Abstract
During forebrain morphogenesis, there is extensive reorganisation of the cells destined to form the eyes, telencephalon and diencephalon. Little is known about the molecular mechanisms that regulate region-specific behaviours and that maintain the coherence of cell populations undergoing specific morphogenetic processes. In this study, we show that the activity of the Eph/Ephrin signalling pathway maintains segregation between the prospective eyes and adjacent regions of the anterior neural plate during the early stages of forebrain morphogenesis in zebrafish. Several Ephrins and Ephs are expressed in complementary domains in the prospective forebrain and combinatorial abrogation of their activity results in incomplete segregation of the eyes and telencephalon and in defective evagination of the optic vesicles. Conversely, expression of exogenous Ephs or Ephrins in regions of the prospective forebrain where they are not usually expressed changes the adhesion properties of the cells, resulting in segregation to the wrong domain without changing their regional fate. The failure of eye morphogenesis in rx3 mutants is accompanied by a loss of complementary expression of Ephs and Ephrins, suggesting that this pathway is activated downstream of the regional fate specification machinery to establish boundaries between domains undergoing different programmes of morphogenesis.
Keywords: Eye field, Neural plate, Morphogenesis, Zebrafish
INTRODUCTION
During the early stages of nervous system development, the neural plate becomes subdivided into different domains along the anterior-posterior and dorsoventral axes by the combined action of a number of signalling pathways and transcription factors (reviewed by Cavodeassi and Houart, 2012; Kiecker and Lumsden, 2012). It is thought that one consequence of fate specification is the establishment of molecular mechanisms to maintain the segregation of discrete cell populations by, for example, the establishment of adhesion codes specific for each domain (reviewed by Dahmann et al., 2011).
Neural plate domains destined to form different CNS structures mostly maintain their relative positions as the neural plate folds up to form the neural tube. This is not the case in the anterior portion of the neural plate (ANP), where telencephalon, eye field and diencephalon undergo extensive reorganisation during early nervous system development (Cavodeassi and Houart, 2012). The most distinctive feature of early ANP morphogenesis is the evagination of cells within the eye field to give rise to the optic vesicles, a morphogenetic process that does not occur at any other level of the neural plate. Despite the complexity of the tissue reorganisations involved, cells within discrete ANP domains largely remain segregated from each other throughout forebrain morphogenesis (Cavodeassi and Houart, 2012). The molecular mechanisms promoting the segregation of ANP domains that undergo different programmes of morphogenesis are not well understood.
The Eph/Ephrin signalling pathway regulates cell and tissue segregation in various contexts during embryonic development (reviewed by Pasquale, 2008), and consequently is a good candidate to regulate cell behaviours during ANP morphogenesis. Both Ephrins and Ephs are membrane-bound proteins, and thus Eph-Ephrin interaction only occurs upon cell-cell contact. This usually results in repulsive responses and in the generation of affinity boundaries between territories (Pasquale, 2008). Such is the case in the hindbrain, where cells in adjacent rhombomeres are kept segregated by Eph/Ephrin signalling at rhombomere boundaries (Pasini and Wilkinson, 2002). Various Ephrins and Ephs are expressed in the ANP (e.g. Cooke et al., 1997) and overexpression of a truncated, dominant-negative form of Epha4a disrupts forebrain development (Xu et al., 1996), suggesting a role for this pathway in forebrain morphogenesis.
Although the identities of genes regulating specific morphogenetic programmes in each domain of the forebrain are largely unknown, it is presumed that such genes would be downstream targets of the transcriptional networks that specify the fate of each domain. The paired-domain homeodomain protein Rx3 is one of a network of transcriptional regulators that specify the eye field (reviewed by Bailey et al., 2004; Beccari et al., 2013). A role for Rx3 in controlling eye morphogenesis is suggested by its failure in rx3 mutants (Loosli et al., 2003; Rembold et al., 2006b; Stigloher et al., 2006; Winkler et al., 2000) and the identification of cxcr4 and nlcam (alcamb - Zebrafish Information Network) as Rx3-regulated genes (Bielen and Houart, 2012; Brown et al., 2010).
In this study we show that Rx3 regulates the complementary expression of Ephs and Ephrins in the zebrafish ANP and that Eph/Ephrin activity subsequently promotes the segregation of the eye field from adjacent domains. Using a variety of approaches to manipulate Eph/Ephrin signalling and the formation of Eph/Ephrin expression interfaces in the ANP, we find that Eph/Ephrin activity at the borders between ANP domains maintains their segregation during forebrain morphogenesis. Indeed, forcing ANP cells to express an inappropriate combination of Eph/Ephrins changes their adhesion properties and leads to their segregation to adjacent ANP domains. We propose that Eph/Ephrin activity helps to prevent the cellular intermixing that would otherwise be likely to occur during the extensive tissue reorganisations that accompany eye formation and ANP morphogenesis.
MATERIALS AND METHODS
Zebrafish lines and husbandry
AB and tupl wild-type zebrafish strains, transgenic lines Tg{rx3::GFP}ET95/1 (Brown et al., 2010; Rembold et al., 2006a) and Tg{emx3::YFP}b1200 (Viktorin et al., 2009) and the mutants chkne2611 [rx3-/- (Stigloher et al., 2006)] and efnb2ahu3393 (Stemple lab; direct submission to ZFIN) were maintained and bred according to standard procedures (Westerfield, 1993). Genotyping of efnb2ahu3393 mutants was by the dCAPS technique (Neff et al., 1998), generating a new restriction site for PshAI associated with the mutant sequence using primers (upstream, 5′-TTTTGATCTAGA - GAGAAATGCGAGT-3′; downstream, 5′-TAGAGGCGTGTCTGCT - TTTGACACCTG-3′) to amplify the sequence around the mutation (J. Cayuso and E. Ober, personal communication). All experiments conform to the guidelines from the European Community Directive and British and Spanish legislation for the experimental use of animals.
Microinjection and cell transplantation
To manipulate the activity of Eph/Ephrins, morpholinos against epha4a [0.5 pmol/embryo (Cooke et al., 2005)], efnb2a [0.5 pmol/embryo (Cooke et al., 2005)] and efnb1 (0.5 pmol/embryo, 5′-TCCACGAGCCGTCACT - TCCAGCCAT-3′; OpenBiosystems), and mRNAs for full-length ephb4a (∼25 pg/embryo), full-length epha4a (∼200 pg/embryo), full-length efnb2a (∼20 pg/embryo) and soluble efnb2a (∼100 pg/embryo) were injected into 1-cell stage embryos. Cell transplantation was performed essentially as described (Cavodeassi et al., 2005). Mid-to-late blastula stage embryos were used as donors of cells to be transplanted into 50-60% epiboly host embryos. When not labelled by a transgene, GFP mRNA (∼50 pg/embryo) was co-injected into donor embryos to identify the transplanted cells. RNAs encoding Lyn-cherry and H2b-RFP (∼50 pg/embryo) were injected to label cell membranes or nuclei, respectively, when required. Manipulated embryos were allowed to develop until the required stage, and then either fixed and prepared for immunostaining/in situ hybridisation, or mounted for live imaging and analysis.
Immunolabelling and mRNA detection
Whole-mount immunolabelling was performed as previously described (Cerveny et al., 2010). The following antibodies were used: chicken anti-GFP (1:1000, Abcam); mouse anti-ZO-1 (Tjp1) (1:400, Molecular Probes); mouse anti-β-catenin (1:400, Sigma); anti-S19-phosphorylated MLC-II (1:100, Cell Signaling Technology); and secondary antibodies (Molecular Probes) coupled to Alexa 488, 543 or 633 fluorophores as required. Membranes were highlighted by incubation with phalloidin coupled to Alexa 488/568 fluorophores (Molecular Probes).
Antisense mRNA probes for in situ hybridisation were synthesised using RNA polymerase (Promega) and digoxigenin-labelled nucleotides (Roche) following manufacturer’s instructions. Whole-mount in situ hybridisations were performed as described (Jülich et al., 2005; Xu et al., 1994). For visible detection, the embryos were incubated with anti-digoxigenin-AP and developed using NBT/BCIP substrates (Roche). For fluorescent detection, embryos were incubated with anti-digoxigenin-POD (Roche) and developed using Cy3-TSA (PerkinElmer) as substrate.
Hanging drop assay
To prepare cells for hanging drop cultures, ∼60-90 Tg{rx3::GFP}ET95/1 embryos at the end of gastrulation were dissociated as described (Kai et al., 2008). Harvested cells were resuspended in 0.3 ml L-15 medium and 25 μl drops were cultured in the lid of a plastic Petri dish at room temperature (∼22/24°C). Drop cultures were allowed to develop for over 24 hours and then mounted for confocal imaging.
Imaging and data processing
Immunostained and in situ hybridised embryos developed with fluorescent substrates were embedded in low melting point agarose (Sigma) at 1-1.5% in PBS for frontal confocal imaging using a 40× (0.8 NA) long working distance water-immersion lens. In some cases embryos were additionally mounted flat in a drop of glycerol and dorsal images were acquired with a 40× (1.3 NA) oil-immersion lens. SPE Leica and Zeiss LSM710 confocal microscopes were used for image acquisition.
Visible in situ hybridisation embryos were mounted flat in a drop of glycerol and dorsal images were acquired with a 20× (0.5 NA) water-immersion lens using a Nikon Eclipse 1000 microscope connected to a digital camera (Jenoptik) and operated by Openlab software (Improvision). Some of these embryos were embedded in OCT (Sakura Fintek) for cryosectioning. Sections (16 μm) were obtained using a Leica cryostat, mounted in glycerol, and imaged with a 40× (1.3 NA) oil-immersion lens.
Embryos for live imaging were embedded in low melting point agarose at 0.8-1% in Embryo Medium and imaged by confocal fluorescence microscopy using Leica SP systems. z-stacks (50-80 μm) were collected every 6-10 minutes for ∼5 hours. Hanging drop cell cultures were transferred to a chamber made with coverslips and silicone grease and imaged with SPE Leica/Zeiss LSM systems.
Raw data from in vivo imaging were processed and analysed with Volocity software (Improvision). Manual cell tracking and drift correction were used when required. Images were exported as TIFF files and all figures were composed using Photoshop (Adobe).
RESULTS
Complementary expression of Ephrins and Ephs in and adjacent to the eye field is lost in rx3-/- mutants
To assess whether Eph/Ephrin signalling is involved in eye morphogenesis, we assessed the expression of these families of genes in the ANP of zebrafish embryos. At least three Ephs (epha4a, ephb4a and epha2) are expressed in territories surrounding the eye field (Fig. 1A,B) (Cooke et al., 1997) and three Ephrins (efnb2a, efnb1 and efna5a) are expressed within the eye field (Fig. 1E,F; supplementary material Fig. S1F) (Thisse et al., 2008; Thisse and Thisse, 2005). This complementary expression of Ephs and Ephrins in the ANP is lost in rx3-/- [chkne2611 (Stigloher et al., 2006)] mutants. epha4a and ephb4a, which are excluded from the wild-type eye field, are homogeneously expressed throughout the ANP of rx3-/- mutants (Fig. 1C,D,G,H; data not shown), similar to other telencephalic markers (Stigloher et al., 2006). The expression of Ephrins, however, seems normal in the rx3-/- mutants (n>100 embryos; not shown). Thus, in rx3-/- mutants there is an overlap in the expression of Ephs and Ephrins that might have consequences for the adhesion properties of eye cells. The repression of epha4a by Rx3 is cell-autonomous, as rx3-/- cells express epha4a when transplanted into wild-type eye fields (Fig. 1I-L).
Fig. 1.
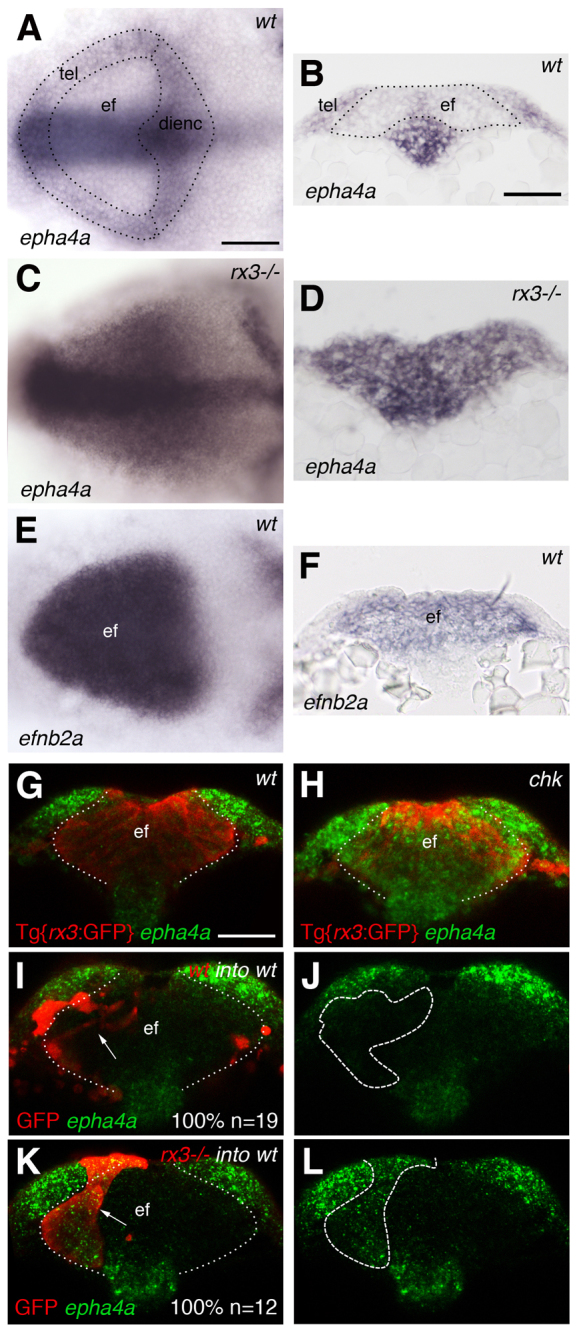
Complementary expression of Ephrins and Ephs in the anterior neural plate (ANP) is lost in zebrafish rx3-/- mutants. (A-F) Whole-mount in situ hybridisations showing the expression in the ANP of epha4a in wild-type (A,B) and rx3-/- (C,D) embryos and of efnb2a in wild-type embryos (E,F). (G,H) Whole-mount in situ hybridisation to detect epha4a expression in the ANP (green) of Tg{rx3:GFP} (G) and Tg{rx3:GFP}; rx3-/- (chk) (H) embryos counterstained for GFP to highlight the eye field (red). (I-L) Transplants of wild-type (I,J) or rx3-/- (K,L) cells (labelled by GFP, red) into wild-type hosts. rx3-/- cells show autonomous activation of epha4a in the eye field (K,L). Arrows (I,K) point to transplanted cells. Dashed/dotted lines outline ANP domains (A), outline the eye field (B,G-I,K) or outline the transplants (J,L). All panels show frontal views with dorsal to the top of 1- to 3-somite stage (ss) embryos, except (A,C,E) which show dorsal views with anterior to the left. ef, eye field; tel, telencephalon; dienc, diencephalon. Scale bars: 50 μm.
The specific Ephs and Ephrins expressed in the ANP physically and/or functionally interact with each other in other signalling contexts (e.g. Cooke et al., 2005; Durbin et al., 1998; Miao and Wang, 2009). Consequently, our results indicate that there is an Eph/Ephrin signalling interface at the boundary between the eye field and adjacent ANP territories and that this interface is disrupted in rx3 mutants in which eyes fail to form properly.
Abrogation of Eph/Ephrin activity leads to defects in the morphogenesis of the ANP
To analyse the role that the complementary expression of Ephs and Ephrins might have during eye morphogenesis, we next manipulated their levels and spatial distribution in the ANP.
Consistent with the high level of redundancy between Ephs and Ephrins (Pasquale, 2008), abrogation of any one Eph or Ephrin by the use of morpholinos did not result in any overt phenotypic outcome (not shown), whereas the concomitant abrogation of at least two led to phenotypes indicative of defective brain morphogenesis. Removing efnb2a and efnb1 simultaneously (efnb2a; efnb1 morphants) leads to mild but reproducible phenotypes in which optic vesicle evagination is slightly delayed and small clumps of cells expressing eye field markers are left behind within the brain (Fig. 2B, compare with wild type in 2A; Table 1). This phenotype is more severe when the activities of three Eph/Ephrin components are concomitantly abrogated (efnb2a; efnb1; epha4a morphants; Fig. 2C).
Fig. 2.
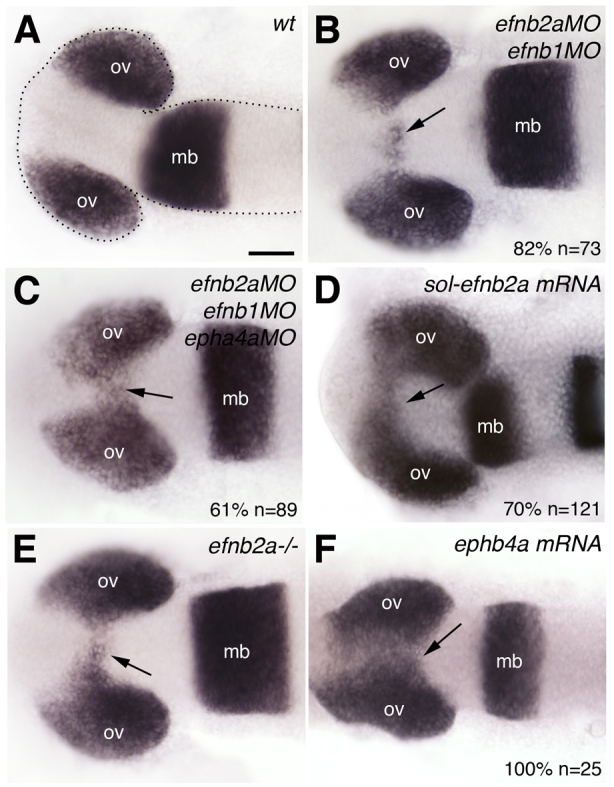
Partial abrogation of Eph/Ephrin activity interferes with optic vesicle evagination. Whole-mount in situ hybridisation in 10-12 ss wild-type zebrafish embryo (A), embryos injected with Eph/Ephrin morpholinos (B,C) or mRNA (D,F), and efnb2a mutant (E). The optic vesicles are labelled by expression of mab21/2. Arrows (B-F) indicate the presence of eye fated cells (labelled by mab21/2) embedded within the forebrain. All panels show dorsal views with anterior to the left. The phenotype shown in C is representative of 61% of the embryos analysed; the remaining 39% are not wild type but show a milder phenotype than that illustrated. The phenotype shown in F is representative for EphA4a and EphB4a misexpressions (epha4a: 70%, n=56; ephb4a: 100%, n=25). mb, midbrain; ov, optic vesicle. Scale bars: 50 μm.
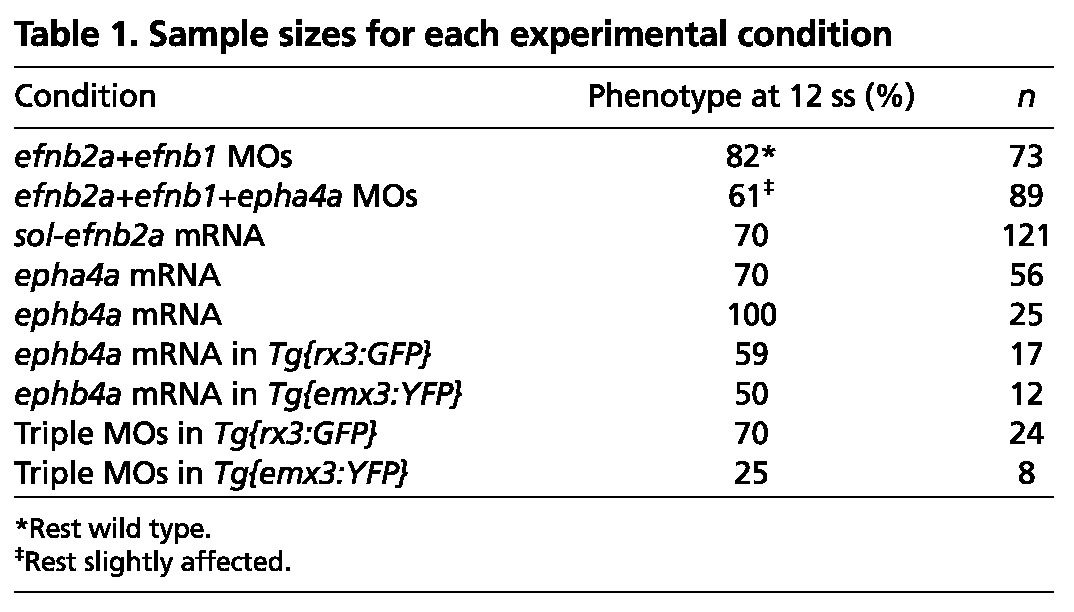
Table 1.
Sample sizes for each experimental condition
A truncated, soluble form of Efnb2a [sol-Efnb2a (Cooke et al., 2001)] competes with a wide array of endogenous Ephrin ligands to bind to Eph receptors, resulting in interference with forward and reverse Eph/Ephrin signalling. As with the morpholino injections, misexpression of sol-efnb2a led to delayed, asymmetric optic vesicle evagination and to the presence of cells expressing eye markers within the brain (Fig. 2D). We also analysed efnb2ahu3393 mutants, which bear a point mutation in position 258 of efnb2a, just prior to the transmembrane domain. This results in a truncated, soluble form of Efnb2a (J. Cayuso and E. Ober, personal communication) similar to that above, which is likely to show a dominant-negative effect. Consistent with this, efnb2ahu3393 mutants show a phenotype similar to that of efnb2a; efnb1 and efnb2a; efnb1; epha4a morphants (Fig. 2E). Finally, misexpression of either epha4a or ephb4a throughout the ANP leads to a phenotype that is more severe than, but qualitatively similar to, those obtained with the previous manipulations, in which the optic vesicles fail to evaginate and eye field cells remain embedded within the forming neural tube (Fig. 2F; data not shown).
Thus, the interference by various means with the level and or spatial localisation of Eph/Ephrin signalling pathway activity leads to defects in optic vesicle evagination. These manipulations do not disrupt the regionalisation of the ANP (supplementary material Fig. S1A-E), and thus the phenotypes are likely to be the result of interference with the morphogenesis of an appropriately regionalised ANP.
A cell affinity boundary at the edge of the eye field
Their complementary expression (Fig. 1) suggests that an interaction between Ephs and Ephrins occurs at the edge the eye field and this would be consistent with the presence of a cell affinity boundary at this location. Other morphogenetic processes associated with boundary formation, such as hindbrain and presomitic mesoderm segmentation in vertebrates, or compartment boundary formation during Drosophila segmentation and imaginal disc development, are associated with the accumulation of F-actin and phosphorylated light chain myosin II (Aliee et al., 2012; Barrios et al., 2003; Cooke et al., 2001; Landsberg et al., 2009; Monier et al., 2010). Indeed, at rhombomere and somite boundaries, the activity of the Eph/Ephrin pathway is upstream of the accumulation of F-actin and phosphorylated light chain myosin II (Cooke et al., 2001; Jülich et al., 2009; Watanabe et al., 2009), suggesting that the localisation of these molecules might be indicative of Eph/Ephrin activity.
Both F-actin and phosphorylated light chain myosin II are present around the margins of the zebrafish eye field (Fig. 3A-H). This localisation of activated actomyosin is accompanied by a concomitant accumulation of β-catenin (supplementary material Fig. S2), which is also seen at somite boundaries (Barrios et al., 2003). These observations support the notion of there being a physical boundary between domains in the ANP (Amack and Manning, 2012; Dahmann et al., 2011). In support of this, cells fated to become eye [labelled by rx3 or Tg{rx3:GFP} expression (Brown et al., 2010; Rembold et al., 2006a)] and telencephalon [labelled by Tg{emx3:YFP} expression (Viktorin et al., 2009)] abut at this region and form a defined interface of expression (Fig. 3I) that is maintained with little or no cell mixing as morphogenesis progresses (Fig. 3I-M, Fig. 4A,D; supplementary material Movie 1). Thus, both eye-fated and telencephalic-fated cells tend to respect the rx3/emx3 boundary throughout eye morphogenesis.
Fig. 3.
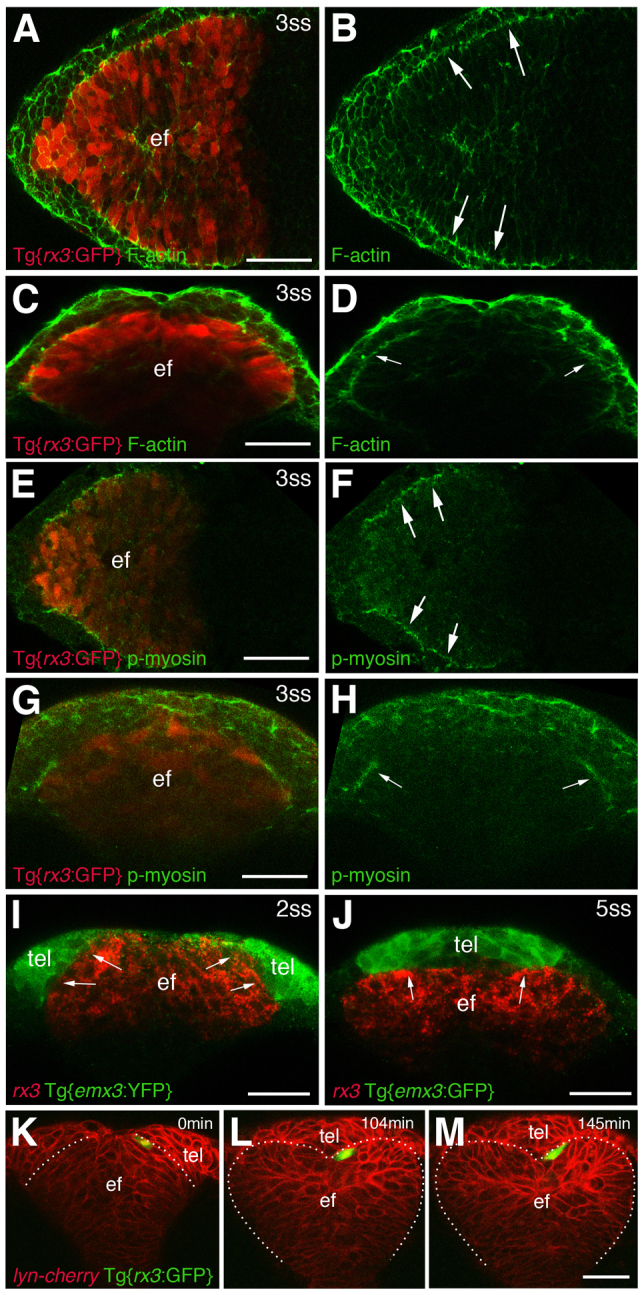
A region of actomyosin activation at the eye field/telencephalic border. (A-H) Whole-mount immunostainings for F-actin and phosphorylated light chain myosin II. Arrows (B,D,F,H) highlight the edge of the eye field. (I,J) Combined in situ hybridisation/immunostaining to detect expression of emx3 (emx3:YFP transgene, green) and rx3 (red). Arrows point at the interface between rx3 and emx3 expression. (K-M) Snapshots taken from supplementary material Movie 1, in which a Tg{rx3:GFP} cell (green) has been transplanted at the edge of the eye field, and its position relative to the boundary then followed over time. The green cell abuts and closely respects the boundary as morphogenesis progresses. The embryo has been counterlabelled with a membrane-tagged form of cherry (Lyn-cherry). All panels show frontal views, except (A,B,E,F) which show dorsal views. ef, eye field; tel, telencephalon. Scale bars: 50 μm.
Fig. 4.
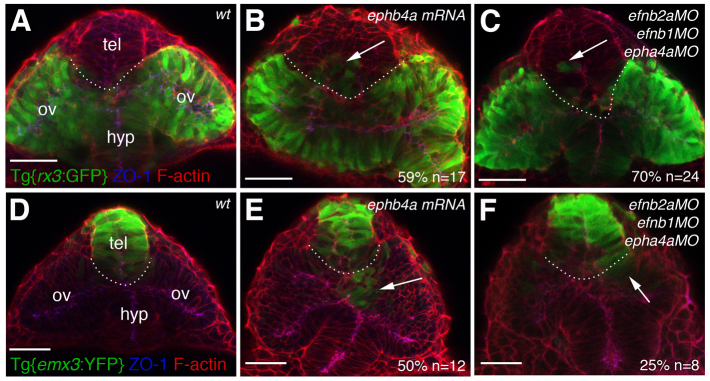
Manipulation of Eph/Ephrin activity leads to defective segregation of eye and telencephalic cells. Frontal views of the forebrain and eyes in Tg{rx3:GFP} (A-C) and Tg{emx3:YFP} (D-F) 10-12 ss wild-type, morphant or mRNA-injected zebrafish embryos immunostained to detect GFP (green), ZO-1 (blue) and F-actin (red). Arrows point at eye fated cells located in the telencephalic domain (B,C) or at telencephalic fated cells located in the optic vesicles (E,F). The dotted lines demarcate the transition between telencephalon and eye. ov, optic vesicle; tel, telencephalon; hyp, hypothalamus. Scale bars: 50 μm.
Manipulation of Eph/Ephrin signalling results in ANP cells failing to respect the eye/telencephalic boundary. To follow the fate of ANP cells after manipulation of Eph/Ephrin signalling, we performed some of the manipulations described above in Tg{rx3:GFP} and Tg{emx3:YFP} transgenic fish, which allowed us to unequivocally identify eye field and telencephalic fates, respectively. Misexpression of ephb4a in these transgenic backgrounds confirms that, in the absence of an Eph/Ephrin interface, groups of eye-fated cells are found in the telencephalic domain (Fig. 4B, compare with wild type in 4A). Conversely, groups of telencephalon-fated cells are found in the optic vesicles (Fig. 4E, compare with wild type in 4D). A similar, but milder, phenotype is seen in efnb2a; efnb1; ephb4a morphants (Fig. 4C,F).
Eph/Ephrin expression controls the segregation properties of ANP cells
In the hindbrain, Eph- and Ephrin-expressing cells segregate from each other and generate an expression interface (Cooke et al., 2005; Xu et al., 1999). To assess whether ANP cells show more affinity toward cells expressing a similar combination of Eph/Ephrins, we transplanted cells ectopically expressing Eph or Ephrins within the ANP, and assessed their ability to integrate in the eye field. We performed the transplantation at early to mid-gastrulation, prior to ANP regionalisation. In these conditions control transplants labelled by GFP always showed a widespread distribution throughout domains in the ANP (100%, n=39; Table 2, Fig. 5A,B).
Table 2.
Sample sizes in transplantation experiments
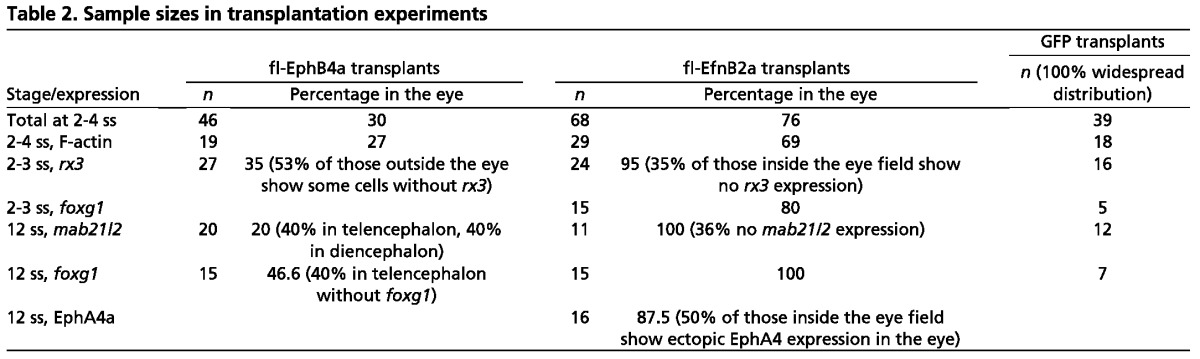
Fig. 5.
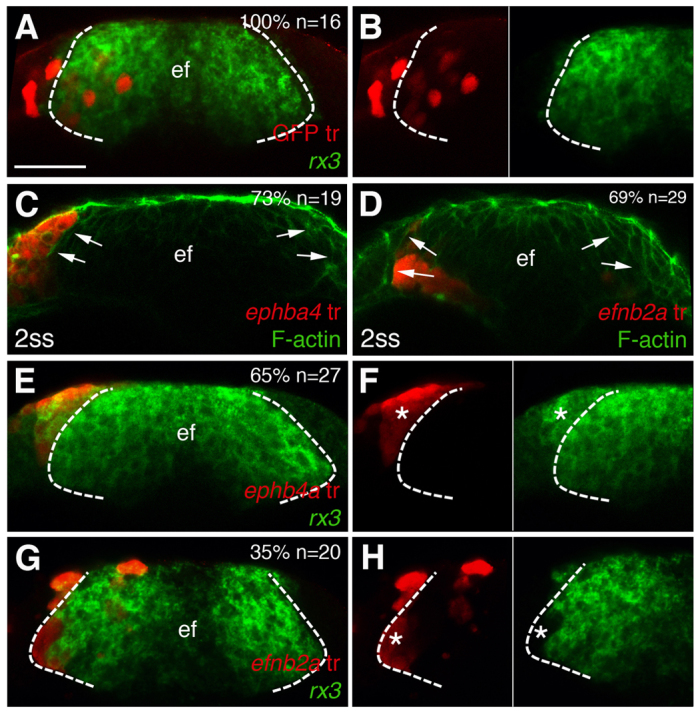
Eph/Ephrin expression influences the segregation of cells between ANP domains. (A-H) Frontal views through the forebrain showing transplants of cells expressing GFP (A,B), ephb4a (C,E,F) or efnb2a (D,G,H) at 1-2 ss, treated to detect rx3 expression in the eye field (A,B,E-H) or accumulation of F-actin at the eye/telencephalic boundary (C,D). Dashed lines (A,B,E-H) demarcate the eye field (ef); arrows (C,D) point at the boundary of the eye. Asterisks (F,H) highlight the transplanted cells. F and H show details from panels E and G, respectively. Scale bars: 50 μm.
Most ephb4a-expressing transplants examined segregated out of the eye field (70%, n=46), often abutting the boundary delineated by F-actin accumulation at the edge of the eye field (Fig. 5C). Some of the transplants analysed remained as very tight clumps within the eye field, potentially minimising their contacts with the surrounding tissue (supplementary material Fig. S3A-A′). Conversely, most efnb2a-expressing transplants examined (76%, n=68) were located within the eye field. Those that were located close to the edge of the eye field abutted the F-actin boundary but never crossed over into the adjacent telencephalic territory (Fig. 5D).
Upon Eph/Ephrin misexpression, transplanted ANP cells tend to segregate into the domain that usually expresses the corresponding Eph or Ephrin, independently of their originally specified regional identity. Indeed, ephb4a-expressing transplants located in the telencephalon and abutting the edge of the eye field maintain expression of eye field markers in at least some of the cells of the transplant (Fig. 5E,F), while some efnb2a-expressing transplants located in the eye field do not show expression of eye field markers (Fig. 5G,H). Thus, the forebrain domain to which ANP cells contribute seems to be determined by the Eph/Ephrins that they express, regardless of their specified identity (as eye or telencephalon): Eph-expressing cells contribute to the telencephalon, even if they express markers of an eye fate, whereas Ephrin-expressing cells contribute to the eye field. Transplanted cells with a mismatch between Eph/Ephrin and regional identity are unable to fully integrate in their new environment. At later developmental stages, ephb4a-expressing transplants located in the telencephalon clump together, express the eye marker mab21/2 (Fig. 6B,B′, compare with the control transplant in 6A,A′) and do not acquire expression of the telencephalic marker foxg1 (supplementary material Fig. S3B-B′). Conversely, efnb2a-expressing cells of likely prospective telencephalic origin located at the edges of the optic vesicles do not acquire expression of mab21/2 (Fig. 6C,C′). Such cells do not seem to express foxg1 (supplementary material Fig. S3C-C′), but do show expression of epha4a, which is usually absent from the eye field (supplementary material Fig. S3D-D′).
Fig. 6.
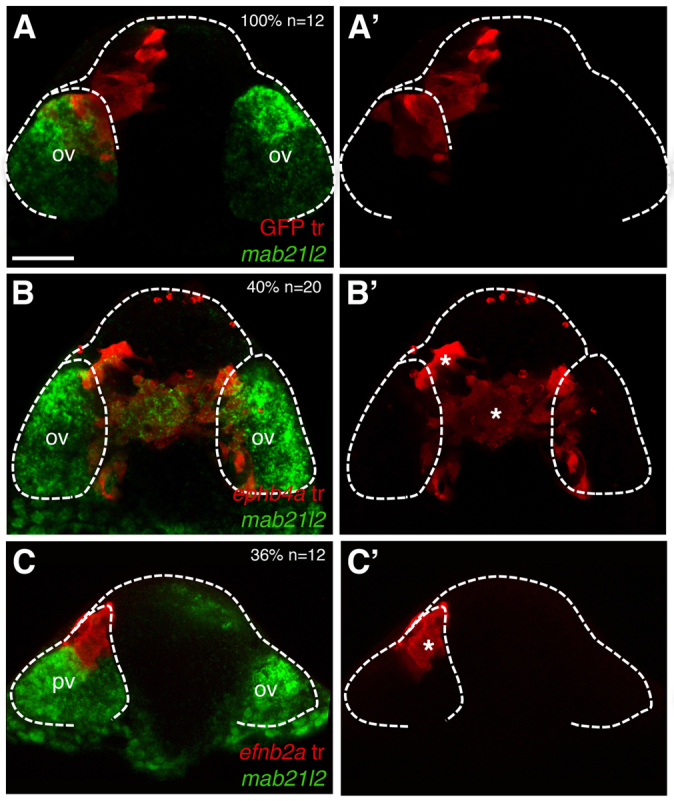
Cell segregation behaviour is independent of cell fate. Frontal views through the forebrain of transplants of cells expressing GFP (A,A′), ephb4a (B,B′) or efnb2a (C,C′) at 10-12 ss, subject to in situ hybridisation to detect mab21/2 in the optic vesicles (ov, green). Dashed lines demarcate the head and the optic vesicles. Asterisks (B′,C′) highlight the transplanted cells. Scale bars: 50 μm.
These results indicate that ANP cells have a specific ‘adhesion code’, according to their regional specification, that leads to the segregation of cells with different prospective fates. Regionalisation of the ANP is initiated during late gastrulation (reviewed by Wilson and Houart, 2004) and so we hypothesised that it would not be until this stage that Eph/Ephrin signalling would lead to regional segregation of cell populations. To determine whether this is the case, we examined the behaviour of ephb4a-expressing cells in the ANP during late gastrulation.
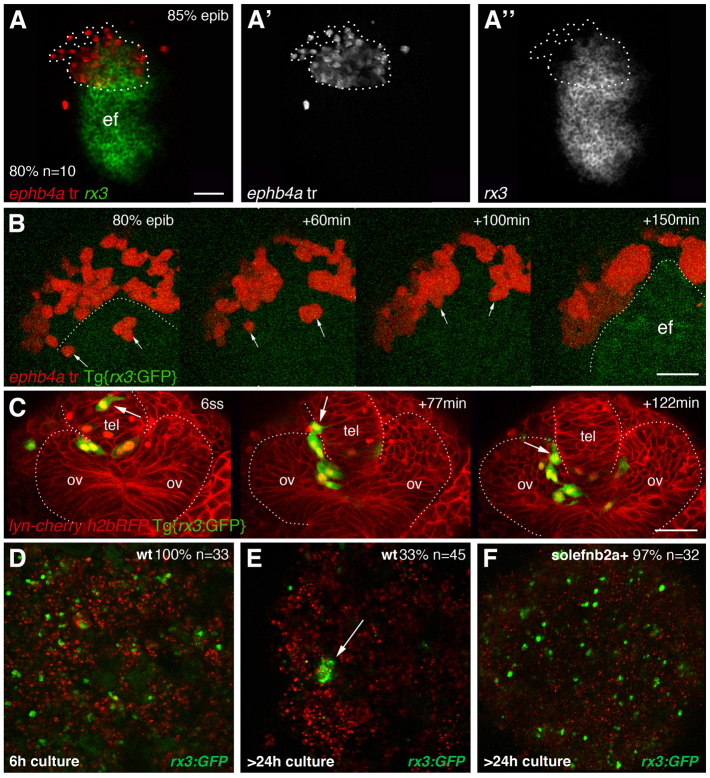
By early somitogenesis, most Eph-expressing cells are located in the prospective telencephalic territory (see above), but at 85% epiboly they straddle the telencephalon/eye field boundary (Fig. 7A-A′), indicating that segregation occurs subsequent to this stage. By following individual and small groups of ephb4a-expressing transplanted cells in high-resolution 4D time-lapse movies, we observed that they actively moved out of the eye field during late gastrulation to coalesce with prospective telencephalic cells adjacent to the rx3:GFP+ domain (Fig. 7B; supplementary material Movie 2; seven movies were analysed). These results indicate that the behaviour of cells changes concomitant with their acquisition of regional identity, resulting in the establishment of discrete morphogenetic domains within the neural plate.
Fig. 7.
Eye field cells actively segregate from cells in surrounding tissues. (A-A′) Dorsal view of the ANP with anterior to the left, showing a transplant of cells expressing ephb4a (red) straddling the eye/telencephalon domain at 80/90% epiboly. (B) Snapshots of the ANP with anterior to the top taken from supplementary material Movie 2, showing ephb4a+ cells (red, arrows) as they move out from the prospective eye field [labelled by GFP (green) and outlined in the last frame]. (C) Snapshots of the evaginating optic vesicles from a frontal view taken from supplementary material Movie 3, showing an rx3:GFP+ cell mislocated in the telencephalon (arrow) as it relocates into the optic vesicle. The host embryo has been counterlabelled by a membrane-tagged form of cherry (red) and the transplanted cells have their nuclei labelled by H2bRFP (red). (D-F) Confocal images of hanging drop cultures of cells obtained from the dissociation of Tg{rx3:GFP} tailbud stage embryos, cultured for 6 hours (D) or more than 24 hours (E,F). Eye field cells from non-manipulated embryos aggregated in small clusters after long culture times (E, arrow), whereas overexpression of sol-efnb2a results in virtually no embryos forming eye field cell aggregates (F). ef, eye field; ov, optic vesicle. Scale bars: 50 μm.
During normal development, there is far less, if any, mixing of cells with different Eph/Ephrin expression profiles and so active movement of cells between domains is less evident. Nevertheless, from examining many movies, we did detect four rx3:GFP+ eye field cells inappropriately positioned within the prospective telencephalon that subsequently migrated into the evaginating optic vesicles (Fig. 7C; supplementary material Movie 3).
As a further assay of the potential differential cohesion/adhesion properties of eye field versus other neural plate cells, we performed hanging drop assays with cells derived from 100% epiboly stage Tg{rx3:GFP} transgenic embryos. In these assays, eye field cells (GFP+) generated small clumps in a subset of the hanging drops, suggesting they had the ability to segregate from surrounding (GFP-) cells after 24 hours (Fig. 7E). From the 45 cultures analysed, 33 were checked after only 6 hours of culture and none showed clumps of GFP+ cells (Fig. 7D), again suggesting that this segregation is an active process. Interference with Eph/Ephrin activity by injection of sol-efnb2a mRNA resulted in only one of the 32 cultures analysed showing GFP+ aggregates of cells, suggesting that Eph/Ephrin activity is required for this segregation to occur (Fig. 7F).
DISCUSSION
This study shows that Eph/Ephrin signalling maintains the segregation between adjacent forebrain territories during morphogenesis. Ephrin and Eph expression is initiated in complementary ANP domains during late gastrulation as a consequence of regional fate acquisition. Subsequently, Eph-Ephrin interaction between cells at domain interfaces results in the generation of boundaries that prevent the cell mixing that would otherwise be likely to occur during the massive cell movements and rearrangements that accompany forebrain development.
Eph/Ephrin activity promotes segregation of eye field cells
Our results show that compromised Eph/Ephrin activity perturbs ANP morphogenesis, and, as a consequence, optic vesicle evagination from the lateral walls of the forebrain is compromised. The severity of the phenotypes that we and others (Xu et al., 1996) have found varies, most likely being dependent upon the extent to which Eph/Ephrin signalling is abrogated. Ephs and Ephrins show considerable functional redundancy (Pasquale, 2008) and, as several are expressed in overlapping patterns in the ANP, there is likely to be involvement of multiple Eph/Ephrins in segregating the eye field from adjacent territories. In vitro and in vivo analyses of the binding preferences of Ephs and Ephrins suggest that all combinations of signalling are possible at the margins of the eye field. There are both type A and type B Ephrins and Ephs expressed at this boundary and thus A-to-A and B-to-B signalling is possible. In addition, Epha4a functionally interacts with Efnb2a and Efnb1 in other contexts (Cooke et al., 2005; Durbin et al., 1998; Miao and Wang, 2009), and the complementary expression of epha2 and efnb1 in some tissues has led to the suggestion that this Eph/Ephrin pair might also be functionally relevant (Miao and Wang, 2009). Recently, it has been shown that the interaction of a given Eph with an Ephrin ligand leads to the formation of multimeric complexes, in which other co-expressed Ephs can participate and become activated, even if they do not directly interact with the Ephrin ligand that promotes complex formation (Janes et al., 2011). Thus, the presence of at least one functional Eph/Ephrin pair at the interface might be enough to lead to the activation of all the Eph receptors that are co-expressed in a given cell, and thus it is likely that activation of all the receptors and ligands expressed at the edge of the eye field can occur.
Misexpression of full-length forms of either a receptor (epha4a/ephb4a) or a ligand (efnb2a) in small groups of cells in the ANP led to the segregation of expressing cells into the domain that matched their Eph/Ephrin expression profile. This behaviour is independent of the original regional identity of the cells. Indeed, we find cells expressing eye field markers segregating into the telencephalon and vice versa. Segregation of transplanted cells occurs by the movement of cells between domains during late gastrulation.
The movement of cells into the domains that match their Eph/Ephrin expression profile can also be observed under normal conditions, as we occasionally observed single eye field cells that were mislocated in the telencephalon relocating into the forming optic vesicles by active cell movement. These results strongly suggest that the segregation of ANP cells into discrete domains is an active process and occurs once the regional fate of the different ANP domains has been established. They further show that, rather than their regional identity, it is the Eph/Ephrin component(s) expressed by a cell that determines the domain into which the cell is accommodated.
Since the complementary expression of Ephrins and Ephs in the ANP is established after regional identity is specified it is unlikely that, in a wild-type situation, cells will extensively move between domains. Rather, we suggest that Eph/Ephrin activity prevents cell mixing between domains once regional identity is established. Previous studies have proposed different models in which Eph/Ephrin activity in the forebrain promotes specific regional identities, and thus would be upstream, and not downstream, of regional fate acquisition. For example, Xu et al. (Xu et al., 1996) proposed that, upon interference with signalling by overexpressing a dominant-negative form of Epha4a, a subset of diencephalic cells was transformed into retinal cells. Moore et al. (Moore et al., 2004) proposed a role for efnb1 in the specification and morphogenesis of the eye field in Xenopus. In their studies, misexpression of efnb1 in one blastomere of the 8-cell stage embryo led to the progeny of that blastomere preferentially populating the retina, and they proposed that efnb1 promotes eye fate by directing the migration of eye field cells to the correct region of the neural plate to become eye (Moore et al., 2004; Lee et al., 2006). Their results and ours are similar in outcome, as we show that the misexpression of efnb2a in small groups of cells leads them to segregate into the eye field, as occurs with efnb1-expressing cells in Xenopus. However, some transplants of Efnb2a+ cells within the eye field do not express eye field markers, showing that they do not always have eye field identity and indicating that the effect of Ephrins on the position of ANP cells is downstream, or independent, of regional fate allocation. Since Moore et al. (Moore et al., 2004) only assessed the position of efnb-expressing cells in the neural plate without assessing their expression of eye field markers, it is unclear whether this is also the case in Xenopus. In summary, despite previous studies proposing a role for the Eph/Ephrin signalling pathway upstream of ANP regional fates, we find that Ephrin signalling is downstream of regional fate allocation.
The role of boundaries of Eph/Ephrin expression in tissue segregation is well established in a wide variety of other experimental contexts and tissues (Barrios et al., 2003; Cooke et al., 2001; Cooke et al., 2005; Durbin et al., 1998; Park et al., 2011; Rohani et al., 2011; Watanabe et al., 2009; Xu et al., 1999). During hindbrain segmentation, for example, Ephs and Ephrins show complementary patterns of expression in alternating rhombomeres, and transplantation approaches showed cell sorting behaviour comparable to that which we describe in the ANP (Cooke et al., 2001; Cooke et al., 2005). In this context, expression of Ephs and Ephrins has been proposed to be controlled downstream of the establishment of rhombomere identity (Cooke et al., 2001; Theil et al., 1998). This is also the case during somite formation (Durbin et al., 2000). Our study thus reinforces the widespread role for Eph/Ephrin signalling in promoting tissue segregation downstream of fate acquisition.
A boundary of Eph/Ephrin activity at the edge of the eye field
Our observations suggest that the Eph/Ephrin pathway is activated at the interface between the eye field and adjacent ANP domains. First, Eph receptors and their ligands are expressed in largely complementary patterns that delineate the ANP domains; thus, Eph to Ephrin interaction can only occur at these interfaces of expression. Second, activated actomyosin accumulates at the interfaces of Eph/Ephrin expression and outlines the margins of the eye field in a manner similar to that which occurs at the interface between Eph-expressing and Ephrin-expressing cells during somite boundary formation and hindbrain segmentation (Cooke et al., 2001; Jülich et al., 2009; Watanabe et al., 2009).
The enrichment of actomyosin cables at boundaries between domains is a feature widely found during development in a variety of tissues. Not only is it present at the interface between rhombomeres and somites, but also at compartmental boundaries in Drosophila embryos and imaginal discs (Aliee et al., 2012; Dahmann et al., 2011; Landsberg et al., 2009; Monier et al., 2010). In all these tissues, the mechanical tension exerted by activated actomyosin maintains cell compartmentalisation. It is thus tempting to speculate that Eph/Ephrin signalling maintains segregation of ANP domains by the modulation of actomyosin activity at the edges of the eye field.
Cell repulsion or cell adhesion during ANP morphogenesis?
Even though our observations indicate that the activity of the Eph/Ephrin signalling pathway in the ANP prevents cell-cell mixing at the edges between ANP domains, Ephrin ligands might also have a cell-cell adhesion role within the eye field. Indeed, Eph-Ephrin interactions have been shown to result in repulsive or adhesive effects depending on the level of activation and the cellular context of the receptor-ligand interaction (reviewed by Janes et al., 2012; Lackmann and Boyd, 2008; Arvanitis et al., 2013). However, since we have not detected expression of any Eph in the eye field, a putative function of efnb1 and efnb2a in promoting cell adhesion might need to be independent of Ephs. Ephrins are proposed to interact functionally with, and become activated by, other signalling systems (Arvanitis and Davy, 2008; Kullander and Klein, 2002). Indeed, a functional interaction between the Fgf signalling pathway and Efnb1 seems to occur at the anterior edge of the eye field in Xenopus, leading to increased cell adhesion (Moore et al., 2004). Efnb1 control of cell adhesion during eye field formation has been shown to require its physical interaction with Dishevelled (Tanaka et al., 2003; Lee et al., 2006) and the activation of a molecular cascade in which many components are shared with the non-canonical Wnt signalling pathway (Lee et al., 2006). We have previously shown that the non-canonical Wnt signalling pathway modulates cell cohesion downstream of Wnt11/Frizzled 5 in the zebrafish eye field (Cavodeassi et al., 2005), suggesting that Ephrin molecules and Wnt11 might functionally interact to control cell adhesion/cohesion in the eye field (Cavodeassi et al., 2005; Lee et al., 2006).
Interaction of Ephrins with other morphogenetic genes in the eye field
Previous studies have suggested roles for Nlcam and Cxcr4 during the morphogenesis of the eye field. These molecules have been proposed to control either the migratory behaviour of eye versus telencephalon cells [Nlcam (Brown et al., 2010)] or the cohesion of eye field cells [Cxcr4 (Bielen and Houart, 2012)]. We show here that the Eph/Ephrin signalling pathway is also a crucial modulator of cell behaviour during ANP morphogenesis, in this case by maintaining the segregation of ANP domains during neural plate morphogenesis.
Nlcam, Cxcr4 and Eph/Ephrins are likely to function downstream of regional fate allocation in the ANP, as their expression seems to be controlled, directly or indirectly, by Rx3, one of the key determinants of eye fate. In the absence of Rx3 function, nlcam, cxcr4 and epha4a/b4a expression is perturbed (this work) (Bielen and Houart, 2012; Brown et al., 2010). It is thus tempting to propose a scenario in which the eye field specification genes control the expression of a battery of morphogenetic regulators, the combinatorial activity of which regulates the behaviour of eye cells (cell migration, cohesion and maintenance of segregation). For example, both Eph/Ephrins and Cxcr4 can modulate cytoskeletal dynamics by controlling the activity of RhoGTPases (Kardash et al., 2010; Kullander and Klein, 2002; Salvucci et al., 2006; Sharfe et al., 2002), and interact with components of the cell-cell and cell-matrix adhesion machinery (Nair and Schilling, 2008; Raz and Mahabaleshwar, 2009). This suggests that functional interactions might exist between these two pathways in those tissues in which they are co-activated (Arvanitis and Davy, 2008; Janes et al., 2012).
Supplementary Material
Acknowledgments
We thank our colleagues in the S.W.W. and P. Bovolenta labs for stimulating discussions and feedback, and Martina Rembold and Jochen Wittbrodt for sharing the Tg{rx3:GFP} fish line with us prior to publication. We are very grateful to Jordi Cayuso and Elke Ober, who provided us with efnb2a mutant embryos and designed the genotyping assay used to identify them, and to Giuseppe Lupo and Gaia Gestri for the efnb1 morpholino. We thank the fish and confocal microscopy facilities at UCL and at the CBMSO for their technical support.
Footnotes
Funding
This work was supported by a Fellowship from the Medical Research Council (MRC)-LMCB PhD Program (to K.I.), and grants from the MRC (to S.W.W., F.C. and G. Gestri), Biotechnology and Biological Sciences Research Council and Welcome Trust (to S.W.W.), the European Community [Zeymorph PCIG11-GA-2012-321788 to F.C.] and the Spanish Government [BFU2011-24701 to F.C.]. F.C. holds a Tenure Track Position from the Spanish Research Council [CSIC, RYC-2010-05656]. Deposited in PMC for immediate release.
Competing interests statement
The authors declare no competing financial interests.
Author contributions
F.C. and S.W.W. concieved and designed the study; K.I. and F.C. performed the experiments; all authors contributed to the interpretation of the results and the writing of the manuscript.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.097048/-/DC1
References
- Aliee M., Röper J. C., Landsberg K. P., Pentzold C., Widmann T. J., Jülicher F., Dahmann C. (2012). Physical mechanisms shaping the Drosophila dorsoventral compartment boundary. Curr. Biol. 22, 967–976 [DOI] [PubMed] [Google Scholar]
- Amack J. D., Manning M. L. (2012). Knowing the boundaries: extending the differential adhesion hypothesis in embryonic cell sorting. Science 338, 212–215 [DOI] [PubMed] [Google Scholar]
- Arvanitis D., Davy A. (2008). Eph/ephrin signaling: networks. Genes Dev. 22, 416–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitis D. N., Béhar A., Tryoen-Tóth P., Bush J. O., Jungas T. H., Vitale N., Davy A. (2013). Ephrin B1 maintains apical adhesion of neural progenitors. Development 140, 2082–2092 [DOI] [PubMed] [Google Scholar]
- Bailey T. J., El-Hodiri H., Zhang L., Shah R., Mathers P. H., Jamrich M. (2004). Regulation of vertebrate eye development by Rx genes. Int. J. Dev. Biol. 48, 761–770 [DOI] [PubMed] [Google Scholar]
- Barrios A., Poole R. J., Durbin L., Brennan C., Holder N., Wilson S. W. (2003). Eph/Ephrin signaling regulates the mesenchymal-to-epithelial transition of the paraxial mesoderm during somite morphogenesis. Curr. Biol. 13, 1571–1582 [DOI] [PubMed] [Google Scholar]
- Beccari L., Marco-Ferreres R., Bovolenta P. (2013). The logic of gene regulatory networks in early vertebrate forebrain patterning. Mech. Dev. 130, 95–111 [DOI] [PubMed] [Google Scholar]
- Bielen H., Houart C. (2012). BMP signaling protects telencephalic fate by repressing eye identity and its Cxcr4-dependent morphogenesis. Dev. Cell 23, 812–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. E., Keller P. J., Ramialison M., Rembold M., Stelzer E. H., Loosli F., Wittbrodt J. (2010). Nlcam modulates midline convergence during anterior neural plate morphogenesis. Dev. Biol. 339, 14–25 [DOI] [PubMed] [Google Scholar]
- Cavodeassi F., Houart C. (2012). Brain regionalization: of signaling centers and boundaries. Dev. Neurobiol. 72, 218–233 [DOI] [PubMed] [Google Scholar]
- Cavodeassi F., Carreira-Barbosa F., Young R. M., Concha M. L., Allende M. L., Houart C., Tada M., Wilson S. W. (2005). Early stages of zebrafish eye formation require the coordinated activity of Wnt11, Fz5, and the Wnt/beta-catenin pathway. Neuron 47, 43–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerveny K. L., Cavodeassi F., Turner K. J., de Jong-Curtain T. A., Heath J. K., Wilson S. W. (2010). The zebrafish flotte lotte mutant reveals that the local retinal environment promotes the differentiation of proliferating precursors emerging from their stem cell niche. Development 137, 2107–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. E., Xu Q., Wilson S. W., Holder N. (1997). Characterisation of five novel zebrafish Eph-related receptor tyrosine kinases suggests roles in patterning the neural plate. Dev. Genes Evol. 206, 515–531 [DOI] [PubMed] [Google Scholar]
- Cooke J., Moens C., Roth L., Durbin L., Shiomi K., Brennan C., Kimmel C., Wilson S., Holder N. (2001). Eph signalling functions downstream of Val to regulate cell sorting and boundary formation in the caudal hindbrain. Development 128, 571–580 [DOI] [PubMed] [Google Scholar]
- Cooke J. E., Kemp H. A., Moens C. B. (2005). EphA4 is required for cell adhesion and rhombomere-boundary formation in the zebrafish. Curr. Biol. 15, 536–542 [DOI] [PubMed] [Google Scholar]
- Dahmann C., Oates A. C., Brand M. (2011). Boundary formation and maintenance in tissue development. Nat. Rev. Genet. 12, 43–55 [DOI] [PubMed] [Google Scholar]
- Durbin L., Brennan C., Shiomi K., Cooke J., Barrios A., Shanmugalingam S., Guthrie B., Lindberg R., Holder N. (1998). Eph signaling is required for segmentation and differentiation of the somites. Genes Dev. 12, 3096–3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin L., Sordino P., Barrios A., Gering M., Thisse C., Thisse B., Brennan C., Green A., Wilson S., Holder N. (2000). Anteroposterior patterning is required within segments for somite boundary formation in developing zebrafish. Development 127, 1703–1713 [DOI] [PubMed] [Google Scholar]
- Janes P. W., Griesshaber B., Atapattu L., Nievergall E., Hii L. L., Mensinga A., Chheang C., Day B. W., Boyd A. W., Bastiaens P. I., et al. (2011). Eph receptor function is modulated by heterooligomerization of A and B type Eph receptors. J. Cell Biol. 195, 1033–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes P. W., Nievergall E., Lackmann M. (2012). Concepts and consequences of Eph receptor clustering. Semin. Cell Dev. Biol. 23, 43–50 [DOI] [PubMed] [Google Scholar]
- Jülich D., Hwee Lim C., Round J., Nicolaije C., Schroeder J., Davies A., Geisler R., Lewis J., Jiang Y. J., Holley S. A.; Tübingen 2000 Screen Consortium (2005). beamter/deltaC and the role of Notch ligands in the zebrafish somite segmentation, hindbrain neurogenesis and hypochord differentiation. Dev. Biol. 286, 391–404 [DOI] [PubMed] [Google Scholar]
- Jülich D., Mould A. P., Koper E., Holley S. A. (2009). Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Development 136, 2913–2921 [DOI] [PubMed] [Google Scholar]
- Kai M., Heisenberg C. P., Tada M. (2008). Sphingosine-1-phosphate receptors regulate individual cell behaviours underlying the directed migration of prechordal plate progenitor cells during zebrafish gastrulation. Development 135, 3043–3051 [DOI] [PubMed] [Google Scholar]
- Kardash E., Reichman-Fried M., Maitre J. L., Boldajipour B., Papusheva E., Messerschmidt E. M., Heisenberg C. P., Raz E. (2010). A role for Rho GTPases and cell-cell adhesion in single-cell motility in vivo. Nat. Cell Biol. 12, 47–53 [DOI] [PubMed] [Google Scholar]
- Kiecker C., Lumsden A. (2012). The role of organizers in patterning the nervous system. Annu. Rev. Neurosci. 35, 347–367 [DOI] [PubMed] [Google Scholar]
- Kullander K., Klein R. (2002). Mechanisms and functions of Eph and ephrin signalling. Nat. Rev. Mol. Cell Biol. 3, 475–486 [DOI] [PubMed] [Google Scholar]
- Lackmann M., Boyd A. W. (2008). Eph, a protein family coming of age: more confusion, insight, or complexity? Sci. Signal. 1, re2 [DOI] [PubMed] [Google Scholar]
- Landsberg K. P., Farhadifar R., Ranft J., Umetsu D., Widmann T. J., Bittig T., Said A., Jülicher F., Dahmann C. (2009). Increased cell bond tension governs cell sorting at the Drosophila anteroposterior compartment boundary. Curr. Biol. 19, 1950–1955 [DOI] [PubMed] [Google Scholar]
- Lee H. S., Bong Y. S., Moore K. B., Soria K., Moody S. A., Daar I. O. (2006). Dishevelled mediates ephrinB1 signalling in the eye field through the planar cell polarity pathway. Nat. Cell Biol. 8, 55–63 [DOI] [PubMed] [Google Scholar]
- Loosli F., Staub W., Finger-Baier K. C., Ober E. A., Verkade H., Wittbrodt J., Baier H. (2003). Loss of eyes in zebrafish caused by mutation of chokh/rx3. EMBO Rep. 4, 894–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H., Wang B. (2009). Eph/ephrin signaling in epithelial development and homeostasis. Int. J. Biochem. Cell Biol. 41, 762–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier B., Pelissier-Monier A., Brand A. H., Sanson B. (2010). An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat. Cell Biol. 12, 60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. B., Mood K., Daar I. O., Moody S. A. (2004). Morphogenetic movements underlying eye field formation require interactions between the FGF and ephrinB1 signaling pathways. Dev. Cell 6, 55–67 [DOI] [PubMed] [Google Scholar]
- Nair S., Schilling T. F. (2008). Chemokine signaling controls endodermal migration during zebrafish gastrulation. Science 322, 89–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff M. M., Neff J. D., Chory J., Pepper A. E. (1998). dCAPS, a simple technique for the genetic analysis of single nucleotide polymorphisms: experimental applications in Arabidopsis thaliana genetics. Plant J. 14, 387–392 [DOI] [PubMed] [Google Scholar]
- Park E. C., Cho G. S., Kim G. H., Choi S. C., Han J. K. (2011). The involvement of Eph-Ephrin signaling in tissue separation and convergence during Xenopus gastrulation movements. Dev. Biol. 350, 441–450 [DOI] [PubMed] [Google Scholar]
- Pasini A., Wilkinson D. G. (2002). Stabilizing the regionalisation of the developing vertebrate central nervous system. BioEssays 24, 427–438 [DOI] [PubMed] [Google Scholar]
- Pasquale E. B. (2008). Eph-ephrin bidirectional signaling in physiology and disease. Cell 133, 38–52 [DOI] [PubMed] [Google Scholar]
- Raz E., Mahabaleshwar H. (2009). Chemokine signaling in embryonic cell migration: a fisheye view. Development 136, 1223–1229 [DOI] [PubMed] [Google Scholar]
- Rembold M., Lahiri K., Foulkes N. S., Wittbrodt J. (2006a). Transgenesis in fish: efficient selection of transgenic fish by co-injection with a fluorescent reporter construct. Nat. Protoc. 1, 1133–1139 [DOI] [PubMed] [Google Scholar]
- Rembold M., Loosli F., Adams R. J., Wittbrodt J. (2006b). Individual cell migration serves as the driving force for optic vesicle evagination. Science 313, 1130–1134 [DOI] [PubMed] [Google Scholar]
- Rohani N., Canty L., Luu O., Fagotto F., Winklbauer R. (2011). EphrinB/EphB signaling controls embryonic germ layer separation by contact-induced cell detachment. PLoS Biol. 9, e1000597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci O., de la Luz Sierra M., Martina J. A., McCormick P. J., Tosato G. (2006). EphB2 and EphB4 receptors forward signaling promotes SDF-1-induced endothelial cell chemotaxis and branching remodeling. Blood 108, 2914–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharfe N., Freywald A., Toro A., Dadi H., Roifman C. (2002). Ephrin stimulation modulates T cell chemotaxis. Eur. J. Immunol. 32, 3745–3755 [DOI] [PubMed] [Google Scholar]
- Stigloher C., Ninkovic J., Laplante M., Geling A., Tannhäuser B., Topp S., Kikuta H., Becker T. S., Houart C., Bally-Cuif L. (2006). Segregation of telencephalic and eye-field identities inside the zebrafish forebrain territory is controlled by Rx3. Development 133, 2925–2935 [DOI] [PubMed] [Google Scholar]
- Tanaka M., Kamo T., Ota S., Sugimura H. (2003). Association of Dishevelled with Eph tyrosine kinase receptor and ephrin mediates cell repulsion. EMBO J. 22, 847–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil T., Frain M., Gilardi-Hebenstreit P., Flenniken A., Charnay P., Wilkinson D. G. (1998). Segmental expression of the EphA4 (Sek-1) receptor tyrosine kinase in the hindbrain is under direct transcriptional control of Krox-20. Development 125, 443–452 [DOI] [PubMed] [Google Scholar]
- Thisse C., Thisse B. (2005). High throughput expression analysis of zf-models consortium clones. ZFIN direct data submission. http://zfin.org
- Thisse B., Wright G. J., Thisse C. (2008). Embryonic and larval expression patterns from a large scale screening for novel low affinity extracellular protein interactions. ZFIN direct data submission. http://zfin.org [DOI] [PMC free article] [PubMed]
- Viktorin G., Chiuchitu C., Rissler M., Varga Z. M., Westerfield M. (2009). Emx3 is required for the differentiation of dorsal telencephalic neurons. Dev. Dyn. 238, 1984–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Sato Y., Saito D., Tadokoro R., Takahashi Y. (2009). EphrinB2 coordinates the formation of a morphological boundary and cell epithelialization during somite segmentation. Proc. Natl. Acad. Sci. USA 106, 7467–7472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. (1993). The Zebrafish Book: a Guide for the Laboratory Use of the Zebrafish (Brachydanio rerio). Eugene, OR: University of Oregon Press; [Google Scholar]
- Wilson S. W., Houart C. (2004). Early steps in the development of the forebrain. Dev. Cell 6, 167–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler S., Loosli F., Henrich T., Wakamatsu Y., Wittbrodt J. (2000). The conditional medaka mutation eyeless uncouples patterning and morphogenesis of the eye. Development 127, 1911–1919 [DOI] [PubMed] [Google Scholar]
- Xu Q., Holder N., Patient R., Wilson S. W. (1994). Spatially regulated expression of three receptor tyrosine kinase genes during gastrulation in the zebrafish. Development 120, 287–299 [DOI] [PubMed] [Google Scholar]
- Xu Q., Alldus G., Macdonald R., Wilkinson D. G., Holder N. (1996). Function of the Eph-related kinase rtk1 in patterning of the zebrafish forebrain. Nature 381, 319–322 [DOI] [PubMed] [Google Scholar]
- Xu Q., Mellitzer G., Robinson V., Wilkinson D. G. (1999). In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature 399, 267–271 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.