Abstract
Voltage-gated Ca2 + channels allow for Ca2 +-dependent intracellular signaling by directly mediating Ca2 + ion influx, by physical coupling to intracellular Ca2 + release channels or functional coupling to other ion channels such as Ca2 + activated potassium channels. L-type Ca2 + channels that comprise the family of Cav1 channels are expressed in many electrically excitable tissues and are characterized by their unique sensitivity to dihydropyridines. In this issue, we summarize genetic defects in L-type Ca2 + channels and analyze their role in human diseases (Ca2 + channelopathies); e.g. mutations in Cav1.2 α1 cause Timothy and Brugada syndrome, mutations in Cav1.3 α1 are linked to sinoatrial node dysfunction and deafness while mutations in Cav1.4 α1 are associated with X-linked retinal disorders such as an incomplete form of congenital stationary night blindness. Herein, we also put the mutations underlying the channel's dysfunction into the structural context of the pore-forming α1 subunit. This analysis highlights the importance of combining functional data with structural analysis to gain a deeper understanding for the disease pathophysiology as well as for physiological channel function. This article is part of a Special Issue entitled: Calcium channels.
Keywords: L-type calcium channel, Channelopathy, Structure–function analysis, Homology modeling
Graphical abstract
Highlights
► Structural homology model for structure–(dys)function analysis in Cav1.x mutants ► Functional ‘hotspots’ for naturally occurring loss- and gain-of-function mutants ► Gain-of-function mutants interfere with voltage sensor coupling to gate opening. ► Intramolecular interaction in LTCC C-termini with pharmacotherapeutic potential
1. Introduction
Voltage-gated calcium channels (VGCCs) govern important physiological functions such as contraction, secretion, neurotransmission, and gene expression in many different cell types by mediating Ca2 + entry into electrically excitable cells in response to membrane depolarization [1]. VGCCs, like other ion channels, do not operate as isolated proteins, but instead form signaling complexes with signaling molecules, receptors, other types of ion channels [2,3]. A central pore-forming α1-subunit determines most of the channel's biophysical and pharmacological properties. In the cell membrane, the calcium channels form a hetero-oligomeric complex with auxiliary β- and α2δ-subunits, and in some cases δ-subunits. VGCCs can be divided into the group of L-type calcium channels (LTCCs, Cav1 family) and non-LTCCs (Cav2 and Cav3 family). We will focus in this review on LTCCs.
The LTCCs comprise the isoforms Cav1.1, Cav1.2, Cav1.3 and Cav1.4. Their functional diversity reaches from excitation–contraction coupling in muscle, to stimulus secretion coupling in sensory and endocrine cells, cardiac pace-making, as well as neuronal firing to learning and memory [1]. They can be distinguished pharmacologically from other VGCCs by their high sensitivity towards organic Ca2 + channel blockers and activators. Among those, dihydropyridines (DHPs) proved especially important because of their very high binding affinity, their high selectivity for LTCCs and their diversity, being both Ca2 + channel blockers (e.g. isradipine, nifedipine) and Ca2 + channel activators (e.g. BayK 8644) [4,5]. The Ca2 + channel blockers in clinical use (as such nifedipine, amlodipine, verapamil and diltiazem) have mainly cardiovascular effects as they preferentially block Cav1.2 channels in the cardiovascular tissue.
The Cav1.3 channels are expressed together with Cav1.2 in several tissues including sinoatrial nodes, heart atria, neurons, chromaffin cells and pancreatic islets. In particular, Cav1.3 serves an important role in the sinoatrial node [6] and in chromaffin cells [7], and they shape neuronal function [8] and help to support pace-making in vulnerable dopaminergic substantia nigra neurons [9]. Importantly the use of DHPs has been shown to be associated with a decreased incidence of Parkinson's disease in humans [10]. In the hippocampus Cav1.2 mediates long-term potentiation, spatial learning and memory [11], whereas Cav1.3 mediates long-term potentiation in the amygdala and participates in the consolidation of fear memory [12].
Cav1.4 channels are predominantly expressed in retinal cells where they play an important role, as evident from mutations in the voltage-gated calcium channel gene CACNA1F encoding Cav1.4 LTCCs that cause several forms of retinal diseases in humans (OMIM: 300071, 300476, 300600). Cav1.4 knock-out mice support this view as these mice show severe visual deficiencies [13,14]. Cav1.4 expression is also reported in dorsal root ganglia neurons [15], mast cells [16] and T-lymphocytes [17].
Cav1.1 channels possess a more restricted expression pattern. This channel is expressed almost exclusively in skeletal muscle cells. Conformational changes of voltage-sensing domains in Cav1.1 are transmitted by a mechanical linkage to the associated ryanodine receptors that release Ca2 + from the sarcoplasmic reticulum [1].
All VGCCs are capable of sensing intracellular Ca2 + levels. This is very likely a safety mechanism to protect cells from calcium overload via activity-dependent feedback mechanisms such as Ca2 +-dependent inactivation (CDI), mediated by C-terminally bound calmodulin [18,19]. Mechanisms that inhibit CDI play an important role in sensory cells (as such for Cav1.4 in retinal cells and for Cav1.3 in auditory cells, see [20]) allowing the inducement of graded and tonic presynaptic depolarization and making neurotransmitter release dependent on sustained activation of presynaptic LTCCs.
Diseases caused by mutations in genes encoding ion channel subunits or their regulatory proteins are referred to as ‘channelopathies’. A large number of distinct channel dysfunctions have been described to be caused by mutations in the channel's 1α subunits. Mutations in LTCCs may result in a loss-of-function, in which the LTCC mediated Ca2 + influx is (much) reduced or completely abolished whereas gain-of-function mutations confer new or enhanced activity. However, apparently such enhanced activity has an unwarranted positive connotation. Gain-of-function mutations may result in enhanced Ca2 + influx, but this increased sensitivity does not necessarily result in improved signaling. Instead, this might result in a loss-of-control of existing Ca2 + signaling pathways.
Here we summarize the role of selected LTCCs in human diseases that are caused by genetic defects in these Ca2 + channels (‘Ca2 + channelopathies’) and we discuss the functional effects of the structural aberrations within the pore-forming α1 subunit that underlie the channel's dysfunction. Table 1 summarizes Cav1.2, Cav1.3 and Cav1.3 α1 subunit related diseases. Mutations in the Cav1.1 channel, leading to hypokalemic periodic paralysis and malignant hyperthermia sensitivity are reviewed elsewhere [21]. We elaborate on common structural ‘hotspots’ that result in either loss- or gain-of-channel function in all the three LTCCs and discuss them in the structural context of a Cav1.4 channel homology model (Fig. 1 and Table 1). The model of the Cav1.4 channel was created using the structure of NavAB (PDB ID: 3RVY, [22]) as a template. The sequences of human Cav1.x and NavAB were first aligned with MUSCLE [23], then models of the Cav1.4 channel were created using MODELLER version 9.8 [24]. The best model based on the DOPE score was selected for analysis [25].
Table 1.
Diseases associated with mutations in Cav1.2, Cav1.3 and Cav1.4 LTCCs.
| LTCC | Disease/syndrome (abbreviation) |
Mutation(s) | Number of affected families/individuals (as far as described clearly in the references) |
|---|---|---|---|
| Cav1.2 | Timothy syndrome (TS) | p.Gly402Ser, p.Gly406Arg (exons 8 and 8A) | 17 patients (4 case studies), refs. 3, 4 |
| Brugada syndrome (BS) | p.Ala39Val, p.Gly490Arg, p.Glu850del, p.Glu1115Lys, p.Glu1829_Gln, 1833dupl, p.Val2041Ile, p.Cys1837Tyr, p.Arg1880Gln, p.Asp2130Asn | 2 patients, ref. 1 | |
| BS & short QT (SQT) | 9 patients, ref. 2 | ||
| Early repolarization syndrome (ERS) | 1 patient, ref. 2 | ||
| Cav1.3 | Sinoatrial node dysfunction and deafness (SANDD) | p.403_404insGly | 2 Pakistani families/6 affected males, 1 affected female, ref. 6 |
| Cav1.4 | Congenital stationary night blindness type 2 (CSNB2) | Exonic mutations (see Table 2) Intronic splice site mutations: IVS4-2A>G, 2387-1(G>C), 2673+3(G>A), 2674-2,3(delCA), 2571+1G>C, IVS24+1G>A, IVS28-1 GCGTC>TGG, 3942+2(T>A), 3942+2(T>A), 4101-1(G>C), IVS40-2A>G |
Missense: 33 families, refs. 7–11, 14, 16, 17, 23, 24 Truncations: 24 families, refs. 7–11, 17, 24 Deletions/insertions: 35 families, refs. 7–12, 21, 23 Splice site mutations: 11 families, refs. 9–11 |
| X-linked retinal disorder (XRD) similar to CSNB2 but more severe phenotype | p.Ile745Thr | 1 New Zealand (Maori) family, ref. 16 | |
| Cone–rod dystrophy (CORDX3) | Splice site mutation: IVS28-1 GCGTC>TGG |
1 Finnish family/7 affected males, 10 female carriers, 33 non-affected family members, ref. 19 | |
| Åland Island eye disease (AIED) | p.del1211–1247 | 1 Finnish family/6 samples from affected males, ref. 21 | |
| Night-blindness-associated transient tonic downgaze (NATTD) | p.Trp349stop, p.Gly359Arg, p.Pro1489Arg | 8 boys, among those 2 pairs of maternally related half-brothers, 2 cousins, and 2 siblings, ref. 24 |
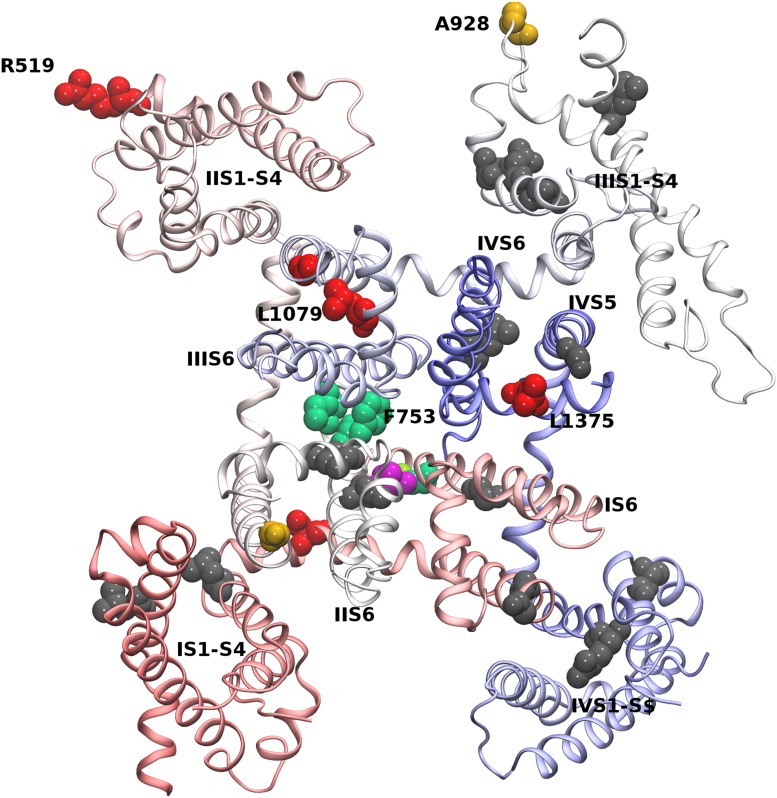
Fig. 1.
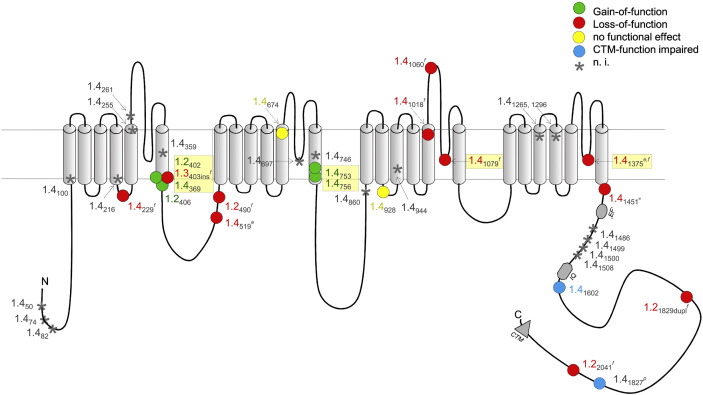
Location of human mutations in Ca2 + channel Cav1.2, Cav1.3 and Cav1.4 α1 subunits. The numbers refer to the position of the mutations in the corresponding LTCC (1.2, Cav1.2; 1.3, Cav1.3; 1.4, Cav1.4). For Cav1.4, only missense and truncation mutants were included, insertion and deletion mutants were omitted for clarity. Colors indicate the observed functional changes. n.i.; not yet investigated. Loss-of-function reported due to: e expression deficiency, f functional defect, p predicted. Abbreviations: ‘EF’, putative EF-hand motif; IQ, IQ-motif; CTM, C-terminal modulator [73]. Potential structure–functional hotspots are highlighted by yellow boxes.
2. Cav1.2-related channelopathies
Cav1.2 is the predominantly expressed LTCC in the cardiovascular system. Its dysfunction can cause severe cardiac diseases in humans often associated with sudden cardiac death. Patients carrying mutations in the CACNA1C gene – that encodes for the Cav1.2 channel – may suffer from Timothy syndrome (TS, [26,27]), Brugada syndrome (BS, [28]), and in some cases are diagnosed together with shorter as normal QT intervals (sQT; [29]) and early repolarization syndrome (ERS, [29]). Among those, TS is a multiorgan disease; the patients may suffer from syndactyly (fusion of fingers and/or toes), immune deficiency, intermittent hypoglycemia, cognitive abnormalities and autism [26,27] in addition to cardiac arrhythmias that are often associated with sudden death. Cav1.2 mutations from patients with BS so far functionally analyzed led to a loss-of-function (Tables 1 and 2) showing a still controversially discussed [28–30] loss-of-trafficking phenotype. In contrary, two mutations in patients suffering from TS show a Cav1.2 channel gain-of-function [26,27]. The ventricular arrhythmias caused by these mutations are severe and the majority of TS patients seldom survived beyond the age of three years. The two mutations were initially identified as de novo mutations. However, they may actually also represent parental mosaicism [27,31]. The low number of reports in the literature of patients/families carrying Cav1.2 (and also Cav1.3) mutations (Table 1) might be due to mild(er) phenotypes that could eventually escape detection in case of somatic mosaicism.
Table 2.
Disease causing mutations in human Cav1.2, Cav1.3 and Cav1.4 alpha1-subunits. Different types of mutations have been reported: M, missense; T, truncation; D, deletion; I, insertion; Dupl, duplication; bp, base pair. Splice site mutations are not included in the table. References: 1: Antzelevitch et al., 2007; 2: Burashnikov et al., 2010; 3: Splawski et al., 2004; 4: Splawski et al., 2005, 5: Etheridge et al., 2011; 6: Baig et al., 2011; 7: Strom et al., 1998; 8: Bech-Hansen et al., 1998; 9: Boycott et al., 2001; 10: Nakamura et al., 2001; 11: Wutz et al., 2002; 12: Jacobi et al., 2003; 13: McRory et al., 2004; 14: Hemara-Wahanui et al., 2005; 15: Hoda et al., 2005; 16: Hope et al., 2005; 17: Zeitz et al., 2005; 18: Hoda et al., 2006; 19: Jalkanen et al., 2006; 20: Singh et al., 2006; 21: Jalkanen et al., 2007; 22: Peloquin et al., 2007; 23: Zeitz et al., 2009; 24: Simonsz et al., 2009. Numbering of mutations refers to the following Genbank accession numbers: Cav1.2: NM_000719, UNIPROT entry number: Q13936, Cav1.3: EU363339, UNIPROT entry number: B0FYA3, Cav1.4: UNIPROT entry number: O60840-1, isoform 1. Numbering referring to Genbank accession number JF701915 in publications showing functional data is added in parenthesis (this isoform contains exon 9a and therefore lacks 11 amino acids). * indicates that loss of channel function is highly predicted.
| Cav1.4 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exon | Type | Loss-of-function | Ref | Exon | Type | Gain-of-function | Ref | Exon | Type | CTM-function impaired | Ref | Exon | Type | Unknown function | Ref |
| 2 | T | p.Arg50stop* | 9 | 8 | M | p.Gly369Asp | 7, 9, 11, 13, 16 | 41 | T | p.Lys1602stop (1591) | 7, 11, 20 | 2 | M | p.Cys74Arg | 11, 24 |
| 2 | D/I | c.151del5* | 11 | 17 | M | p.Phe753Cys (742) |
11, 23 | 46 | T | p.Arg1827stop (1816) predicted20 | 11 | 6 | M | p.Gly261Arg | 11 |
| 2 | T | p.Arg82stop* | 9, 11 | 17 | M | p.Ile756Thr (745) |
14, 16 | 7 | D/I | c.951del3bp | 9 | ||||
| 4 | D/I | c.271del4 bp/ins34 bp, del/ins net 30 bp* | 9, 10 | 8 | M | p.Gly359Arg | 24 | ||||||||
| 6 | M | p.Ser229Pro | 11, 15 | 16 | M | p.Asn746Thr | 23 | ||||||||
| 7 | D/I | c.904insG* (709) | 10 | 21 | M | p.Leu860Pro [849] | 11 | ||||||||
| 7 | D/I | c.935delA* | 23 | 23 | M | p.Asp944Tyr | 23 | ||||||||
| 8 | T | Trp360stop | 24 | 28 | M | p.Glu1145Lys | 23 | ||||||||
| 9 | D/I | c.1218delC* | 8, 9 | 29 | M | p.Arg1182Pro | 11 | ||||||||
| 10 | T | p.Gln428stop* | 11 | 31 | M | p.Ser1265Ile | 17 | ||||||||
| 13 | M | p.Arg519Gln (508) | 7, 9, 18, 23 | 33 | M | p.Arg1296Ser | 17 | ||||||||
| 14 | T | p.Arg625stop* | 9, 11 | 38 | M | p.Leu1486Pro | 23 | ||||||||
| 15 | T | p.Arg691stop* | 17 | 38 | M | p.Pro1492Ala | 23 | ||||||||
| 21 | T | p.Arg895stop* | 8, 9, 17 | 38 | M | p.Cys1499Arg | 11 | ||||||||
| 24 | T | p.Arg969stop* | 7, 11 | 38 | M | p.Pro1500Arg | 11, 24 | ||||||||
| 24 | T | p.Arg978stop* | 10 | 39 | M | p.Leu1508Pro | 11 | ||||||||
| 25 | M | p.Gly1018Arg (1007) | 11, 23 | 47 | D/I | c.5665delC | 9 | ||||||||
| 27 | D/I | c.3158delG* | 9 | ||||||||||||
| 27 | D/I | c.3166-3167insC* (c.3133-3134insC) | 7, 8, 9, 11 | ||||||||||||
| 27 | M | p.Arg1060Trp (1049) | 7, 11, 22 | ||||||||||||
| 27 | M | p.Leu1079Pro (1068) | 11, 15 | ||||||||||||
| 29 | D/I | c.3504del2bp | 23 | ||||||||||||
| 30 | D/I | p.del1222-1258 | 21 | ||||||||||||
| 27 | M | p.Leu1079Pro (1068) | 11, 15 | ||||||||||||
| 29 | D/I | c.3504del2bp | 23 | ||||||||||||
| 30 | D/I | p.del1222-1258 | 21 | ||||||||||||
| 27 | M | p.Leu1079Pro (1068) | 11, 15 | ||||||||||||
| 29 | D/I | c.3504del2bp | 23 | ||||||||||||
| 30 | D/I | p.del1222-1258 | 21 | ||||||||||||
| Cav1.2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exon | Type | Loss-of-function | Ref | Exon | Type | Gain-of-function | Ref | Exon | Type | Unknown function | Ref |
| 2 | M | p.Ala39Val | 1 | 8 | M | p.Gly402Ser | 4 | 19 | D/I | p.Glu850del | 2 |
| 10 | M | p.Gly490Arg | 1 | 8 | M | p.Gly406Arg | 4 | 26 | M | p.Glu1115Lys | 2 |
| 43 | Dupl | p.Glu1829_Gln 1833dupl | 2 | 8A | M | p.Gly406Arg | 3, 5 | 42/43 | M | p.Cys1837Tyr | 2 |
| 46 | M | p.Val2041Ile | 2 | ||||||||
| Cav1.3 | |||
|---|---|---|---|
| Exon | Type | Loss-of-function | Ref |
| 8B | D/I | p.403_404insGly (c.1208_1209insGGG) | 6 |
The gain-of-function of a glycine-to-serine mutation at position 402 (Gly402Ser) at the cytoplasmic end of segment IS6 (Fig. 1) resulted in a strong reduction of voltage-dependent inactivation (VDI, [26]). A similar effect that included a slight shift in the voltage-dependence of activation was observed for a glycine-to-arginine substitution just four amino acids downstream at position 406 (Gly406Arg) in helix 6. The change of channel function was irrespective of the co-expressed β-subunit [27,32]. Importantly the same mutation at position 406 can be located in exon 8A [VNDAV-coding exon] where it results in a relatively mild phenotype of Timothy syndrome (named TS1 in [26]) compared to its occurrence in the alternative exon 8 (MQDAM-coding exon). The latter more severe variant named TS2 [27] can be rationalized by the higher expression of exon 8 in the heart and brain (80% versus 20% exon 8A [27]). Neither heterozygous nor homozygous TS2-like mice were viable. Most likely the predominant expression of exon 8 in the brain and heart resulted in a lethally high level of mutated channels [33]. However, heterozygous mice that still carried the inverted neomycin cassette in exon 8A (TS2-neo) survived through adulthood. It is unclear, whether or not heterozygous TS2-neo mice better tolerated the mutation because the neo-cassette lowered expression levels of the mutated channel; supporting biochemical data are missing. Interestingly behavioral phenotyping showed that TS2-neo mice have normal general health and activity but show a markedly restricted, repetitive and preservative behavior, altered social behavior, altered ultrasonical vocalization as well as enhanced tone-cued and contextual memory following fear conditioning despite displaying normal anxiety levels [33]. These data suggest that these mice also show autism-related behavior corresponding to core aspects of autism and autism spectrum disorders seen in humans.
Kinetic models suggested that the functional consequences of an almost complete loss of VDI of Cav1.2 channels in cardiac cells results in a prolongation of action potential duration and in increased calcium transients [34]. This correlates well with the observed prolongation in the QT interval in the affected individuals. Indeed such effects are evident from Ca2 + signaling experiments in Timothy syndrome cardiomyocytes derived from human skin cells of Timothy syndrome patients that were reprogrammed and induced to produce pluripotent stem cells. Mutation Gly406Arg led to significantly larger and prolonged Ca2 + elevations suggesting that channel inactivation is important for maintaining timing and amplitude of the ventricular Ca2 + release [35]. Similar effects were also observed in iPSC-derived neuronal cells on action potential widening [36]. Thus, drugs that directly interfere with the Cav1.2 inactivation gating mechanism are likely to improve cardiac arrhythmias as well as other severe symptoms affecting TS patients. Yarotskyy and colleagues [37] previously found in heterologously expressed Cav1.2-Gly406Arg channels that roscovitine [originally developed as a selective blocker of cyclin-dependent kinases [38] that was undergoing phase II clinical trials as an anticancer drug] enhances VDI [39,40]. Therefore, roscovitine could have a potential to restore the electrical and calcium signaling properties of cardiomyocytes from Timothy syndrome patients [35].
Other pro-arrhythmic factors beyond the loss of VDI in TS have been attributed to aberrant Cav1.2 phosphorylation. Spontaneous mode 2 gating that appeared to depend on CaMKII-dependent protein phosphorylation was observed in a rabbit Cav1.2-Gly436Arg mutation (homologue to the human mutation Gly406Arg) that involved the downstream serine residue 439 [41]. Rat ventricular myocytes (lenti-)virally transduced with the mutant Cav1.2-Gly406Arg channel exhibited increased CaMKII sensitivity. CaMKII inhibitors reversed action potential prolongation in TS myocytes [42]. Transgenic mice that also expressed the rabbit homolog mutation (named in Cav1.2-LQT8 in reference [43]) expectedly showed slow calcium current inactivation in both ventricular myocytes and mouse embryonic fibroblasts. Normal calcium current inactivation was restored in myocytes from mice that were crossed with mice lacking the A-Kinase anchoring protein AKAP150 [43]. In addition channel expression and spatial distribution in the ventricular myocyte was changed and the channels seemed to form multiple clusters in the sacrolemma. The authors suggested a mechanism that requires AKAP150 during defective inactivation and for coupled gating events in Cav1.2 TS mutant myocytes previously described by Navedo and colleagues [44]. Such mechanism implies coupling of Cav1.2 channels by transient interactions between neighboring channels via their intracellular C-termini. The mechanism per se however is still controversially discussed in the field [44–48].
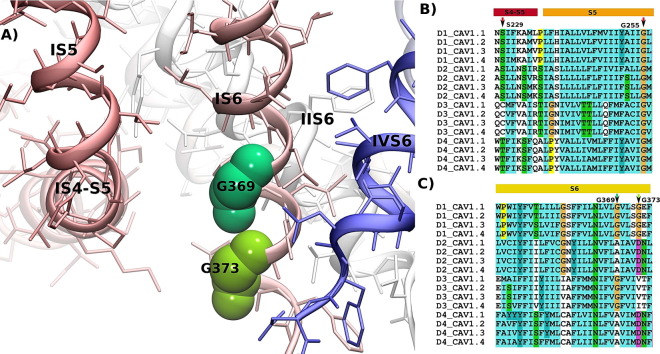
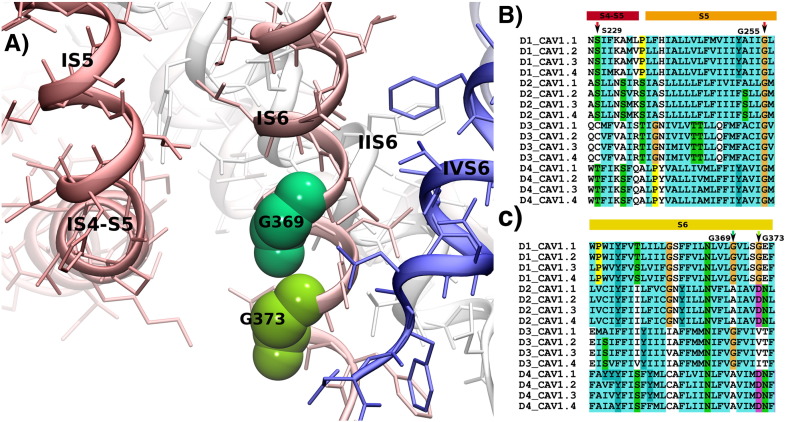
The two amino acid residues Gly402 and Gly406 identified a region forming a critical “hotspot” for channel gating (Figs. 1 and 3). Interestingly, the Gly402 corresponds to Gly369 in Cav1.4 α1 subunits. This residue is mutated to arginine in patients with the incomplete form of congenital stationary night blindness (CSNB2) and was found to strongly inhibit VDI [49–51]. Moreover, the insertion of an additional glycine residue at position 403 in Cav1.3 α1 subunits causes a loss-of-function mutation in patients that are affected with sinoatrial node dysfunction and deafness (SANDD) [52].
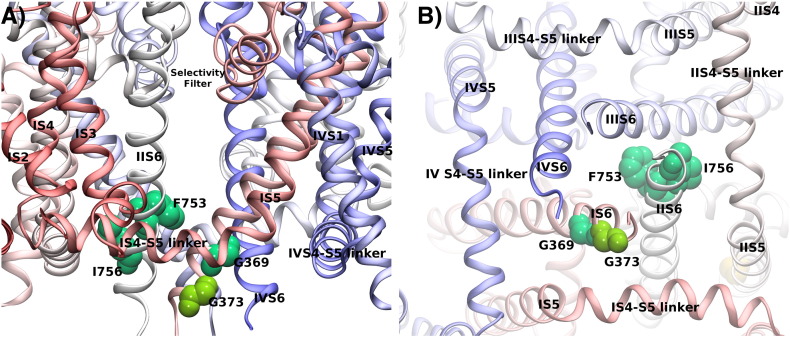
Fig. 3.
Hotspot of mutations in Cav1.x channels. Panel A shows the structure of Cav1.4 with a focus on the interface between the IS4–S5 linker and IS6 at the intracellular side. Residues at position Gly369 (corresponding to position 402 in Cav1.2 and 403 in Cav1.3) and Gly373 (which corresponds to position Gly406 in Cav1.2) at the end of S6 are highlighted. A glycine residue (indicated as Gly255 in Cav1.4) at the end of S5 is highly conserved among transmembrane domains (D1–D4) in all Cav1.x channels. Panel B shows the alignment of the S4–S5 linker and the S5 transmembrane helices in D1–D4 of human Cav1.x sequences. Panel C shows the alignment of the S6 helices of the Cav1.x channels. Arrows indicate the position of hotspot residues. Residue color coding follows the convention of Clustal (http://www.jalview.org/help/html/colourSchemes/clustal.html). Sequence numbers are given as observed in the first TM domain of Cav1.4.
Glycine residues have special properties in transmembrane helices as their side chain consists of only a hydrogen atom; they i) allow close association of helices as e.g. observed in the GXXXG helix–helix crossing motif, and ii) introduce flexibility in helices by providing a bending point. A GXXXG sequence is present in the IS6 helix of Cav1.x channels. The respective residues corresponding to Gly369 in the IIS6–IVS6 helices of Cav1.4 channels are A755, G1134 and A1438. These residues interface with the S4–S5 linker helix, suggesting that a small residue like glycine or alanine (the second smallest residue) is required. The side chain at this position interacts with the end of the S4–S5 linker helix in both states of the gate: in the closed gate as found in NavAB [22] and in the open gate as observed in Kv1.2–2.1 [53]. It is conceivable that tight coupling of the S4–S5 linker to residue Gly369 is required for correct gate opening. A change in function by mutation at position Gly402 (in Cav1.2) or Gly369 (in Cav1.4) showing altered channel opening by interfering with this interaction would therefore also be expected from the model.
3. Cav1.3-related channelopathy
Until now only one human disease syndrome resulting from a mutation in the CACNA1D gene – encoding the Cav1.3 α1 subunit – was reported. The reason for this low incidence could be that mutations cause embryonically lethal dysfunction in the homozygous state or that the phenotypes are so mild that they pass unnoticed in the heterozygous state as observed in Cav1.3 knock-out mice [6]. Members of two consanguineous families carried an insertion mutation that introduced an additional glycine residue at position 403 (403_404insGly) in the highly conserved, alternatively spliced region in domain I. This insertion occurs in the S6 helix, next to the channel pore (Fig. 1, position indicated by Gly369 of the Cav1.4 channel in Figs. 3A, C and 4B). The resulting mutant channel is non-conducting and shows abnormal voltage-dependent gating [52]. The patients presented with a congenital cardiac (sinoatrial node arrhythmia at rest) and auditory (severe to profound deafness) phenotype. These data manifested a similar role of Cav1.3 in humans and mice, because mouse models predicted no clinical symptoms in heterozygous patients, but showed congenital hearing impairment and sinoatrial node dysfunction in homozygous individuals [6]. Cav1.3 channels have also been reported to mediate Ca2 + oscillations underlying autonomous pace-making in dopaminergic substantia nigra neurons in mice. Such mechanism renders these neurons more susceptible to neurotoxicity [9,54] and therefore suggests a delicate role of Cav1.3 channels in the pathophysiology of Parkinson's disease. No symptoms have been yet observed in patients that would indicate a central neuronal disease (which might also be because neuronal consequences have not yet become apparent due to the youth of the patients; age 15–24). Cav1.3 channels seem prominently expressed in the mouse retina [12]. They regulate the light peak of the electroretinogram (ERG) [55] and might contribute at least partly to the b-wave component in the ERG [12]. Ophthalmological investigation of patients, however, did not indicate any visual defect to light. Structural differences between mouse and human retinas could explain the diverging observations. The human mutation 403_404insGly was only found in exon 8B, not in the more widely expressed exon 8A [52]. If exon 8A would be the predominant splice variant in the retina, then this could also well explain the absence of visual disturbances in the patients.
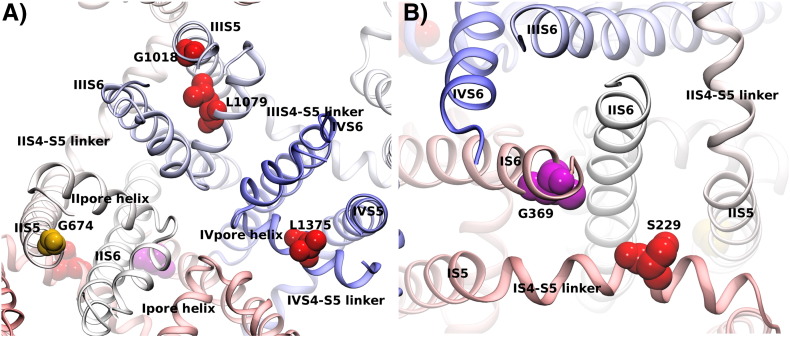
Fig. 4.
Loss-of-function mutations. The position of loss-of-function mutations are highlighted on the Cav1.4 channel model. Panel A displays a view on the channel from the cell exterior, while panel B shows the channel from the cytosolic site with a focus on the central S6 helices. Loss-of-function mutations described for Cav1.4 are shown in red. The position of the insertion mutation in Cav1.3 at position 403_404 is highlighted in magenta by the corresponding residue G369 in Cav1.4.
The reported Cav1.3 glycine insertion affects a functionally sensitive region at the cytoplasmic end of the inner pore-lining S6 helix in domain I. Insertions of residues in α-helices can have two outcomes: the creation of a π-helical element with 5 instead of 4 residues per helical turn that is larger and at the same time more flexible, or a register shift that results in a 100° rotation of all downstream residues. In either case we expect a dramatic change in function. The larger size of the π-helical element would directly shrink the pore in the gating region. The increase in flexibility could have an additional and potentially more dramatic effect, as it might disengage the S6 helix motion of gate opening that is triggered by the voltage-sensor bending of the otherwise rigid S6 helix. This could result in a closed gate, driven by hydrophobic interactions to the other S6 segment within the gate region, despite a gate opening conformational change in the voltage sensor. If instead the insertion of the glycine induces a register shift, then the helix S6 would present to its interaction partner downstream of the insertion the residue adjacent to the one found in the wildtype channel. Therefore every interaction with the S6 helices in the other 3 domains would change and would affect the interaction with the S4–S5 linker helix. Thus, this mutation could directly compromise the structural communication with the voltage-sensor and prevent pore opening.
4. Cav1.4-related channelopathies
Several X-linked visual disorders that share a variety of clinical symptoms have been associated with mutations in the CACNA1F gene — that encodes for the Cav1.4 α1 subunit. As such Åland Island eye disease (AIED, [56]), cone–rod dystrophy (CORDX3, [57]), X-linked retinal disorder (XRD, [58]), night blindness-associated transient tonic downgaze (NATTD, [59]) and incomplete congenital stationary night blindness (iCSNB, CSNB2, [49,50,60–62]) have been reported. The majority of mutations were identified among patients originally diagnosed with CSNB 2.
CSNB2 is characterized by variable and often mild clinical symptoms. The term is however misleading because night blindness is not necessarily the major complaint. Typical symptoms in CSNB2 are moderately low visual acuity, myopia, nystagmus and variable levels of night blindness but one or more of these symptoms may be absent [60]. CSNB2 is therefore diagnosed on the basis of ERG abnormalities. CSNB2 patients show an abnormal dim scotopic ERG and a typical negative bright-flash ERG which has large a-waves, but severely reduced b-waves. Oscillatory potentials are also missing [63]. This ERG phenotype is compatible with a defect in neurotransmission within the retina between photoreceptors and second-order neurons, very similar to the phenotype described in Cav1.4 knock-out mice [13]. Upon light absorption in the photoreceptor outer segments the closure of cGMP-gated cation channels hyperpolarizes the cells to below − 55 mV [64]. In the dark, photoreceptors depolarize to a resting membrane potential of − 36 to − 40 mV, thereby enhancing tonic neurotransmitter (glutamate) release downstream [65]. Release occurs at specialized ribbon-type synapses where LTCCs are the predominant channels controlling neurotransmitter secretion at the ribbon synapses of retinal photoreceptors [66,67] and of cochlear inner hair cells [6,68,69]. Only a few channels that activate rapidly at relatively negative voltages (< − 40 mV, [66,70,71]) and inactivate slowly are needed to support tonic release [66]. In cultured mammalian cells, heterologously expressed Cav1.4 currents indeed activate rapidly, open at negative membrane potentials and thereby allow the channel to conduct Ca2 + at potentials negative to − 40 mV. In addition, the Cav1.4 currents show slow VDI accompanied by complete absence of calcium-dependent inactivation (CDI) during depolarizing pulses [72,73]. Peloquin and colleagues observed that the inactivation kinetics were accelerated at near physiological temperatures but the window current was still preserved [74]. Dysfunction of Cav1.4 channels at release sites of mammalian photoreceptors in the outer plexiform layer is expected to decrease also photoreceptor neurotransmitter release capacity and impair signaling to second-order retinal neurons [75]. Cav1.4 may also contribute to the LTCC currents measured in bipolar cell terminals as indicated by the Cav1.4 immunostaining in mouse inner plexiform [12,13,76–78].
So far more than 50 structural aberrations were identified in the Cav1.4 α1 subunit gene of CSNB2 patients (Table 2). CSNB2 mainly affects males, because of the X-linked condition of Cav1.4 channel dysfunction. However, if the CACNA1F gene is subject to X-inactivation, then heterozygote females can be affected as well [79] by showing cellular mosaicism for healthy/mutant Cav1.4 channels. As outlined in Table 2 and Fig. 2, we can classify three groups of Cav1.4 mutations on the basis of their functional effects: i) loss-of-function mutations, ii) gain-of-function mutations and iii) mutations causing impaired C-terminal modulator (CTM) function (described below). It is not completely understood how the different functional channel phenotypes can all result in defective retinal synaptic transmission underlying CSNB2 symptoms. A majority of mutations are predicted to cause severe structural changes so that they are unlikely to from functional channels, often due to premature truncation (Table 2, marked with *). Nonsense-mediated mRNA decay eliminates mRNA containing pre-mature stop codons in regions followed by splice sites at a distance of 50–55 nucleotides downstream [80]. Therefore, the truncated Cav1.4 channels might not even be expressed. Other point mutations abolish channel activity. Among those, no channel activity was observed for mutations Ser229Pro, Gly1018Arg, Arg1060Trp and Leu1079Pro mutations expressed in Xenopus laevis oocytes (Ser229Pro, Leu1079Pro, [51]) or tsA-201 cells (Gly1018Arg, Arg1060Trp [81]) although their α1 subunits were expressed at levels indistinguishable from wildtype channels. Only in the Leu1079Pro mutant channel current could be elicited in the presence of the calcium channel activator BayK8644 (BayK), showing slightly faster inactivation kinetics compared to wildtype + BayK [51]. Ser229 is located in the middle of the IS4–S5 linker (Fig. 3B). Mutation Ser229Pro in the center of the IS4–S5 linker helix exchanges the polar amino acid serine with the helix breaking, but hydrophobic amino acid proline. Proline has helix destabilization properties, as it misses the amide proton essential for the helix stabilizing backbone hydrogen bond to the carbonyl oxygen of the amino acid in the preceding helical turn. In addition, the serine to proline mutation increases the hydrophobicity at position 229, which is oriented towards the gate and interacts with the IIS6 helix (Fig. 4B). This change in property could therefore have a stabilizing effect, counteracting the helix destabilization and therefore explaining why expression was not significantly affected. Signaling of the voltage sensor depends on a mechanical communication transmitted by the S4S–S5 linker helix. Ser229Pro adds a kink or alternatively shortens the S4–S5 helix by one helical turn, thereby most likely interrupting the mechanical communication in both cases.
Fig. 2.
Cav1.4 channel mutation summary. The position of all listed mutants is shown in an overview of the transmembrane domain of the Cav1.4 channel, seen from the extracellular site. The channel sequence is color coded from N- to C-terminus from red to blue. Residues that show a loss-of-function mutation are indicated in red; residues, in which the mutation is accompanied by a gain-of-function, are depicted in green; mutation of residues shown in orange does not change channel function; residues, of which the effect of mutation has not yet been experimentally described, are shown in gray.
Residues Gly1018 and Leu1079 come into close proximity in the tertiary structure (see Fig. 4A) of the Cav1.4 ion channel. Gly1018 is found at the extracellular end of IIIS5, interfacing with IIIS6. The mutation Gly1018Arg introduces the positively charged arginine instead of the small glycine. Water exposed arginines have been previously described to support folding of transmembrane proteins [82–84]. The Leu1079Pro mutation exchanges one hydrophobic amino acid by another, but destabilizes the beginning of the domain III pore helix. Both mutations, the stark increase in the side chain size of the Gly1018Arg mutation and the helix binding properties of the Leu1079Pro mutation, could potentially change the geometry of the selectivity filter and block ionic flux.
Reduced protein expression was seen in mutants Arg519Gln and Leu1375His in transfected tsA-201 cells; this effect appeared to be temperature-dependent [85]. The authors found no changes in gating properties for Arg519Gln and minor changes affecting inactivation properties and channel recovery (Leu1375His) when the mutated channels were heterologously expressed in X. laevis oocytes. The expression deficit observed for mutant Arg519Gln might be explained by its critical position at the end of the amphipathic I–II linker, interfacing with the end of the IIS2 helix (Fig. 2). An arginine in this position could play a dual role, namely interact with the negatively charged phosphate group of phospholipids and at the same time stabilize the negative dipole of the C-terminus of helix IIS2. A glutamine in the Arg519Gln mutation cannot fulfill the same role.
Similarly to the pore mutation Leu1079Pro, the mutation Leu1375His is located in domain IV in almost the same position of the pore helix (relative shift by one residue). In both cases a leucine in the core of the domain is affected. While the mutation Leu1079Pro maintains the hydrophobic property of residue 1079, mutation Leu1375His introduces a bulky polar side chain. The reduced expression could potentially be attributed to this change. Nevertheless the Leu1375His mutation should maintain the structural integrity of the pore helix that possibly explains the only minor functional changes.
The truncation mutant Trp1451stop, which could theoretically form a functional channel because it contains all transmembrane segments and a portion of the C-terminal tail, did not express at the protein level in one study [51] but was indicated to normally express in another study (mutation W1459stop in [16]).
Remarkably, McRory and colleagues found that the two missense mutations Gly674Asp and Ala928Asp exerted no detectable changes in the activation, inactivation, or conductance properties of expressed Cav1.4 channels [16]. Residue Ala928 can be found outside the membrane within the intracellular IIIS2–S3 loop (Fig. 2). An alanine-to-aspartic acid mutation can probably be accommodated in this water exposed loop. The mutation of Gly674Asp (Fig. 4A) is located at the end of helix IIS5. This glycine is conserved in all four S4 helices of the human Cav1.x channels (see Fig. 3B; the corresponding residue G255 in first transmembrane domain of Cav1.4 is indicated). It is intriguing, that the corresponding Gly1018Arg mutation strongly affects function (see above), while the Gly674Asp mutation is functionally silent (Fig. 4A).
The gain-of-function mutations reported so far promote enhanced Ca2 + entry through the channel by vastly slowed VDI accompanied by a pronounced depolarizing shift in the voltage-dependence activation [16,51,79,81]. The gain-of-function mutations Gly369Arg, Phe753Cys and Ile756Thr might therefore significantly increase Ca2 + influx during illumination at negative voltages (at around − 55 mV physiologically). At the same time this effect would reduce the increase upon depolarization (at around − 35 mV physiologically) leading to a reduced dynamic range in the photoreceptor. Accordingly, also the glutamate release might be changed and could explain why synaptic transmission is reduced in CSNB2 retinas. Alternatively the expected increased calcium influx might trigger retinal cell death and thereby cause an overall loss-of-function. Neither hypothesis is yet corroborated. The hyperpolarizing shift in Cav1.4 channel activation (around 30 mV) was most pronounced in mutation Ile756Thr, which was identified in a New Zealand family. The affected family members showed an unusual severity of the phenotype and an association with intellectual disability in males, and also heterozygote female family members had clinical and ERG abnormalities [58,79]. A pronounced negative shift in channel activation was also reported for the mutation Gly369Arg [51]. The group of McRory found only a slight, though statistically significant increase in the slope factor of the activation curve and a less pronounced shift of the half-activation potential with Ca2 + as compared to Ba2 + used as charge carrier for the mutation Gly369Arg ([16]; possible reasons for this finding have been discussed in detail in reference [51]). A pronounced channel gating activity is however well supported from the structural model that we provide. As depicted in Fig. 5, residue Gly369 is clearly located at the channel gate. As suggested above, a small residue is proposed to be required in position 369 to allow efficient gate closing, while larger residues would collide with the amphiphilic S4–S5 linker helix. Residue Phe753 is a very hydrophobic (hydrophobicity score 4.80) pore oriented residue (Figs. 2 and 5), while cysteine shows a hydrophobicity score of 0.49 [86]. A reduction of hydrophobicity and residue size in the center of the hydrophobic constriction zone of the gate by the mutation Phe753Cys will most likely increase the open probability of the mutant channel. Residue Ile756 interfaces with the adjacent IIIS6 helix and with the IIIS4–S5 linker helix. Also the Ile756Thr mutation changes hydrophobicity (Ile = 3.48 vs. Thr = 0.65) and residue size and might therefore increase the open probability of the channel by destabilizing the closed conformation, as the polar threonine residue cannot form the same stable interactions with the adjacent S6 and the S4–S5 linker as Ile756 can.
Fig. 5.
Gain-of-function mutations. The gain-of-function mutations are highlighted in green on the Cav1.4 channel model. Panel A shows a side view of the transmembrane domain of the channel, while in panel B the cytosolic side of the channel is exposed. The three gain of function mutations of Cav1.4 are shown in dark green, while residue G373, corresponding to G402 in Cav1.2, is shown in light green.
Truncation mutations in the Cav1.4 C-terminus downstream of the CDI machinery — either natural, by alternative splicing [87] or introduced by an artificial mutation [73,88] are of particular interest because i) they develop an apparent gain-of-function due to a hyperpolarizing shift of the Cav1.4 mediated window current, ii) they might reduce Ca2 + influx due to occurrence of CDI [73,88] and iii) in analogy to recently published data on short Cav1.3 channels [89], enhanced open probability is expected in truncated Cav1.4 channels. Which of these processes dominates the phenotype of Cav1.4 mutants with impaired CTM function is still unclear as available experimental data remain inconclusive and because in vivo models do not yet exist. The analysis of the truncation mutation Lys1602stop (named K1591X in references [21,73,88]) uncovered that the CDI in Cav1.4 channels is an intrinsic channel property that depends on the active suppression by a C-terminal inhibitory domain. Notably Singh and colleagues found that deletion of the C-terminal domain not only restored robust CDI but also induced a strong hyperpolarizing shift of the voltage-dependence of Cav1.4 activation. Accordingly, this domain was termed “C-terminal modulator” to emphasize this dual regulatory effect. Similar to Cav1.2 [90] (and as observed also for Cav1.3 [89]) channels, this intrinsic C-terminal modulation in Cav1.4 channels seems to rely on a binding interaction between a pair of positively charged residues in a putative α-helical region immediately downstream of the IQ-motif and a set of three negatively charged residues in the distal end of the C-terminus (Fig. 1) in the CTM domain. The critical residues comprising the CTM (and ICDI, as named by Wahl-Schott and colleagues [88]) were restricted to a highly conserved stretch of about 25 amino acid residues within the distal C-terminus.
The discovery of the gating modulator in the C-terminus of Cav1.4 channels (Cav1.4-CTM) raises an interesting question on whether this mode of controlling channel function could be developed into a therapeutic concept for the treatment of patients suffering from channelopathies. Although partial blockade of mutated Cav1.4 channels seems to be a possibility to modulate these channels, such an approach is not practicable in vivo because antagonist drugs such as the DHP isradipine [72] are 10- to 20-fold more potent in blocking Cav1.2 compared to Cav1.4 channels. Therefore the higher concentration required for blocking Cav1.4 channels is expected to cause major Cav1.2-mediated unwanted side effects. As obvious from the above section, a ‘pharmacological knock-out’ of these channels is largely disadvantageous due to the expected cardiac side effect. Instead of modulating channel function with drugs that interact directly with the channel pore and thereby inhibit ion permeation, molecules that exert their modulatory effects by changing regulatory channel interactions could serve as study tools, but may even be of therapeutic value. One could speculate that mimicking the Cav1.4–CTM interaction would shift the voltage- and calcium-dependence of Cav1.4 gating back to WT levels in truncated channels and vice versa induction of CDI via interference with the Cav1.4–CTM interaction could have beneficial effects in gain-of-function mutations at least within a limited range.
5. Conclusions
In this review we developed a Cav1.4 structural homology model with the aim to elucidate the structure–(dys)function relationship in Cav1.x channelopathy mutants in the structural context. We assigned potential functional ‘hotspots’ for naturally occurring loss- and gain-of-function mutants. The vast majority of loss-of-function mutations are nested in core channel folding domains whereas all the gain-of-function mutations sit at the inner mouth of the channel pore and these gain-of-function mutants interfere with coupling between the voltage sensor (via the S4–S5 linker) and the channel gate. Cav1.4 mutations with impaired CTM function led previously to the discovery of a novel inhibitory feedback mechanism in LTCCs that depends on a gating modifier in the C-terminus. This opened a new field of LTCC research that uses this intramolecular modulator as study tool to adjust intracellular Ca2 + activity. This property might even bear the potential for a therapeutic application.
Acknowledgements
The work of the authors is supported by the Austrian Science Fund (P-22528, SFB 4402 to AK) and the Medical University of Vienna.
Footnotes
This article is part of a Special Issue entitled: Calcium channels.
References
- 1.Catterall W.A., Perez-Reyes E., Snutch T.P., Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure–function relationships of voltage-gated calcium channels. Pharmacol. Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 2.Turner R.W., Anderson D., Zamponi G.W. Signaling complexes of voltage-gated calcium channels. Channels (Austin) 2011;5:440–448. doi: 10.4161/chan.5.5.16473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altier C., Dubel S.J., Barrere C., Jarvis S.E., Stotz S.C., Scott J.D., Nargeot J., Zamponi G.W., Bourinet E. AKAP79 modulation of L-type channels involves disruption of intramolecular interactions in the CaV1.2 subunit. Channels (Austin) 2012;6:157–165. doi: 10.4161/chan.20865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Striessnig J., Grabner M., Mitterdorfer J., Hering S., Sinnegger M.J., Glossmann H. Structural basis of drug binding to L calcium channels. Trends Pharmacol. Sci. 1998;19:108–115. doi: 10.1016/s0165-6147(98)01171-7. [DOI] [PubMed] [Google Scholar]
- 5.Striessnig J. Pharmacology, structure and function of cardiac L-type Ca2 + channels. Cell. Physiol. Biochem. 1999;9:242–269. doi: 10.1159/000016320. [DOI] [PubMed] [Google Scholar]
- 6.Platzer J., Engel J., Schrott-Fischer A., Stephan K., Bova S., Chen H., Zheng H., Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2 + channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 7.Marcantoni A., Vandael D.H., Mahapatra S., Carabelli V., Sinnegger-Brauns M.J., Striessnig J., Carbone E. Loss of Cav1.3 channels reveals the critical role of L-type and BK channel coupling in pacemaking mouse adrenal chromaffin cells. J. Neurosci. 2010;30:491–504. doi: 10.1523/JNEUROSCI.4961-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson P.A., Tkatch T., Hernandez-Lopez S., Ulrich S., Ilijic E., Mugnaini E., Zhang H., Bezprozvanny I., Surmeier D.J. G-protein-coupled receptor modulation of striatal Cav1.3 L-type Ca2 + channels is dependent on a Shank-binding domain. J. Neurosci. 2005;25:1050–1062. doi: 10.1523/JNEUROSCI.3327-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzman J.N., Sanchez-Padilla J., Chan C.S., Surmeier D.J. Robust pacemaking in substantia nigra dopaminergic neurons. J. Neurosci. 2009;29:11011–11019. doi: 10.1523/JNEUROSCI.2519-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker C., Jick S.S., Meier C.R. Use of antihypertensives and the risk of Parkinson disease. Neurology. 2008;70:1438–1444. doi: 10.1212/01.wnl.0000303818.38960.44. [DOI] [PubMed] [Google Scholar]
- 11.Moosmang S., Haider N., Klugbauer N., Adelsberger H., Langwieser N., Muller J., Stiess M., Marais E., Schulla V., Lacinova L., Goebbels S., Nave K.A., Storm D.R., Hofmann F., Kleppisch T. Role of hippocampal Cav1.2 Ca2 + channels in NMDA receptor-independent synaptic plasticity and spatial memory. J. Neurosci. 2005;25:9883–9892. doi: 10.1523/JNEUROSCI.1531-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busquet P., Nguyen N.K., Schmid E., Tanimoto N., Seeliger M.W., Ben-Yosef T., Mizuno F., Akopian A., Striessnig J., Singewald N. CaV1.3 L-type Ca2 + channels modulate depression-like behaviour in mice independent of deaf phenotype. Int. J. Neuropsychopharmacol. 2010;13:499–513. doi: 10.1017/S1461145709990368. [DOI] [PubMed] [Google Scholar]
- 13.Mansergh F., Orton N.C., Vessey J.P., Lalonde M.R., Stell W.K., Tremblay F., Barnes S., Rancourt D.E., Bech-Hansen N.T. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum. Mol. Genet. 2005;14:3035–3046. doi: 10.1093/hmg/ddi336. [DOI] [PubMed] [Google Scholar]
- 14.Doering C.J., Rehak R., Bonfield S., Peloquin J.B., Stell W.K., Mema S.C., Sauve Y., McRory J.E. Modified Ca(v)1.4 expression in the Cacna1f(nob2) mouse due to alternative splicing of an ETn inserted in exon 2. PLoS One. 2008;3:e2538. doi: 10.1371/journal.pone.0002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakami M., Nakagawasai O., Fujii S., Kameyama K., Murakami S., Hozumi S., Esashi A., Taniguchi R., Yanagisawa T., Tan-no K., Tadano T., Kitamura K., Kisara K. Antinociceptive action of amlodipine blocking N-type Ca2 + channels at the primary afferent neurons in mice. Eur. J. Pharmacol. 2001;419:175–181. doi: 10.1016/s0014-2999(01)00985-2. [DOI] [PubMed] [Google Scholar]
- 16.McRory J.E., Hamid J., Doering C.J., Garcia E., Parker R., Hamming K., Chen L., Hildebrand M., Beedle A.M., Feldcamp L., Zamponi G.W., Snutch T.P. The CACNA1F gene encodes an L-type calcium channel with unique biophysical properties and tissue distribution. J. Neurosci. 2004;24:1707–1718. doi: 10.1523/JNEUROSCI.4846-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotturi M.F., Jefferies W.A. Molecular characterization of L-type calcium channel splice variants expressed in human T lymphocytes. Mol. Immunol. 2005;42:1461–1474. doi: 10.1016/j.molimm.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Alseikhan B.A., DeMaria C.D., Colecraft H.M., Yue D.T. Engineered calmodulins reveal the unexpected eminence of Ca2 + channel inactivation in controlling heart excitation. Proc. Natl. Acad. Sci. U. S. A. 2002;99:17185–17190. doi: 10.1073/pnas.262372999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halling D.B., Aracena-Parks P., Hamilton S.L. Regulation of voltage-gated Ca2 + channels by calmodulin. Sci. STKE. 2005;2005:re15. doi: 10.1126/stke.3152005re15. [DOI] [PubMed] [Google Scholar]
- 20.Striessnig J. C-terminal tailoring of L-type calcium channel function. J. Physiol. 2007;585:643–644. doi: 10.1113/jphysiol.2007.147140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Striessnig J., Bolz H.J., Koschak A. Channelopathies in Cav1.1, Cav1.3, and Cav1.4 voltage-gated L-type Ca2 + channels. Pflugers Arch. 2010;460:361–374. doi: 10.1007/s00424-010-0800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Payandeh J., Scheuer T., Zheng N., Catterall W.A. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sali A., Blundell T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 25.Shen M.Y., Sali A. Statistical potential for assessment and prediction of protein structures. Protein Sci. 2006;15:2507–2524. doi: 10.1110/ps.062416606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Splawski I., Timothy K.W., Decher N., Kumar P., Sachse F.B., Beggs A.H., Sanguinetti M.C., Keating M.T. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc. Natl. Acad. Sci. U. S. A. 2005;102:8089–8096. doi: 10.1073/pnas.0502506102. (discussion 8086-8088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Splawski I., Timothy K.W., Sharpe L.M., Decher N., Kumar P., Bloise R., Napolitano C., Schwartz P.J., Joseph R.M., Condouris K., Tager-Flusberg H., Priori S.G., Sanguinetti M.C., Keating M.T. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Antzelevitch C., Pollevick G.D., Cordeiro J.M., Casis O., Sanguinetti M.C., Aizawa Y., Guerchicoff A., Pfeiffer R., Oliva A., Wollnik B., Gelber P., Bonaros E.P., Jr., Burashnikov E., Wu Y., Sargent J.D., Schickel S., Oberheiden R., Bhatia A., Hsu L.F., Haissaguerre M., Schimpf R., Borggrefe M., Wolpert C. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115:442–449. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burashnikov E., Pfeiffer R., Barajas-Martinez H., Delpon E., Hu D., Desai M., Borggrefe M., Haissaguerre M., Kanter R., Pollevick G.D., Guerchicoff A., Laino R., Marieb M., Nademanee K., Nam G.B., Robles R., Schimpf R., Stapleton D.D., Viskin S., Winters S., Wolpert C., Zimmern S., Veltmann C., Antzelevitch C. Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm. 2010;7:1872–1882. doi: 10.1016/j.hrthm.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simms B.A., Zamponi G.W. The Brugada syndrome mutation A39V does not affect surface expression of neuronal rat Cav1.2 channels. Mol. Brain. 2012;5:9. doi: 10.1186/1756-6606-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Etheridge S.P., Bowles N.E., Arrington C.B., Pilcher T., Rope A., Wilde A.A., Alders M., Saarel E.V., Tavernier R., Timothy K.W., Tristani-Firouzi M. Somatic mosaicism contributes to phenotypic variation in Timothy syndrome. Am. J. Med. Genet. A. 2011;155A:2578–2583. doi: 10.1002/ajmg.a.34223. [DOI] [PubMed] [Google Scholar]
- 32.Barrett C.F., Tsien R.W. The Timothy syndrome mutation differentially affects voltage- and calcium-dependent inactivation of CaV1.2 L-type calcium channels. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2157–2162. doi: 10.1073/pnas.0710501105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bader P.L., Faizi M., Kim L.H., Owen S.F., Tadross M.R., Alfa R.W., Bett G.C., Tsien R.W., Rasmusson R.L., Shamloo M. Mouse model of Timothy syndrome recapitulates triad of autistic traits. Proc. Natl. Acad. Sci. U. S. A. 2011;108:15432–15437. doi: 10.1073/pnas.1112667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faber G.M., Silva J., Livshitz L., Rudy Y. Kinetic properties of the cardiac L-type Ca2 + channel and its role in myocyte electrophysiology: a theoretical investigation. Biophys. J. 2007;92:1522–1543. doi: 10.1529/biophysj.106.088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yazawa M., Hsueh B., Jia X., Pasca A.M., Bernstein J.A., Hallmayer J., Dolmetsch R.E. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471:230–234. doi: 10.1038/nature09855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasca S.P., Portmann T., Voineagu I., Yazawa M., Shcheglovitov A., Pasca A.M., Cord B., Palmer T.D., Chikahisa S., Nishino S., Bernstein J.A., Hallmayer J., Geschwind D.H., Dolmetsch R.E. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat. Med. 2011;17:1657–1662. doi: 10.1038/nm.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yarotskyy V., Gao G., Peterson B.Z., Elmslie K.S. The Timothy syndrome mutation of cardiac CaV1.2 (L-type) channels: multiple altered gating mechanisms and pharmacological restoration of inactivation. J. Physiol. 2009;587:551–565. doi: 10.1113/jphysiol.2008.161737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meijer L., Borgne A., Mulner O., Chong J.P., Blow J.J., Inagaki N., Inagaki M., Delcros J.G., Moulinoux J.P. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 39.Yarotskyy V., Elmslie K.S. Roscovitine, a cyclin-dependent kinase inhibitor, affects several gating mechanisms to inhibit cardiac L-type (Ca(V)1.2) calcium channels. Br. J. Pharmacol. 2007;152:386–395. doi: 10.1038/sj.bjp.0707414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yarotskyy V., Gao G., Du L., Ganapathi S.B., Peterson B.Z., Elmslie K.S. Roscovitine binds to novel L-channel (CaV1.2) sites that separately affect activation and inactivation. J. Biol. Chem. 2010;285:43–53. doi: 10.1074/jbc.M109.076448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erxleben C., Liao Y., Gentile S., Chin D., Gomez-Alegria C., Mori Y., Birnbaumer L., Armstrong D.L. Cyclosporin and Timothy syndrome increase mode 2 gating of CaV1.2 calcium channels through aberrant phosphorylation of S6 helices. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3932–3937. doi: 10.1073/pnas.0511322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thiel W.H., Chen B., Hund T.J., Koval O.M., Purohit A., Song L.S., Mohler P.J., Anderson M.E. Proarrhythmic defects in Timothy syndrome require calmodulin kinase II. Circulation. 2008;118:2225–2234. doi: 10.1161/CIRCULATIONAHA.108.788067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng E.P., Yuan C., Navedo M.F., Dixon R.E., Nieves-Cintron M., Scott J.D., Santana L.F. Restoration of normal L-type Ca2 + channel function during Timothy syndrome by ablation of an anchoring protein. Circ. Res. 2011;109:255–261. doi: 10.1161/CIRCRESAHA.111.248252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Navedo M.F., Cheng E.P., Yuan C., Votaw S., Molkentin J.D., Scott J.D., Santana L.F. Increased coupled gating of L-type Ca2 + channels during hypertension and Timothy syndrome. Circ. Res. 2010;106:748–756. doi: 10.1161/CIRCRESAHA.109.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fallon J.L., Halling D.B., Hamilton S.L., Quiocho F.A. Structure of calmodulin bound to the hydrophobic IQ domain of the cardiac Ca(v)1.2 calcium channel. Structure. 2005;13:1881–1886. doi: 10.1016/j.str.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 46.Fallon J.L., Baker M.R., Xiong L., Loy R.E., Yang G., Dirksen R.T., Hamilton S.L., Quiocho F.A. Crystal structure of dimeric cardiac L-type calcium channel regulatory domains bridged by Ca2 +* calmodulins. Proc. Natl. Acad. Sci. U. S. A. 2009;106:5135–5140. doi: 10.1073/pnas.0807487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim E.Y., Rumpf C.H., Van Petegem F., Arant R.J., Findeisen F., Cooley E.S., Isacoff E.Y., Minor D.L., Jr. Multiple C-terminal tail Ca(2 +)/CaMs regulate Ca(V)1.2 function but do not mediate channel dimerization. EMBO J. 2010;29:3924–3938. doi: 10.1038/emboj.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Findeisen F., Tolia A., Arant R., Kim E.Y., Isacoff E., Minor D.L., Jr. Calmodulin overexpression does not alter Cav1.2 function or oligomerization state. Channels (Austin) 2011;5:320–324. doi: 10.4161/chan.5.4.16821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strom T.M., Nyakatura G., Apfelstedt-Sylla E., Hellebrand H., Lorenz B., Weber B.H., Wutz K., Gutwillinger N., Ruther K., Drescher B., Sauer C., Zrenner E., Meitinger T., Rosenthal A., Meindl A. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat. Genet. 1998;19:260–263. doi: 10.1038/940. [DOI] [PubMed] [Google Scholar]
- 50.Boycott K.M., Pearce W.G., Musarella M.A., Weleber R.G., Maybaum T.A., Birch D.G., Miyake Y., Young R.S., Bech-Hansen N.T. Evidence for genetic heterogeneity in X-linked congenital stationary night blindness. Am. J. Hum. Genet. 1998;62:865–875. doi: 10.1086/301781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoda J.C., Zaghetto F., Koschak A., Striessnig J. Congenital stationary night blindness type 2 mutations S229P, G369D, L1068P, and W1440X alter channel gating or functional expression of Cav1.4 L-type Ca2 + channels. J. Neurosci. 2005;25:252–259. doi: 10.1523/JNEUROSCI.3054-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baig S.M., Koschak A., Lieb A., Gebhart M., Dafinger C., Nurnberg G., Ali A., Ahmad I., Sinnegger-Brauns M.J., Brandt N., Engel J., Mangoni M.E., Farooq M., Khan H.U., Nurnberg P., Striessnig J., Bolz H.J. Loss of Ca(v)1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat. Neurosci. 2011;14:77–84. doi: 10.1038/nn.2694. [DOI] [PubMed] [Google Scholar]
- 53.Long S.B., Tao X., Campbell E.B., MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 54.Chan C.S., Guzman J.N., Ilijic E., Mercer J.N., Rick C., Tkatch T., Meredith G.E., Surmeier D.J. ‘Rejuvenation’ protects neurons in mouse models of Parkinson's disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- 55.Wu J., Marmorstein A.D., Striessnig J., Peachey N.S. Voltage-dependent calcium channel CaV1.3 subunits regulate the light peak of the electroretinogram. J. Neurophysiol. 2007;97:3731–3735. doi: 10.1152/jn.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jalkanen R., Bech-Hansen N.T., Tobias R., Sankila E.M., Mantyjarvi M., Forsius H., de la Chapelle A., Alitalo T. A novel CACNA1F gene mutation causes Aland Island eye disease. Invest. Ophthalmol. Vis. Sci. 2007;48:2498–2502. doi: 10.1167/iovs.06-1103. [DOI] [PubMed] [Google Scholar]
- 57.Jalkanen R., Mantyjarvi M., Tobias R., Isosomppi J., Sankila E.M., Alitalo T., Bech-Hansen N.T. X linked cone–rod dystrophy, CORDX3, is caused by a mutation in the CACNA1F gene. J. Med. Genet. 2006;43:699–704. doi: 10.1136/jmg.2006.040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hope C.I., Sharp D.M., Hemara-Wahanui A., Sissingh J.I., Lundon P., Mitchell E.A., Maw M.A., Clover G.M. Clinical manifestations of a unique X-linked retinal disorder in a large New Zealand family with a novel mutation in CACNA1F, the gene responsible for CSNB2. Clin. Experiment. Ophthalmol. 2005;33:129–136. doi: 10.1111/j.1442-9071.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- 59.Simonsz H.J., Florijn R.J., van Minderhout H.M., Bergen A.A., Kamermans M. Nightblindness-associated transient tonic downgaze (NATTD) in infant boys with chin-up head posture. Strabismus. 2009;17:158–164. doi: 10.3109/09273970903396893. [DOI] [PubMed] [Google Scholar]
- 60.Boycott K.M., Pearce W.G., Bech-Hansen N.T. Clinical variability among patients with incomplete X-linked congenital stationary night blindness and a founder mutation in CACNA1F. Can. J. Ophthalmol. 2000;35:204–213. doi: 10.1016/s0008-4182(00)80031-9. [DOI] [PubMed] [Google Scholar]
- 61.Boycott K.M., Maybaum T.A., Naylor M.J., Weleber R.G., Robitaille J., Miyake Y., Bergen A.A., Pierpont M.E., Pearce W.G., Bech-Hansen N.T. A summary of 20 CACNA1F mutations identified in 36 families with incomplete X-linked congenital stationary night blindness, and characterization of splice variants. Hum. Genet. 2001;108:91–97. doi: 10.1007/s004390100461. [DOI] [PubMed] [Google Scholar]
- 62.Bech-Hansen N.T., Naylor M.J., Maybaum T.A., Pearce W.G., Koop B., Fishman G.A., Mets M., Musarella M.A., Boycott K.M. Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat. Genet. 1998;19:264–267. doi: 10.1038/947. [DOI] [PubMed] [Google Scholar]
- 63.Tremblay F., Laroche R.G., De Becker I. The electroretinographic diagnosis of the incomplete form of congenital stationary night blindness. Vision Res. 1995;35:2383–2393. doi: 10.1016/0042-6989(95)00006-l. [DOI] [PubMed] [Google Scholar]
- 64.Witkovsky P., Schmitz Y., Akopian A., Krizaj D., Tranchina D. Gain of rod to horizontal cell synaptic transfer: relation to glutamate release and a dihydropyridine-sensitive calcium current. J. Neurosci. 1997;17:7297–7306. doi: 10.1523/JNEUROSCI.17-19-07297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corey D.P., Dubinsky J.M., Schwartz E.A. The calcium current in inner segments of rods from the salamander (Ambystoma tigrinum) retina. J. Physiol. 1984;354:557–575. doi: 10.1113/jphysiol.1984.sp015393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bartoletti T.M., Jackman S.L., Babai N., Mercer A.J., Kramer R.H., Thoreson W.B. Release from the cone ribbon synapse under bright light conditions can be controlled by the opening of only a few Ca(2 +) channels. J. Neurophysiol. 2011;106:2922–2935. doi: 10.1152/jn.00634.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mercer A.J., Chen M., Thoreson W.B. Lateral mobility of presynaptic L-type calcium channels at photoreceptor ribbon synapses. J. Neurosci. 2011;31:4397–4406. doi: 10.1523/JNEUROSCI.5921-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zampini V., Johnson S.L., Franz C., Lawrence N.D., Munkner S., Engel J., Knipper M., Magistretti J., Masetto S., Marcotti W. Elementary properties of CaV1.3 Ca(2 +) channels expressed in mouse cochlear inner hair cells. J. Physiol. 2010;588:187–199. doi: 10.1113/jphysiol.2009.181917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brandt A., Striessnig J., Moser T. CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J. Neurosci. 2003;23:10832–10840. doi: 10.1523/JNEUROSCI.23-34-10832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heidelberger R., Matthews G. Calcium influx and calcium current in single synaptic terminals of goldfish retinal bipolar neurons. J. Physiol. 1992;447:235–256. doi: 10.1113/jphysiol.1992.sp019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.von Gersdorff H., Matthews G. Calcium-dependent inactivation of calcium current in synaptic terminals of retinal bipolar neurons. J. Neurosci. 1996;16:115–122. doi: 10.1523/JNEUROSCI.16-01-00115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koschak A., Reimer D., Walter D., Hoda J.C., Heinzle T., Grabner M., Striessnig J. Cav1.4a1 subunits can form slowly inactivating dihydropyridine-sensitive L-type Ca2 + channels lacking Ca2 +-dependent inactivation. J. Neurosci. 2003;23:6041–6049. doi: 10.1523/JNEUROSCI.23-14-06041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh A., Hamedinger D., Hoda J.C., Gebhart M., Koschak A., Romanin C., Striessnig J. C-terminal modulator controls Ca2 +-dependent gating of Cav1.4 L-type Ca2 + channels. Nat. Neurosci. 2006;9:1108–1116. doi: 10.1038/nn1751. [DOI] [PubMed] [Google Scholar]
- 74.Peloquin J.B., Doering C.J., Rehak R., McRory J.E. Temperature dependence of Cav1.4 calcium channel gating. Neuroscience. 2008;151:1066–1083. doi: 10.1016/j.neuroscience.2007.11.053. [DOI] [PubMed] [Google Scholar]
- 75.Jarsky T., Tian M., Singer J.H. Nanodomain control of exocytosis is responsible for the signaling capability of a retinal ribbon synapse. J. Neurosci. 2010;30:11885–11895. doi: 10.1523/JNEUROSCI.1415-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morgans C.W. Localization of the alpha(1F) calcium channel subunit in the rat retina. Invest. Ophthalmol. Vis. Sci. 2001;42:2414–2418. [PubMed] [Google Scholar]
- 77.Morgans C.W., Gaughwin P., Maleszka R. Expression of the alpha1F calcium channel subunit by photoreceptors in the rat retina. Mol. Vis. 2001;7:202–209. [PubMed] [Google Scholar]
- 78.Berntson A., Taylor W.R., Morgans C.W. Molecular identity, synaptic localization, and physiology of calcium channels in retinal bipolar cells. J. Neurosci. Res. 2003;71:146–151. doi: 10.1002/jnr.10459. [DOI] [PubMed] [Google Scholar]
- 79.Hemara-Wahanui A., Berjukow S., Hope C.I., Dearden P.K., Wu S.B., Wilson-Wheeler J., Sharp D.M., Lundon-Treweek P., Clover G.M., Hoda J.C., Striessnig J., Marksteiner R., Hering S., Maw M.A. A CACNA1F mutation identified in an X-linked retinal disorder shifts the voltage dependence of Cav1.4 channel activation. Proc. Natl. Acad. Sci. U. S. A. 2005;102:7553–7558. doi: 10.1073/pnas.0501907102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maquat L.E. Nonsense-mediated mRNA decay: splicing, translation and mRNP dynamics. Nat. Rev. Mol. Cell Biol. 2004;5:89–99. doi: 10.1038/nrm1310. [DOI] [PubMed] [Google Scholar]
- 81.Peloquin J.B., Rehak R., Doering C.J., McRory J.E. Functional analysis of congenital stationary night blindness type-2 CACNA1F mutations F742C, G1007R, and R1049W. Neuroscience. 2007;150:335–345. doi: 10.1016/j.neuroscience.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 82.Loo T.W., Bartlett M.C., Clarke D.M. Insertion of an arginine residue into the transmembrane segments corrects protein misfolding. J. Biol. Chem. 2006;281:29436–29440. doi: 10.1074/jbc.C600209200. [DOI] [PubMed] [Google Scholar]
- 83.Loo T.W., Bartlett M.C., Clarke D.M. Arginines in the first transmembrane segment promote maturation of a P-glycoprotein processing mutant by hydrogen bond interactions with tyrosines in transmembrane segment 11. J. Biol. Chem. 2008;283:24860–24870. doi: 10.1074/jbc.M803351200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Loo T.W., Bartlett M.C., Clarke D.M. Identification of residues in the drug translocation pathway of the human multidrug resistance P-glycoprotein by arginine mutagenesis. J. Biol. Chem. 2009;284:24074–24087. doi: 10.1074/jbc.M109.023267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoda J.C., Zaghetto F., Singh A., Koschak A., Striessnig J. Effects of congenital stationary night blindness type 2 mutations R508Q and L1364H on Cav1.4 L-type Ca2 + channel function and expression. J. Neurochem. 2006;96:1648–1658. doi: 10.1111/j.1471-4159.2006.03678.x. [DOI] [PubMed] [Google Scholar]
- 86.Wilce M.C.J., Aguilar M.I., Hearn M.T.W. Physicochemical basis of amino-acid hydrophobicity scales — evaluation of 4 new scales of amino-acid hydrophobicity coefficients derived from RP-HPLC of peptides. Anal. Chem. 1995;67:1210–1219. [Google Scholar]
- 87.Tan G.M., Yu D., Wang J., Soong T.W. Alternative splicing at C terminus of Ca(V)1.4 calcium channel modulates calcium-dependent inactivation, activation potential, and current density. J. Biol. Chem. 2012;287:832–847. doi: 10.1074/jbc.M111.268722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wahl-Schott C., Baumann L., Cuny H., Eckert C., Griessmeier K., Biel M. Switching off calcium-dependent inactivation in L-type calcium channels by an autoinhibitory domain. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15657–15662. doi: 10.1073/pnas.0604621103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bock G., Gebhart M., Scharinger A., Jangsangthong W., Busquet P., Poggiani C., Sartori S., Mangoni M.E., Sinnegger-Brauns M.J., Herzig S., Striessnig J., Koschak A. Functional properties of a newly identified C-terminal splice variant of Cav1.3 L-type Ca2 + channels. J. Biol. Chem. 2011;286:42736–42748. doi: 10.1074/jbc.M111.269951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hulme J.T., Yarov-Yarovoy V., Lin T.W., Scheuer T., Catterall W.A. Autoinhibitory control of the Cav1.2 channel by its proteolytically processed distal C-terminal domain. J. Physiol. 2006;576(Pt 1):87–102. doi: 10.1113/jphysiol.2006.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]