Abstract
Lipid droplets (LD) are the main depot of non-polar lipids in all eukaryotic cells. In the present study we describe isolation and characterization of LD from the industrial yeast Pichia pastoris. We designed and adapted an isolation procedure which allowed us to obtain this subcellular fraction at high purity as judged by quality control using appropriate marker proteins. Components of P. pastoris LD were characterized by conventional biochemical methods of lipid and protein analysis, but also by a lipidome and proteome approach. Our results show several distinct features of LD from P. pastoris especially in comparison to Saccharomyces cerevisiae. P. pastoris LD are characterized by their high preponderance of triacylglycerols over steryl esters in the core of the organelle, the high degree of fatty acid (poly)unsaturation and the high amount of ergosterol precursors. The high phosphatidylinositol to phosphatidylserine of ~ 7.5 ratio on the surface membrane of LD is noteworthy. Proteome analysis revealed equipment of the organelle with a small but typical set of proteins which includes enzymes of sterol biosynthesis, fatty acid activation, phosphatidic acid synthesis and non-polar lipid hydrolysis. These results are the basis for a better understanding of P. pastoris lipid metabolism and lipid storage and may be helpful for manipulating cell biological and/or biotechnological processes in this yeast.
Abbreviations: TG, triacylglycerols; SE, steryl esters; LD, lipid droplets; PA, phosphatidic acid; LP, lysophospholipids; PI, phosphatidylinositol; PS, phosphatidylserine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; DMPE, dimethyl-PE; CL, cardiolipin; DMCD, 4,14-dimethyl-cholesta-8,24-dienol; MS, mass spectrometry; GFP, green fluorescent protein; WT, wild type
Keywords: Lipid droplet, Triacylglycerol, Steryl ester, Lipidome, Proteome, Pichia pastoris
Highlights
► We isolated and characterized lipid droplets from P. pastoris for the first time. ► Lipidome and proteome analysis of P. pastoris lipid droplets were performed. ► Lipid droplets from P. pastoris are different from S. cerevisiae lipid droplets. ► P. pastoris lipid droplets contain much triacylglycerols but little steryl esters. ► A large number of P. pastoris lipid droplet proteins are involved in lipid metabolism.
1. Introduction
The methylotrophic yeast Pichia pastoris is widely used for heterologous protein expression [1–4]. Despite its extensive commercial use the cell biological characterization of this yeast is lacking behind. For this reason our laboratory initiated a systematic approach to investigate P. pastoris organelles with the emphasis on the characterization of biomembranes and lipids [5,6]. Despite the progress which we made a number of subcellular compartments remained uncharacterized so far. As an example, isolation and characterization of lipid droplets (LD) from P. pastoris have not yet been reported. LD, also named lipid particles or oil bodies are specific subcellular compartments which gained much interest recently regarding their emerging role in health and disease [7]. They mainly function as depots of excess lipids (sterols and fatty acids) in the biological inert form of triacylglycerols (TG) and steryl esters (SE), but also contribute to non-polar lipid synthesis and mobilization [8]. LD are supposed to originate from the ER by a budding process, although steps and mechanism(s) leading to the biogenesis of this organelle are still a matter of dispute. Alternative models for LD formation have also been proposed (for reviews, see Refs. [9–14]). Recent studies in Saccharomyces cerevisiae advocated structural and functional connection between ER and LD and proposed the possibility of protein exchange between these two compartments [15].
The general structure of LD is similar in all eukaryotic cells (for reviews, see [8,9]). Yeast LD consist of a hydrophobic core formed by TG and SE encompassed by a phospholipid monolayer with a small number of proteins embedded [16,17]. Most recently, more than 90 proteins were allocated to LD from S. cerevisiae [18]. Many of these polypeptides participate in lipid metabolism, such as phosphatidate and sterol synthesis [19,20], fatty acid activation [21–23], and TG and SE synthesis/lipolysis [24–30]. Besides lipid metabolic functions several other functions unrelated to lipid metabolism were assigned to LD, such as storage and sequestration of protein aggregates and incorrectly folded proteins [9,31].
TG and SE comprise the highly hydrophobic core of LD. In S. cerevisiae, these two major non-polar lipids are synthesized by four enzymes [32]. TG are formed via acylation of diacylglycerols (DAG) with the fatty acid moiety derived from different sources. The diacylglycerol:phospholipid acyltransferase Lro1p possesses phospholipase A2 (B) and acyltransferase activities and catalyzes TG formation in an acyl-CoA-independent manner utilizing phospholipids, especially phosphatidylethanolamine as acyl donor [33,34]. The second TG synthesizing enzyme, Dga1p, esterifies DAG in an acyl-CoA-dependent way and requires activated fatty acids as co-substrates. Lro1p is found exclusively in the ER whereas Dga1p is dually localized to LD and ER [35]. Are1p and Are2p from S. cerevisiae are two homologous SE synthases [36,37]. They are mainly present in the ER and esterify sterols with fatty acids using acyl-CoA as fatty acid donor [20]. Are2p has the major acyl CoA:sterol acyltransferase activity in S. cerevisiae and predominantly forms esters of ergosterol, the final product of the sterol biosynthetic pathway in yeast. Are1p esterifies ergosterol precursors as well as ergosterol and has elevated activity under hypoxic conditions [36,38–40]. Noteworthy, only one acyl CoA:sterol acyltransferase, Are2p, has been annotated in the P. pastoris genome database [41].
In the present report we extend our knowledge about LD biochemistry and cell biology to P. pastoris and compare these data to the well-established model yeast S. cerevisiae. The strategy to characterize LD from P. pastoris cells included (i) isolation of highly pure organelles; (ii) conventional biochemical analysis of lipid components; and (iii) mass spectrometric (MS) analysis of lipids and proteins. Especially the lipidome and proteome studies allowed us to investigate LD from P. pastoris at the molecular level which may become highly relevant for biotechnological applications.
2. Experimental procedures
2.1. Strains and culture conditions
P. pastoris X33 (MATa, Mut+, His−) and P. pastoris X33_GFP-ERG6 (MATa, Mut+, His+) strains were used throughout this study. Cells were grown under aerobic conditions to the early stationary phase (26 h) at 30 °C in YPD medium containing 1% yeast extract (Oxoid), 2% peptone (Oxoid) and 2% glucose (Merck). Media were inoculated to a starting OD600 of 0.1 from precultures grown aerobically for 48 h in YPD medium at 30 °C.
2.2. Construction of GFP-PpErg6p expression vector
The primer pair (GFP-fwd CGCGGATCCGCGTTTTTGTAGAAATGTCTTG GTGTCCTCGTCCAATCAGGTAGCCATCTCTG and GFP-rev ATAGTTTAGCGGCC GCCTCGAGCCCGGGATTTAAATACTTGTACAATTCATCCATGCCATGTGTAATCCCAGCAGCAGT) was used for amplifying a GAP promoter fused to a Cy3-GFP open reading frame lacking the stop codon. The PCR product was inserted into BamHI and NotI restriction sites at the multiple cloning site of the pPIC3.5 plasmid. Primer GFP-rev also contained additional recognition sites enabling N-terminal fusion of GFP to genes of interest. The PpERG6 open reading frame was amplified from genomic DNA using the forward primer ERG6-fwd (5′-GCGCGATTTAAATATGACTACCT CTACAACTGAACAAG-3′) which contained a SmiI site and the reverse primer ERG6-rev (5′-CAATGCGGCCGCTTATTTGGCATCCAATGGTTTTC-3′) with a NotI site. PpERG6 was inserted within the corresponding sites of the aforementioned vector behind the GFP gene. The quality of the final construct was confirmed by sequencing.
2.3. Yeast cell transformation
For transformation experiments, the expression vector described above was linearized by cutting within the 5′ AOX1 promoter fragment with SacI restriction endonuclease (Fermentas). The DNA was introduced into P. pastoris competent cells by electroporation as described by Lin-Cereghino et al. [42] with the aid of a MicroPulser™ Electroporator (Bio-Rad). Transformed cells were transferred to plates containing 0.67% yeast nitrogen base without amino acids, 2% glucose, 0.4 mg/l biotin and 2% agar and incubated at 30 °C for 2–3 days until colonies appeared. Transformants were checked by PCR for the presence of the GFP-PpERG6 open reading frame. Successful expression of the GFP-PpErg6p fusion product was confirmed by fluorescence microscopy and Western blot analysis (see below).
2.4. Fluorescent microscopy
P. pastoris cells were grown on YPD medium to the early stationary phase (26 h). Cells from 1 ml culture were harvested by centrifugation, washed once with deionized water, stained for 10 min with 10 μg/ml Nile Red (Sigma) and analyzed using a Zeiss Axiovert 35 microscope with a 100-fold oil immersion objective. Detection ranges of 450–490 nm for Nile Red and 510–520 nm for GFP were used. Images were taken with a CCD camera.
2.5. Subcellular fractionation
Lipid droplets (LD) from P. pastoris were obtained at high purity from cells grown to the early stationary phase as described previously [16,43] with minor modifications. Briefly, cells were grown aerobically on YPD to the early stationary phase (26 h), harvested by centrifugation and washed with deionized water. Cells were converted to spheroplasts using Zymolyase 20T (Seikagaku Corporation, Japan). Spheroplasts (~ 90 g) were suspended in buffer A (10 mM MES/Tris, pH 6.9, 12% Ficoll 400, 0.2 mM EDTA, 1 mM PMSF) and disintegrated using a Dounce Homogenizer (30 strokes) on ice. After centrifugation at 6,000g for 5 min the supernatant was removed and the pellet was homogenized and centrifuged again as described above. Combined supernatants (homogenate) in buffer A were centrifuged at 12,000g for 15 min. The resulting supernatant was put into an Ultra-Clear Centrifuge Tube (Beckman), overlaid with buffer A and centrifuged at 141,000g for 45 min using a swing out rotor AH-629 (Sorvall). The resulting floating layer was collected, overlaid with buffer B (10 mM MES/Tris, pH 6.9, 8% Ficoll 400, 0.2 mM EDTA, 1 mM PMSF) and ultracentrifuged at 141,000g for 30 min. The floating layer was collected again, homogenized, transferred to the bottom of the ultracentrifuge tube filled with buffer C (0.25 M sorbitol, 10 mM MES/Tris, pH 6.9, 0.2 mM EDTA) and centrifuged at 141,000g for 90 min. The resulting top layer represents highly pure lipid droplets.
Isolation of other subcellular fractions used in this study was described before [6,7,43]. For the isolation of mitochondria, microsomes and cytosol, spheroplasts (~ 25 g) were suspended in 10 mM Tris–HCl, pH 7.4, containing 0.6 M mannitol, homogenized with a Dounce Homogenizer (15 strokes) and centrifuged at 4,000g for 5 min. The supernatant was collected and the pellet was homogenized and centrifuged again. Combined supernatants were centrifuged at 12,000g for 15 min. The supernatant was used for microsomal and cytosol preparations (see below). The pellet was re-suspended in the same buffer and centrifuged at 4,000g for 5 min. The resulting supernatant was centrifuged at 12,000g for 10 min. The final pellet represents the mitochondria fraction. Combined supernatants from the previous steps were centrifuged at 20,000g, 30,000g and 40,000g for 30 min each. Pellet fractions of 30,000g and 40,000g centrifugation steps correspond to 30,000 g and 40,000 g microsomes. The final supernatant is cytosol including 100,000 g microsomes. The quality of subcellular fractions was routinely tested by Western blot analysis (see below).
2.6. Protein analysis
Proteins were quantified by the method of Lowry et al. [44] using bovine serum albumin as a standard. Proteins were precipitated with trichloroacetic acid and solubilized in 0.1% SDS, 0.1 M NaOH prior to quantification with the exception of LP proteins which were precipitated and de-lipidated according to the method of Wessel and Flügge [45].
SDS–PAGE was performed by the method of Laemmli [46] using 12.5% SDS gels. Samples were denaturated at 37 °C to avoid aggregation of membrane proteins. Proteins were visualized by staining with Coomassie Blue. Western blot analysis was performed according to Haid and Suissa [47]. Primary rabbit antibodies used in this study were directed against Por1p, Pma1p, Erg6p and GAPDH from S. cerevisiae, and against the 75-kDa microsomal marker protein (75-ER marker) from P. pastoris. The 75 kDa microsomal protein appears as a typical band of microsomal fractions on SDS–polyacrylamide gels. The function of this protein is unknown. For immunization the respective band was excised from a preparative SDS–polyacrylamide gel, and the protein was electro-eluted and injected into rabbits using standard procedures. Mouse antiserum against GFP was purchased from Roche. Peroxidase-conjugated secondary antibody and enhanced chemiluminescent signal detection reagents (SuperSignal™, Pierce) were used to visualize immunoreactive bands.
2.7. Mass spectrometry of proteins
Protein (20 μg) from the LD fraction was precipitated following the protocol of Wessel and Fluegge [45]. Dried samples were dissolved in digestion buffer (25 mM triethylammonium bicarbonate). After addition of 0.375 μg porcine trypsin to each sample, the digestion was performed for 18 h at 37 °C with gentle agitation. The reaction was stopped by adding 1 μl TFA (trifluoroacetic acid) to each sample. Prior to sample analysis volumes were decreased to 10 μl by vacuum centrifugation.
Peptide separation was performed on a Proxeon Biosystems EASY-nLC™ system coupled with a SunCollect MALDI Spotting device (SunChrom, Germany). The MALDI Spotting was done by mixing the LC-eluent with matrix solution containing 3 mg/ml alpha-cyano-4-hydroxycinnamic acid (Bruker Daltonics, Bremen, Germany) dissolved in 70% MeCN and 0.1% TFA. The final mixture was spotted every 20 s on a blank LC-MALDI insert metal target. All MS and MS/MS were acquired on a 4800 TOF/TOF™ Analyzer (ABSciex, Darmstadt, Germany). For protein and peptide identification an in-house Mascot database search engine v2.2.03 (Matrix Science Ltd.) and the Swissprot Protein Database were used.
2.8. Blast analysis of lipid droplet protein sequences
Amino acid sequences of P. pastoris LD proteins were obtained from Universal Protein Resource Knowledgebase—UniProtKB (http://www.uniprot.org/) and used for blast analysis. For the identification of orthologs from S. cerevisiae, the online blast tool of the Saccharomyces Genome Database (SGD) (http://www.yeastgenome.org/cgi-bin/blast-sgd.pl) was applied. Candidate orthologs with functions similar to those annotated for previously detected LD proteins and homologies/similarities in amino acid sequences were selected.
2.9. Lipid extraction and analysis
Lipids from yeast cells were extracted as described by Folch et al. [48]. For quantification of non-polar lipids, extracts were applied to Silica Gel 60 plates with the aid of a sample applicator (CAMAG, Automatic TLC Sampler 4, Muttenz, Switzerland), and chromatograms were developed in an ascending manner by a two-step developing system. First, light petroleum/diethyl ether/acetic acid (25/25/1; per vol.) was used as mobile phase, and chromatograms were developed to half-distance of the plate. Then, plates were dried briefly and chromatograms were further developed to the top of the plate using light petroleum/diethyl ether (49/1; v/v) as the second mobile phase. To visualize separated bands, TLC plates were dipped into a charring solution consisting of 0.63 g MnCl2 × 4 H2O, 60 ml water, 60 ml methanol and 4 ml of concentrated sulfuric acid, briefly dried, and heated at 105 °C for 40 min. Visualized lipids together with ergosterol, triolein and cholesteryl ester as standards were quantified by densitometric scanning at 400 and 600 nm using a Shimadzu scanner CS-930.
For phospholipid analysis, lipids from homogenate and LD were loaded on silica gel 60 plates (Merck, Darmstadt, Germany), separated by two dimensional TLC (2D-TLC) using chloroform/methanol/25%NH3 (65/35/5; per vol.) as first, and chloroform/acetone/methanol/acetic acid/water (50/20/10/10/5; per vol.) as second developing solvent system. Phospholipids were stained with iodine vapor, scraped off and quantified by the method of Broekhyuse [49]. The same method was applied for quantification of total phospholipids obtained as bands from one dimensional TLC using light petroleum/diethyl ether/acetic acid (25/25/1; per volume) as developing system.
Fatty acids were analyzed by gas–liquid chromatography (GLC). Lipid extracts prepared as described above were subjected to methanolysis using 2.5% H2SO4 in methanol and converted to methyl esters. Fatty acid methyl esters were separated using a Hewlett-Packard 6890 gas chromatograph equipped with an HP-INNOWax capillary column (15 m × 0.25 mm inner diameter × 0.50 μm film thickness) and helium as carrier gas. Fatty acids were identified by comparison to commercial fatty acid methyl ester standards (NuCheck, Inc., Elysian, MN).
Individual sterols from total cell extracts (homogenates) or LD were identified and quantified by GLC/MS after alkaline hydrolysis of lipid extracts [50]. GLC/MS was performed on a Hewlett-Packard 5890 gas chromatograph equipped with a mass selective detector (HP 5972), using an HP5-MS capillary column (30 m × 0.25 mm × 0.25 μm film thickness). Aliquots of 1 μl were injected in the splitless mode at 270 °C injection temperature with helium as a carrier gas at a flow rate of 0.9 ml/min in constant flow mode. The following temperature program was used: 1 min at 100 °C, 10 °C/min to 250 °C, and 3 °C/min to 310 °C. Mass spectra were acquired in scan mode (scan range 200–550 atomic mass units) with 3.27 scans/s. Sterols were identified based on their mass fragmentation pattern.
2.10. Lipidome analysis by LC–MS
Lipids were extracted as described above and dissolved in chloroform/methanol (1/1; v/v) spiked with a set of 28 internal standards. Internal standardization and data acquisition by HPLC coupled to a FT-ICR-MS hybrid mass spectrometer (LTQ-FT, Thermo Scientific) was described previously in much detail by Fauland et al. [51]. Data processing takes into account exact mass and retention time and was performed by Lipid Data Analyzer according to Hartler et al. [52].
3. Results
3.1. Isolation of lipid droplets from P. pastoris and quality control
Lipid droplets (LD) are lipid storage compartments present in all types of eukaryotic cells including various yeast species. Here we describe isolation and characterization of LD from the industrial yeast P. pastoris to broaden our fundamental knowledge of the cell biology and the lipid metabolism of this important microorganism. For the isolation of LD from P. pastoris cells we adapted and optimized a protocol which had been established previously for S. cerevisiae [16]. The procedure is described in detail in Experimental procedures section.
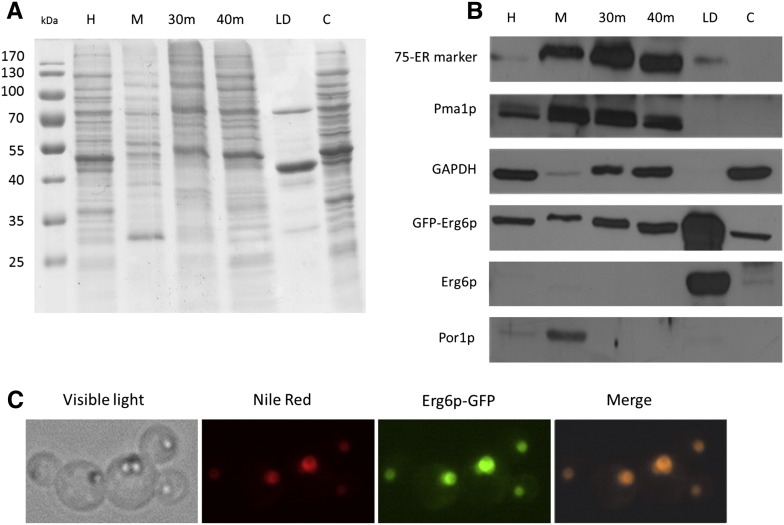
For the quality control of isolated LD from P. pastoris cellular fractions were separated by SDS–polyacrylamide electrophoresis (Fig. 1A) and tested by Western blot analysis (Fig. 1B). As marker protein for P. pastoris LD we used Erg6p and Erg6-GFP. Localization of the Erg6-GFP-hybrid protein to LD was confirmed by fluorescence microscopy and co-staining with Nile Red (Fig. 1C). Western blot analysis (see Fig. 1B) revealed high enrichment of Erg6p and GFP-Erg6p in isolated LD fractions. Not surprisingly, the overexpressed GFP-Erg6 hybrid was also found in microsomal fractions at lower concentration as had been shown previously for Erg6p in S. cerevisiae [24]. Cross-contamination of P. pastoris LD with other subcellular fractions was only marginal. Hence, the isolation protocol was efficient and could be used as the basis for biochemical characterization of P. pastoris LD.
Fig. 1.
Quality control of isolated lipid droplets. A—Protein pattern of lipid droplet fraction and control fractions (10 μg protein). Homogenate (H), lipid droplets (LD), 30,000 g microsomes (30 m), 40,000 g microsomes (40 m), cytosol (C) and mitochondria (M) fractions were loaded onto an SDS–polyacrylamide gel. B—Western blot analysis. Antisera against Pichia pastoris 75-ER marker protein (microsomal marker); Pma1p, plasma membrane H+-ATPase (plasma membrane marker); GAPDH, glyceraldehyde-3-phosphate dehydrogenase (cytosolic marker); GFP-Erg6p, green fluorescent protein fused to Erg6p (LD marker); Erg6p, ∆(24)-sterol C-methyltransferase (LD marker); and Por1p, mitochondrial porin (mitochondrial marker) were applied. C—Fluorescent imaging. Cells were grown on glucose at 30 °C for 26 h, stained with Nile Red and subjected to fluorescent microscopy. The Erg6p-GFP-signal coincides with Nile Red stained LD.
3.2. Non-polar storage lipids of lipid droplets from P. pastoris
Non-polar lipids are the major components of LD. In yeast, non-polar lipids accumulate at substantial amounts in the stationary growth phase. For this reason, P. pastoris cells grown on glucose were harvested at the early stationary phase (26 h) and LD were analyzed. TG (~ 55–65 mg/mg LD protein) comprised more than 90% of all non-polar lipids from LD. The amount of SE (~ 3–5 mg/mg LD protein) was low resulting in an approximate TG to SE ratio of ~ 15. Only traces of free sterols were detected in LD. This finding is in sharp contrast to LD from S. cerevisiae where SE and TG are present at equal amounts [16]. However, the high TG to SE ratio in P. pastoris is reminiscent to LD from the yeast Yarrowia lipolytica [53] but also to mammalian adipocytes [54].
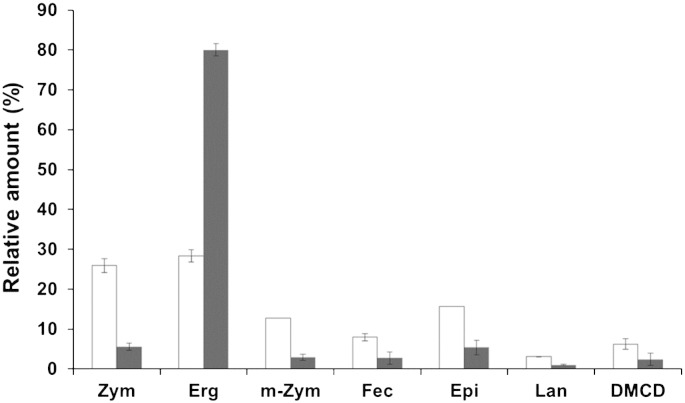
Whereas in S. cerevisiae the excess of sterols formed is converted to substantial amounts of SE stored in LD [36,37] the obviously small amount of sterols formed in P. pastoris appears to be the reason for moderate occurrence of SE. However, accumulation of ergosterol precursors in LD from P. pastoris is similar to S. cerevisiae. As can be seen from Fig. 2, the sterol composition of P. pastoris LD mainly derived from the esterified form as SE is completely different from total cell extracts. In homogenates, the final product of the sterol biosynthetic pathway, ergosterol, is strongly enriched and comprises up to 80% of total sterols. Only small amounts of ergosterol precursors were found in total cell extracts. In contrast, LD contain ergosterol only at 30% of total sterol, but sterol intermediates are present at large quantities. In LD, the concentration of zymosterol is comparable to ergosterol (~ 26%), and also episterol, 4-methylzymosterol, fecosterol, episterol, lanosterol and 4,14-dimethylcholesta-8,24-dienol are strongly enriched over the homogenate.
Fig. 2.
Sterol composition of lipid droplets. Cells were grown on glucose at 30 °C to the stationary phase (26 h). Lipid extracts from LD (white bars) and homogenates (grey bars) were subjected to MS analysis of sterols. Amounts of individual sterols are shown as percentage of total sterols. Zym, Zymosterol; Erg, ergosterol; m-Zym, 4-methylzymosterol; Fec, fecosterol; Epi, episterol; Lan, lanosterol; DMCD, 4,14-dimethylcholesta-8,24-dienol. Data are mean values of three independent experiments. Error bars indicate standard deviation.
3.3. Phospholipid analysis
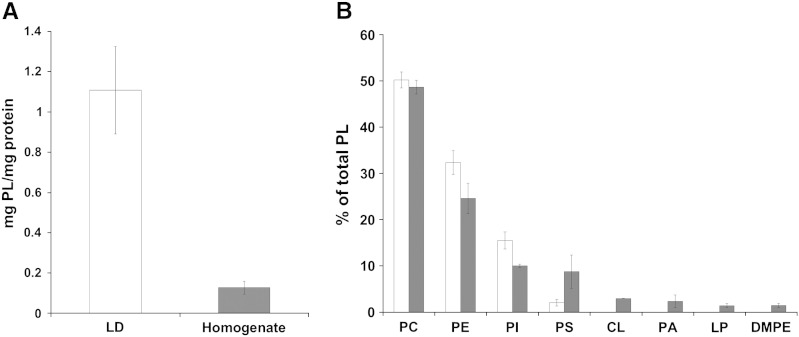
A phospholipid monolayer forms the surface membrane of LD and shields the highly hydrophobic particle from the aqueous environment. This surface membrane is assumed to be very important for the maintenance and the structure of LD [55]. Fig. 3 shows a comparison of phospholipid compositions from P. pastoris cell homogenate and LD. First, the ratio of phospholipids to proteins was found to be very high in LD which is, however, mainly result of the low abundance of proteins in LD (Fig. 3A). The ratio of TG to total phospholipids was ~ 60 indicating the low abundance of the latter lipid class in LD and at the same time confirming the high purity of isolated LD fractions. The pattern of major phospholipids in LD roughly reflected total cell extracts, although certain differences are noteworthy (Fig. 3B). Whereas phosphatidylcholine (PC) and phosphatidylethanolamine (PE) are major phospholipids of both LD and homogenate, the ratio of phosphatidylinositol (PI) to phosphatidylserine (PS) differs strongly. This PI to PS ratio is ~ 1 in the homogenate and ~ 7.5 in LD. Other phospholipid species such as cardiolipin (CL), phosphatidic acid (PA), lysophosphatidic acid (LPA) and dimethyl-phosphatidylethanolamine (DMPE) were only present at trace amounts in LD.
Fig. 3.
Phospholipid pattern of lipid droplets. Cells were grown on glucose at 30 °C to stationary phase (26 h). Lipid extracts were analyzed for phospholipids as described in the experimental section. A—Total phospholipids in LD and homogenate. B—Relative distribution of individual phospholipids in LD (white bars) and homogenates (grey bars). Phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidylserine (PS), cardiolipin (CL), phosphatidic acid (PA), lysophospholipids (LP), dimethylphosphatidylethanolamine (DMPE), are shown as percentage of total phospholipids. Data are mean values of at least three independent experiments. Error bars indicate standard deviation.
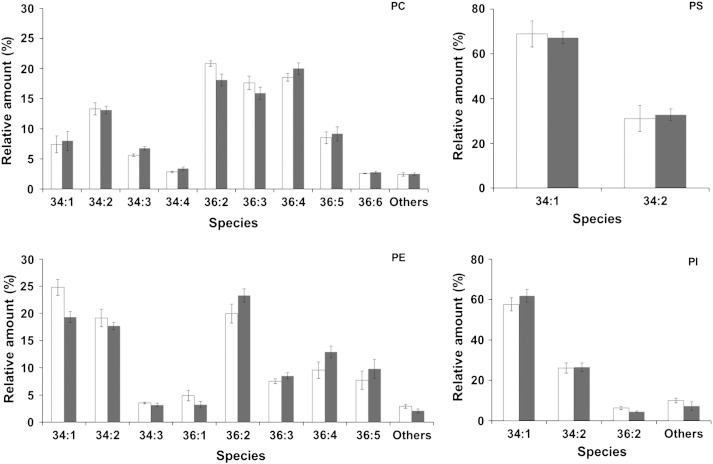
Besides conventional phospholipid analysis we extended our studies to mass spectrometric analysis of PC, PE, PI and PS (Fig. 4). This analysis revealed that there were only minor differences in the phospholipid species patterns of homogenate and LD. The vast majority of all phospholipid species in both samples comprised C36 and C34 species. The fact that the entire phospholipid species profile of LD follows the homogenate supports the notion postulated in a study with S. cerevisiae [18] that there is no species selective transfer of phospholipids to LD. This result also suggests that proteins of the LD surface membrane do not require a specific phospholipid species milieu. However, the difference in the PI/PS ratio as mentioned above has to be kept in mind.
Fig. 4.
Phospholipid species composition. Lipid extracts from homogenate (grey bars) and LD (white bars) were analyzed by LC–MS for phospholipid species of phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI) and phosphatidylserine (PS). Data are mean values of at least three independent experiments. Error bars indicate standard deviation.
When examining individual phospholipids of P. pastoris homogenate and LD fractions we observed peculiar properties of the different phospholipid classes. PC is the phospholipid with the largest variety of species in both homogenates and LD. Noteworthy, PC occurs in the form of C36:2 and several polyunsaturated species such as C36:3, C36:4 and C36:5. The species pattern of PE is markedly different from PC. The most prominent PE species are C36:2, C34:1 and C34:2. Interestingly, in both homogenate and LD PI is presented mostly as C34:1 and C34:2 species. The simplest species pattern was observed with PS being restricted to C34:1 and C34:2. Similar results were obtained with S. cerevisiae LD where C34:1 was the major PI species and PS was mostly present in its C34:1 and C34:2 form [18].
3.4. Fatty acids analysis of lipid droplets
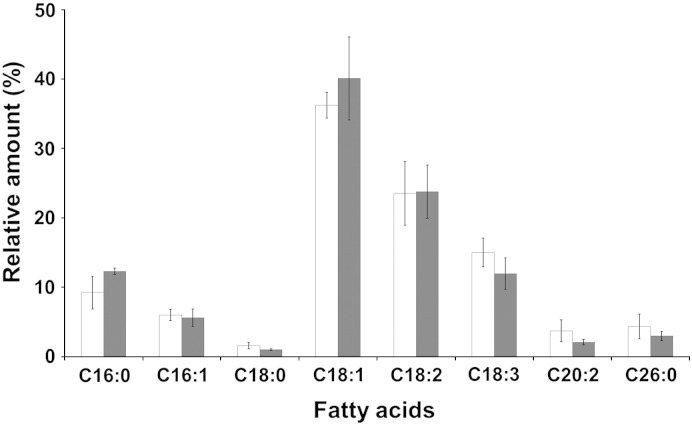
The vast majority of fatty acids in LD are stored in the form of TG and SE and only a minor part is present in the surface phospholipid monolayer. To address the specificity of the lipid storage machinery in P. pastoris we analyzed the fatty acid composition of LD. As can be seen from Fig. 5, oleic (C18:1), linoleic (C18:2), linolenic (C18:3) and palmitic (C16.0) acids are dominant in both LD and homogenate. Other fatty acids are present only at minor amounts in both samples. This finding indicates that in P. pastoris the distribution of fatty acid species is not specific for LD and reflects the total cell fatty acid pattern. We can exclude from these data that P. pastoris enzymes synthesizing non-polar lipids have strong fatty acid substrate specificity. This result is in contrast to the two TG synthases from S. cerevisiae, Dga1p and Lro1p, which have a clear preference for unsaturated fatty acids, especially C16:1 and C18:1 [18,56]. With that respect, P. pastoris LD are different from other yeasts such as S. cerevisiae [18] and Y. lipolytica [53], but similar to plant oil bodies which also contain marked amounts of polyunsaturated fatty acids [57].
Fig. 5.
Fatty acid pattern of lipid droplets. Lipid extracts from LD (white bars) and homogenate (grey bars) were analyzed by GC-FID for fatty acid composition. The amounts of individual fatty acids in homogenate and LD are shown as % of total fatty acids. Data are mean values of at least three independent experiments. Error bars indicate standard deviation.
3.5. P. pastoris lipid droplet proteome
To complete the characterization of P. pastoris LD we performed a proteome analysis of this compartment. For this investigation we utilized direct MS as outlined in detail in Experimental procedures section. Proteins were identified through annotation in the Universal Protein Resource Knowledgebase (http://www.uniprot.org/) and assigned to putative functions according to blast and motif searches. Additionally, we identified S. cerevisiae orthologs with their cellular localization and biological functions. Data are summarized in Table 1.
Table 1.
Proteome of P. pastoris lipid droplets. LD were isolated from cells grown on glucose to stationary phase (26 h, 30 °C). Proteins were subjected to MS analysis (see Experimental procedures). Blast analysis of amino acid sequences revealed S. cerevisiae orthologs and the degree of homology. Localization and biological processes inferred from homology are shown. Databases used were Universal Protein Resource Knowledgebase—UniProtKB (http://www.uniprot.org/20121203) and Saccharomyces Genome Database—SGD (http://www.yeastgenome.org/20121203). C, cytosol; M, mitochondria; PM, plasma membrane; ER, endoplasmic reticulum; LD, lipid droplets; E, endosomes; G, Golgi, N, nucleus; R, ribosome.
| UniProtKB ID | Submitted name (UniProtKB) | S. cerevisiae ortholog (homology %) | S. cerevisiae ortholog localization (SGD) | Biological process inferred from homology (SGD) |
|---|---|---|---|---|
| C4R4C9 | Delta(24)-sterol C-methyltransferase | Erg6p (67%) | ER/LD/M | Ergosterol biosynthetic process |
| C4R1R9 | Long chain fatty acyl-CoA synthetase with a preference for C12:0-C16:0 fatty acids | Faa1p (59%) | LD/M/PM | Long-chain fatty acid transport, long-chain fatty-acyl-CoA metabolic process |
| C4R3L8 | Squalene epoxidase, catalyzes the epoxidation of squalene to 2,3-oxidosqualene | Erg1p (53%) | ER/LD | Sterol metabolism |
| C4QXK6 | 3-ketosphinganine reductase, catalyzes the second step in phytosphingosine synthesis | Tsc10p (38%) | C/ER/M | 3-Keto-sphinganine metabolic process, sphingolipid biosynthetic process |
| C4QX24 | Acyl-coenzymeA:ethanol O-acyltransferase | Eht1p (37%) | LD/M | Medium-chain fatty acid biosynthetic/catabolic process |
| C4R403 | Steryl ester hydrolase | Tgl1p (42%) | LD | Cellular lipid metabolic process, sterol metabolic process |
| C4R0I8 | Lanosterol synthase, an essential enzyme that catalyzes the cyclization of squalene 2,3-epoxide | Erg7p (60%) | ER/LD/PM | Ergosterol biosynthetic process |
| C4R6T8 | Putative acyltransferase with similarity to Eeb1p and Eht1p | Eeb1p (35%), Eht1p (35%) | LD/M | Medium-chain fatty acid biosynthetic/catabolic process |
| C4QVA2 | Protein component of the small (40S) ribosomal subunit | Rps3p (82%) | R | Translation (RNA binding) |
| F2QTD3 | Uncharacterized membrane protein YLR326W | Ylr326wp (28%) | Unknown | Unknown |
| C4QV50 | Conserved ribosomal protein P0 similar to rat P0, human P0, and E. coli L10e | Rpp0p (71%) | R | Cytoplasmic translation, ribosomal large subunit assembly, translational elongation |
| C4R1Z2 | Mitochondrial porin (Voltage-dependent anion channel), outer membrane protein | Por1p (47%) | M | Apoptotic process, cell redox homeostasis, transport, ion transport, mitochondrion organization |
| C4QVF8 | Putative uncharacterized protein | Not found | Unknown | Unknown |
| C4QVE4 | 1-Acyl-sn-glycerol-3-phosphate acyltransferase | Slc1p (50%) | LD | Glycerophospholipid biosynthetic process |
| C4R196 | 3-Keto sterol reductase | Erg27p (63%) | ER/M | Ergosterol biosynthetic process |
| C4R2N5 | ATP synthase subunit beta | Atp2p (87%) | M | ATP synthesis coupled proton transport |
| C4QZB0 | Elongation factor 1-alpha | Tef1p (89%) | R/C | Translational elongation, tRNA export from nucleus |
| C4R4Y8 | ATP synthase subunit alpha | Atp1p (88%) | M | ATP synthesis coupled proton transport |
| C4QXD6 | Fatty acid transporter and very long-chain fatty acyl-CoA synthetase | Fat1p (49%) | ER/LD/PM | Long-chain fatty acid transport, very long chain fatty acid metabolic process |
| C4QXC1 | Putative fatty aldehyde dehydrogenase | Hfd1p (43%) | E/LD/M | Cellular aldehyde metabolic process |
| C4R2Z6 | NADPH-dependent 1-acyl dihydroxyacetone phosphate reductase | Ayr1p (52%) | ER/LD/C/M | Phosphatidic acid biosynthetic process |
| C4QW07 | Steryl ester hydrolase, one of three gene products (Yeh1p, Yeh2p, Tgl1p) | Tgl1p (39%) | LD | Cellular lipid metabolic process, sterol metabolic process |
| C4R760 | Major ADP/ATP carrier of the mitochondrial inner membrane | Pet9p (85%) | M | Respiration |
| F2QNQ8 | Transcriptional repressor OPI1 | Opi1p (27%) | ER/N | Unfolded protein response, regulation of transcription, phospholipid biosynthetic process |
| C4QYN4 | 40S ribosomal protein subunit | Rps29ap (88%) | R | Cytoplasmic translation |
| C4QW21 | Protein involved in ER-associated protein degradation | Ubx2p (25%) | ER/M | ER-associated protein catabolic process, proteasomal protein degradations, protein secretion |
| C4R7R7 | Protein with similarity to oxidoreductases, found in lipid particles | Env9p (48%) | LD | Vacuolar protein processing, vacuole organization |
| C4QYK0 | 40S ribosomal protein S0 nearly identical to Rps0Bp | Rps0ap (84%) | R | Structural constituent of ribosome |
| C4R7L9 | Putative uncharacterized protein | Tom5p (39%) | M | Protein targeting to mitochondrion |
| C4QVS9 | Plasma membrane H+-ATPase, pumps protons out of the cell | Pma1p (86%) | PM | Proton transport, regulation of pH |
| F2QT41 | Alcohol dehydrogenase class-3 | Yim1p (30%) | LD/C/M | Response to DNA damage stimulus |
| F2QYU4 | AN1-type zinc finger protein YNL155W | Ynl155wp (30%) | C/N | Unknown |
| C4R4C3 | Mitochondrial matrix ATPase | Ssc1p (82%) | M | Protein import into mitochondrial matrix, protein folding |
| C4QXL9 | GTPase, similar to Ypt51p and Ypt53p and to mammalian rab5 | Vps21p (54%) | E | Endocytosis, protein targeting to vacuole |
| C4QWQ5 | Prenyltransferase, required for cell viability | Nus1p (47%) | LD/ER | Protein glycosylation |
| C4R3N7 | Secretory vesicle-associated Rab GTPase essential for exocytosis | Ypt1p (49%) | ER/G/M | ER to Golgi vesicle-mediated transport |
The function of most P. pastoris LD proteins identified here is hypothetical and derived from bioinformatic analysis (http://www.uniprot.org/20121203). However, detected proteins can be divided in two groups depending on association with lipid metabolism or being involved in other cellular processes. The first group includes the most abundant proteins of the P. pastoris LD proteome such as enzymes involved in ergosterol, phospholipid and sphingolipid biosynthesis as well as fatty acid metabolism, fatty acid transport and activation, and lipolysis. These findings are in line with proteomic studies of S. cerevisiae LD performed recently in our laboratory [18]. The second group of P. pastoris LD proteins identified here is not associated with lipid metabolism, but with ribosomal translation, energy metabolism and mitochondria function. Although our LD preparations from P. pastoris were shown to be of high quality, contamination with other subcellular fractions cannot be completely excluded. However, recent studies showed structural and functional interactions between LD and other organelles [58–62] which are of physiological relevance. The possible involvement of proteins found in this study in such processes will be subject to individual investigations.
4. Discussion
During the investigation presented here we established a reliable technique for the isolation of highly pure LD from the industrial yeast P. pastoris which enabled us to analyze lipidome and proteome of this organelle. Our results confirm in general our current knowledge of yeast LD biochemistry, although several peculiarities in lipid and protein composition of LD from P. pastoris were observed. A specific and remarkable feature of P. pastoris LD is the high prevalence for TG over SE in its hydrophobic core. This result is in strong contrast to S. cerevisiae LD which contain equivalent amounts of TG and SE [16]. The other difference between P. pastoris and S. cerevisiae LD is the unselective occurrence of poly/unsaturated fatty acids in the former microorganism. This finding is in line with our studies of other P. pastoris organelles, e.g. with peroxisomes and mitochondria [5,6], which did not show any specific fatty acid targeting either. Thus, the high degree of fatty acid unsaturation appears to apply to most P. pastoris organelles.
Proteome analysis of P. pastoris LD revealed a distinct set of LD associated proteins. Based on homologies to S. cerevisiae a number of these proteins were identified. However, functions of some of these newly identified proteins remained unassigned. Interestingly, the total number of P. pastoris LD proteins found in this study was rather low compared to investigations with S. cerevisiae [18], mammalian cells [59,63,64] and plant cells [65–67]. We can speculate that P. pastoris LD require only a minimal set of proteins to maintain structure and function of this organelle, and that functions of LD from this yeast are not diverse from other organisms [68–70]. Our results also demonstrate that LD from P. pastoris contribute to lipid metabolism (see Table 1) as has been shown before for LD from other cell types. Individual features or functions of LD from different species may be explained by differences in the proteome. From this view point “unusual” or unidentified proteins in the LP proteome from P. pastoris may become important. The functional relevance of such proteins will have to be tested rather on an individual than on a global basis. Proteome analysis as presented here may set the stage for such studies.
Acknowledgments
This work was supported by the Austrian Science Fund FWF, Translational Research Project TRP009 and DK Molecular Enzymology W901-B05 to G.D.
References
- 1.Cregg J.M., Cereghino J.L., Shi J., Higgins D.R. Recombinant protein expression in Pichia pastoris. Mol. Biotechnol. 2000;16:23–52. doi: 10.1385/MB:16:1:23. [DOI] [PubMed] [Google Scholar]
- 2.Daly R., Hearn M.T. Expression of heterologous proteins in Pichia pastoris: a useful experimental tool in protein engineering and production. J. Mol. Recognit. 2005;18:119–138. doi: 10.1002/jmr.687. [DOI] [PubMed] [Google Scholar]
- 3.Macauley-Patrick S., Fazenda M.L., McNeil B., Harvey L.M. Heterologous protein production using the Pichia pastoris expression system. Yeast. 2005;22:249–270. doi: 10.1002/yea.1208. [DOI] [PubMed] [Google Scholar]
- 4.Li P., Anumanthan A., Gao X.G., Ilangovan K., Suzara V.V., Duzgunes N., Renugopalakrishnan V. Expression of recombinant proteins in Pichia pastoris. Appl. Biochem. Biotechnol. 2007;142:105–124. doi: 10.1007/s12010-007-0003-x. [DOI] [PubMed] [Google Scholar]
- 5.Wriessnegger T., Gübitz G., Leitner E., Ingolic E., Cregg J., de la Cruz B.J., Daum G. Lipid composition of peroxisomes from the yeast Pichia pastoris grown on different carbon sources. Biochim. Biophys. Acta. 2007;1771:455–461. doi: 10.1016/j.bbalip.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Wriessnegger T., Leitner E., Belegratis M.R., Ingolic E., Daum G. Lipid analysis of mitochondrial membranes from the yeast Pichia pastoris. Biochim. Biophys. Acta. 2009;1791:166–172. doi: 10.1016/j.bbalip.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg A.S., Coleman R.A., Kraemer F.B., McManaman J.L., Obin M.S., Puri V., Yan Q.W., Miyoshi H., Mashek D.G. The role of lipid droplets in metabolic disease in rodents and humans. J. Clin. Invest. 2011;121:2102–2110. doi: 10.1172/JCI46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olofsson S.O., Bostrom P., Andersson L., Rutberg M., Perman J., Boren J. Lipid droplets as dynamic organelles connecting storage and efflux of lipids, Biochim. Biophys. Acta. 2009;1791:448–458. doi: 10.1016/j.bbalip.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Cheng J., Suzuki M., Shinohara Y. Lipid droplets: a classic organelle with new outfit. Histochem. Cell Biol. 2008;130:263–279. doi: 10.1007/s00418-008-0449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalantari F., Bergeron J.J., Nilsson T. Biogenesis of lipid droplets—how cells get fatter. Mol. Membr. Biol. 2010;27:462–468. doi: 10.3109/09687688.2010.538936. [DOI] [PubMed] [Google Scholar]
- 11.Murphy D.J., Vance J. Mechanisms of lipid-body formation. Trends Biochem. Sci. 1999;24:109–115. doi: 10.1016/s0968-0004(98)01349-8. [DOI] [PubMed] [Google Scholar]
- 12.Ploegh H.L. A lipid-based model for the creation of an escape hatch from the endoplasmic reticulum. Nature. 2007;448:435–438. doi: 10.1038/nature06004. [DOI] [PubMed] [Google Scholar]
- 13.Robenek H., Buers I., Hofnagel O., Robenek M.J., Troyer D., Severs N.J. Compartmentalization of proteins in lipid droplet biogenesis. Biochim. Biophys. Acta. 2009;1791:408–418. doi: 10.1016/j.bbalip.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Waltermann M., Hinz A., Robenek H., Troyer D., Reichelt R., Malkus U., Galla H.J., Kalscheuer R., Stoveken T., von Landenberg P., Steinbuchel A. Mechanism of lipid-body formation in prokaryotes: how bacteria fatten up. Mol. Microbiol. 2005;55:750–763. doi: 10.1111/j.1365-2958.2004.04441.x. [DOI] [PubMed] [Google Scholar]
- 15.Jacquier N., Choudhary V., Mari M., Toulmay A., Reggiori F., Schneiter R. Lipid droplets are functionally connected to the endoplasmic reticulum in Saccharomyces cerevisiae. J. Cell Sci. 2011;124:2424–2437. doi: 10.1242/jcs.076836. [DOI] [PubMed] [Google Scholar]
- 16.Leber R., Zinser E., Zellnig G., Paltauf F., Daum G. Characterization of lipid particles of the yeast, Saccharomyces cerevisiae. Yeast. 1994;10:1421–1428. doi: 10.1002/yea.320101105. [DOI] [PubMed] [Google Scholar]
- 17.Athenstaedt K., Zweytick D., Jandrositz A., Kohlwein S.D., Daum G. Identification and characterization of major lipid particle proteins of the yeast Saccharomyces cerevisiae. J. Bacteriol. 1999;181:6441–6448. doi: 10.1128/jb.181.20.6441-6448.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grillitsch K., Connerth M., Köfeler H., Arrey T.N., Rietschel B., Wagner B., Karas M., Daum G. Lipid particles/droplets of the yeast Saccharomyces cerevisiae revisited: lipidome meets proteome. Biochim. Biophys. Acta. 2011;1811:1165–1176. doi: 10.1016/j.bbalip.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Athenstaedt K., Daum G. Phosphatidic acid, a key intermediate in lipid metabolism. Eur. J. Biochem. 1999;266:1–16. doi: 10.1046/j.1432-1327.1999.00822.x. [DOI] [PubMed] [Google Scholar]
- 20.Zinser E., Paltauf F., Daum G. Sterol composition of yeast organelle membranes and subcellular distribution of enzymes involved in sterol metabolism. J. Bacteriol. 1993;175:2853–2858. doi: 10.1128/jb.175.10.2853-2858.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson D.R., Knoll L.J., Levin D.E., Gordon J.I. Saccharomyces cerevisiae contains four fatty acid activation (FAA) genes: an assessment of their role in regulating protein N-myristoylation and cellular lipid metabolism. J. Cell Biol. 1994;127:751–762. doi: 10.1083/jcb.127.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duronio R.J., Knoll L.J., Gordon J.I. Isolation of a Saccharomyces cerevisiae long chain fatty acyl:CoA synthetase gene (FAA1) and assessment of its role in protein N-myristoylation. J. Cell Biol. 1992;117:515–529. doi: 10.1083/jcb.117.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watkins P.A., Lu J.F., Steinberg S.J., Gould S.J., Smith K.D., Braiterman L.T. Disruption of the Saccharomyces cerevisiae FAT1 gene decreases very long-chain fatty acyl-CoA synthetase activity and elevates intracellular very long-chain fatty acid concentrations. J. Biol. Chem. 1998;273:18210–18219. doi: 10.1074/jbc.273.29.18210. [DOI] [PubMed] [Google Scholar]
- 24.Sorger D., Athenstaedt K., Hrastnik C., Daum G. A yeast strain lacking lipid particles bears a defect in ergosterol formation. J. Biol. Chem. 2004;279:31190–31196. doi: 10.1074/jbc.M403251200. [DOI] [PubMed] [Google Scholar]
- 25.Oelkers P., Cromley D., Padamsee M., Billheimer J.T., Sturley S.L. The DGA1 gene determines a second triglyceride synthetic pathway in yeast. J. Biol. Chem. 2002;277:8877–8881. doi: 10.1074/jbc.M111646200. [DOI] [PubMed] [Google Scholar]
- 26.Athenstaedt K., Daum G. YMR313c/TGL3 encodes a novel triacylglycerol lipase located in lipid particles of Saccharomyces cerevisiae. J. Biol. Chem. 2003;278:23317–23323. doi: 10.1074/jbc.M302577200. [DOI] [PubMed] [Google Scholar]
- 27.Athenstaedt K., Daum G. Tgl4p and Tgl5p, two triacylglycerol lipases of the yeast Saccharomyces cerevisiae are localized to lipid particles. J. Biol. Chem. 2005;280:37301–37309. doi: 10.1074/jbc.M507261200. [DOI] [PubMed] [Google Scholar]
- 28.Mullner H., Deutsch G., Leitner E., Ingolic E., Daum G. YEH2/YLR020c encodes a novel steryl ester hydrolase of the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2005;280:13321–13328. doi: 10.1074/jbc.M409914200. [DOI] [PubMed] [Google Scholar]
- 29.Koffel R., Tiwari R., Falquet L., Schneiter R. The Saccharomyces cerevisiae YLL012/YEH1, YLR020/YEH2, and TGL1 genes encode a novel family of membrane-anchored lipases that are required for steryl ester hydrolysis. Mol. Cell. Biol. 2005;25:1655–1668. doi: 10.1128/MCB.25.5.1655-1668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jandrositz A., Petschnigg J., Zimmermann R., Natter K., Scholze H., Hermetter A., Kohlwein S.D., Leber R. The lipid droplet enzyme Tgl1p hydrolyzes both steryl esters and triglycerides in the yeast, Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2005;1735:50–58. doi: 10.1016/j.bbalip.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Fei W., Wang H., Fu X., Bielby C., Yang H. Conditions of endoplasmic reticulum stress stimulate lipid droplet formation in Saccharomyces cerevisiae. Biochem. J. 2009;424:61–67. doi: 10.1042/BJ20090785. [DOI] [PubMed] [Google Scholar]
- 32.Czabany T., Athenstaedt K., Daum G. Synthesis, storage and degradation of neutral lipids in yeast. Biochim. Biophys. Acta. 2007;1771:299–309. doi: 10.1016/j.bbalip.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Oelkers P., Tinkelenberg A., Erdeniz N., Cromley D., Billheimer J.T., Sturley S.L. A Lecithin cholesterol acyltransferase-like gene mediates diacylglycerol esterification in yeast. J. Biol. Chem. 2000;275:15609–15612. doi: 10.1074/jbc.C000144200. [DOI] [PubMed] [Google Scholar]
- 34.Dahlqvist A., Stahl U., Lenman M., Banas A., Lee M., Sandager L., Ronne H., Stymne S. Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6487–6492. doi: 10.1073/pnas.120067297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorger D., Daum G. Synthesis of triacylglycerols by the acyl-coenzyme A:diacyl-glycerol acyltransferase Dga1p in lipid particles of the yeast Saccharomyces cerevisiae. J. Bacteriol. 2002;184:519–524. doi: 10.1128/JB.184.2.519-524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang H., Bard M., Bruner D.A., Gleeson A., Deckelbaum R.J., Aljinovic G., Pohl T.M., Rothstein R., Sturley S.L. Sterol esterification in yeast: a two-gene process. Science. 1996;272:1353–1356. doi: 10.1126/science.272.5266.1353. [DOI] [PubMed] [Google Scholar]
- 37.Yu C., Kennedy N.J., Chang C.Y., Rothblatt J.A. Molecular cloning and characterization of two isoforms of Saccharomyces cerevisiae acyl-CoA:sterol acyltransferase. J. Biol. Chem. 1996;271:24157–24163. doi: 10.1074/jbc.271.39.24157. [DOI] [PubMed] [Google Scholar]
- 38.Zweytick D., Leitner E., Kohlwein S.D., Yu C., Rothblatt J., Daum G. Contribution of Are1p and Are2p to steryl ester synthesis in the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 2000;267:1075–1082. doi: 10.1046/j.1432-1327.2000.01103.x. [DOI] [PubMed] [Google Scholar]
- 39.Jensen-Pergakes K., Guo Z., Giattina M., Sturley S.L., Bard M. Transcriptional regulation of the two sterol esterification genes in the yeast Saccharomyces cerevisiae. J. Bacteriol. 2001;183:4950–4957. doi: 10.1128/JB.183.17.4950-4957.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valachovic M., Klobucnikova V., Griac P., Hapala I. Heme-regulated expression of two yeast acyl-CoA:sterol acyltransferases is involved in the specific response of sterol esterification to anaerobiosis. FEMS Microbiol. Lett. 2002;206:121–125. doi: 10.1111/j.1574-6968.2002.tb10996.x. [DOI] [PubMed] [Google Scholar]
- 41.Kuberl A., Schneider J., Thallinger G.G., Anderl I., Wibberg D., Hajek T., Jaenicke S., Brinkrolf K., Goesmann A., Szczepanowski R., Puhler A., Schwab H., Glieder A., Pichler H. High-quality genome sequence of Pichia pastoris CBS7435. J. Biotechnol. 2011;154:312–320. doi: 10.1016/j.jbiotec.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 42.Lin-Cereghino J., Wong W.W., Xiong S., Giang W., Luong L.T., Vu J., Johnson S.D., Lin-Cereghino G.P. Condensed protocol for competent cell preparation and transformation of the methylotrophic yeast Pichia pastoris. Biotechniques. 2005;38:44–48. doi: 10.2144/05381BM04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zinser E., Daum G. Isolation and biochemical characterization of organelles from the yeast, Saccharomyces cerevisiae. Yeast. 1995;11:493–536. doi: 10.1002/yea.320110602. [DOI] [PubMed] [Google Scholar]
- 44.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 45.Wessel D., Flügge U.I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 46.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 47.Haid A., Suissa M. Immunochemical identification of membrane proteins after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Methods Enzymol. 1983;96:192–205. doi: 10.1016/s0076-6879(83)96017-2. [DOI] [PubMed] [Google Scholar]
- 48.Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 49.Broekhuyse R.M. Phospholipids in tissues of the eye. I. Isolation, characterization and quantitative analysis by two-dimensional thin-layer chromatography of diacyl and vinyl-ether phospholipids. Biochim. Biophys. Acta. 1968;152:307–315. doi: 10.1016/0005-2760(68)90038-6. [DOI] [PubMed] [Google Scholar]
- 50.Quail M.A., Kelly S.L. The extraction and analysis of sterols from yeast. Methods Mol. Biol. 1996;53:123–131. doi: 10.1385/0-89603-319-8:123. [DOI] [PubMed] [Google Scholar]
- 51.Fauland A., Koefeler H., Troetzmueller M., Knopf A., Hartler J., Eberl A., Chitraju C., Lankmayr E., Spener F. A comprehensive method for lipid profiling by liquid chromatography-ion cyclotron resonance mass spectrometry. J. Lipid Res. 2011;52:2314–2322. doi: 10.1194/jlr.D016550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hartler J., Trötzmüller M., Chitraju C., Spener F., Köfeler H.C., Thallinger G.G. Lipid Data Analyzer: unattended identification and quantitation of lipids in LC–MS data. Bioinformatics. 2011;27:572–577. doi: 10.1093/bioinformatics/btq699. [DOI] [PubMed] [Google Scholar]
- 53.Athenstaedt K., Jolivet P., Boulard C., Zivy M., Negroni L., Nicaud J.M., Chardot T. Lipid particle composition of the yeast Yarrowia lipolytica depends on the carbon source. Proteomics. 2006;6:1450–1459. doi: 10.1002/pmic.200500339. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki M., Shinohara Y., Ohsaki Y., Fujimoto T. Lipid droplets: size matters. J. Electron Microsc. (Tokyo) 2011;60:101–116. doi: 10.1093/jmicro/dfr016. [DOI] [PubMed] [Google Scholar]
- 55.Tauchi-Sato K., Ozeki S., Houjou T., Taguchi R., Fujimoto T. The surface of lipid droplets is a phospholipid monolayer with a unique fatty acid composition. J. Biol. Chem. 2002;277:44507–44512. doi: 10.1074/jbc.M207712200. [DOI] [PubMed] [Google Scholar]
- 56.Czabany T., Wagner A., Zweytick D., Lohner K., Leitner E., Ingolic E., Daum G. Structural and biochemical properties of lipid particles from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2008;283:17065–17074. doi: 10.1074/jbc.M800401200. [DOI] [PubMed] [Google Scholar]
- 57.Horn P.J., Ledbetter N.R., James C.N., Hoffman W.D., Case C.R., Verbeck G.F., Chapman K.D. Visualization of lipid droplet composition by direct organelle mass spectrometry. J. Biol. Chem. 2011;286:3298–3306. doi: 10.1074/jbc.M110.186353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pu J., Ha C.W., Zhang S., Jung J.P., Huh W.K., Liu P. Interactomic study on interaction between lipid droplets and mitochondria. Protein Cell. 2011;2:487–496. doi: 10.1007/s13238-011-1061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H., Wang Y., Li J., Yu J., Pu J., Li L., Zhang H., Zhang S., Peng G., Yang F., Liu P. Proteome of skeletal muscle lipid droplet reveals association with mitochondria and apolipoprotein A-I. J. Proteome Res. 2011;10:4757–4768. doi: 10.1021/pr200553c. [DOI] [PubMed] [Google Scholar]
- 60.Wolinski H., Kolb D., Hermann S., Koning R.I., Kohlwein S.D. A role for seipin in lipid droplet dynamics and inheritance in yeast. J. Cell Sci. 2011;124:3894–3904. doi: 10.1242/jcs.091454. [DOI] [PubMed] [Google Scholar]
- 61.Binns D., Januszewski T., Chen Y., Hill J., Markin V.S., Zhao Y., Gilpin C., Chapman K.D., Anderson R.G., Goodman J.M. An intimate collaboration between peroxisomes and lipid bodies. J. Cell Biol. 2006;173:719–731. doi: 10.1083/jcb.200511125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu P., Bartz R., Zehmer J.K., Ying Y.S., Zhu M., Serrero G., Anders R.G. Rab-regulated interaction of early endosomes with lipid droplets. Biochim. Biophys. Acta. 2007;1773:784–793. doi: 10.1016/j.bbamcr.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larsson S., Resjö S., Gomez M.F., James P., Holm C. Characterization of the lipid droplet proteome of a clonal insulin-producing β-cell line (INS-1 832/13) J. Proteome Res. 2012;11:1264–1273. doi: 10.1021/pr200957p. [DOI] [PubMed] [Google Scholar]
- 64.Ding Y., Wu Y., Zeng R., Liao K. Proteomic profiling of lipid droplet-associated proteins in primary adipocytes of normal and obese mouse. Acta Biochim. Biophys. Sin. (Shanghai) 2012;44:394–406. doi: 10.1093/abbs/gms008. [DOI] [PubMed] [Google Scholar]
- 65.Jolivet P., Roux E., d'Andrea S., Davanture M., Negroni L., Zivy M., Chardot T. Protein composition of oil bodies in Arabidopsis thaliana ecotype WS. Plant Physiol. Biochem. 2004;42:501–509. doi: 10.1016/j.plaphy.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 66.Katavic V., Agrawal G.K., Hajduch M., Harris S.L., Thelen J.J. Protein and lipid composition analysis of oil bodies from two Brassica napus cultivars. Proteomics. 2006;6:4586–4598. doi: 10.1002/pmic.200600020. [DOI] [PubMed] [Google Scholar]
- 67.Moellering E.R., Benning C. RNA interference silencing of a major lipid droplet protein affects lipid droplet size in Chlamydomonas reinhardtii. Eukaryot. Cell. 2010;9:97–106. doi: 10.1128/EC.00203-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fujimoto T., Parton R.G. Not just fat: the structure and function of the lipid droplet. Cold Spring Harb. Perspect. Biol. 2011;3:a004838. doi: 10.1101/cshperspect.a004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chapman K.D., Dyer J.M., Mullen R.T. Biogenesis and functions of lipid droplets in plants: thematic review series: lipid droplet synthesis and metabolism: from yeast to man. J. Lipid Res. 2012;53:215–226. doi: 10.1194/jlr.R021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buers I., Hofnagel O., Ruebel A., Severs N.J., Robenek H. Lipid droplet associated proteins: an emerging role in atherogenesis. Histol. Histopathol. 2011;26:631–642. doi: 10.14670/HH-26.631. [DOI] [PubMed] [Google Scholar]