297:406–408. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- Llinás R, Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. Journal of Physiology. 1981;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher W, Hönack D, Fassbender CP, Nolting B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. III. Pentylenetetrazole seizure models. Epilepsy Research. 1991;8:171–189. doi: 10.1016/0920-1211(91)90062-k. [DOI] [PubMed] [Google Scholar]
- Martin FC, Thu Le A, Handforth A. Harmaline-induced tremor as a potential preclinical screening method for essential tremor medications. Movement Disorders. 2005;20:298–305. doi: 10.1002/mds.20331. [DOI] [PubMed] [Google Scholar]
- Miwa H, Hama K, Kajimoto Y, Kondo T. Effects of zonisamide on experimental tremors in rats. Parkinsonism and Related Disorders. 2008;14:33–36. doi: 10.1016/j.parkreldis.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Molineux ML, McRory JE, McKay BE, Hamid J, Mehaffey WH, Rehak R, Snutch TP, Zamponi GW, Turner RW. Specific T-type calcium channel isoforms are associated with distinct burst phenotypes in deep cerebellar nuclear neurons. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5555–5560. doi: 10.1073/pnas.0601261103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima YM, Pereverzev A, Schneider T, Covey DF, Lingle CJ. Blockade of Ba2+ current through human α1E channels by two steroid analogs, (+)-ACN and (+)-ECN. Neuropharmacology. 1999;38:843–855. doi: 10.1016/s0028-3908(99)00013-1. [DOI] [PubMed] [Google Scholar]
- Ortinski PI, Lu C, Takagaki K, Fu Z, Vicini S. Expression of distinct α subunits of GABAA receptor regulates inhibitory synaptic strength. Journal of Neurophysiology. 2004;92:1718–1727. doi: 10.1152/jn.00243.2004. [DOI] [PubMed] [Google Scholar]
- Park SJ, Park SJ, Lee MJ, Rhim H, Kim Y, Lee JH, Chung BY, Lee JY. Synthesis and SAR studies of a novel series of T-type calcium channel blockers. Bioorganic & Medicinal Chemistry. 2006;14:3502–3511. doi: 10.1016/j.bmc.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, Van Deusen AL, Vitko I. Molecular pharmacology of human Cav3.2 T-type Ca2+ channels: block by antihypertensives, antiarrhythmics, and their analogs. Journal of Pharmacology and Experimental Therapeutics. 2009;328:621–627. doi: 10.1124/jpet.108.145672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrenko AB, Tsujita M, Kohno T, Sakimura K, Baba H. Mutation of α1G T-type calcium channels in mice does not change anesthetic requirements for loss of the righting reflex and minimum alveolar concentration but delays the onset of anesthetic induction. Anesthesiology. 2007;106:1177–1185. doi: 10.1097/01.anes.0000267601.09764.e6. [DOI] [PubMed] [Google Scholar]
- Rock DM, Macdonald RL, Taylor CP. Blockade of sustained repetitive action potentials in cultured spinal cord neurons by zonisamide (AD 810, CI 912), a novel anticonvulsant. Epilepsy Research. 1989;3:138–143. doi: 10.1016/0920-1211(89)90041-7. [DOI] [PubMed] [Google Scholar]
- Shipe WD, Barrow JC, Yang ZQ, Lindsley CW, Yang FV, Schlegel KA, Shu Y, Rittle KE, Bock MG, Hartman GD, Tang C, Ballard JE, Kuo Y, Adarayan ED, Prueksaritanont T, Zrada MM, Uebele VN, Nuss CE, Connolly TM, Doran SM, Fox SV, Kraus RL, Marino MJ, Graufelds VK, Vargas HM, Bunting PB, Hasbun-Manning M, Evans RM, Koblan KS, Renger JJ. Design, synthesis, and evaluation of a novel 4-aminomethyl-4-fluoropiperidine as a T-type Ca2+ channel antagonist. Journal of Medicinal Chemistry. 2008;51:3692–3695. doi: 10.1021/jm800419w. [DOI] [PubMed] [Google Scholar]
- Sinton CM, Krosser BI, Walton KD, Llinás RR. The effectiveness of different isomers of octanol as blockers of harmaline-induced tremor. Pflügers Archiv. 1989;414:31–36. doi: 10.1007/BF00585623. [DOI] [PubMed] [Google Scholar]
- Stöhr T, Lekieffre D, Freitag J. Lacosamide, the new anticonvulsant, effectively reduces harmaline-induced tremors in rats. European Journal of Pharmacology. 2008;589:114–116. doi: 10.1016/j.ejphar.2008.06.038. [DOI] [PubMed] [Google Scholar]
- Todorovic SM, Lingle CJ. Pharmacological properties of T-type Ca2+ current in adult rat sensory neurons: effects of anticonvulsant and anesthetic agents. Journal of Neurophysiology. 1998;79:240–252. doi: 10.1152/jn.1998.79.1.240. [DOI] [PubMed] [Google Scholar]
- Todorovic SM, Prakriya M, Nakashima YM, Nilsson KR, Han M, Zorumski CF, Covey DF, Lingle CJ. Enantioselective blockade of T-type Ca2+ current in adult rat sensory neurons by a steroid that lacks γ-aminobutyric acid-modulatory activity. Molecular Pharmacology. 1998;54:918–927. doi: 10.1124/mol.54.5.918. [DOI] [PubMed] [Google Scholar]
- Topaktas S, Onur R, Dalkara T. Calcium channel blockers and essential tremor. European Neurology. 1987;27:114–119. doi: 10.1159/000116142. [DOI] [PubMed] [Google Scholar]
- Vanover KE, Suruki M, Robledo S, Huber M, Wieland S, Lan NC, Gee KW, Wood PL, Carter RB. Positive allosteric modulators of the GABAA receptor: differential interaction of benzodiazepines and neuroactive steroids with ethanol. Psychopharmacology (Berlin) 1999;141:77–82. doi: 10.1007/s002130050809. [DOI] [PubMed] [Google Scholar]
- Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABAA receptor α1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. Journal of Neuroscience. 2001;21:3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfong A, Schultz R. Zonisamide for absence seizures. Epilepsy Research. 2005;64:31–34. doi: 10.1016/j.eplepsyres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Yang ZQ, Barrow JC, Shipe WD, Schlegel KA, Shu Y, Yang FV, Lindsley CW, Rittle KE, Bock MG, Hartman GD, Uebele VN, Nuss CE, Fox SV, Kraus RL, Doran SM, Connolly TM, Tang C, Ballard JE, Kuo Y, Adarayan ED, Prueksaritanont T, Zrada MM, Marino MJ, Graufelds VK, DiLella AG, Reynolds IJ, Vargas HM, Bunting PB, Woltmann RF, Magee MM, Koblan KS, Renger JJ. Discovery of 1,4-substituted piperidines as potent and selective inhibitors of T-type calcium channels. Journal of Medicinal Chemistry. 2008;51:6471–6477. doi: 10.1021/jm800830n. [DOI] [PubMed] [Google Scholar]