Abstract
In yeast like in many other eukaryotes, fatty acids are stored in the biologically inert form of triacylglycerols (TG) and steryl esters (SE) as energy reserve and/or as membrane building blocks. In the present study, we identified gene products catalyzing formation of TG and SE in the methylotrophic yeast Pichia pastoris. Based on sequence homologies to Saccharomyces cerevisiae, the two diacylglycerol acyltransferases Dga1p and Lro1p and one acyl CoA:sterol acyltransferase Are2p from P. pastoris were identified. Mutants bearing single and multiple deletions of the respective genes were analyzed for their growth phenotype, lipid composition and the ability to form lipid droplets. Our results indicate that the above mentioned gene products are most likely responsible for the entire TG and SE synthesis in P. pastoris. Lro1p which has low fatty acid substrate specificity in vivo is the major TG synthase in this yeast, whereas Dga1p contributes less to TG synthesis although with some preference to utilize polyunsaturated fatty acids as substrates. In contrast to S. cerevisiae, Are2p is the only SE synthase in P. pastoris. Also this enzyme exhibits some preference for certain fatty acids as judged from the fatty acid profile of SE compared to bulk lipids. Most interestingly, TG formation in P. pastoris is indispensable for lipid droplet biogenesis. The small amount of SE synthesized by Are2p in a dga1∆lro1∆ double deletion mutant is insufficient to initiate the formation of the storage organelle. In summary, our data provide a first insight into the molecular machinery of non-polar lipid synthesis and storage in P. pastoris and demonstrate specific features of this machinery in comparison to other eukaryotic cells, especially S. cerevisiae.
Keywords: Acyltransferase, Triacylglycerol, Steryl ester, Lipid droplet, Pichia pastoris
Highlights
-
•
We identified acyltransferases involved in non-polar lipid synthesis of P. pastoris.
-
•
Dga1p is an acyl-CoA dependent, Lro1p an acyl-CoA independent triglyceride synthase.
-
•
Both enzymes have limited substrate specificity; Lro1p has higher activity than Dga1p.
-
•
Are2p is the only acyl CoA:sterol acyltransferase of P. pastoris.
-
•
A triplet mutant deleted of all three acyltransferases lacks lipid droplets.
1. Introduction
The methylotrophic yeast Pichia pastoris is widely used in industry for the production of heterologously expressed proteins [1–4]. The intense commercial interest in this yeast has raised various questions about its cell biology. A deeper knowledge of biomembranes, organelle physiology and lipid metabolism of this microorganism can obviously contribute to a better understanding of protein processing and secretion routes through the endomembrane system in the host cells. For this reason, we started a systematic approach to investigate organelles from P. pastoris with emphasis on their membrane lipid and protein composition [5–7].
Most recently, we characterized for the first time the lipidome and proteome of lipid droplets (LD) from P. pastoris, the storage organelle for the non-polar lipids triacylglycerols (TG) and steryl esters (SE) [5]. In the present study we extended these investigations to the identification of genes and gene products performing synthesis of non-polar lipids in this yeast and characterized their contribution to lipid storage in LD. Enzymes which synthesize non-polar lipids in yeast were so far best characterized in Saccharomyces cerevisiae [8–13] and Yarrowia lipolytica [14,15]. In S. cerevisiae, the two TG synthases Lro1p and Dga1p and the two SE synthases Are1p and Are2p are typical representatives of these enzyme classes.
TG are typically formed by acylation of diacylglycerols (DAG) with fatty acids. In S. cerevisiae, the diacylglycerol:phospholipid acyltransferase Lro1p with its phospholipase A2/B and acyltransferase activities forms TG in an acyl-CoA-independent manner utilizing phospholipids as acyl donors [8,9]. In contrast, the diacylglycerol acyltransferase Dga1p utilizes activated fatty acids as co-substrates to esterify DAG in an acyl-CoA-dependent reaction [10,11]. Lro1p is a component of the endoplasmic reticulum (ER) whereas Dga1p was found to be dually located to LD and the ER [11]. It has been reported that Dga1p and Lro1p from baker's yeast have a certain preference for unsaturated fatty acids, especially C16:1 and C18:1 [16,17]. We showed recently that the fatty acid composition of TG from P. pastoris reflects rather the total cellular pool of fatty acids [5] suggesting moderate fatty acid specificity of the enzymes involved in synthesis.
The two homologous SE synthesizing enzymes from S. cerevisiae, Are1p and Are2p, are present in the ER [12,13,18,19]. Both enzymes esterify sterols using acyl-CoAs as co-substrates. Are2p is the major acyl CoA:sterol acyltransferase in S. cerevisiae and preferentially forms esters of ergosterol, the final product of the sterol biosynthetic pathway in this yeast. Are1p esterifies sterol precursors with similar efficiency as ergosterol and is more active under hypoxic conditions [13,20,21]. In our previous study [5] we showed that SE in LD from P. pastoris are present as esters of ergosterol and ergosterol precursors in comparable amounts.
TG and SE are synthesized in the ER and stored in the core of specialized organelles, the LD [22]. The basic structure of LD is similar in different cell types and consists of a non-polar lipid core surrounded by a phospholipid monolayer with a small but distinct set of proteins embedded. Most recently, proteome and lipidome analyses of LD from P. pastoris identified the major components of this compartment [5]. In contrast to S. cerevisiae, LD from P. pastoris contain much TG but only a small amount of SE. It was hypothesized that the low amount of SE was due to the limited capacity of P. pastoris to form sterols. The lipid composition of LD was reminiscent of the yeast Y. lipolytica [23] but also of mammalian adipocytes [24].
Interestingly, in P. pastoris the presence of the two diacylglycerol acyltransferases Dga1p and Lro1p but only of one acyl CoA:sterol acyltransferase, Are2p, has been predicted by homologies [25]. The aim of the present study was to identify and characterize these non-polar lipid synthesizing enzymes from P. pastoris at the molecular and biochemical level, to investigate their contribution to TG and SE synthesis and study their influence on LD biogenesis. For this purpose, we constructed strains lacking the putative P. pastoris diacylglycerol acyltransferases, Dga1p and Lro1p and the acyl CoA:sterol acyltransferase, Are2p. Single, double and triple deletion mutants were subjected to growth phenotype, lipid and organelle analysis. The results presented here provide molecular information about the network of non-polar lipid synthesis and storage in P. pastoris and further contribute to our understanding of cell and molecular biology of this microorganism.
2. Experimental procedures
2.1. Strains and culture conditions
P. pastoris strain CBS7435ku70his4 (MATa, Mut+, His−) (kindly provided by A. Glieder) [26] considered as wild type and its derivative strains lro1∆ (MATa, Mut+, His−), dga1∆ (MATa, Mut+, His−), are2∆ (MATa, Mut+, His+), dga1∆lro1∆ (MATa, Mut+, His−), lro1∆are2∆ (MATa, Mut+, His+), and dga1∆lro1∆are2∆ (MATa, Mut+, His+) were used throughout this study. Cells were grown under aerobic conditions to the early stationary phase (26 h) at 30 °C in YPD medium containing 1% yeast extract (Oxoid), 2% peptone (Oxoid) and 2% glucose (Merck); and in YPO medium containing 1% yeast extract (Oxoid), 2% peptone (Oxoid), 0.2% oleic acid, and 0.02% TWEEN® 40 (Sigma-Aldrich). Media were inoculated to a starting OD600 of 0.1 from precultures grown aerobically for 48 h in YPD at 30 °C.
2.2. Strain constructions
For the analysis of protein sequences of P. pastoris Dga1p (C4R3W3), Lro1p (C4R1G7) and Are2p (F2QWB7) we made use of annotations in UniProtKB/TrEMBL (protein entries shown in brackets). Alignment with S. cerevisiae and Y. lipolytica orthologs with Clustal Omega algorithm [27] and common domain search in the Pfam protein families databases [28] was performed with tools available at KEGG (Kyoto Encyclopedia of Genes and Genomes) database resource (http://www.genome.jp/kegg/).
DNA from wild type P. pastoris CBS7435∆Ku70 strain was isolated employing the Yeast DNA Kit (Omega bio-tek) and used for applications described below. The construction of acyltransferase deletion mutants was based on the gene deletion strategy described by Wach [29]. Deletion cassettes either contained antibiotic resistance markers or auxotrophy genes as core selection markers flanked by 5′ and 3′ adjacent regions of target genes. The homologous recombination strategy replaced target genes by selection marker genes. All primers used in this study are listed in Table 1.
Table 1.
List of primers used in this study.
| Nr | Sequence from 5′ to 3′ end |
|---|---|
| 1 | CTAGTGATATAGCAACAGATGTTGTTG |
| 2 | CGCCTTAATTAACCCGGGGATCCGAGGTGAAAGGCTGACGGCTCAAG |
| 3 | CCATCCAGTTTAAACGAGCTCGAATTCTGTTTACATCTGTGAGTTGTAAAC |
| 4 | GCTGATCAGGATGGTCAGC |
| 5 | AGGTTTGCCGTTATTCTGGG |
| 6 | GTGTGTGGGGGATCCGCACAAACGAAGGTTTTATCGCCAGTTTGCGGA |
| 7 | CGCTCGAAGGCTTTAATTTGCAAGCTGGAGTTGAGCCAGTATCTTTTTTA |
| 8 | AGATGTCAACTGAAACAGAAGATGG |
| 9 | CGTATGCAGGTAGCAAGGGAAATGTCATCATTAAATGGAGGTGAAAGTTTG |
| 10 | TACTTGTATCGGCATTACACAGCC |
| 11 | CAGAGACTGGATGTTGCGGTATTC |
| 12 | CGTATGGAGAAACTGGGACTTATTTAAGTGTTTTAAATAGGGATATAC |
| 13 | ATGACATTTCCCTTGCTACCTGCATACG |
| 14 | TTAAATAAGTCCCAGTTTCTCCATACG |
| 15 | ATGCAACTACGGAAAAGAGG |
| 16 | GTTTAACTCTGCAGATCCCAG |
| 17 | ATGCCTGAAAAGAAGAACAGTCG |
| 18 | AGGATCCAGCATTAAACCTCTC |
| 19 | ATGCCGATCCCAGTGGCGAG |
| 20 | CTAAGTATAGAGAGCACAAGAGGC |
| 21 | CAAACGGGGTTACTATTTCC |
| 22 | AAAATCACTCGCATCAACCA |
| 23 | GAGAATTAAAAGGTAGATACGC |
| 24 | TCAGTCCTGCTCCTCGGCCACGAAGTGCACGC |
| 25 | CATTCATCGGATAAAACTGTGCTGC |
| 26 | CTGTGGCTTCCTTATCGACCC |
| 27 | CATTCCATCCATGGATAACAGG |
The cassette for deletion of LRO1 was produced using overlap extension PCR strategy. Long 5′ (593 bp) and 3′ (600 bp) flanking regions of LRO1 gene were amplified from genomic DNA using primers 1, 2 and 3, 4, respectively. Then, these flanking regions were fused with the Kanamycin resistance gene together with promoter and terminator parts from pFA6a-kanMX6 plasmid in one overlap extension (OE) PCR with the outermost primers 1 and 4, resulting in the LRO1 deletion cassette (2673 bp). After column spin purification the cassette was ready for yeast transformation.
For construction of the DGA1 gene deletion cassette 5′ (540 bp) and 3′ (426 bp) flanking regions of the DGA1 gene were amplified from genomic DNA using primers 5, 6 and 7, 8, respectively. Then flanking regions were fused with the Zeocin resistance gene together with promoter and terminator parts (1194 bp) from pPICz/B plasmid (Invitrogen) in one OE PCR with the outermost primers 5 and 8, resulting in the DGA1 deletion cassette (2160 bp). After column spin purification the cassette was ready to use.
To create an ARE2 gene deletion cassette, long 5′ (551 bp) and 3′ (551 bp) flanking regions of ARE2 gene were amplified from genomic DNA using primers 9, 10 and 11, 12, respectively. The HIS4 open reading frame (2536 bp) was amplified from pPIC3.5 K plasmid (Invitrogen) using primers 13 and 14. PCR products were column purified with NucleoSpin® Extract II kit (genXpress) and fused in a sequential overlap extension resulting in the ARE2 deletion cassette (3885 bp). After column spin purification the cassette was ready to use.
All deletion cassettes obtained as described above were utilized for transformation of the P. pastoris wild type strain CBS7435∆Ku70. Transformations were performed following the protocol of Lin-Cereghino et al. [30]. In brief, 50–70 μl electro-competent cells mixed with 1–2 μg of the respective purified deletion cassette were subjected to electroporation with a MicroPulser™ (BIO-RAD), incubated for 2 h in 1 ml 1 M sorbitol/YPD (1:1; v:v), centrifuged, resuspended in 100 μl of supernatant and incubated on appropriate selection plates for 48–60 h at 30 °C until colonies appeared.
Gene deletions were verified by PCR and sequencing. For this purposes, two sets of primers for each gene deletion were designed. The first set was complementary to the ends of the full or almost full length ORFs. Primers 15 and 16 correspond to LRO1, 17 and 18 to DGA1, and 19 and 20 to ARE2. The second set of primers was designed as follows. One primer binds 50–100 bases outside the putative integration place. The other primer binds inside the cassette to its selection marker part. The presence and the correct size of the resulting PCR product confirmed the correct locus of integration of the cassette. Primers 21 and 22 were designed to confirm the presence of the LRO1 deletion cassette, primers 23 and 24 for the DGA1 deletion cassette, and primers 25 and 14 for the ARE2 deletion cassette. DNA from non-transformed wild type cells was used as a negative control.
Clones which did not show a signal for target ORFs but the correct signal for the integration event were subjected to sequencing. Sequencing primer 26 was used for proving LRO1 deletion, 27 for DGA1 deletion, and 10 for ARE2 deletion. Confirmed single mutants were used for generating double and triple deletion mutants by sequential deletions using the same procedure described above for each single deletion.
2.3. Analytical methods
Proteins were quantified by the method of Lowry et al. [31] using bovine serum albumin as a standard. Prior to quantification, proteins from yeast cell homogenates were precipitated with trichloroacetic acid and solubilized in 0.1% SDS, 0.1 M NaOH.
Lipids from homogenates of yeast cells grown to the early stationary phase (26 h) were extracted as described by Folch et al. [32]. For quantification of non-polar lipids, extracts were loaded onto Silica Gel 60 plates with the aid of a sample applicator (CAMAG, Automatic TLC Sampler 4, Muttenz, Switzerland), and chromatograms were developed in an ascending manner by a two-step developing system. First, light petroleum/diethyl ether/acetic acid (25/25/1; per vol.) was used as mobile phase and chromatograms were developed to half-distance of the plate. Then, plates were dried briefly and chromatograms were further developed to the top of the plate using light petroleum/diethyl ether (49/1; v/v) as the second mobile phase. To visualize separated bands, TLC plates were dipped into a charring solution consisting of 0.63 g MnCl2 x · 4 H2O, 60 ml water, 60 ml methanol and 4 ml of concentrated sulfuric acid, briefly dried and heated to 105 °C for 40 min. Visualized bands were scanned at 400 nm and quantified using triolein and cholesteryl ester as standards. The amounts of non-polar lipids were related to 1 mg total cell protein.
Fatty acids were analyzed by gas liquid chromatography (GLC). Total cell lipid extracts were prepared as described above. For the analysis of the fatty acid composition of individual non-polar lipids, TLC-separated TG and SE were scrapped off the plate, re-extracted and dried. Then, samples were subjected to methanolysis using 2.5% H2SO4 in methanol and fatty acids were converted to methyl esters. Fatty acid methyl esters were separated using a Hewlett-Packard 6890 gas chromatograph equipped with an HP-INNOWax capillary column (15 m × 0.25 mm inner diameter × 0.50 μm film thickness) and helium as carrier gas. Fatty acids were identified by comparison to commercial fatty acid methyl ester standards (NuCheck, Inc., Elysian, MN).
2.4. Microscopy
For fluorescence microscopy, P. pastoris cells were grown on YPD medium to the early stationary phase (26 h). Cells from 1 ml culture were harvested by centrifugation, washed once with deionized water, stained for 10 min with 5 μl (10 μg/ml) of the lipid droplet specific dye AC-201 [2-(2,6-diisopropylphenyl)-4-(ethylamino)-5,6,7-trifluoroisoindole-1,3-dione], a structural analog of trifluoroaminophthalimide [33] and analyzed using a LEICA DM LB2 microscope with a 100-fold oil immersion objective and excitation filter BP 515–560 nm. Images were taken with Leica DFC 350 FX camera.
For electron microscopy, cells were harvested in the early stationary phase by centrifugation and washed 3 times with double distilled water. Subsequently, cells were fixed for 5 min in a 1% aqueous solution of KMnO4 at room temperature, washed with double distilled water and fixed in a 1% aqueous solution of KMnO4 for 20 min again. Fixed cells were washed four times in distilled water and incubated in 0.5 % aqueous uranyl acetate overnight at 4 °C. Samples were then dehydrated for 20 min each in a graded series of 50%, 70%, 90%, and 100% ethanol. Pure ethanol was then changed to propylene oxide, and specimen were gradually infiltrated with increasing concentrations (30%, 50%, 70% and 100%) of Agar 100 epoxy resin (Agar Scientific Ltd., Stansted, England) mixed with propylene oxide for a minimum of 3 h per step. Samples were embedded in pure, fresh Agar 100 epoxy resin and polymerized at 60 °C for 48 h. Ultrathin sections of 80 nm were stained for 3 min with lead citrate and viewed with a Philips CM 10 transmission electron microscope.
3. Results
3.1. Identification of non-polar lipid forming acyltransferases from Pichia pastoris
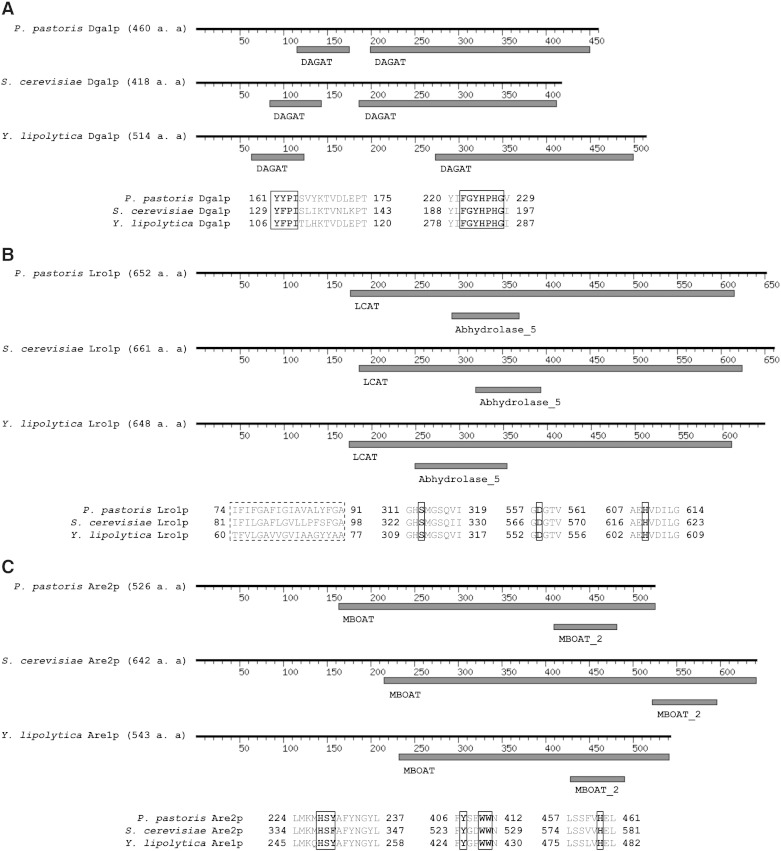
Protein sequences of P. pastoris Dga1p, Lro1p and Are2p were aligned with orthologs from S. cerevisiae and Y. lipolytica [8–15] and analyzed for their conserved domain architecture (Fig. 1) as described in the method section. UniProtKB entries are shown in brackets.
Fig. 1.
Sequence alignment of non-polar lipid synthesizing enzymes from P. pastoris.
A: Domain architecture of Dga1p from P. pastoris, S. cerevisiae and Y. lipolytica. Common diacylglycerol acyltransferase domains (DAGAT) are retrieved from Pfam database. Multiple protein sequence alignments show two highly conserved yeast Dga1p amino acid motifs (in bold, marked with squares). B: Domain architecture of Lro1p from P. pastoris, S. cerevisiae and Y. lipolytica. Common lecithin:cholesterol acyltransferase (LCAT) and α/β hydrolase (Abhydrolase_5) domains are retrieved from Pfam database. Aligned amino acid sequences of transmembrane domains are marked with dashed squares and active site vicinity motifs with potential active site catalytic triade (in bold) are shown within squares. C: Domain architecture of Are2p from P. pastoris, S. cerevisiae and Y. lipolytica. Common membrane bound O-acyl transferase family domains (MBOAT) are retrieved from Pfam database. Protein sequence alignment shows conserved active site motifs with crucial amino acids (in bold, marked with squares). Numbers indicate amino acid positions.
Diacylglycerol acyltransferase, Dga1p from P. pastoris (C4R3W3) has a total amino acid sequence identity of 40% with Dga1p from S. cerevisiae (Q08650) and 33% identity with Dga1p from Y. lipolytica (Q6C3R2). Similar to its orthologs, Dga1p from P. pastoris has two diacylglycerol acyltransferase (DAGAT) domains at amino acid positions 115–175 and 199–450 and the highly conserved DAGAT motifs 161YFPI164and 222FGYHPHG228 [34] (Fig. 1A).
The whole amino acid sequence of the phospholipid:diacylglycerol acyltransferase Lro1p from P. pastoris (C4R1G7) has 49% identity with S. cerevisiae (P40345) and 52% with Y. lipolytica (Q6C5M4) orthologs. As its orthologs, it contains a large lecithin:cholesterol acyltransferase (LCAT) domain at amino acids 176–615 and an α/β hydrolase-fold at amino acid positions 292–369 (Fig. 1B). Lro1p from P. pastoris has also a predicted transmembrane domain at amino acid positions 74–91, which has similarity to the transmembrane domains of S. cerevisiae [35] and Y. lipolytica orthologs. Noteworthy, the amino acids of P. pastoris Lro1p in positions Ser313, Asp558, and His609 are conserved between orthologs and correspond to the active site of S. cerevisiae Lro1p. Ser324 is the potential site of acyl ester intermediate formation, and Asp567 and His618 are predicted to be involved in the charge relay system (UniProtKB).
The acyl-CoA:sterol acyltransferase Are2p (F2QWB7) from P. pastoris has 40% identity with the S. cerevisiae (P53629) and 42.5% identity with the Y. lipolytica (Q6C2M6) ortholog (Fig. 1C). As its homologs, it has two membrane associated O-acyl transferase family domains between amino acids 163–525 and 409–481. The conserved amino acid motifs of P. pastoris Are2p at positions 224–237 and 406–412 are similar to two sites of S. cerevisiae Are2p providing affinity for oleoyl-CoA [36]. Additionally, the P. pastoris enzyme has a conserved motif at positions 457–461 where His459 corresponds to His579 of the potential active site of the S. cerevisiae ortholog.
3.2. Growth phenotype of P. pastoris strains deficient in non-polar lipid synthetic enzymes
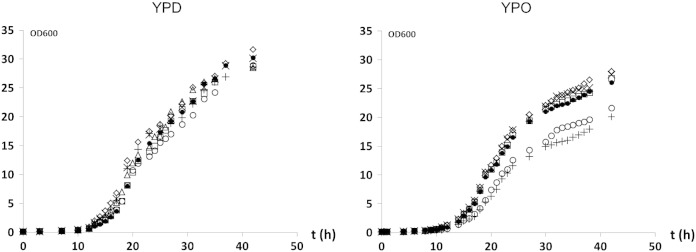
To address possible general physiological effects caused by the lack of non-polar lipid biosynthetic enzymes in P. pastoris we analyzed the growth phenotype of lro1∆, dga1∆ and are2∆ single deletion strains; dga1∆lro1∆ and lro1∆are2∆ double deletion mutants; and the dga1∆lro1∆are2∆ (TM) triple deletion mutant when grown on glucose and oleic acid, respectively (Fig. 2). Growth of all mutants was similar to wild type when glucose was used as a carbon source. When cells were grown on oleic acid, the dga1∆lro1∆ double mutant lacking both diacylglycerol acyltransferases Dga1p and Lro1p and the dga1∆lro1∆are2∆ triple mutant showed slight growth retardation, whereas single deletion mutants grew like wild type.
Fig. 2.
Growth of P. pastoris mutants bearing defects in non-polar lipid synthesis.
A yeast pre-culture grown in YPD for 48 h was used to inoculate fresh YPD or YPO media at a starting OD600 of 0.1. Cells were incubated at 30 °C with vigorous shaking. At time points indicated aliquots were withdrawn, cells were washed in 0.5% fatty acid-free BSA, and absorbance was measured. ◇, WT; □, lro1∆; △, dga1∆; ×, are2∆; ●, lro1∆are2∆;
○, dga1∆lro1∆; +, TM (dga1∆lro1∆are2∆).
3.3. Non-polar lipid analysis of deletion mutants
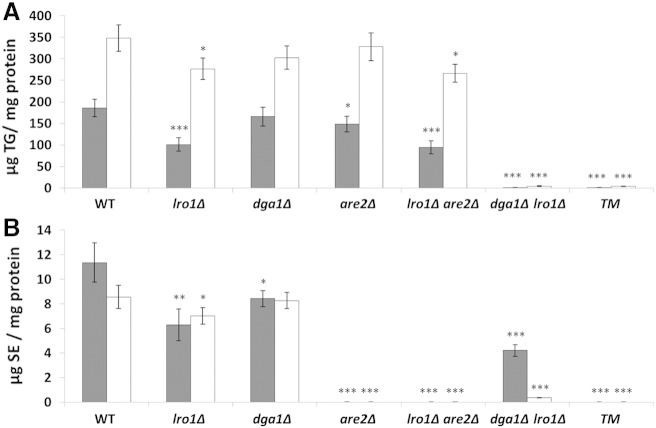
To confirm the predicted function of gene products encoded by LRO1, DGA1 and ARE2 from P. pastoris we measured the amounts of TG and SE in total cell extracts of deletion mutants and wild type strain grown on glucose or oleic acid (Fig. 3). In strains grown on glucose, deletion of LRO1 led to a 46% decrease of TG compared to wild type, whereas in a dga1∆ mutant only a moderate decrease of TG was observed (Fig. 3A). Interestingly, the TG level was also slightly decreased in a strain deleted of ARE2. The dga1∆lro1∆ double mutant lacking both predicted TG synthases was practically devoid of TG similar to the TM where all three genes encoding for non-polar lipid synthesizing enzymes were missing. Essentially the same results were obtained with cells grown on oleate. Also on this carbon source deletion of LRO1 led to highest reduction of the TG amount, and strains deleted of both DGA1 and LRO1 as well as the TM were practically devoid of TG.
Fig. 3.
Non-polar lipid composition in P. pastoris strains deleted of DGA1, LRO and ARE2.
Cells were grown at 30 °C on YPD or YPO media, respectively, to the early stationary phase. Total cell lipids were extracted and analyzed by TLC as described in the methods section.
A: TG content in deletion strains grown on glucose (gray bars) and oleate (white bars). B: SE content in deletion strains grown on glucose (gray bars) and oleate (white bars). Data are mean values of at least three independent experiments. Error bars indicate the standard deviation. Significance was calculated by Student's t-test (one tailed, unpaired). p values are as follows: * < 0.05; ** < 0.01; *** < 0.005.
Surprisingly, cellular SE levels were also decreased by deletion of DGA1 and LRO1 (Fig. 3B). Also in this case, the effect was stronger in the lro1∆ mutant than in dga1∆. In a dga1∆lro1∆ double mutant the SE level was ~ 40 % of wild type grown on glucose, and only ~ 10% of wild type when oleate was used as a carbon source. Not unexpectedly, deletion of ARE2 alone or in combination with DGA1 and LRO1 (TM) led to a complete loss of SE in cells grown on both carbon sources.
Based on these results, Lro1p appears to be the major and Dga1p the minor TG synthase in P. pastoris under conditions tested. It is very unlikely that these two gene products catalyze SE formation, because the single deletion of ARE2 led to a more or less complete loss of SE. The decreased amounts of SE in lro1∆ and dga1∆ strains as well as the slightly reduced level of TG in the are2∆ mutant can rather be explained by a negative feedback control to fatty acid synthesis caused by the lack of non-polar lipid producing enzymes. The occurrence of trace amounts of TG and SE in deletion mutants described above may serve as a hint that further enzymes capable of synthesizing non-polar lipids although with minor capacity exist in P. pastoris.
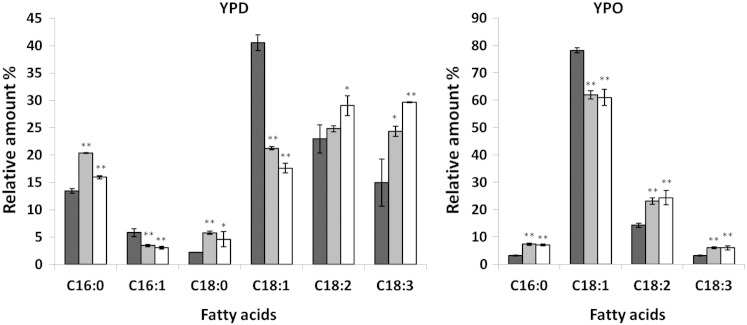
The major fatty acids found in P. pastoris lipids from cells grown under standard conditions are palmitic (C16:0), palmitoleic (C16:1), stearic (C18:0), oleic (C18:1), linoleic (C18:2) and linolenic acid (C18:3) [5–7]. In dga1∆ and are2∆ single mutants the fatty acid pattern was similar to wild type and only in lro1∆ a slight increase in C18:3 and a decrease of C18:1 was observed (data not shown). In the dga1∆lro1∆ double mutant and in the dga1∆lro1∆are2∆ TM, however, fatty acid patterns were markedly changed (Fig. 4). Growth of both mutants on YPD led to elevation of polyunsaturated fatty acids C18:2 and C18:3 accompanied by a strong decrease in oleic acid (C18:1). At the same time, the amount of palmitic acid (C16:0) increased in both mutants although more pronounced in dga1∆lro1∆ than in TM. When cells were grown on YPO, oleic acid was the major fatty acid found in lipid extracts of all strains for obvious reasons. Also under these conditions, in dga1∆lro1∆ and TM the relative amounts of polyunsaturated fatty acids increased in comparison to wild type. In particular, a 1.6-fold increase in C18:2 and a 2-fold increase in C18:3 were observed. In both strains, the amounts of C16:0 increased, whereas reduced levels of C18:1 were detected. Thus, it appears that the capability of fatty acid storage in the form of SE and TG has a strong impact on the fatty acid pattern in P. pastoris.
Fig. 4.
Fatty acid profile of P. pastoris dga1∆lro1∆ and dga1∆lro1∆are2∆ strains.
Total cell lipid extracts from wild type (black bars), dga1∆lro1∆ (gray bars) and dga1∆lro1∆are2∆ TM (white bars) strains grown on YPD or YPO to the early stationary phase were analyzed by GLC for fatty acid composition. The amounts of individual fatty acids are shown as % of total fatty acids. Data are mean values of at least three independent experiments. Error bars indicate the standard deviation. Significance was calculated by Student's t-test (one tailed, unpaired). p values are as follows: * < 0.05; ** < 0.005.
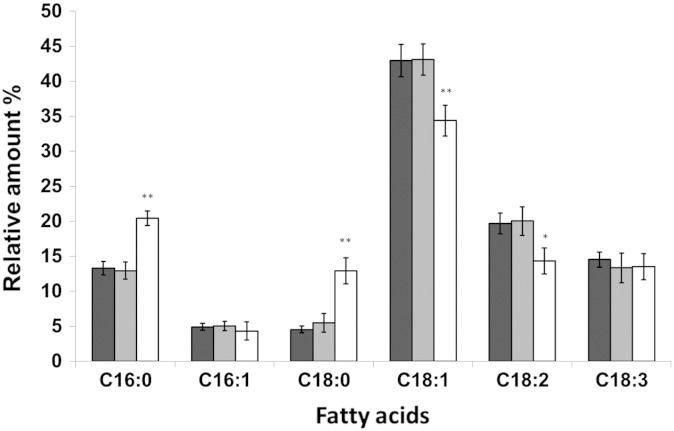
To address the substrate specificity of LRO1 and DGA1 gene products toward fatty acids we performed fatty acid analysis of TG isolated from lro1∆ and dga1∆ single deletion mutants and compared the patterns to wild type (Fig. 5). This analysis showed that in dga1∆ where only Lro1p is active the fatty acid pattern of TG was identical to wild type indicating low substrate selectivity of Lro1p. In the lro1∆ mutant bearing Dga1p as the only TG synthesizing enzyme the fatty acid pattern of TG was changed toward more saturated species. A 1.5-fold increase in C16:0 and a more than 2 fold increase in the relative amount of C18:0 were observed at the expense of C18:1 and C18:2. Although Dga1p appears to be the minor contributor to total TG synthesis in P. pastoris (see Fig. 3) and only marginally affects the total cellular fatty acid composition (see Fig. 4) this enzyme appears to be more specific for acyl CoA substrates than Lro1p.
Fig. 5.
Fatty acid composition of triacylglycerols from dga1∆ and lro1∆.
TG from wild type (black bars), dga1∆ (gray bars) and lro1∆ (white bars) strains grown on YPD to the early stationary phase were analyzed for their fatty acid composition as described in the methods section. The amounts of individual fatty acids are shown as % of total fatty acids. Data are mean values of at least three independent experiments. Error bars indicate the standard deviation. Significance was calculated by Student's t-test (one tailed, unpaired). p values are as follows: * < 0.01; ** < 0.005.
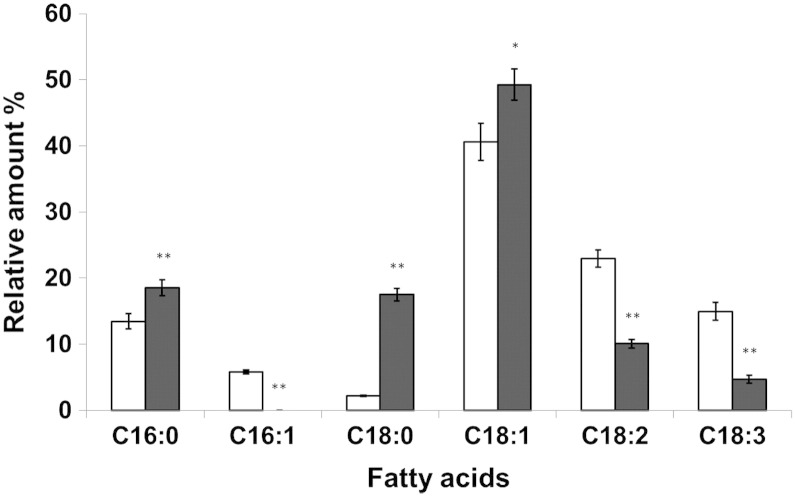
As described above the ARE2 gene product appears to be the only SE synthase in P. pastoris. To test for the substrate specificity of Are2p in vivo we analyzed the fatty acid pattern of SE and compared it to total cell lipid extracts from wild type. Fig. 6 shows that in SE the relative amount of stearic acid (C18:0) was 8-fold higher than in bulk lipids and the amount of C16:0 was slightly increased. Surprisingly, C16:1 was completely missing in SE and the amounts of polyunsaturated fatty acids C18:2 and C18:3 were markedly lowered. These data suggest that Are2p has a rather strong preference for certain fatty acid substrates. The specificity of Are2p from P. pastoris for sterols used as the second substrate for the formation of SE can be deduced from the sterol pattern of SE present in LD [5]. These results showed that similar to S. cerevisiae the Are2p from P. pastoris can utilize both ergosterol and its precursors as substrates.
Fig. 6.
Fatty acid composition of steryl esters from wild type.
P. pastoris wild type was grown on YPD to the early stationary phase. Total cellular lipids (white bars) and SE (black bars) were analyzed for their fatty acid composition. Amounts of individual fatty acids are shown as % of total fatty acids. Data are mean values of at least three independent experiments. Error bars indicate the standard deviation. Significance was calculated by Student's t-test (one tailed, unpaired). p values are as follows: * < 0.01; ** < 0.005.
3.4. Microscopic inspection of mutants compromised in non-polar lipid synthesis
To test the ability of P. pastoris mutants bearing defects in non-polar lipid synthesizing enzymes to store lipids in the form of LD microscopic inspections were performed. For this purpose, wild type and mutants were grown on glucose or oleate, respectively, to the stationary phase and stained with the LD specific lipophilic dye AC-201 [2-(2,6-diisopropylphenyl)-4-(ethylamino)-5,6,7-trifluoroisoindole-1,3-dione] [33]. Fluorescence microscopy (Fig. 7) revealed that single deletion mutants formed LD similar to wild type independently of the carbon source. However, the dga1∆lro∆ double mutant lacking both TG synthases and the dga1∆lro1∆are2∆ TM deleted of all three genes encoding non-polar lipid synthesizing enzymes did not form LD at all. These observations suggested that TG are indispensable for LD formation in P. pastoris and the small amount of SE which is present in dga1∆lro1∆ is not sufficient for LD biogenesis.
Fig. 7.
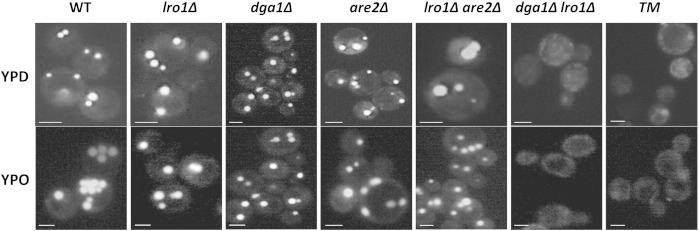
Fluorescence microscopy of P. pastoris strains compromised in non-polar lipid synthesis.
Wild type (WT), single mutants (lro1∆, dga1∆, and are2∆), double mutants (lro1∆are2∆ and dga1∆lro1∆) and triple mutant (TM, dga1∆lro1∆are2∆) were grown on YPD or YPO at 30° to early stationary phase (26 h), stained with AC-201 [2-(2,6-diisopropylphenyl)-4-(ethylamino)-5,6,7-trifluoroisoindole-1,3-dione] and subjected to fluorescent microscopy as described in the methods section. Green fluorescence highlights lipid droplets. Scale bars, 2.5 μm.
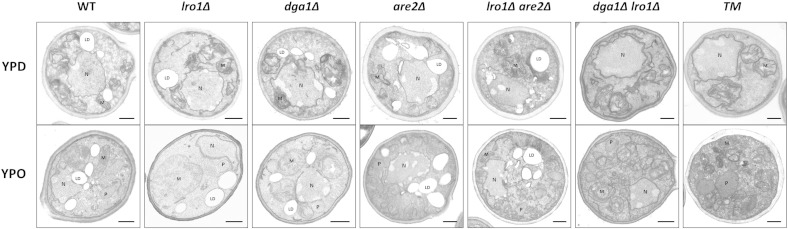
To unambiguously prove the role of LRO1 and DGA1 gene products in the process of LD formation and to investigate in addition the general cellular structure of non-polar lipid synthesis deficient strains, we performed electron microscopic studies of mutants grown on YPD and YPO (Fig. 8). The appearance of LD in all strains tested perfectly matched data obtained with fluorescence microscopy. Also these experiments showed that dga1∆lro1∆ and TM strains are unable to form LD. Furthermore, no major differences in the cell structure of mutants and wild type were observed at least under growth conditions used in this study.
Fig. 8.
Electron microscopy of P. pastoris strains compromised in non-polar lipid synthesis.
Pictures show electron micrographs of KMnO4-fixed wild type (WT), single mutants (lro1∆, dga1∆, and are2∆), double mutants (lro1∆are2∆ and dga1∆lro1∆) and the triple mutant (TM, dga1∆lro1∆are2∆) grown on YPD or YPO, respectively, at 30° to the early stationary phase. Details of the experimental procedures are described in the methods section. Abbreviations indicate specific organelles: LD — lipid droplets, M — mitochondria, P — peroxisomes, N — nucleus. Scale bars, 0.5 μm.
4. Discussion
In the present study we identified gene products catalyzing formation of non-polar lipids in the methylotrophic yeast P. pastoris. Bioinformatic analysis identified two diacylglycerol acyltransferases Dga1p and Lro1p and one acyl-CoA:sterol acyltransferase Are2p. The proposed catalytic functions of these proteins were tested by using single, double and triple deletion mutants and analyzing growth phenotype, lipid composition and cell structure.
Under standard growth conditions with glucose as the sole carbon source the growth behavior of all generated mutants was similar to wild type. This result is in agreement with data obtained with S. cerevisiae before [8,11,12]. However, a marked difference between these two yeast species was observed when oleate was used as a carbon source. Whereas in S. cerevisiae strains lacking the ability to form non-polar lipids [37,38] growth on oleate led to a lipotoxic effect, growth retardation of P. pastoris dga1∆lro1∆ and dga1∆lro1∆are2∆ on this carbon sources was only moderate. Most likely this effect is due to efficient induction of peroxisome proliferation and fatty acid β-oxidation upon growth of P. pastoris on fatty acids [6] which is much less pronounced in S. cerevisiae.
Another marked difference to S. cerevisiae is the contribution of Dga1p and Lro1p from P. pastoris to TG synthesis. Whereas in P. pastoris Lro1p is the more potent diacylglycerol acyltransferase, Dga1p is the major contributor to TG synthesis in S. cerevisiae [16]. P. pastoris mutants dga1∆lro1∆ and dga1∆lro1∆are2∆ bearing deletions of both diacylglycerol acyltransferases contained only traces of TG indicating that these two gene products are the major or even the only TG synthase in P. pastoris. Our molecular biological and biochemical analyses also suggested that P. pastoris in contrast to S. cerevisiae harbors only one acyl-CoA:sterol acyltransferase, the gene product of ARE2. P. pastoris is a low sterol producing yeast [6,7] which also contains only small amounts of SE. Lack of SE in P. pastoris are2∆ strongly suggests that the respective gene product is the only SE biosynthetic enzyme in this yeast. Notably, however, deletion of LRO1 gene alone or in combination with DGA1 also resulted in a marked decrease of SE. We assume that in the absence of TG synthetic enzymes fatty acid synthesis may be reduced resulting in an indirect negative effect on SE production. Similar observations were made with S. cerevisiae [39]. Interestingly, growth of P. pastoris on oleate did not considerably affect SE synthesis as has been shown in S. cerevisiae [39] where SE biosynthetic enzymes are inhibited in the presence of oleate.
Since non-polar lipids are the core components of LD it was not surprising that deletion of the respective biosynthetic genes from P. pastoris affected the formation of this lipid storage organelle. Our microscopic inspections showed that single deletions of acyltransferases changed neither number nor size of LD significantly. However, a dga1∆lro1∆ double deletion strain completely lacked LD. Obviously, the low amount of SE produced in this strain was not sufficient for LD biogenesis. In contrast, S. cerevisiae strains grown on glucose form LD even if only one SE biosynthetic enzyme is active [16]. Electron microscopy of P. pastoris deletion mutants grown on YPD or YPO did not show altered organelle structure. Thus, a putative lipotoxic stress caused by the presence of oleate in the medium does not harm P. pastoris cells. Previous studies had demonstrated that the endoplasmic reticulum in S. cerevisiae was highly proliferated when an excess of fatty acids was present in a dga1∆lro1∆are1∆are2∆ mutant grown on oleate [38]. This is not the case in P. pastoris. The more robust utilization of fatty acids as a source of energy in P. pastoris may explain these findings. This view was supported by the finding that the levels of total and individual phospholipids from P. pastoris mutants compromised in non-polar lipid biosynthesis grown either on YPD or YPO remained unaffected (our own unpublished results).
The absence of TG in P. pastoris dga1∆lro1∆ and in dga1∆lro1∆are2∆ had a marked effect on the bulk membrane fatty acid composition (see Fig. 4). In both mutants the amount of polyunsaturated fatty acids was enhanced. At present, we can only speculate about a possible feedback regulation of non-polar lipid formation on fatty acid synthesis and modification. The slight preferences of TG and SE biosynthetic enzymes for certain fatty acids cannot explain the observed effects with bulk fatty acids.
In conclusion, our results presented here describe for the first time molecular components of the non-polar lipid biosynthetic machinery of P. pastoris. Interestingly, some of these results parallel data obtained with S. cerevisiae, whereas other findings unveil marked differences in the synthesis of TG and SE in the two yeast species. One striking example is the robust utilization of fatty acids in P. pastoris even in the absence of non-polar biosynthetic enzymes. Thus, results reported here expand our current knowledge of P. pastoris lipid metabolism and shed some light on lipid storage and LD formation in this yeast, but also provide more general evidence for the variety of lipid metabolic processes in different types of cells.
Acknowledgements
We would like to thank László Puskás for providing the AC-201 lipid droplet dye and G. Glieder for providing yeast strains. This work was supported by the Austrian Science Fund (FWF), Translational Research Project TRP009 and the DK Molecular Enzymology W901-B05 to G.D., and by the Austrian BMWFJ, BMVIT, SFG, Standortagentur Tirol and ZIT through the Austrian FFG-COMET-Funding Program.
References
- 1.Cregg J.M., Cereghino J.L., Shi J., Higgins D.R. Recombinant protein expression in Pichia pastoris. Mol. Biotechnol. 2000;16:23–52. doi: 10.1385/MB:16:1:23. [DOI] [PubMed] [Google Scholar]
- 2.Daly R., Hearn M.T. Expression of heterologous proteins in Pichia pastoris: a useful experimental tool in protein engineering and production. J. Mol. Recognit. 2005;18:119–138. doi: 10.1002/jmr.687. [DOI] [PubMed] [Google Scholar]
- 3.Macauley-Patrick S., Fazenda M.L., McNeil B., Harvey L.M. Heterologous protein production using the Pichia pastoris expression system. Yeast. 2005;22:249–270. doi: 10.1002/yea.1208. [DOI] [PubMed] [Google Scholar]
- 4.Li P., Anumanthan A., Gao X.G., Ilangovan K., Suzara V.V., Duzgunes N., Renugopalakrishnan V. Expression of recombinant proteins in Pichia pastoris. Appl. Biochem. Biotechnol. 2007;142:105–124. doi: 10.1007/s12010-007-0003-x. [DOI] [PubMed] [Google Scholar]
- 5.Ivashov V.A., Grillitsch K., Koefeler H., Leitner E., Baeumlisberger D., Karas M., Daum G. Lipidome and proteome of lipid droplets from the methylotrophic yeast Pichia pastoris. Biochim. Biophys. Acta. 2013;1831:282–290. doi: 10.1016/j.bbalip.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wriessnegger T., Gübitz G., Leitner E., Ingolic E., Cregg J., de la Cruz B.J., Daum G. Lipid composition of peroxisomes from the yeast Pichia pastoris grown on different carbon sources. Biochim. Biophys. Acta. 2007;1771:455–461. doi: 10.1016/j.bbalip.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Wriessnegger T., Leitner E., Belegratis M.R., Ingolic E., Daum G. Lipid analysis of mitochondrial membranes from the yeast Pichia pastoris. Biochim. Biophys. Acta. 2009;1791:166–172. doi: 10.1016/j.bbalip.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Oelkers P., Tinkelenberg A., Erdeniz N., Cromley D., Billheimer J.T., Sturley S.L. A lecithin cholesterol acyltransferase-like gene mediates diacylglycerol esterification in yeast. J. Biol. Chem. 2000;275:15609–15612. doi: 10.1074/jbc.C000144200. [DOI] [PubMed] [Google Scholar]
- 9.Dahlqvist A., Stahl U., Lenman M., Banas A., Lee M., Sandager L., Ronne H., Stymne S. Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6487–6492. doi: 10.1073/pnas.120067297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oelkers P., Cromley D., Padamsee M., Billheimer J.T., Sturley S.L. The DGA1 gene determines a second triglyceride synthetic pathway in yeast. J. Biol. Chem. 2002;277:8877–8881. doi: 10.1074/jbc.M111646200. [DOI] [PubMed] [Google Scholar]
- 11.Sorger D., Daum G. Synthesis of triacylglycerols by the acyl-coenzyme A:diacylglycerol acyltransferase Dga1p in lipid particles of the yeast Saccharomyces cerevisiae. J. Bacteriol. 2002;184:519–524. doi: 10.1128/JB.184.2.519-524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu C., Kennedy N.J., Chang C.Y., Rothblatt J.A. Molecular cloning and characterization of two isoforms of Saccharomyces cerevisiae acyl-CoA:sterol acyltransferase. J. Biol. Chem. 1996;271:24157–24163. doi: 10.1074/jbc.271.39.24157. [DOI] [PubMed] [Google Scholar]
- 13.Zweytick D., Leitner E., Kohlwein S.D., Yu C., Rothblatt J., Daum G. Contribution of Are1p and Are2p to steryl ester synthesis in the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 2000;267:1075–1082. doi: 10.1046/j.1432-1327.2000.01103.x. [DOI] [PubMed] [Google Scholar]
- 14.Athenstaedt K. YALI0E32769g (DGA1) and YALI0E16797g (LRO1) encode major triacylglycerol synthases of the oleaginous yeast Yarrowia lipolytica. Biochim. Biophys. Acta. 2011;1811:587–596. doi: 10.1016/j.bbalip.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beopoulos A., Haddouche R., Kabran P., Dulermo T., Chardot T., Nicaud J.M. Identification and characterization of DGA2, an acyltransferase of the DGAT1 acyl-CoA:diacylglycerol acyltransferase family in the oleaginous yeast Yarrowia lipolytica. New insights into the storage lipid metabolism of oleaginous yeasts. Appl. Microbiol. Biotechnol. 2012;93:1523–1537. doi: 10.1007/s00253-011-3506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czabany T., Wagner A., Zweytick D., Lohner K., Leitner E., Ingolic E., Daum G. Structural and biochemical properties of lipid particles from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2008;283:17065–17074. doi: 10.1074/jbc.M800401200. [DOI] [PubMed] [Google Scholar]
- 17.Grillitsch K., Connerth M., Köfeler H., Arrey T.N., Rietschel B., Wagner B., Karas M., Daum G. Lipid particles/droplets of the yeast Saccharomyces cerevisiae revisited: lipidome meets proteome. Biochim. Biophys. Acta. 2011;1811:1165–1176. doi: 10.1016/j.bbalip.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang H., Bard M., Bruner D.A., Gleeson A., Deckelbaum R.J., Aljinovic G., Pohl T.M., Rothstein R., Sturley S.L. Sterol esterification in yeast: a two-gene process. Science. 1996;272:1353–1356. doi: 10.1126/science.272.5266.1353. [DOI] [PubMed] [Google Scholar]
- 19.Zinser E., Paltauf F., Daum G. Sterol composition of yeast organelle membranes and subcellular distribution of enzymes involved in sterol metabolism. J. Bacteriol. 1993;175:2853–2858. doi: 10.1128/jb.175.10.2853-2858.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen-Pergakes K., Guo Z., Giattina M., Sturley S.L., Bard M. Transcriptional regulation of the two sterol esterification genes in the yeast Saccharomyces cerevisiae. J. Bacteriol. 2001;183:4950–4957. doi: 10.1128/JB.183.17.4950-4957.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valachovic M., Klobucnikova V., Griac P., Hapala I. Heme-regulated expression of two yeast acyl-CoA:sterol acyltransferases is involved in the specific response of sterol esterification to anaerobiosis. FEMS Microbiol. Lett. 2002;206:121–125. doi: 10.1111/j.1574-6968.2002.tb10996.x. [DOI] [PubMed] [Google Scholar]
- 22.Olofsson S.O., Bostrom P., Andersson L., Rutberg M., Perman J., Boren J. Lipid droplets as dynamic organelles connecting storage and efflux of lipids. Biochim. Biophys. Acta. 2009;1791:448–458. doi: 10.1016/j.bbalip.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Athenstaedt K., Jolivet P., Boulard C., Zivy M., Negroni L., Nicaud J.M., Chardot T. Lipid particle composition of the yeast Yarrowia lipolytica depends on the carbon source. Proteomics. 2006;6:1450–1459. doi: 10.1002/pmic.200500339. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki M., Shinohara Y., Ohsaki Y., Fujimoto T. Lipid droplets: size matters. J. Electron Microsc. (Tokyo) 2011;60:101–116. doi: 10.1093/jmicro/dfr016. [DOI] [PubMed] [Google Scholar]
- 25.Kuberl A., Schneider J., Thallinger G.G., Anderl I., Wibberg D., Hajek T., Jaenicke S., Brinkrolf K., Goesmann A., Szczepanowski R., Puhler A., Schwab H., Glieder A., Pichler H. High-quality genome sequence of Pichia pastoris CBS7435. J. Biotechnol. 2011;154:312–320. doi: 10.1016/j.jbiotec.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Näätsaari L., Mistlberger B., Ruth C., Hajek T., Hartner F.S., Glieder A. Deletion of the Pichia pastoris KU70 homologue facilitates platform strain generation for gene expression and synthetic biology. PLoS One. 2012;7:e39720. doi: 10.1371/journal.pone.0039720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sievers F., Wilm A., Dineen D.G., Gibson T.J., Karplus K., Li W., Lopez R., McWilliam H., Remmert M., Söding J., Thompson J.D., Higgins D.G. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Punta M., Coggill P.C., Eberhardt R.Y., Mistry J., Tate J., Boursnell C., Pang N., Forslund K., Ceric G., Clements J., Heger A., Holm L., Sonnhammer E.L., Eddy S.R., Bateman A., Finn R.D. The Pfam protein families database. Nucleic Acids Res. 2012;40:D290–D301. doi: 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wach A. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast. 1996;12:259–265. doi: 10.1002/(SICI)1097-0061(19960315)12:3%3C259::AID-YEA901%3E3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 30.Lin-Cereghino J., Wong W.W., Xiong S., Giang W., Luong L.T., Vu J., Johnson S.D., Lin-Cereghino G.P. Condensed protocol for competent cell preparation and transformation of the methylotrophic yeast Pichia pastoris. Biotechniques. 2005;38:44–48. doi: 10.2144/05381BM04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 32.Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 33.Puskás L.G., Fehér L.Z., Vizler C., Ayaydin F., Rásó E., Molnár E., Magyary I., Kanizsai I., Gyuris M., Madácsi R., Fábián G., Farkas K., Hegyi P., Baska F., Ozsvári B., Kitajka K. Polyunsaturated fatty acids synergize with lipid droplet binding thalidomide analogs to induce oxidative stress in cancer cells. Lipids Health Dis. 2010;9:56. doi: 10.1186/1476-511X-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Q., Siloto R.M., Snyder C.L., Weselake R.J. Functional and topological analysis of yeast acyl-CoA:diacylglycerol acyltransferase 2, an endoplasmic reticulum enzyme essential for triacylglycerol biosynthesis. J. Biol. Chem. 2011;286:13115–13126. doi: 10.1074/jbc.M110.204412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosal A., Banas A., Ståhl U., Dahlqvist A., Lindqvist Y., Stymne S. Saccharomyces cerevisiae phospholipid:diacylglycerol acyl transferase (PDAT) devoid of its membrane anchor region is a soluble and active enzyme retaining its substrate specificities. Biochim. Biophys. Acta. 2007;1771:1457–1463. doi: 10.1016/j.bbalip.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Guo Z., Cromley D., Billheimer J.T., Sturley S.L. Identification of potential substrate-binding sites in yeast and human acyl-CoA sterol acyltransferases by mutagenesis of conserved sequences. J. Lipid Res. 2001;42:1282–1291. [PubMed] [Google Scholar]
- 37.Kohlwein S.D., Petschnigg J. Lipid-induced cell dysfunction and cell death: lessons from yeast. Curr. Hypertens. Rep. 2007;9:455–461. doi: 10.1007/s11906-007-0084-5. [DOI] [PubMed] [Google Scholar]
- 38.Petschnigg J., Wolinski H., Kolb D., Zellnig G., Kurat C.F., Natter K., Kohlwein S.D. Good fat, essential cellular requirements for triacylglycerol synthesis to maintain membrane homeostasis in yeast. J. Biol. Chem. 2009;284:30981–30993. doi: 10.1074/jbc.M109.024752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Connerth M., Czabany T., Wagner A., Zellnig G., Leitner E., Steyrer E., Daum G. Oleate inhibits steryl ester synthesis and causes liposensitivity in yeast. J. Biol. Chem. 2010;285:26832–26841. doi: 10.1074/jbc.M110.122085. [DOI] [PMC free article] [PubMed] [Google Scholar]