Abstract
Background
Follicular variant of papillary thyroid carcinoma (FV-PTC) has been increasingly diagnosed in recent years. However, little is known about its clinical behavior. The purpose of this study was to determine the disease characteristics of FV-PTC, and to compare it with classical papillary thyroid carcinoma (C-PTC) and follicular thyroid carcinoma (FTC).
Methods
All cases of C-PTC, FV-PTC, and FTC larger than 1 cm in the Surveillance, Epidemiology and End Results (SEER) Cancer Database from 1988 to 2007 were identified. Tumor behavior and patient survival were compared among these three groups. Different risk factors for disease-specific mortality in each group were evaluated by multivariate analysis.
Results
More than 36,000 surgical cases were identified, including 21,796 C-PTCs, 10,740 FV-PTCs, and 3958 FTCs. Extrathyroidal extension and lymph-node metastases were more common in FV-PTC than in FTC, but significantly less common than in C-PTC (p<0.0001). Distant metastasis rates were present in 2% of patients with FV-PTC, in 1% with C-PTC, and in 4% with FTC (p<0.0001). The 10-year disease-specific survival for patients with FV-PTC was 98%, similar to C-PTC (97%) but better than FTC (94%, p<0.0001). Being over the age of 45 years remained a strong risk factor for disease-specific mortality in both FV-PTC and C-PTC, while the presence of extrathyroidal extension and distant metastases were stronger predictors of disease-specific mortality in FV-PTC than in C-PTC.
Conclusions
FV-PTC is a common variant of PTC. Its clinical behavior is unique and represents an intermediate entity with clinical features that are between C-PTC and FTC. Interestingly, despite the variations in clinical behavior, the long-term outcome of these patients remains excellent and similar to C-PTC.
Introduction
Thyroid cancer is one of the most rapidly increasing malignancies in the United States. The incidence rate of thyroid cancer in 2007 almost doubled since 1997, climbing to 11.99 per 100,000 (1). Approximately 90% of thyroid cancer cases are well differentiated, and are usually classified as papillary thyroid carcinoma (PTC) or follicular thyroid carcinoma (FTC) based on the predominant histology (2). Over the past decade, it has been recognized that only about half of the PTC cases are of classical type (C-PTC), whereas the remaining 50% of cases are made up of various histologic variants (3,4). These PTC variants, while sharing similar nuclear and cytological features, are often diagnosed on the basis of their distinct histopathologic features (5).
Among all PTC variants, follicular variant of papillary thyroid carcinoma (FV-PTC) is the most common subtype of PTC, constituting between 9% and 22.5% of all PTC cases (4,6–8). In a more recent study, it has been found that FV-PTC accounts for up to 41% of PTC (9). FV-PTC was first described by Crile and Hazard in 1953 (10). Microscopically, it predominantly has a follicular architecture, which is lined by cells with nuclear features of papillary carcinoma (11). With the dramatic increasing incidence of thyroid cancer, FV-PTC has been increasingly diagnosed in recent years (1,12,13).
Despite the high incidence, the clinical behavior and outcomes of FV-PTC remain controversial, making it a challenge to establish a standard treatment protocol. Since FV-PTC contains a mixed histology of both PTC and FTC, it has been speculated that it inherits the features from both types and is thus more aggressive than C-PTC (14). Supporting evidence has been provided by some groups, who have documented that FV-PTC has a greater tendency for pulmonary metastases compared with C-PTC (6,15). Additionally, bone metastases have been reported in FV-PTC cases, although they are not very common (16,17). Regarding the prognosis, persistent and progressive disease rates were reported as 15% and 8% respectively in FV-PTC, which were much higher than those in C-PTC and FTC patients (18). On the contrary, in many other series, FV-PTC has been noted to have a less aggressive clinical behavior in terms of a lower incidence of lymph-node and distant metastasis while having a similar or even better long-term survival than C-PTC (3,7,9,19,20).
The lack of consensus in previous studies could be due to relative small sample sizes and the short mean duration of follow-up. Due to the nature of well-differentiated thyroid cancer, including PTC and FTC, larger patient populations are required with longer-term follow-up times to evaluate prognosis and risk factors for mortality. Most of the studies on FV-PTC have reported only a single institutional experience, and none have included more than a few thousand patients. The purpose of this study was to determine the disease characteristics of FV-PTC, compare its clinical behavior and survival, as well as risk factors with C-PTC and FTC in a large patient population from a national database.
Materials and Methods
Database
A retrospective cohort study was performed using data from the Surveillance, Epidemiology and End Results (SEER) Cancer Database (www.seer.cancer.gov) maintained by the National Cancer Institute. The characteristics and representativeness of the SEER database have been discussed previously (21–24). The data set used in the current study was released in April 2010, based on the November 2009 submission. Current SEER registries, representing 26% of the U.S. population, consist of the states of Connecticut, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico, and Utah; the metropolitan areas of Atlanta, Detroit, San Francisco-Oakland, Seattle-Puget Sound, and San Jose-Monterey; and the Alaska Native Tumor Registry, rural Georgia, Greater California, and Los Angeles County. Cancer incidence and survival data were collected from the above 17 population-based cancer registries. Aside from data on patient demographics, primary tumor site, tumor pathology, stage at diagnosis, and first course of treatment were also routinely collected by SEER registries. The University of Wisconsin institutional review board deemed this project to be exempt from review.
Case definition
All patients with PTC or FTC diagnosed between 1988 and 2007 were identified using a primary tumor site code of C739 (thyroid) in combination with the International Classification of Disease for Oncology, third edition (ICD-O-3) (25). Histology codes listed below were used to select patients of each category accordingly.
PTC included (i) C-PTC: 8050 (papillary carcinoma not otherwise specified, NOS), 8260 (papillary adenocarcinoma, NOS), 8343 (papillary carcinoma, encapsulated); (ii) FV-PTC: 8340 (papillary carcinoma, follicular variant); and (iii) other variants: 8052 (papillary squamous cell carcinoma), 8130 (papillary transitional cell carcinoma), 8342 (papillary carcinoma, oxyphilic cell), 8344 (papillary carcinoma, columnar cell).
FTC included 8330 (follicular adenocarcinoma, NOS), 8331 (follicular adenocarcinoma well differentiated), 8332 (follicular adenocarcinoma trabecular), and 8335 (follicular carcinoma, minimal invasive).
Only patients with a tumor size of more than 1 cm were included in the current study cohort. Patients with more than one primary site of malignancy were excluded. Cases without surgery and pathological diagnoses were also excluded from this analysis.
Data analysis
After identification of the study cohort in the SEER database, we first compared the demographics, tumor features, and treatment among the patients with C-PTC, FV-PTC, and FTC. The continuous variables were presented as mean±standard deviation (SD). Significance of the differences was calculated either by chi-square test for categorical variables or one way analysis of variance (ANOVA) for continuous variables.
The 10- and 15-year overall survivals were estimated using the Kaplan–Meier method, as well as disease-specific survivals (DSS). Tests of survival equality were performed during the survival analysis by the log-rank test. For multivariate analysis, Cox proportional hazards model was applied to assess the association between potential predictors and disease-specific mortality (26). The potential predictors were all clinically relevant and selected based on several different staging systems, including pTNM and AMES (27,28), for well-differentiated thyroid cancer. All the factors in the multivariate analysis, including tumor size and age, were analyzed as categorical variables. Age was analyzed either using a cut-off of 47 years or using 5- or 10-year increments. Hazard ratios (HR) with confidence intervals [CI] were used to quantify the strength of the association between predictors and mortality. All statistical analyses were performed using SAS v9.2 (SAS Institute, Cary, NC). All tests were two-sided, and a p value <0.05 was defined as statistically significant.
Results
In the SEER database, there were more than 95,000 thyroid cancer records. Our selection criteria identified 32,882 PTC patients and 3958 FTC patients after excluding microcarcinomas. All of the selected patients had surgery as the initial treatment. Among all the PTCs, 21,796 (66%) were C-PTC, 10,740 (33%) were FV-PTC, while the rest (1%) were of other variants. Other variants of PTC were excluded from further analysis.
Patient characteristics, tumor features, and treatments
The demographics, clinicopathologic features, and treatment of the three histologic subgroups are compared in Table 1. Age and sex distribution were similar among FV-PTC (46 years; 78% female), C-PTC (44 years; 76% female), and FTC patients (48 years; 73% female). The majority of FV-PTC patients were Caucasian (81%), comparable with C-PTC (82%) and FTC (79%).
Table 1.
Demographics, Clinicopathologic Features, and Treatment in Classical and Follicular Variant of Papillary Thyroid Carcinoma and in Follicular Thyroid Carcinoma
| Characteristic | C-PTC (21,796) | FV-PTC (10,740) | FTC (3958) |
|---|---|---|---|
| Age at diagnosis (years±SD) | 44±15 | 46±15 | 48±17 |
| Sex | |||
| Male (%) | 5126 (24) | 2320 (22) | 1075 (27) |
| Female (%) | 16,670 (76) | 8420 (78) | 2883 (73) |
| Ethnicity | |||
| Caucasian (%) | 17,783 (82) | 8724 (81) | 3114 (79) |
| African American (%) | 844 (4) | 792 (7) | 383 (10) |
| Others (%) | 3035 (14) | 1152 (11) | 434 (11) |
| Unknown (%) | 134 (0.6) | 72 (0.6) | 27 (0.7) |
| Tumor size (cm±SD) | 2.6±1.8 | 2.8±1.9 | 3.7±2.3 |
| Extrathyroidal extension | |||
| Yes (%) | 5499 (25) | 1624 (15) | 376 (9) |
| No (%) | 16,041 (74) | 8983 (84) | 3465 (88) |
| Unknown (%) | 256 (1) | 133 (1) | 117 (3) |
| Lymph-node metastasis | |||
| Yes (%) | 7420 (34) | 1668 (16) | 85 (2) |
| No (%) | 14,318 (66) | 9041 (84) | 3859 (98) |
| Unknown (%) | 58 (0.3) | 31 (0.3) | 14 (0.4) |
| Distant metastasis | |||
| Yes (%) | 260 (1) | 165 (2) | 158 (4) |
| No (%) | 21,536 (99) | 10,575 (98) | 3800 (96) |
| Total thyroidectomy | |||
| Yes (%) | 19,925 (91) | 9161 (85) | 2899 (73) |
| No (%) | 1871 (9) | 1579 (15) | 1059 (27) |
| Radioactive iodine | |||
| Yes (%) | 12,721 (58) | 6021 (56) | 1931 (49) |
| No (%) | 9075 (42) | 4719 (44) | 2027 (51) |
p Values were calculated by either chi-square test for categorical variables or one way ANOVA for continuous variables. All p values among the three groups for different variables were less than 0.0001.
C-PTC, classical papillary thyroid carcinoma; FV-PTC, follicular variant of papillary thyroid carcinoma; FTC, follicular thyroid carcinoma.
The mean size of the primary tumor of FV-PTC was 2.8±1.9 cm, relatively smaller than that of FTC (3.7 cm) but larger than that of C-PTC (2.6 cm). Extrathyroidal extension was more common in FV-PTC than in FTC (15% vs. 9%), but significantly less common than in C-PTC (25%, p<0.0001). The incidence of pathological confirmed lymph-node metastases was 16% in FV-PTC, which was eight times more common in comparison to FTC (2%), but less than half the rate seen in C-PTC (34%, p<0.0001). Distant metastases were present in 2% of patients with FV-PTC, 1% in C-PTC, and 4% in FTC (p<0.0001).
Total thyroidectomies were performed in 91% of patients with C-PTC, 85% with FV-PTC, and 73% with FTC (p<0.0001). Radioactive iodine ablations were administrated to more than half of patients with C-PTC (58%) and FV-PTC (56%). Less than half of patients (49%) in the FTC group received radioactive iodine ablation (p<0.0001).
Overall and disease-specific survival
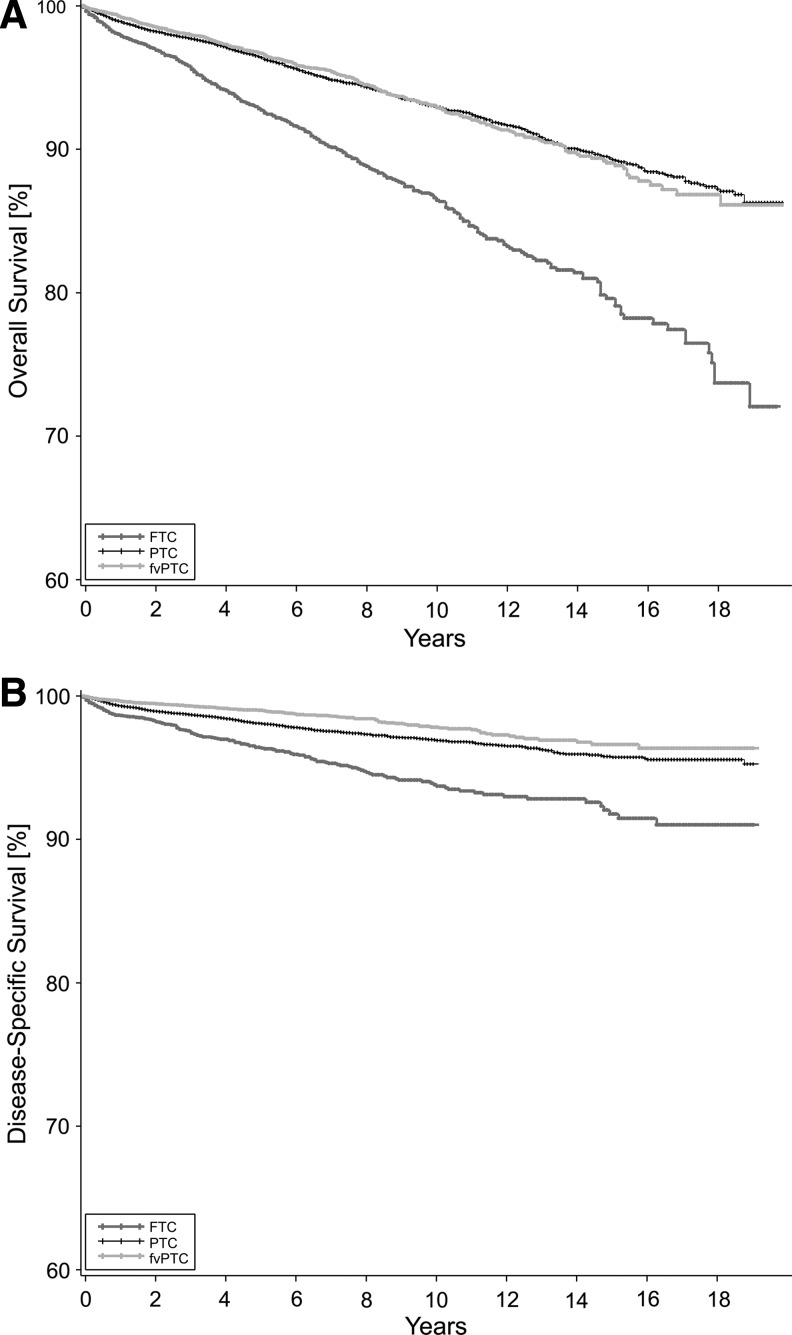
The 10-year and 15-year overall survival, as well as DSS rates, are summarized in Table 2. The median follow-up times for PTC, FV-PTC, and FTC were 4.8 (0–19.9), 4.6 (0–19.9), and 4.7 (0–19.9) years respectively. The overall survival for FV-PTC was almost the same as that for C-PTC (p=0.533; Fig. 1A). Compared with these two subgroups, FTC patients revealed a significantly poorer overall survival (p<0.0001). The DSS for patients with FV-PTC was also similar to C-PTC and better than FTC (Fig. 1B). At the same time, the outcomes were also compared between minimal invasive FTC and FV-PTC. FV-PTC and minimal invasive FTC showed very similar overall survival and DSS (p=0.612 and 0.110 respectively).
Table 2.
Overall and Disease-Specific Survival in Patients with Classical and Follicular Variant of Papillary Thyroid Carcinoma and in Follicular Thyroid Carcinoma
| Survival | C-PTC | FV-PTC | FTC |
|---|---|---|---|
| 10-year overall | 93% | 93% | 87% |
| 15-year overall | 89% | 89% | 79% |
| 10-year disease-specific | 97% | 98% | 94% |
| 15-year disease-specific | 96% | 97% | 92% |
FIG. 1.
(A) Kaplan–Meier estimates for overall survival in three groups of patients (p<0.0001, between follicular thyroid carcinoma (FTC) and papillary thyroid carcinoma (PTC); p<0.0001 between FTC and follicular variant of papillary thyroid carcinoma (FV-PTC); p=0.533 between PTC and FV-PTC, by log-rank test). (B) Kaplan–Meier estimates for disease-specific survival of patients with PTC, FV-PTC, and FTC (p<0.0001 between each two groups, by log-rank test).
Multivariate survival analyses for risk factors of disease-specific mortality
Cox proportional hazards models were applied to quantify the prognostic significance of demographic, tumor factors, and treatment with adjustment to competing risk factors (Table 3). Being over the age of 45 years remained the strongest risk factor for disease-specific mortality in both FV-PTC and C-PTC (HR=18 and 23 respectively; p<0.0001). It also significantly impacted the mortality of FTC but with a relatively lower HR of around 8 (p<0.0001). The impacts of age increment on disease-specific mortality were assessed as well. The HR for each 10-year increase in age was 2.3 in both C-PTC and FV-PTC groups, which was a little higher compared with FTC (HR=1.8, p<0.0001). Moreover, the HR for the five-year age increment was calculated as 1.5, 1.5, and 1.3 for C-PTC, FV-PTC, and FTC respectively (p<0.0001). Male sex did not increase the risk for disease-specific mortality in any of the three subgroups. Each tumor feature, such as tumor size, extension, or metastases, had a significant impact on disease-specific mortality, but with different power depending on the histological types. FV-PTC patients with extrathyroidal extension of their tumor or distant metastases had higher risks (HR=8, p<0.0001, and HR=5, p=0.0005, respectively) to develop disease-specific mortality compared with C-PTC patients (HR=5 and HR=4 respectively; p<0.0001). The presence of distant metastases served as a very strong predictive factor for unfavorable survival in FTC patients as well (HR=8, p<0.0001). The presence of lymph-node metastases was a better predictor of disease-specific mortality for FTC (HR=6, p<0.0001) than for C-PTC or FV-PTC (HR=3 and 2 respectively). A tumor size larger than 3 cm had a moderate predictive value for mortality among all three groups.
Table 3.
Multivariate Analysis for the Risk Factors of Disease-Specific Mortality in Classical and Follicular Variant of Papillary Thyroid Carcinoma and in Follicular Thyroid Carcinoma
| |
HR [CI] |
||
|---|---|---|---|
| Risk factors | C-PTC | FV-PTC | FTC |
| Age >45years | 23.2 [14.5–36.9]* | 17.6 [7.1–43.5]* | 8.0 [4.0–16.0]* |
| Male sex | 1.2 [0.9–1.5] | 1.4 [0.9–2.1] | 0.9 [0.6–1.4] |
| Tumor size >3.0 cm | 3.1 [2.5–3.9]* | 3.7 [2.4–5.9]* | 2.4 [1.4–4.0]* |
| Extrathyroidal extension | 4.4 [3.4–5.7]* | 8.1 [5.1–13.1]* | 3.8 [2.5–5.9]* |
| Lymph node metastases | 2.5 [2.0–3.1]* | 1.8 [1.2–2.8]* | 5.8 [3.3–10.3]* |
| Distant metastases | 3.8 [2.0–7.2]* | 5.4 [2.1–13.8]* | 7.5 [3.8–14.7]* |
| Total thyroidectomy | 0.6 [0.4–0.8]* | 0.8 [0.4–1.4] | 1.1 [0.7–1.7] |
| Radioactive iodine | 0.7 [0.5–0.8]* | 0.9 [0.6–1.4] | 0.7 [0.4–1.0] |
p Values were obtained by Cox proportional hazards model; *p<0.05.
HR, hazard ratio; CI, confidence interval.
It was noticed in this multivariate analysis (Table 3) that C-PTC patients who underwent total thyroidectomy or radioactive iodine ablation had a significantly lower risk for disease-specific mortality (HR=0.6 and 0.7 respectively). However, total thyroidectomy and administration of radioactive iodine ablation did not significantly benefit the DSS in FV-PTC patients (HR=0.8 and 0.9 respectively; p>0.05 for both variables). Similarly, total thyroidectomy and radioactive iodine ablation did not provide a significant survival benefit for FTC patients (HR=1.1 and 0.7 respectively; p>0.05 for both variables).
Discussion
As a very common variant of papillary carcinoma, FV-PTC has been increasingly diagnosed in recent years. However, the clinical behavior and long-term outcome of FV-PTC has remained controversial based on the previous literature. Most published series documenting clinicopathologic features and outcomes of FV-PTC have suffered from drawbacks such as inclusion of relatively few cases, the lack of long-term follow-up, and single institutional bias (7,18–20,29). Therefore, it is necessary to obtain a more comprehensive view of FV-PTC from population-based studies. The present study, using a national database, compared the clinicopathologic features and long-term survival of FV-PTC with that of C-PTC and FTC from more than 36,000 patients.
FV-PTC accounted for around one third of all PTC cases in this study after excluding microcarcinomas, which confirmed FV-PTC as the major subtype of papillary carcinoma. Previous studies have reported the percentage of FV-PTC in relation to all PTC to vary widely from 9% to 41% (4,6–9). This big difference among the series could result from inconsistent inclusion and exclusion criteria. In two studies reporting FV-PTC as a relative low proportion of around 10%, microcarcinomas were categorized as a separate subtype (3,4). Microcarcinomas were regarded as part of FV-PTC in another study, which documented a higher percentage of FV-PTC (9). To obtain a clear picture of FV-PTC, we excluded all microcarcinomas in this analysis, which may help to avoid an impact from occult tumors on the disease outcome of the whole subgroup. The other important reason for the variant percentages of FV-PTC documented in the literature can be the institutional variation on the diagnostic criteria for FV-PTC. Although several studies have tried to use well-defined histopathologic features to improve the consistency in diagnosing the subtypes of PTC (3,9), the diagnosis of FV-PTC is variable even among experienced thyroid pathologists (30). Since variation due to human factors is sometimes unavoidable, a large sample size is one of the best ways to compensate for it.
In term of tumor features and behavior, FV-PTC seems to be quite a unique entity according to our data when compared with C-PTC and FTC. It has been debatable whether FV-PTC acts more aggressively than C-PTC. Several studies have observed less frequent lymph-node or distant metastases in FV-PTC compared with C-PTC (9,19), while FV-PTC has been considered more aggressive in another study showing evidence of higher rates of extensive extrathyroidal local spread, bilateral lesions, and vascular invasion than C-PTC (18). However, we demonstrated in this systematic study that FV-PTC represented an intermediate entity with clinical features that are between C-PTC and FTC: the mean tumor size of FV-PTC was slightly larger than that of C-PTC, but smaller than FTC; extrathyroidal extension and lymph-node metastases were more common in FV-PTC than in FTC, but less common than in C-PTC; distant metastasis were present in 2% of patients with FV-PTC, which doubled the rate in C-PTC, but was only half of the rate in FTC.
Although the clinical behavior of FV-PTC varied from C-PTC, the long-term outcome of these patients remains excellent and was similar to C-PTC. In the current study, the 10-year and 15-year overall survivals for FV-PTC were 93% and 89% respectively, which were quite similar to the survival rate of C-PTC, but were much better than that of FTC. The 10-year DSS of FV-PTC was even better than that of C-PTC. However, there is no doubt that FV-PTC is not homogeneous (31), and 2% of the FV-PTC patients with higher-risk tumors did die of the disease. We found all the risk factors in the conventional staging systems for well-differentiated thyroid carcinoma remained significant in the three histologic subgroups, but with a different risk index after the factors of treatment were controlled. The high-risk profile for FV-PTC was similar to that for C-PTC. However, it was noticeable that FV-PTC patients who presented with extrathyroidal extension or distant metastases had a higher risk of developing disease-specific mortality than C-PTC patients with the same presentation, suggesting that FV-PTC is not biologically identical to C-PTC.
Additionally, we found that total thyroidectomy and radioiodine ablation benefitted the DSS significantly in C-PTC patients, while they did not benefit the survival for FV-PTC and FTC patients. Since 1997, many studies have reported the outcomes of PTC and FTC patients who were subjected to radioactive iodine ablation after bilateral lobe resection. It was recognized later on that most of these patients are truly at “low risk” of developing life-threatening recurrences (32). This may explain our finding that the inappropriate selection criteria during the late 1990s could have failed to identify the true high-risk FV-PTC or FTC patients, which largely masked the significant effectiveness of total thyroidectomy and radioactive iodine ablation. Besides, the differences of the tumor biological and clinical features among these three types of thyroid cancer could contribute to this observation. Since C-PTC is often multifocal (33), total thyroidectomy could be more effective for C-PTC patients than for FTC patients. Radioactive iodine ablation may benefit C-PTC more than FV-PTC due to more patients with iodine avid tumors in the C-PTC group. Additionally, in our study, fewer patients in the FV-PTC group were treated with total thyroidectomy compared to the C-PTC group, which may have impacted both the receipt and efficacy of radioactive iodine ablation.
Despite the above findings, this study does possess some limitations. As a large administrative database, the SEER database cannot capture every subtle factor, some of which may be critical for clinicians. Factors such as the presence of invasive or encapsulated tumors may impact outcomes (5,34,35). It has been reported that noninvasive encapsulated FV-PTC could be quite indolent and the same as invasive encapsulated FV-PTC without distant metastasis (34). In addition, we had little information on disease-free survival due to the lack of recurrence records in the database. Moreover, the variability of the diagnostic criteria and level of scrutiny from different eras and institutions may cause a portion of cases to be misclassified in the SEER database.
The molecular profiles of FV-PTC and C-PTC tumors have been extensively studied recently. It was reported that encapsulated FV-PTC had a molecular profile absent of BRAF mutations while the infiltrated FV-PTC, which is a more aggressive phenotype, frequently harbored BRAF mutations (36). Using microarray expression analysis, 15 transcripts, including CD14, CD74, and DPP6, were identified as being significantly associated with FV-PTC morphology compared with C-PTC (37). These studies suggest that the specific molecular markers or their combination may be helpful to determine the character and prognosis of the disease. If this is supported by further evidence, routine collection of these variables into the national database could be considered in future.
In conclusion, FV-PTC is a very common variant of PTC, constituting about one third of all cases. Its clinical behavior is unique and represents an intermediate entity with clinical features that are between C-PTC and FTC. Interestingly, despite the variations in clinical behavior, the long-term outcome of these patients remains excellent and similar to C-PTC.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.National Cancer Institute 2010 SEER cancer statistics review 1975–2007. http://seer.cancer.gov/csr/1975_2007/browse_csr.php?section=26&page=sect_26_table.05.html. [Mar 6;2011 ]. http://seer.cancer.gov/csr/1975_2007/browse_csr.php?section=26&page=sect_26_table.05.html
- 2.Jossart GH. Clark OH. Well-differentiated thyroid cancer. Curr Probl Surg. 1994;31:933–1012. doi: 10.1016/0011-3840(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 3.Lang BH. Lo CY. Chan WF. Lam AK. Wan KY. Classical and follicular variant of papillary thyroid carcinoma: a comparative study on clinicopathologic features and long-term outcome. World J Surg. 2006;30:752–758. doi: 10.1007/s00268-005-0356-7. [DOI] [PubMed] [Google Scholar]
- 4.Lam AK. Lo CY. Lam KS. Papillary carcinoma of thyroid: a 30-yr clinicopathological review of the histological variants. Endocr Pathol. 2005;16:323–330. doi: 10.1385/ep:16:4:323. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd RV. Buehler D. Khanafshar E. Papillary thyroid carcinoma variants. Head Neck Pathol. 2011;5:51–56. doi: 10.1007/s12105-010-0236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carcangiu ML. Zampi G. Pupi A. Castagnoli A. Rosai J. Papillary carcinoma of the thyroid. A clinicopathologic study of 241 cases treated at the University of Florence, Italy. Cancer. 1985;55:805–828. doi: 10.1002/1097-0142(19850215)55:4<805::aid-cncr2820550419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Tielens ET. Sherman SI. Hruban RH. Ladenson PW. Follicular variant of papillary thyroid carcinoma. A clinicopathologic study. Cancer. 1994;73:424–431. doi: 10.1002/1097-0142(19940115)73:2<424::aid-cncr2820730230>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 8.Sebastian SO. Gonzalez JM. Paricio PP. Perez JS. Flores DP. Madrona AP. Romero PR. Tebar FJ. Papillary thyroid carcinoma: prognostic index for survival including the histological variety. Arch Surg. 2000;135:272–277. doi: 10.1001/archsurg.135.3.272. [DOI] [PubMed] [Google Scholar]
- 9.Zidan J. Karen D. Stein M. Rosenblatt E. Basher W. Kuten A. Pure versus follicular variant of papillary thyroid carcinoma: clinical features, prognostic factors, treatment, and survival. Cancer. 2003;97:1181–1185. doi: 10.1002/cncr.11175. [DOI] [PubMed] [Google Scholar]
- 10.Crile G., Jr Hazard JB. Relationship of the age of the patient to the natural history and prognosis of carcinoma of the thyroid. Ann Surg. 1953;138:33–38. doi: 10.1097/00000658-195307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosai J. Carcangiu ML. Delellis RA. Follicular carcinomas., Papillary carcinomas. In: Rosai J, editor; Sobin LH, editor. Atlas of Tumor Pathology: Tumors of the Thyroid Gland. Armed Forces Institute of Pathology; Washington, DC: 1992. pp. 49–121. [Google Scholar]
- 12.Davies L. Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 13.Chen AY. Jemal A. Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 14.LiVolsi VA. Asa SL. The demise of follicular carcinoma of the thyroid gland. Thyroid. 1994;4:233–236. doi: 10.1089/thy.1994.4.233. [DOI] [PubMed] [Google Scholar]
- 15.Chang HY. Lin JD. Chou SC. Chao TC. Hsueh C. Clinical presentations and outcomes of surgical treatment of follicular variant of the papillary thyroid carcinomas. Jpn J Clin Oncol. 2006;36:688–693. doi: 10.1093/jjco/hyl093. [DOI] [PubMed] [Google Scholar]
- 16.Cardenas MG. Kini S. Wisgerhof M. Two patients with highly aggressive macrofollicular variant of papillary thyroid carcinoma. Thyroid. 2009;19:413–416. doi: 10.1089/thy.2008.0178. [DOI] [PubMed] [Google Scholar]
- 17.Liu L. Venkataraman G. Salhadar A. Follicular variant of papillary thyroid carcinoma with unusual late metastasis to the mandible and the scapula. Pathol Int. 2007;57:296–298. doi: 10.1111/j.1440-1827.2007.02092.x. [DOI] [PubMed] [Google Scholar]
- 18.Hagag P. Hod N. Kummer E. Cohenpour M. Horne T. Weiss M. Follicular variant of papillary thyroid carcinoma: clinical-pathological characterization and long-term follow-up. Cancer J. 2006;12:275–282. doi: 10.1097/00130404-200607000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Passler C. Prager G. Scheuba C. Niederle BE. Kaserer K. Zettinig G. Niederle B. Follicular variant of papillary thyroid carcinoma: a long-term follow-up. Arch Surg. 2003;138:1362–1366. doi: 10.1001/archsurg.138.12.1362. [DOI] [PubMed] [Google Scholar]
- 20.Burningham AR. Krishnan J. Davidson BJ. Ringel MD. Burman KD. Papillary and follicular variant of papillary carcinoma of the thyroid: initial presentation and response to therapy. Otolaryngol Head Neck Surg. 2005;132:840–844. doi: 10.1016/j.otohns.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Nathan H. Pawlik TM. Limitations of claims and registry data in surgical oncology research. Ann Surg Oncol. 2008;15:415–423. doi: 10.1245/s10434-007-9658-3. [DOI] [PubMed] [Google Scholar]
- 22.Gaur P. Leary C. Yao JC. Thymic neuroendocrine tumors: a SEER database analysis of 160 patients. Ann Surg. 2010;251:1117–1121. doi: 10.1097/SLA.0b013e3181dd4ec4. [DOI] [PubMed] [Google Scholar]
- 23.Yu XM. Wan Y. Sippel RS. Chen H. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann Surg. 2011;254:653–660. doi: 10.1097/SLA.0b013e318230036d. [DOI] [PubMed] [Google Scholar]
- 24.Schneider DF. Chen H. Sippel RS. The impact of lymph node ratio on survival in papillary thyroid cancer. Ann Surg Oncol. doi: 10.1245/s10434-012-2802-8. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fritz AG. Third. World Health Organization; Geneva: 2000. International Classification of Disease for Oncology: ICD-O. [Google Scholar]
- 26.Harrell FE., Jr Lee KL. Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Sherman SI. Thyroid carcinoma. Lancet. 2003;361:501–511. doi: 10.1016/s0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 28.Cady B. Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947–953. [PubMed] [Google Scholar]
- 29.LiVolsi VA. Pure versus follicular variant of papillary thyroid carcinoma: clinical features, prognostic factors, treatment, survival. Cancer. 2003;1997;98:1997–1998. doi: 10.1002/cncr.11750. author reply. [DOI] [PubMed] [Google Scholar]
- 30.Lloyd RV. Erickson LA. Casey MB. Lam KY. Lohse CM. Asa SL. Chan JK. DeLellis RA. Harach HR. Kakudo K. LiVolsi VA. Rosai J. Sebo TJ. Sobrinho-Simoes M. Wenig BM. Lae ME. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol. 2004;28:1336–1340. doi: 10.1097/01.pas.0000135519.34847.f6. [DOI] [PubMed] [Google Scholar]
- 31.Daniels GH. What if many follicular variant papillary thyroid carcinomas are not malignant? A review of follicular variant papillary thyroid carcinoma and a proposal for a new classification. Endocr Pract. 2011;17:768–787. doi: 10.4158/EP10407.RA. [DOI] [PubMed] [Google Scholar]
- 32.Hay ID. Selective use of radioactive iodine in the postoperative management of patients with papillary and follicular thyroid carcinoma. J Surg Oncol. 2006;94:692–700. doi: 10.1002/jso.20696. [DOI] [PubMed] [Google Scholar]
- 33.Pitt SC. Sippel RS. Chen H. Contralateral papillary thyroid cancer: does size matter? Am J Surg. 2009;197:342–347. doi: 10.1016/j.amjsurg.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivera M. Tuttle RM. Patel S. Shaha A. Shah JP. Ghossein RA. Encapsulated papillary thyroid carcinoma: a clinico-pathologic study of 106 cases with emphasis on its morphologic subtypes (histologic growth pattern) Thyroid. 2009;19:119–127. doi: 10.1089/thy.2008.0303. [DOI] [PubMed] [Google Scholar]
- 35.Liu J. Singh B. Tallini G. Carlson DL. Katabi N. Shaha A. Tuttle RM. Ghossein RA. Follicular variant of papillary thyroid carcinoma: a clinicopathologic study of a problematic entity. Cancer. 2006;107:1255–1264. doi: 10.1002/cncr.22138. [DOI] [PubMed] [Google Scholar]
- 36.Rivera M. Ricarte-Filho J. Knauf J. Shaha A. Tuttle M. Fagin JA. Ghossein RA. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs. infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010;23:1191–1200. doi: 10.1038/modpathol.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finn SP. Smyth P. Cahill S. Streck C. O'Regan EM. Flavin R. Sherlock J. Howells D. Henfrey R. Cullen M. Toner M. Timon C. O'Leary JJ. Sheils OM. Expression microarray analysis of papillary thyroid carcinoma and benign thyroid tissue: emphasis on the follicular variant and potential markers of malignancy. Virchows Arch. 2007;450:249–260. doi: 10.1007/s00428-006-0348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]