Abstract
G-protein activated inwardly rectifying K+ channels (GIRKs) of the heterotetrameric GIRK1/GIRK4 composition mediate IK + ACh in atrium and are regulated by cAMP dependent protein kinase (PKA). Phosphorylation of GIRK1/GIRK4 complexes promotes the activation of the channel by the G-protein Gβγ-dimer (“heterologous facilitation”). Previously we reported that 3 serines/threonines (S/Ts) within the GIRK1 subunit are phosphorylated by the catalytic subunit of PKA (PKA-cs) in-vitro and are responsible for the acute functional effects exerted by PKA on the homooligomeric GIRK1F137S (GIRK1⁎) channel. Here we report that homooligomeric GIRK4WT and GIRK4S143T (GIRK4⁎) channels are clearly regulated by PKA phosphorylation. Heterooligomeric channels of the GIRK1S385CS401CT407C/GIRK4WT composition, where the GIRK1 subunit is devoid of PKA mediated phosphorylation, exhibited reduced but still significant acute effects (reduction during agonist application was ≈ 49% compared to GIRK1WT/GIRK4WT). Site directed mutagenesis of truncated cytosolic regions of GIRK4 revealed four serines/threonines (S/Ts) that were heavily phosphorylated by PKA-cs in vitro. Two of them were found to be responsible for the acute effects exerted by PKA in vivo, since the effect of cAMP injection was reduced by ≈ 99% in homooligomeric GIRK4⁎T199CS412C channels. Coexpression of GIRK1WT/GIRK4T199CS412C reduced the acute effect by ≈ 65%. Only channels of the GIRK1S385CS401CT407C/GIRK4T199CS412C composition were practically devoid of PKA mediated effects (reduction by ≈ 97%), indicating that both subunits contribute to the heterologous facilitation of IK + ACh.
Keywords: GIRK1, GIRK4, PKA, cAMP, Heterologous facilitation
Graphical abstract
Highlights
► Phosphorylation of GIRKs is the prerequisite for activation by G-proteins (=heterologous facilitation). ► Direct phosphorylation by PKA was found to be responsible for GIRK4 regulation. ► GIRK4 subunits contribute substantially to regulation of heterooligomeric GIRK1/GIRK4 channels by PKA in vivo. ► Mechanistic insight how G-protein/effector interaction is modulated by effector phosphorylation is provided.
1. Introduction
The indirect, inhibitory action of numerous neurotransmitter molecules is mediated by G-protein activated inwardly rectifying potassium channels (GIRKs). GIRKs are triggered by G-protein coupled receptors (GPCRs) via G-protein α- and βγ-subunits. In electrically excitable tissue GIRKs contribute to autonomous regulation of the heartbeat, nociception and hormone secretion [1,2]. In electrically non-excitable tissues other important roles of GIRKs such as platelet activation [3], insulin secretion [4] and sperm function [5] have also been unravelled. According to this outstanding importance, single nucleotide polymorphisms or pathological mutations in one of the four human genes encoding GIRK subunit isoforms are causally related to various diseases, including primary aldosteronism and hypertension, alcohol abuse, atrial fibrillation, Andersen & Barter syndromes and congenital hyperinsulinism [6–10]. On the structural level functional GIRK channels are formed as homo- or heterotetrameric complexes [11]. The pore-forming tetramer is associated with G-protein α- and βγ-subunits, GPCRs coupling to different classes of G-proteins, protein kinases and -phosphatases, regulators of G-protein signalling, adenylate cyclase and cytoskeletal compounds in order to form membrane-subdomain localized signalling complexes with specific functional properties [12–20]. According to this complex supramolecular organisation, GIRK channels are not simply regulated by G-protein subunits alone, but additional regulatory pathways mediated by phosphatitylinositole-4,5-bisphosphate, protein kinases and Na+ ions, also exist [1]. GIRK channels, produced by homooligomeric GIRK2 and heterooligomeric GIRK1/GIRK2 or GIRK1/GIRK4 composition, were found to be regulated by cAMP dependent protein kinase (PKA; [21]). In the case of IK + ACh, the atrial GIRK1/GIRK4 complex, PKA phosphorylation was found to provide an “on-switch” for the activation of IK + ACh by Gβγ itself, whereas protein phosphatase 2A (PP2A) generated GIRKs that were insensitive to Gβγ [22]. Our laboratory investigated this outstanding regulation at the single channel level [23] and later on succeeded to identify the structural determinant on the GIRK1 subunit in the form of three serine/threonines (S/Ts) within the cytosolic carboxyl-terminus (CT; [24,25]). So far, most GIRK complexes studied in the context of PKA regulation were of heterooligomeric nature. Therefore, the aim of the current study was to investigate whether GIRK4 subunits also participate in PKA mediated regulation of GIRK tetrameric complexes. Here we report that GIRK4 homooligomeric channels are regulated by PKA phosphorylation, identify two S/Ts within the GIRK4 structure that are responsible for the robust increase in both basal and agonist induced current and show that the GIRK4 subunit plays a significant role in the PKA regulation of heterooligomeric GIRK1/GIRK4 complexes.
2. Materials and methods
2.1. Reagents
Glutathione Sepharose 4B was from GE Healthcare Europe GmbH, Vienna, Austria (27-4574-01). SpCAMPS and RpCAMPS were from Sigma-Aldrich, St. Louis, MO, USA (A166, A165, respectively). [32P]ATP[γP] was from GE Healthcare Europe GmbH, Vienna, Austria (25001748). Catalytic subunit of cAMP dependent protein kinase (PKA-cs) was purchased from Boehringer Ingelheim GmbH, Ingelheim, Germany (1529-307). All other reagents used were of reagent grade.
2.2. Solutions (concentrations in mmole.L− 1 unless stated otherwise)
Phosphorylation buffer (PhB): HEPES (25), MgCl2 (5), EGTA (5), Tween-20 (0.05%) buffered with NaOH to pH: 7.4. 4 × SDS loading buffer: Tris (400), sucrose (20% w/v), SDS (4% w/v), mercaptoethanole (20% v/v) titrated with HCl to pH: 6.8. Coomassie staining solution: Coomassie blue (0.04‰ w/v), methanol (50% v/v), acetic acid (10% v/v). Destain I: methanol (50% v/v), acetic acid (10% v/v). Destain II: methanol (5% v/v), acetic acid (7% v/v). ND96: NaCl (96), KCl (2), MgCl2 (1), CaCl2 (1), HEPES (5) buffered with NaOH to pH: 7.4. NDE: same as ND96 but CaCl2 was 1.8 and pyruvate (2.5) and antibiotics (0.1% v/v; from 1000 × stock from Sigma-Aldrich (G-1397)) were added. HK: KCl (96), NaCl (2), MgCl2 (1), CaCl2 (1), HEPES (5) buffered with KOH to pH: 7.4.
2.3. Genetic engineering
Amplification of plasmids in bacteria, purification, linearization, ligation and site directed mutagenesis (using the DpnI method) were performed using standard procedures [26]. Plasmids with inserts encoding the different proteins have been already described: m2R [21], rGIRK1 & hGIRK4 [27]. cRNA was synthesised according to [28]. Phosphorylation deficient mutations of heterooligomeric rGIRK1WT were produced using the identical primers as described for the homooligomeric rGIRK1⁎ construct [25]. The primers used for site directed mutagenesis of potential S/Ts within the hGIRK4 sequence are listed in supplementary Table S1. Plasmids for production of recombinant truncated intracellular regions of hGIRK4 fused to GST were produced according to [25] using suitable primers are listed in supplementary Table S2. All constructs and mutations were verified by sequencing.
2.4. Recombinant protein purification
Constructs were transfected into BL-21(RIL) competent cells (Stratagene, La Jolla, CA, USA). Overexpression and purification were achieved as previously described [29]. Purified recombinant protein was quantitated by the method of Bradford [30], diluted to a concentration of 1 μg. μL− 1, aliquots were shock frozen in liquid N2 and stored at − 70 °C until used.
2.5. In-vitro phosphorylation
In-vitro phosphorylation of recombinant protein was performed as described [25]. Shortly, 1 μg protein was subjected to phosphorylation in-vitro by PKA-cs using [32P]ATP[γP] as co-substrate. Subsequently the protein was submitted to SDS-PAGE [31] on a 12% gel. Gels were stained with Coomassie blue, dried and scanned densitometrically. Quantitative autoradiography was performed using the Storm Phosphor imager (GE Healthcare Europe GmbH, Vienna, Austria). Incorporated radioactivity was normalized to the total amount of protein as detected by Coomassie stain (= relative specific radioactivity).
2.6. Xenopus laevis oocyte expression
The Xenopus laevis oocyte expression system was used exactly as described [32]. The amounts of RNA injected per oocyte are given in parenthesis (in ng per oocyte): m2R (2.5); GIRK1WT (0.01–0.25); GIRK1S385CS401CT407C (1); GIRK4WT (0.01–2.5); GIRK4⁎ (0.01); GIRK4⁎S75C (0.01); GIRK4⁎S191C (0.165); GIRK4⁎T199C (0.165); GIRK4⁎S382C/A/E (0.01–10); GIRK4⁎S412C (1); GIRK4⁎S418A (0.01); GIRK4⁎T199CS412C (1); GIRK4S75C (0.25); GIRK4T199C (0.165); GIRK4S412C (0.165) and GIRK4T199CS412C (1).
2.7. Electrophysiology
Oocytes were studied following 3–5 day incubation at 19 °C in NDE. Superfusion with ND96 and HK (with and w/o 10− 5 mole/L acetylcholine) was performed at 21 °C while the membrane potential was set to − 80 mV and currents were recorded with the two electrode voltage clamp technique (TEVC) using agarose cushion electrodes [33] in connection with the Gene Clamp 500 amplifier (Molecular Devices Germany GmbH (Biberach an der Riss, Germany)). Cytosolic injections of cAMP and analogues (30–60 pmole per oocyte) during recording were performed as described [21]. The acute effect of cAMP injection was quantitated by normalizing the current increase, induced by cAMP injection (IACh) to the basal current before cAMP injection was performed (IHK; see Fig. 1A for illustration). Thus, both in the case when cAMP was injected in the absence or in the presence of agonist, the cAMP effect is given by: . Current traces were low pass filtered at 10 Hz and digitized at a sampling rate of 50 Hz using the digidata 1322A interface (Molecular Devices (Germany) GmbH, Biberach an der Riss, Germany) connected to a MS-Windows compatible computer using the pClamp9.2 software (Molecular Devices (Germany) GmbH, Biberach an der Riss, Germany).
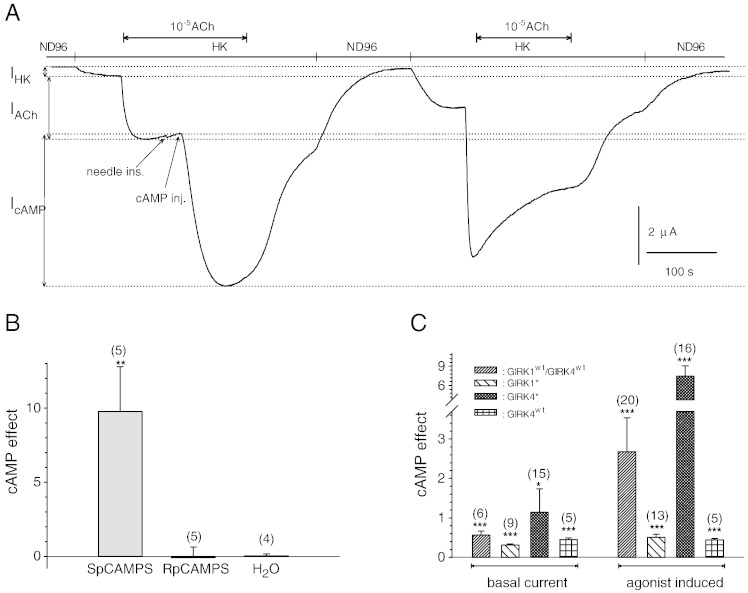
Fig. 1.
Homooligomeric GIRK4 channels are regulated by PKA.
A.: Original current recording derived from an oocyte injected with cRNA encoding GIRK4 and m2R. Membrane potential was kept constant at − 80 mV. Basal current (IHK) was induced by changing from the physiological extracellular medium (ND96) to a medium containing 96 mmole/L K+ (HK). G-protein activation was achieved by perfusion with HK containing 10− 5 mole/L acetylcholine. An injection needle was inserted during agonist activation. In order to activate PKA 3 pmole cAMP were injected into the cytosol of the oocyte (both insertion and injection are indicated by an arrow). After washout of agonist and HK, both IHK and IACh were elicited again in order to demonstrate the increase of currents through GIRK4 complexes.
B.: Statistical analysis of the effect of injection of SpCAMPS, RpCAMPS and water on agonist induced currents through homooligomeric GIRK4S143T complexes. Number of individual oocytes tested in parenthesis above each bar. **: the mean value deviates statistically significant from both RpCAMPS and H2O at the p < 0.01 level.
C.: Statistical analysis of the effect of cAMP injection on basal currents and agonist induced currents through homooligomeric and heterooligomeric GIRK channel complexes. Number of individual oocytes tested in parenthesis above each bar. *, (***): the mean value deviates statistically significant from zero at the p < 0.05 (0.001) level.
2.8. Statistical analysis
Results from given experimental groups were compared for statistical significance using ANOVA or Student's t-Test, where appropriate (Sigma Plot 12.0; Systat Software Inc., San Jose, CA 95110 USA). In some instances, the calculated average value of current increase in percent was tested for a significant difference from zero by assuming Gaussian distribution [34].
3. Results
3.1. Homooligomeric GIRK4 channel complexes are regulated by PKA
In order to investigate a potential contribution of the GIRK4 subunit, the homooligomeric mutation S143T [35] was introduced into the human GIRK4 sequence resulting in the human GIRK4⁎ subunit. GIRK4⁎ channels were expressed and studied in the Xenopus laevis oocyte expression system. Interestingly, cytosolic injections of cAMP led to a significant increase of both agonist induced and basal current through homooligomeric GIRK4⁎ channels (Fig. 1A). To further substantiate PKA catalysed phosphorylation as being responsible, the non-hydrolysable cAMP analogues SpCAMPS (activatory to PKA) and RpCAMPS (inhibitory to PKA) were injected. SpCAMPS, but not RpCAMPS, led to considerable augmentation of agonist induced currents, suggesting that PKA was responsible for the rapid and substantial effect caused by cAMP injections (Fig. 1B). Comparison of cAMP effects on GIRK1/GIRK4 complexes of different subunit composition revealed that both homooligomeric GIRK1⁎ (GIRK1F137S), GIRK4⁎ (GIRK4S143T) and GIRK4WT complexes as well as heterooligomeric GIRK1WT/GIRK4WT complexes were regulated by cAMP injection resulting in an increase of agonist as well as basal current (Fig. 1C). This finding is in line with previous results on the regulation of GIRK1/GIRK4 complexes by PKA, but indicates that the GIRK4 subunit may play an additional, hitherto unrecognized, role.
3.2. Identification of the structural determinant on the GIRK4 subunit
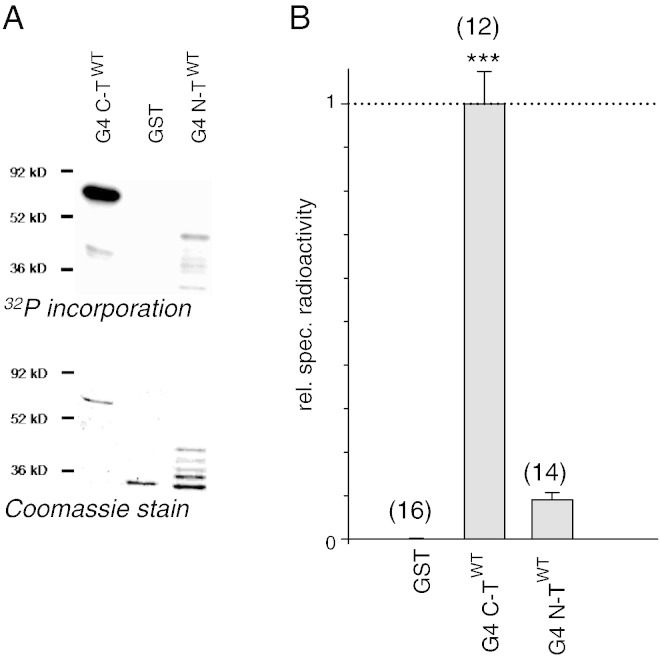
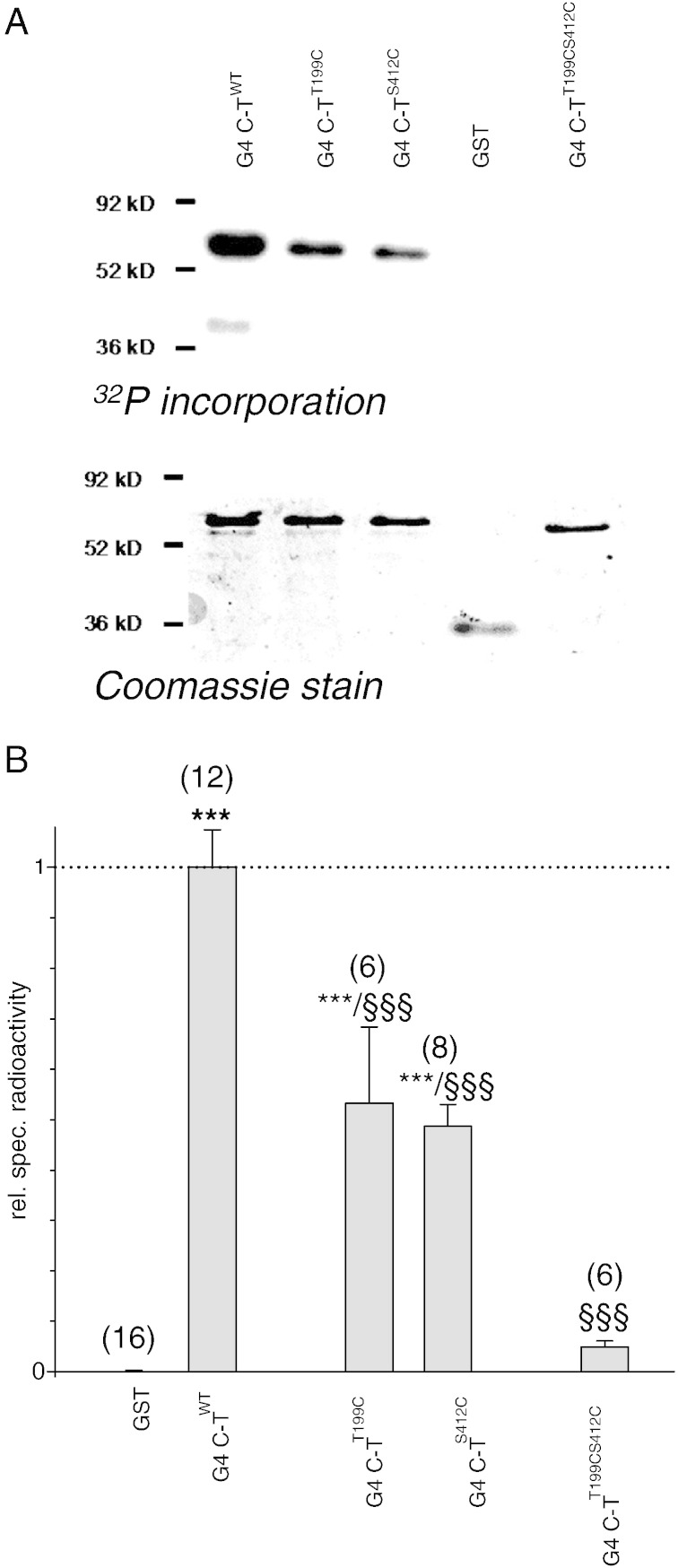
Since GIRK4 channels revealed themselves susceptible to regulation by cAMP, it was of interest to investigate whether the cytosolic regions of GIRK4 subunits are targets for PKA mediated phosphorylation. Recombinant GST fusion proteins with the C- and the N-terminal regions of GIRK4 were used as substrates for phosphorylation by the catalytic subunit of PKA (PKA-cs) in-vitro. Both intracellular regions were identified to be substrates for this enzyme, the C-terminus being phosphorylated considerably stronger, when compared to the N-terminus (Fig. 2). This contrasts previous findings where exclusively the GIRK1, but not the GIRK4 subunit, was found to be a substrate for PKA [22]. In this case, however, the IK + ACh complex was immunoprecipitated from bovine atrium. Besides species differences, a possible explanation for the failure to phosphorylate GIRK4 in-vitro could be high intrinsic levels of PKA phosphorylation in atrial tissue per se in contrast to the recombinant protein produced and purified by bacteria in the prevailing study. In order to more closely localize S/Ts responsible for PKA phosphorylation in-vitro, the GIRK4 C-terminus was further truncated into 4 smaller regions and subjected to PKA-cs phosphorylation in-vitro. In contrast to GIRK1, where the three S/Ts responsible for PKA phosphorylation are localized within a narrow stretch of no more than 22 amino acids within the C-terminus [25], phosphorylation sites within GIRK4 were dispersed all over the C-terminus, the proximal and distal parts being more prominently phosphorylated when compared to the intermediate region (supplementary Figs. S1A). S/Ts within the truncated fusion proteins complying to the PKA consensus sequence R/K-[X]1–2-S/T were mutated to cysteines (in some cases, when the introduction of artificial disulphide bonds was regarded to be a potential problem, the resulting point mutation was alanine). The mutant constructs were subjected to phosphorylation in vitro which was compared to the phosphorylation of the corresponding WT construct. In case the 32P incorporation of a mutant construct was still considerable, additional S/Ts other than the consensus were also mutated and the corresponding mutant construct subjected to phosphorylation, until its in-vitro phosphorylation was negligible. Finally, based on the effect of mutation of individual S/Ts within a construct, the relative contribution of each individual mutation to the phosphorylation of the entire cytosolic region (N–T plus C–T truncations) was calculated. Finally, four amino acids, T199, S382, S412 and S4128, contributed most prominently to the phosphorylation of the cytosolic parts of GIRK4 (supplementary Figs. S1B). In order to investigate whether T199, S382, S412 or S418 plays a functional role in the heterologous facilitation of GIRK currents by PKA, point mutations were introduced into the entire GIRK4⁎ sequence and the corresponding homooligomeric channel complex was tested for functional cAMP regulation. The N-terminal amino acids S75 and S191 (which had been reported to be essential for PKC regulation [36]) were mutated as controls. Mutation of S382 to C (or A or E) was lethal to channel function as we were unable to record significant currents from oocytes injected with GIRK4⁎S382C(A,E) cRNA. When cRNA encoding a N-terminal fusion protein of eGFP and GIRK4S143TS382C was injected into oocytes, no fluorescence could be detected, indicating that mutation of S382 prohibited GIRK4 synthesis (data not shown). Hence we were unable to study this S382 in further detail. From the remaining three amino acids, T199 and S412 exhibited profound and significant ad hoc effects on PKA regulation, while mutation S418A had no influence (see supplementary Figs. S2B). The impact of T199 and S412 on cAMP regulation was further investigated in the homooligomeric GIRK4⁎ complex. While single mutation of T199 almost completely abolished the effect of cAMP injections on agonist induced K+ currents, a single mutation of S412 also greatly reduced functional effects, when compared to WT. Hence we conclude that both amino acids contribute. Accordingly, simultaneous mutation of both amino acids led to homooligomeric channels that were completely insensitive to PKA modulation (Fig. 3). Since both T199 and S412 exerted profound and significant ad hoc effects upon PKA regulation, these two amino acids were mutated in the full-length C-terminal construct and the effect on in vitro phosphorylation was tested. Individual single mutations of T199 and S412 reduced the level of 32P incorporation catalysed by PKA-cs significantly and to a similar extent, while simultaneous mutation of the two amino acids abolished phosphorylation of the entire C–T almost completely (Fig. 4).
Fig. 2.
Phosphorylation of the cytosolic regions of GIRK4 by PKA in-vitro.
A.: upper panel: Autoradiogram showing 32P incorporation of the GIRK4 C- and N-terminal fusion proteins following incubation with PKA-cs and ATP-γ-32P. Lower panel: Coomassie stain of the electrophoresis gel shown in upper panel.
B: Statistical analysis of 32P incorporation into N- and C-terminus. The number of individual phosphorylation experiments is shown in parenthesis above each bar. ***: the mean value deviates statistically significant from GST at the p < 0.001 level.
Fig. 3.
Role of T199 and S412 in the heterologous facilitation of homooligomeric GIRK4⁎ channels.
A.: Original current recording derived from an oocyte injected with cRNA encoding GIRK4⁎T199CS412C and m2R. Membrane potential was kept constant at − 80 mV. After induction of basal current (IHK) by superfusion with HK and G-protein activation by 10− 5 mole/L acetylcholine 5 pmole cAMP were injected into the cytosol oocyte (injection is indicated by an arrow).
B.: Statistical analysis of the effect of cAMP injection on agonist induced currents through GIRK4⁎WT and mutant channels. Number of individual oocytes tested in parenthesis above each bar. ***: the mean value deviates statistically significant from GIRK4⁎WT at the p < 0.001 level.
Fig. 4.
Effect of T199 and S412 on PKA-cs catalysed 32P incorporation into the GIRK4 C-terminus.
A.: Upper panel: Autoradiogram showing the effect of T199 and S412 on 32P incorporation into the GIRK4 C-terminal fusion protein (upper panel). Lower panel: Coomassie stain of the electrophoresis gel shown in upper panel.
B.: Statistical analysis of 32P incorporation into WT and mutant forms of the GIRK4 C-terminus. The number of individual phosphorylation experiments is shown in parenthesis above each bar. ***: the mean value deviates statistically significant from zero at the p < 0.001 level. §§§: the mean value deviates statistically significant from WT at the p < 0.001 level.
3.3. Molecular basis of the regulation of the heterooligomeric IK + ACh complex by PKA
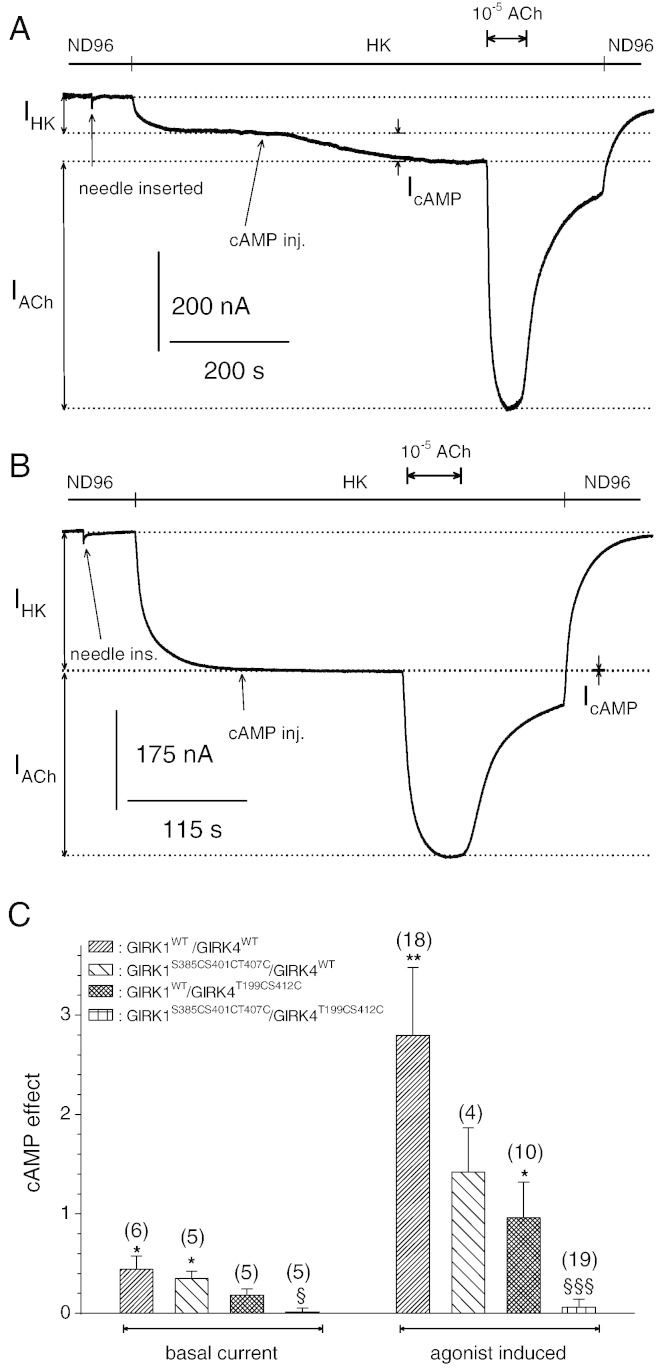
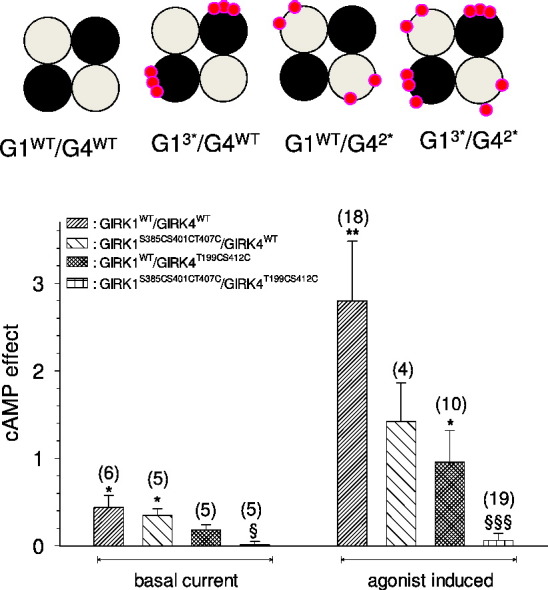
Since PKA catalyses the phosphorylation of both the GIRK1 and the GIRK4 subunits and both homooligomeric ion channel complexes are functionally affected by this enzyme, it was of interest to assess the role of each subunit type to the heterologous facilitation of the heterooligomeric IK + ACh complex that plays an essential role in the regulation of the heartbeat by the autonomous nervous system [37], but presumably is also important in the central nervous system [38]. Combinations of wild type and phosphorylation deficient GIRK1S385S401CT407C and GIRK4T199CS412C subunits were coexpressed and their regulation by cAMP injections was compared. The importance of PKA catalysed phosphorylation of both subunits is underlined by the fact that incorporation of either the phosphorylation-deficient GIRK1S385S401CT407C or the GIRK4T199CS412C subunit into the heterooligomeric complex resulted in a considerable reduction of cAMP facilitation of currents, but it was still clearly detectable. Only GIRK1/4 complexes comprising both types of phosphorylation-deficient subunits were completely devoid of facilitation mediated by cAMP injection (Fig. 5). Hence we conclude that both subunits contribute to the heterologous facilitation of G-protein activation by PKA. At the molecular level five S/Ts, namely T199 and S412 on the GIRK4 subunit and S385, S401 and T407 on the GIRK1 subunit are required to produce the regulatory action.
Fig. 5.
Role of individual subunits in the heterologous facilitation of heterooligomeric IK,ACh channels.
A: Original current recording derived from an oocyte injected with cRNA encoding GIRK1WT, GIRK4WT and m2R. Membrane potential was kept constant at − 80 mV. After induction of basal current (IHK) by superfusion with HK, 4 pmole cAMP were injected into the cytosol of the oocyte (both insertion of needle and injection are indicated by an arrow).
B: similar to 5 A, but the oocyte was injected with cRNA encoding the phosphorylation deficient GIRK1S385CS401CT407C and GIRK4T199CS412C subunits.
C: Statistical analysis of the effect of cAMP injection on basal and agonist induced currents through heterooligomeric GIRK1/GIRK4 WT and mutant channel complexes. Number of individual oocytes tested in parenthesis above each bar. *, (**): the mean value deviates significantly from zero at the p < 0.05 (0.01) level. §, (§§§): the mean value deviates statistically significant from GIRK1WT/GIRK4WT at the p < 0.05 (0.001) level.
4. Discussion
The present study provides clear evidence that phosphorylation of the GIRK4 subunit plays an important role in the regulation of heterooligomeric GIRK1/GIRK4 channel complexes by PKA. PKA phosphorylation exerted robust stimulation of currents mediated by homooligomeric GIRK4 channels. Threonine-199 and serine-412 were identified as the structural determinant being responsible for ≈ 95% of phosphorylation of the cytosolic GIRK4 C-terminus catalysed by PKA in-vitro and ≈ 99% of the acute effect exerted by cAMP injections on homooligomeric GIRK4 channels in-vivo. Within the heterooligomeric GIRK1/GIRK4 complex, T199 and S412 contribute to ≈ 65% to the acute effect induced by PKA when coexpressed with the GIRK1WT subunit. Acute PKA effects were practically absent (reduction to ≈ 3% vs. WTs) in heterotetrameric channels composed of both phosphorylation-deficient GIRK1 and GIRK4 subunits.
Specificity of S/T protein kinases is determined to a great extent by 3-D structure of the protein substrate and hence is completely understood in selected cases only [39]. In addition to 3-D structure, molecular determinants within the primary sequence near the target S/T exist that favour PKA phosphorylation. All 3 S/Ts that have been identified previously within the GIRK1 sequence [25] conform to the classical PKA consensus sequence –R/K1–2-X-S/T-, that is known to occur in about 95% of PKA substrates [40]. While T199 within GIRK4 also represents a “classical” PKA consensus sequence, S412 does not. According to [39] basic residues (R/K) at positions − 4 to − 7 can compensate, at least partially, for the lack of arginyl residues at positions − 2 and/or − 3 and indeed a K is located at position − 5 in the case of S412. Obviously K407, together with the 3-D structure of the GIRK4 C-T, renders S412 susceptible to PKA. Noteworthy, the nearby located S418 conforms to the classical consensus sequence described above and was phosphorylated by PKA-cs in our in vitro assay to an extent comparable to S412. Site-directed mutagenesis of S418 revealed, however, that this amino acid was not involved in the acute PKA effects described here. We conclude that S418 is either not phosphorylated by PKA in-vivo for reasons of 3-D structure or its phosphorylation is responsible, at least partially, for another hitherto unrecognized effect mediated by PKA.
Partial 3-D structures are available for GIRK1 [41,42] and GIRK2 [11]. Since the structures do not comprise the flexible distal C-termini where the determinants within GIRK1 and S412 of GIRK4 are located, it is at present not possible to understand mechanistically how PKA phosphorylation promotes GIRK activation by Gβγ via these S/Ts. T199, however, is covered by the crystal structures currently available and we may speculate about its mechanistical contribution to heterologous facilitation. The amino acid corresponding to T199 in GIRK4 is conserved throughout all four GIRK subunits (see Fig. 6) and lies within the trans membrane-domain/cytosolic-domain (TMD–CTD) linker that is essential for the control of GIRK1 and GIRK2 gating by G-proteins. According to the crystal structure of GIRK2, T204 contributes essentially to the stabilization of the closed conformation of the G-loop gate via a hydrogen bond with Y78 of the adjacent GIRK2 subunit. Since both amino acids are conserved between GIRK2 and GIRK4 (T199 and Y53 in GIRK4), the consequence of phosphorylation of T199 would be the disruption of Y53/T199 interaction what in turn would destabilize the G-loop gates closed conformation and promote its opening in GIRK4 homooligomeric channels.
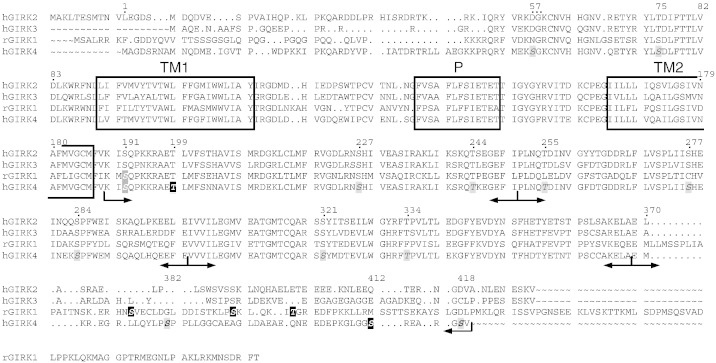
Fig. 6.
Primary structure alignment of the four GIRK subunit isoforms at the protein level.
S/Ts in the GIRK4 sequence are highlighted in grey (italics: no major effect on phosphorylation in-vitro; italics/bold: major effect on phosphorylation in-vitro, but no functional consequence) or black (both profound effect on phosphorylation in-vitro and on heterologous facilitation). S/Ts that have been found to be important for heterologous facilitation in the GIRK1 sequence are also highlighted in black [25]. S191 that has been reported to be responsible for PKC regulation is marked in darker grey (both GIRK1 and GIRK4; [36]). Arrows indicate the truncations of the C-terminus used for phosphorylation in vitro (suppl. Figs. S1). TM1, TM2 and P denote the trans membrane and the pore helices, respectively.
Protein phosphorylation has been shown to play important roles in the regulation of GIRK channel function in physiology and pathophysiology. Several excitatory neurotransmitters, including glutamate, inhibit GIRKs via PTX insensitive G-proteins involving the PLC/PKC pathway [43,44]. The underlying mechanism has been identified as PKC-catalysed phosphorylation of two serines located in the cytosolic part of the GIRK1 and GIRK4 C-termini [36]. Another instance is the phosphorylation of serine 9 in the cytosolic N-terminus of GIRK2 via a protein kinase that is at present unknown. Protein phosphatase 1 mediated dephosphorylation of this serine results in increased surface localisation of GIRK2 at excitatory synapses and dendritic spines and represents an essential mechanism resulting in depotentiation of long term potentiation (LTP), an important form of excitatory synapse plasticity [45,46]. Regulation of GIRK1/GIRK4 heterooligomeric channels via PKA is at present well documented although its physiological role remains unidentified. In the case of atrial IK + ACh one may speculate that the heterologous facilitation may represent a mechanism for stabilizing the membrane resting potential during increased speed of conduction and contractility that is required to prevent re-entry of excitation and fibrillation. Another, pathophysiological, function may be indicated by the ß-adrenergic stimulation of growth of cancer cells that has been shown to be linked to GIRK1 RNA expression [47]. GIRK1/GIRK4 channels are also known to exist in the CNS and sparse evidence about possible functional roles, derived from knockout mice, exists [38]. No data are hitherto available on possible roles of PKA regulation of GIRK1/GIRK4 channels in the brain.
Homooligomeric GIRK4 channel complexes have been shown to exist in native atrial tissue [48]. Functional single IK + ACh channels presumably formed by GIRK4 homooligomers, but with aberrant kinetics and fast desensitisation, have been observed in atrial myocytes derived from GIRK1 k.o. mice [49]. Furthermore, Nikolov & Ivanova-Nikolova, were able to observe atypical small-conductance IK + ACh channels in atrial myocytes, possibly formed by GIRK4 homooligomers that were regulated by PKA [50]. Regulation of homooligomeric GIRK4 channels may represent a potentially important physiological regulation mechanism in atrium but possibly also in other tissues.
Acknowledgements
We thank T. DeVaney (Graz; Austria) for correcting the English language. W.S. is supported by a grant from the Austrian Research Foundation (FWF; P22974-B19).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bbamem.2012.12.016.
Appendix A. Supplementary data
Supplementary material.
References
- 1.Luscher C., Slesinger P.A. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat. Rev. Neurosci. 2010;11:301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christensen H.R., Zeng Q., Murawsky M.K., Gregerson K.A. Estrogen regulation of the dopamine-activated GIRK channel in pituitary lactotrophs: implications for regulation of prolactin release during the estrous cycle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R746–R756. doi: 10.1152/ajpregu.00138.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shankar H., Kahner B.N., Prabhakar J., Lakhani P., Kim S., Kunapuli S.P. G-protein-gated inwardly rectifying potassium channels regulate ADP-induced cPLA(2) activity in platelets through Src family kinases. Blood. 2006;108:3027–3034. doi: 10.1182/blood-2006-03-010330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwanir S., Reuveny E. Adrenaline-induced hyperpolarization of mouse pancreatic islet cells is mediated by G protein-gated inwardly rectifying potassium (GIRK) channels. Pflugers Arch. 2008;456:1097–1108. doi: 10.1007/s00424-008-0479-4. [DOI] [PubMed] [Google Scholar]
- 5.Yi Y.J., Sung D.Y., Millette C., Sutovsky M., Kennedy C., Sutovsky P., Thompson W., Thomas K. Sperm GIRK2-containing K + inward rectifying channels participate in sperm capacitation and fertilization. Syst. Biol. Reprod. Med. 2011;57:296–308. doi: 10.3109/19396368.2011.631685. [DOI] [PubMed] [Google Scholar]
- 6.Hibino H., Inanobe A., Furutani K., Murakami S., Findlay I., Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol. Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 7.Choi M., Scholl U.I., Yue P., Bjoerklund P., Zhao B., Nelson-Williams C., Ji W., Cho Y., Patel A., Men C.J., Lolis E., Wisgerhof M.V., Geller D.S., Mane S., Hellman P., Westin G., Akerstrom G., Wang W., Carling T., Lifton R.P. K + channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–772. doi: 10.1126/science.1198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jabbari J., Olesen M.S., Holst A.G., Nielsen J.B., Haunso S., Svendsen J.H. Common polymorphisms in KNCJ5 are associated with early-onset lone atrial fibrillation in Caucasians. Cardiology. 2011;118:116–120. doi: 10.1159/000323840. [DOI] [PubMed] [Google Scholar]
- 9.Clarke T.K., Laucht M., Ridinger M., Wodarz N., Rietschel M., Maier W., Lathrop M., Lourdusamy A., Zimmermann U.S., Desrivieres S., Schumann G. KCNJ6 is associated with adult alcohol dependence and involved in gene × early life stress interactions in adolescent alcohol drinking. Neuropsychopharmacology. 2011;36:1142–1148. doi: 10.1038/npp.2010.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada K., Iwayama Y., Toyota T., Ohnishi T., Ohba H., Maekawa M., Yoshikawa T. Association study of the KCNJ3 gene as a susceptibility candidate for schizophrenia in the Chinese population. Hum. Genet. 2012;131:443–451. doi: 10.1007/s00439-011-1089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whorton M.R., MacKinnon R. Crystal structure of the mammalian GIRK2 K + channel and gating regulation by G proteins, PIP2, and sodium. Cell. 2011;147:199–208. doi: 10.1016/j.cell.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berlin S., Keren-Raifman T., Castel R., Rubinstein M., Dessauer C.W., Ivanina T., Dascal N. G alpha(i) and G beta gamma jointly regulate the conformations of a G beta gamma effector, the neuronal G protein-activated K + channel (GIRK) J. Biol. Chem. 2010;285:6179–6185. doi: 10.1074/jbc.M109.085944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikolov E.N., Ivanova-Nikolova T.T. Coordination of membrane excitability through a GIRK1 signaling complex in the atria. J. Biol. Chem. 2004;279:23630–23636. doi: 10.1074/jbc.M312861200. [DOI] [PubMed] [Google Scholar]
- 14.Nikolov E.N., Ivanova-Nikolova T.T. Dynamic integration of alpha-adrenergic and cholinergic signals in the atria — role of G protein-regulated inwardly rectifying K + channels. J. Biol. Chem. 2007;282:28669–28682. doi: 10.1074/jbc.M703677200. [DOI] [PubMed] [Google Scholar]
- 15.Berlin S., Tsemakhovich V.A., Castel R., Ivanina T., Dessauer C.W., Keren-Raifman T., Dascal N. Two distinct aspects of coupling between G alpha(i) protein and G protein-activated K + channel (GIRK) revealed by fluorescently labeled G alpha(i3) protein subunits. J. Biol. Chem. 2011;286:33223–33235. doi: 10.1074/jbc.M111.271056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubinstein M., Peleg S., Berlin S., Brass D., Dascal N. G alpha(i3) primes the G protein-activated K + channels for activation by coexpressed G beta gamma in intact Xenopus oocytes. J. Physiol. (Lond.) 2007;581:17–32. doi: 10.1113/jphysiol.2006.125864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubinstein M., Peleg S., Berlin S., Brass D., Keren-Raifman T., Dessauer C.W., Ivanina T., Dascal N. Divergent regulation of GIRK1 and GIRK2 subunits of the neuronal G protein gated K + channel by G alpha(GDP)(i) and G beta gamma. J. Physiol. (Lond.) 2009;587:3473–3491. doi: 10.1113/jphysiol.2009.173229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavine N., Ethier N., Oak J.N., Pei L., Liu F., Trieu P., Rebois R.V., Bouvier M., Hebert T.E., Van Tol H.H.M. G protein-coupled receptors form stable complexes with inwardly rectifying potassium channels and adenylyl cyclase. J. Biol. Chem. 2002;277:46010–46019. doi: 10.1074/jbc.M205035200. [DOI] [PubMed] [Google Scholar]
- 19.Ciruela F., Fernandez-Duenas V., Sahlholm K., Fernandez-Alacid L., Nicolau J.C., Watanabe M., Lujan R. Evidence for oligomerization between GABA(B) receptors and GIRK channels containing the GIRK1 and GIRK3 subunits. Eur. J. Neurosci. 2010;32:1265–1277. doi: 10.1111/j.1460-9568.2010.07356.x. [DOI] [PubMed] [Google Scholar]
- 20.Schwarzer S., Nobles M., Tinker A. Do Caveolae have a role in the fidelity and dynamics of receptor activation of G-protein-gated inwardly rectifying potassium channels? J. Biol. Chem. 2010;285:27817–27826. doi: 10.1074/jbc.M110.103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullner C., Vorobiov D., Bera A.K., Uezono Y., Yakubovich D., Frohnwieser-Steinecker B., Dascal N., Schreibmayer W. Heterologous facilitation of G protein-activated K + channels by beta-adrenergic stimulation via cAMP-dependent protein kinase. J. Gen. Physiol. 2000;115:547–557. doi: 10.1085/jgp.115.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medina I., Krapivinsky G., Arnold S., Kovoor P., Krapivinsky L., Clapham D.E. A switch mechanism for G ss gamma activation of I-KACh. J. Biol. Chem. 2000;275:29709–29716. doi: 10.1074/jbc.M004989200. [DOI] [PubMed] [Google Scholar]
- 23.Mullner C., Yakubovich D., Dessauer C.W., Platzer D., Schreibmayer W. Single channel analysis of the regulation of GIRK1/GIRK4 channels by protein phosphorylation. Biophys. J. 2003;84:1399–1409. doi: 10.1016/S0006-3495(03)74954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rusinova R., Shen Y.M., Dolios G., Padovan J., Yang H.Y., Kirchberger M., Wang R., Logothetis D. Mass spectrometric analysis reveals a functionally important PKA phosphorylation site in a Kir3 channel subunit. Pflugers Arch. 2009;458:303–314. doi: 10.1007/s00424-008-0628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullner C., Steinecker B., Gorischek A., Schreibmayer W. Identification of the structural determinant responsible for the phosphorylation of G-protein activated potassium channel 1 by cAMP-dependent protein kinase. FEBS J. 2009;276:6218–6226. doi: 10.1111/j.1742-4658.2009.07325.x. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J., Russel D.W. 3rd ed. CSH; Cold Spring harbour: 2001. Molecular Cloning. [Google Scholar]
- 27.Wagner V., Stadelmeyer E., Riederer M., Regitnig P., Gorischek A., DeVaney T., Schmidt K., Tritthart H.A., Hirschberg K., Bauernhofer T., Schreibmayer W. Cloning and characterisation of GIRK1 variants resulting from alternative RNA editing of the KCNJ3 gene transcript in a human breast cancer cell line. J. Cell. Biochem. 2010;110:598–608. doi: 10.1002/jcb.22564. [DOI] [PubMed] [Google Scholar]
- 28.Dascal N., Lotan I. Expression of exogenous ion channels and neurotransmitter receptors in RNA-injected Xenopus oocytes. In: Longstaff A.R., P., editors. vol. 13. Humana Press; Totowa, NJ: 1992. pp. 205–225. (Methods in Neurobiology). [Google Scholar]
- 29.Poparic I., Schreibmayer W., Schoser B., Desoye G., Gorischek A., Miedl H., Hochmeister S., Binder J., Quasthoff S., Wagner K., Windpassinger C., Malle E. Four and a half LIM protein 1C (FHL1C): a binding partner for voltage-gated potassium channel K-v1.5. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradford M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 31.Laemmli U.K. Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 32.Hofer D., Lohberger B., Steinecker B., Schmidt K., Quasthoff S., Schreibmayer W. A comparative study of the action of tolperisone on seven different voltage dependent sodium channel isoforms. Eur. J. Pharmacol. 2006;538:5–14. doi: 10.1016/j.ejphar.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 33.Schreibmayer W., Lester H.A., Dascal N. Voltage clamping of Xenopus-Laevis oocytes utilizing agarose-cushion electrodes. Pflugers Arch. 1994;426:453–458. doi: 10.1007/BF00388310. [DOI] [PubMed] [Google Scholar]
- 34.Spiegel M.R. Schaum Publishing Co.; New York: 1961. Theory and Problems of Statistics. [Google Scholar]
- 35.Vivaudou M., Chan K.W., Sui J.L., Jan L.Y., Reuveny E., Logothetis D.E. Probing the G-protein regulation of GIRK1 and GIRK4, the two subunits of the K-ACh channel, using functional homomeric mutants. J. Biol. Chem. 1997;272:31553–31560. doi: 10.1074/jbc.272.50.31553. [DOI] [PubMed] [Google Scholar]
- 36.Mao J.Z., Wang X.R., Chen F.X., Wang R.P., Rojas A., Shi Y., Piao H., Jiang C. Molecular basis for the inhibition of G protein-coupled inward rectifier K + channels by protein kinase C. Proc. Natl. Acad. Sci. U. S. A. 2004;101:1087–1092. doi: 10.1073/pnas.0304827101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wickman K., Nemec J., Gendler S.J., Clapham D.E. Abnormal heart rate regulation in GIRK4 knockout mice. Neuron. 1998;20:103–114. doi: 10.1016/s0896-6273(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 38.Wickman K., Karschin C., Karschin A., Picciotto M.R., Clapham D.E. Brain localization and behavioral impact of the G-protein-gated K + channel subunit GIRK4. J. Neurosci. 2000;20:5608–5615. doi: 10.1523/JNEUROSCI.20-15-05608.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinna L.A., Ruzzene M. How do protein kinases recognize their substrates? Biochim. Biophys. Acta, Mol. Cell Res. 1996;1314:191–225. doi: 10.1016/s0167-4889(96)00083-3. [DOI] [PubMed] [Google Scholar]
- 40.Kennelly P.J., Krebs E.G. Consensus sequences as substrate-specificity determinants for protein-kinases and protein phosphatases. J. Biol. Chem. 1991;266:15555–15558. [PubMed] [Google Scholar]
- 41.Nishida M., MacKinnon R. Structural basis of inward rectification: cytoplasmic pore of the G protein-gated inward rectifier GIRK1 at 1.8 angstrom resolution. Cell. 2002;111:957–965. doi: 10.1016/s0092-8674(02)01227-8. [DOI] [PubMed] [Google Scholar]
- 42.Nishida M., Cadene M., Chait B.T., MacKinnon R. Crystal structure of a Kir3.1-prokaryotic Kir channel chimera. EMBO J. 2007;26:4005–4015. doi: 10.1038/sj.emboj.7601828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill J.J., Peralta E.G. Inhibition of a G(i)-activated potassium channel (GIRK1/4) by the G(q)-coupled m1 muscarinic acetylcholine receptor. J. Biol. Chem. 2001;276:5505–5510. doi: 10.1074/jbc.M008213200. [DOI] [PubMed] [Google Scholar]
- 44.Sharon D., Vorobiov D., Dascal N. Positive and negative coupling of the metabotropic glutamate receptors to a G protein-activated K + channel, GIRK, in Xenopus oocytes. J. Gen. Physiol. 1997;109:477–490. doi: 10.1085/jgp.109.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chung H.J., Ge W.P., Qian X., Wiser O., Jan Y.N., Jan L.Y. G protein-activated inwardly rectifying potassium channels mediate depotentiation of long-term potentiation. Proc. Natl. Acad. Sci. U. S. A. 2009;106:635–640. doi: 10.1073/pnas.0811685106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung H.J., Qian X., Ehlers M., Jan Y.N., Jan L.Y. Neuronal activity regulates phosphorylation-dependent surface delivery of G protein-activated inwardly rectifying potassium channels. Proc. Natl. Acad. Sci. U. S. A. 2009;106:629–634. doi: 10.1073/pnas.0811615106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plummer H.K., Dhar M.S., Cekanova M., Schuller H.M. Expression of G-protein inwardly rectifying potassium channels (GIRKs) in lung cancer cell lines. BMC Cancer. 2005;5 doi: 10.1186/1471-2407-5-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corey S., Clapham D.E. Identification of native atrial G-protein-regulated inwardly rectifying K(+) (GIRK4) channel homomultimers. J. Biol. Chem. 1998;273:27499–27504. doi: 10.1074/jbc.273.42.27499. [DOI] [PubMed] [Google Scholar]
- 49.Bettahi H., Marker C.L., Roman M.I., Wickman K. Contribution of the Kir3.1 subunit to the muscarinic-gated atrial potassium channel I-KACh. J. Biol. Chem. 2002;277:48282–48288. doi: 10.1074/jbc.M209599200. [DOI] [PubMed] [Google Scholar]
- 50.Nikolov E.N., Ivanova-Nikolova T.T. Functional characterization of a small conductance GIRK channel in rat atrial cells. Biophys. J. 2004;87:3122–3136. doi: 10.1529/biophysj.103.039487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.