Abstract
High refractive index and transparency of the eye lens require uniformly shaped and precisely aligned lens fiber cells. During lens development, equatorial epithelial cells undergo cell-to-cell alignment to form meridional rows of hexagonal cells. The mechanism that controls this morphogenesis from randomly packed cuboidal epithelial cells to highly organized hexagonal fiber cells remains unknown. In Epha2-/- mouse lenses, equatorial epithelial cells fail to form precisely aligned meridional rows; moreover, the lens fulcrum, where the apical tips of elongating epithelial cells constrict to form an anchor point before fiber cell differentiation and elongation at the equator, is disrupted. Phosphorylated Src-Y424 and cortactin-Y466, actin and EphA2 cluster at the vertices of wild-type hexagonal epithelial cells in organized meridional rows. However, phosphorylated Src and phosphorylated cortactin are not detected in disorganized Epha2-/- cells with altered F-actin distribution. E-cadherin junctions, which are normally located at the basal-lateral ends of equatorial epithelial cells and are diminished in newly differentiating fiber cells, become widely distributed in the apical, lateral and basal sides of epithelial cells and persist in differentiating fiber cells in Epha2-/- lenses. Src-/- equatorial epithelial cells also fail to form precisely aligned meridional rows and lens fulcrum. These results indicate that EphA2/Src signaling is essential for the formation of the lens fulcrum. EphA2 also regulates Src/cortactin/F-actin complexes at the vertices of hexagonal equatorial cells for cell-to-cell alignment. This mechanistic information explains how EphA2 mutations lead to disorganized lens cells that subsequently contribute to altered refractive index and cataracts in humans and mice.
Keywords: Eph, Ephrin, Lens, Cataracts
INTRODUCTION
Cataracts, defined as any lens opacity, remain the leading cause of blindness (Asbell et al., 2005). The lens is an avascular, transparent, highly refractive and biconvex structure that focuses light images onto the retina. The lens is composed of a monolayer of epithelial cells that cover the anterior hemisphere of bulk elongated fibers and is wrapped by a basement membrane called the lens capsule. The mechanisms that regulate lens growth, homeostasis and lifelong transparency are still not well understood. During early development, the posterior cells of the lens vesicle elongate to form primary fibers while the anterior cells differentiate into epithelial cells. Throughout life, lens epithelial cells continually differentiate into secondary fiber cells at the lens equator (Piatigorsky, 1981). High refractive index and lifelong transparency of the lens rely on the precise alignment of lens fiber cells that are overlaid on previous generations of fiber cells in a concentric manner. Cell-to-cell organization starts when equatorial epithelial cells undergo morphogenesis to form aligned meridional rows of hexagonal cells just before fiber cell differentiation and elongation at the lens equator (Bassnett et al., 1999).
One of the key events during lens development is the hexagonal shape and packing geometry of lens fiber cells. Lens fiber cells, which are hexagonal in cross-section, are packed tightly to eliminate intercellular space, establish a high refractive index and minimize light scattering (Kuszak, 1995). The structural organization of contractile and adhesive elements (N-cadherin/actin/myosin) may be required for cell packing and appropriate migration and alignment of elongating fiber cells (Bassnett et al., 1999). N-cadherin/actin complexes form a hexagonal lattice in fiber cell basal ends with the contractile proteins myosin and caldesmon, which are localized at the center of the fiber cell (Bassnett et al., 1999). F-actin bundles of one cell are aligned with those in the next. Paxillin, myosin light chain kinase (MLCK) and focal adhesion kinase (FAK) are also detected in fiber cell basal membrane complexes. Tropomodulin 1 is also required for maintaining the hexagonal geometry of interior lens fiber cells in mice (Nowak et al., 2009). However, it is not known which mechanisms control the initiation of lens equatorial cell-to-cell alignment when randomly packed pre-equatorial epithelial cells become organized into the meridional rows of hexagonal cells at the lens equator.
Recent studies, including our work, have reported that EphA2 or ephrin A5 mutations cause cataracts with variable severity or incomplete penetrance in humans and mice (Cheng and Gong, 2011; Cooper et al., 2008; Jun et al., 2009; Kaul et al., 2010; Masoodi et al., 2012; Park et al., 2012; Shi et al., 2012; Shiels et al., 2008; Sundaresan et al., 2012; Tan et al., 2011; Zhang et al., 2009). Bidirectional signals mediated by membrane-anchored ephrins and Eph receptor tyrosine kinases play important roles in a broad range of cell-cell recognition events, including axon pathfinding, early segmentation and organ morphogenesis, by modulating cell repulsive or adhesive signals through multiple downstream proteins, such as Ras/Rho, MAP kinase, Akt or FAK, that are crucial for intracellular signal transduction and cytoskeletal dynamics (Arvanitis and Davy, 2008; Himanen et al., 2007; Kullander and Klein, 2002). Eph receptor kinases mediate forward signaling in one cell while ephrin ligands transmit reverse signaling in the adjacent cell (Davy et al., 1999; Holland et al., 1996). The Eph family of receptor tyrosine kinases includes 16 members, divided into EphA (1 to 10) and EphB (1 to 6) kinases. The ephrin family of ligands is divided into ephrin-A (1 to 5) and ephrin-B (1 to 4 and 6). EphA receptors preferentially bind to glycosyl-phosphatidylinositol (GPI)-anchored ephrin-A ligands, whereas EphB receptors bind to transmembrane ephrin-B ligands. Each receptor interacts with multiple ligands and vice versa. In addition, cross interactions between EphA and ephrin-B or between EphB and ephrin-A can also occur. For example, EphA4 and EphB2 interact with ephrin B2 and ephrin A5, respectively (Himanen et al., 2004; Takemoto et al., 2002). The complementary or overlapping expression pattern of Ephs and ephrins suggests diverse functions of Eph-ephrin signaling in tissue development and homeostasis (Poliakov et al., 2004). Eph-ephrin bidirectional signals have emerged as a major cell-cell contact-dependent communication to coordinate not only developmental processes but also the normal physiology and homeostasis of mature organs. Altered Eph-ephrin function contributes to a variety of diseases (Pasquale, 2008).
Eph-ephrin signaling is essential for lens transparency. Mutations in EPHA2 cause human congenital dominant (Park et al., 2012; Zhang et al., 2009) and recessive (Kaul et al., 2010) cataracts. In addition, non-synonymous SNPs of EPHA2 have been linked to age-related cortical cataracts in humans (Masoodi et al., 2012; Shiels et al., 2008; Sundaresan et al., 2012; Tan et al., 2011). In mice, the loss of EphA2 disrupts the structure and organization of lens fiber cells associated with altered N-cadherin adhesion junctions (Cheng and Gong, 2011; Jun et al., 2009) as well as causing an increased stress response as reflected by elevated Hsp27 (Hspb2) levels (Jun et al., 2009). Epha2-/- knockout lenses have significant changes in refractive index (Shi et al., 2012). Efna5-/- mice develop severe age-related nuclear cataracts (Cooper et al., 2008) or anterior cataracts (Cheng and Gong, 2011) depending on the strain background, and these differ from Epha2-/- lens phenotypes. It is unclear how EphA2 or ephrin A5 mutations lead to these pathological changes or why variable lens phenotypes occur in Epha2-/- or Efna5-/- mice.
In this study, we demonstrate that EphA2 is required for the formation of lens fulcrum and organized meridional rows of hexagonal, differentiating, lens equatorial epithelial cells. The loss of EphA2 results in the absence of the lens fulcrum, disorganized meridional rows, altered equatorial epithelial cell shape and, ultimately, disrupted alignment of lens fiber cells. EphA2 is essential for the activation and phosphorylation of Src and cortactin, which in turn recruit F-actin to the vertices of the equatorial hexagonal cells. This Eph/Src signaling cascade controls lens equatorial cell morphogenesis during development. A lack of EphA2 abolishes Src activation and other downstream regulation to disrupt the lens fulcrum and the alignment of newly differentiating hexagonal cells at the lens equator, which subsequently contributes to the disruption of lens refractive index and transparency.
MATERIALS AND METHODS
Mice
Mouse care and breeding were performed according to approved protocols (UC Berkeley Animal Care and Use Committee and Fred Hutchinson Cancer Research Center Institutional Animal Care and Use Committee) and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Epha2-/- mice were acquired from The Jackson Laboratory (strain #: 006028). The Epha2-/- mice were genotyped according to the method provided by The Jackson Laboratory using a standard PCR method (Gong et al., 1997). Src-/- mice were generated as previously described (Soriano et al., 1991). Src+/- mice were intercrossed to generate Src-/- mice. Knockout mice were genotyped using three primers: Exon 2, 5′-AGCAACAAGAGCAAGCCCAAGGAC-3′; Exon 2.3, 5′-GTGACGGTGTCCGAGGAGTTGAAG-3′; and 9603, 5′-TCATAGCCGAATAGCCTCTCCAC-3′. PCR was performed with the following program: 93°C for 3 minutes; then 40 cycles of 93°C for 35 seconds and 49°C for 40 seconds; and finally 65°C for 2 minutes. This PCR produced a 200 bp band from the Src WT allele and a 410 bp band from the Src knockout allele.
Imaging of GFP-positive live lenses
GFP-positive (GFP+) transgenic WT mice, in which GFP expression is under the chicken β-actin promoter (Okabe et al., 1997), were mated with Epha2-/- mice to generate GFP+ knockout mice, which were screened as previously described (Cheng and Gong, 2011). Mutant and WT mice with one copy of the GFP transgene were used for image analysis. Fresh intact GFP+ lenses from postnatal day (P) 21 mice were dissected in DMEM without Phenol Red immediately before imaging. Images of lens epithelial and fiber cells with a mosaic GFP expression pattern were collected using a Zeiss LSM700 confocal microscope. Lenses were maintained in DMEM on the stage of the confocal microscope. z-stack images of the lens equator were collected with 1 μm z-steps. ZEN 2010 software (Zeiss) was used to analyze equatorial epithelial and fiber cells and create three-dimensional reconstructions.
Immunohistochemistry
Frozen lens sections from P14 mice were processed and collected as previously described (Gong et al., 1997) for immunostaining. Lens capsule flat-mounts from P21 mice were prepared using a previously described protocol (Cheng and Gong, 2011; Sugiyama et al., 2010). Anti-EphA2 (R&D Systems), anti-β-actin (Sigma-Aldrich), anti-E-cadherin (Invitrogen), anti-cortactin (Millipore), anti-cortactin-pY466 (Invitrogen) and anti-Src-pY416 (equivalent residue is Y424 for mouse; Cell Signaling) primary antibodies, appropriate fluorescent secondary antibodies (Jackson ImmunoResearch Laboratories) and phalloidin-Rhodamine (Invitrogen) were used. Samples were mounted with DAPI VectorShield mounting medium (Vector Laboratories). Confocal and z-stack images were collected using a Zeiss LSM700 confocal microscope. Staining was repeated at least three times, and representative results are shown.
Wheat germ agglutinin staining
Rhodamine-conjugated wheat germ agglutinin (WGA; Vector Laboratories) was used to stain P21 whole fixed lenses for confocal imaging. WGA was previously shown to stain the plasma membranes of lens epithelial and fiber cells (Bond et al., 1996). Enucleated eyeballs with a small posterior opening were fixed in fresh 4% paraformaldehyde for 30 minutes on ice. Eyeballs were then briefly washed twice with cold 1× PBS and stored overnight in 1× PBS at room temperature before processing. Lenses were carefully dissected from fixed eyeballs and placed in blocking solution (3% BSA, 3% normal goat serum, 0.3% Triton X-100) for 15 minutes at room temperature. Lenses were then placed in DAPI VectorShield mounting medium for 30 minutes at room temperature. After washing twice with 1× PBS, lenses were finally placed in a 1:10 dilution of WGA (in 1× PBS) for 30 minutes at room temperature. Lenses were washed again in 1× PBS twice before imaging on a Zeiss LSM700 confocal microscope as described above.
Quantification of immunostaining signal intensity
Confocal images of EphA2, β-actin, E-cadherin, cortactin, cortactin-pY466 and Src-pY424 staining in WT hexagonal equatorial epithelial cells were analyzed to compare the signal intensity at cell vertices versus the broad/short sides of the cells. Three separate staining samples for each antibody were evaluated. Each image was first exported in grayscale and then cropped to the same size. A heat map for each image was generated in ImageJ (NIH) using the HeatMap Histogram plug-in. Heat maps were pseudocolored between purple (0) and red (255) for signal intensity. A circular area (1.6 μm in diameter or 2.01 μm2 in area) was marked at each vertex and along each side of a cell. Mean intensities at the vertices and on the broad and short sides of three individual cells were collected from each image. A total of nine cells were analyzed for each antibody, and mean intensities and standard deviation were calculated and plotted in Excel (Microsoft). Student’s t-test was used to determine significance (P<0.001).
RESULTS
EphA2 plays an important role in the formation of meridional rows at the lens equator
To elucidate the role of EphA2 in the lens, we first examined lens cell morphology in live GFP+ wild-type (WT) and Epha2-/- lenses using a laser confocal microscope. In the WT lens, equatorial epithelial cells with typical mosaic GFP expression became hexagonal and organized into meridional rows (Fig. 1A, arrowheads), and newly differentiating fiber cells were straight, uniform in width and aligned precisely (Fig. 1C). By contrast, the majority of Epha2-/- equatorial epithelial cells were not hexagonal and failed to form organized meridional rows (Fig. 1B, arrows). In addition, underlying fiber cells in the Epha2-/- lens were wavy, variable in width and misaligned (Fig. 1D). Three-dimensional reconstruction of z-stacks through the lens equator demonstrated, in both anterior-posterior and transverse views of lens bow regions, that normal elongated fiber cells were precisely overlaid in WT lenses (Fig. 1E,G). The lens fulcrum (Sugiyama et al., 2009) or modiolus (Zampighi et al., 2000) is a distinct region at the lens equator where the apical tips of elongating epithelial cells constrict to form an anchor point before fiber cell elongation in the WT lens (Fig. 1E, arrowhead). Epha2-/- lenses had an abnormal lens fulcrum (Fig. 1F, arrowheads) and irregularly shaped and disorganized fiber cells (Fig. 1H). Thus, a loss of EphA2 resulted in a disruption of the lens fulcrum (also see Figs 5, 6, 7) and a failure of equatorial epithelial cells to fully convert into hexagonal cells to form organized meridional rows.
Fig. 1.
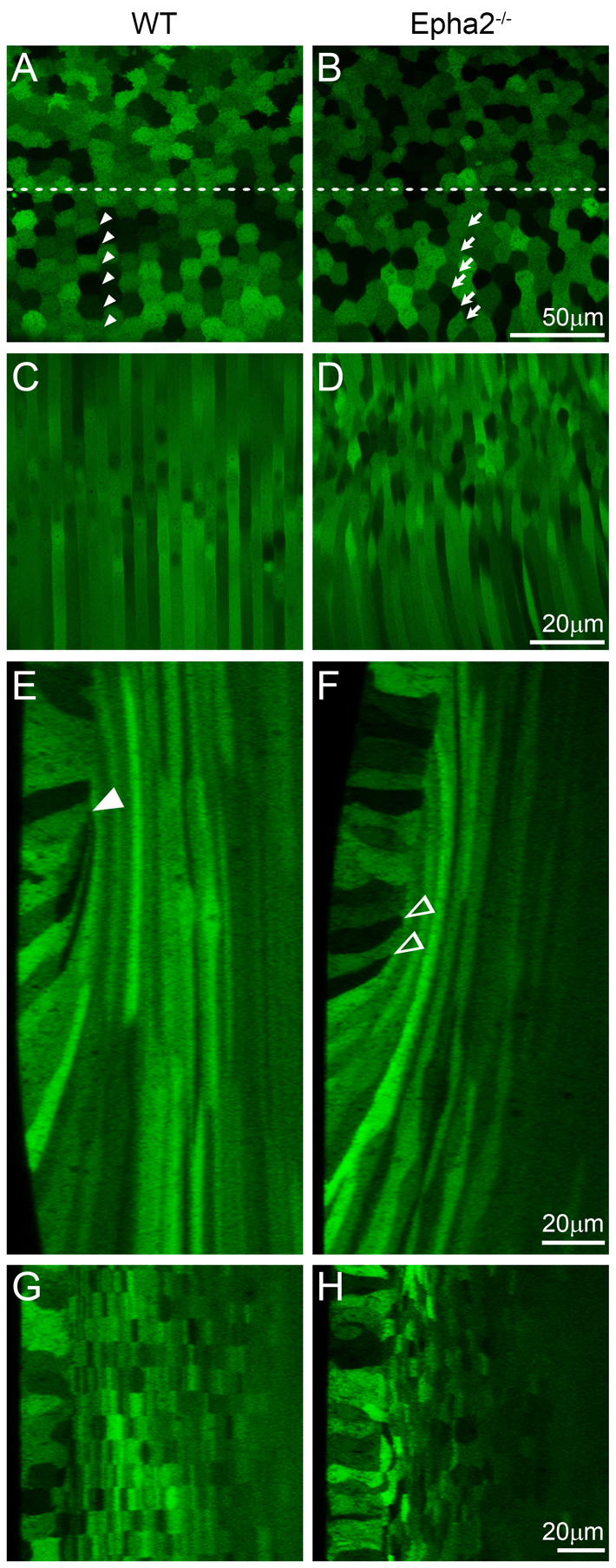
Confocal images of the equatorial region of P21 GFP+ wild-type and Epha2-/- mouse lenses. (A-D) The wild-type (WT) lens shows the typical mosaic GFP expression pattern in the equatorial epithelial cells (A), with hexagonal cells organized into meridional rows (below the dashed line, arrowheads) and straight and organized peripheral differentiating fiber cells (C). By contrast, equatorial epithelial cells in the Epha2-/- lens lack organized meridional rows (B, arrows), and underlying fiber cells are wavy and disorganized (D). (E,F) Three-dimensional reconstruction of z-stacks in the anterior-posterior view through the WT lens reveals organized fiber cells in the bow region (E), whereas a comparable reconstruction through the Epha2-/- lens demonstrates disorganization of peripheral fiber cells with variable cell width (F). The WT lens fulcrum is indicated by the arrowhead (E); in the Epha2-/- lens, multiple points of apical constriction are observed (F, arrowheads). (G,H) Reconstruction of z-stacks through the transverse view shows that WT fiber cells are neatly organized into straight rows (G), whereas Epha2-/- fiber cells are misaligned (H). The epithelial cells are on the left in G and H.
Fig. 5.
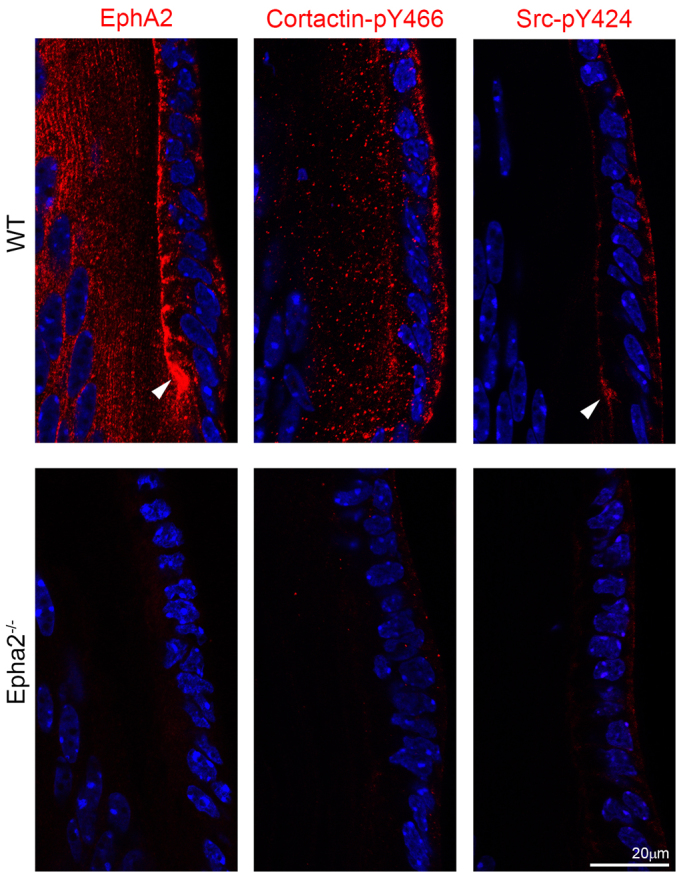
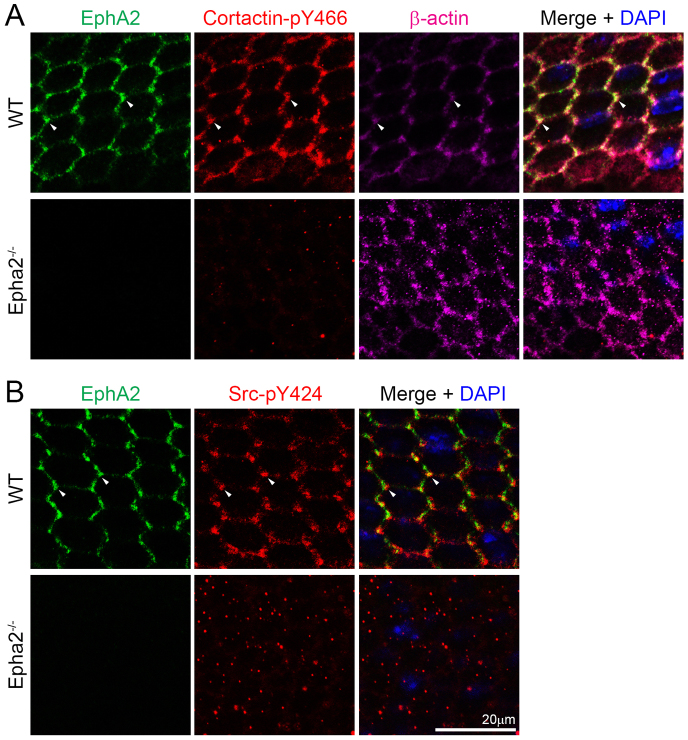
EphA2, phosphorylated cortactin and phosphorylated Src staining in P14 WT and Epha2-/- frozen lens sections. EphA2 (left) and cortactin-pY466 (middle) are present in WT epithelial and fiber cells, and Src-pY424 (right) signal is predominant in WT epithelial cells. EphA2 and Src-pY424 are enriched at the lens fulcrum (arrowheads). By contrast, Epha2-/- lens epithelial and fiber cells have only weak cortactin-pY466 and Src-pY424 staining.
Fig. 6.
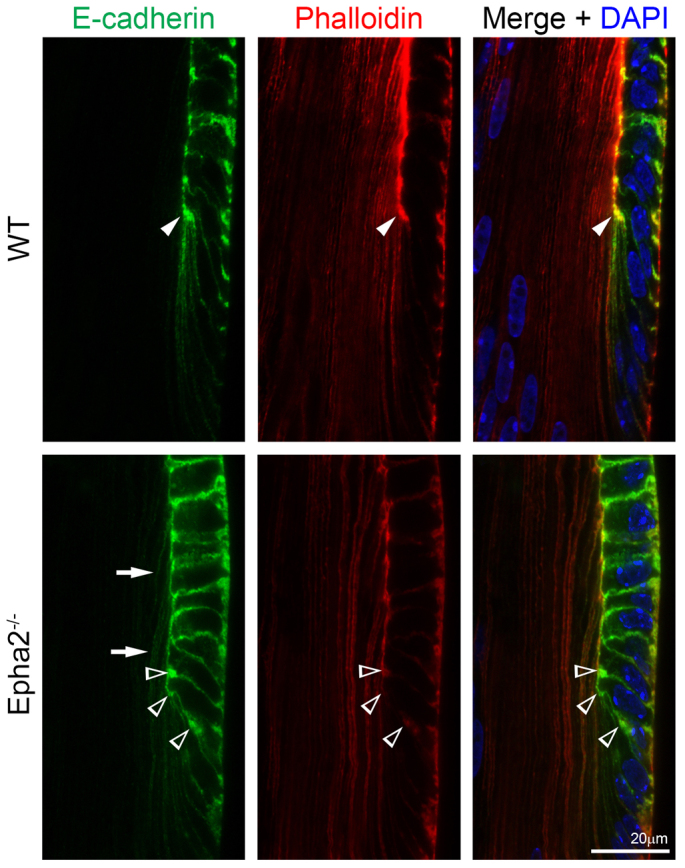
E-cadherin and phalloidin staining of P14 WT and Epha2-/- frozen lens sections. Double immunolabeling of E-cadherin (green) and with phalloidin (F-actin, red) in frozen lens sections demonstrates that E-cadherin and F-actin are highly enriched only at the lens fulcrum (arrowheads) in the WT lens section, but form multiple aggregates in the Epha2-/- lens section (open arrowheads). Abnormal E-cadherin signal is observed in Epha2-/- lens fiber cells (arrows).
Fig. 7.
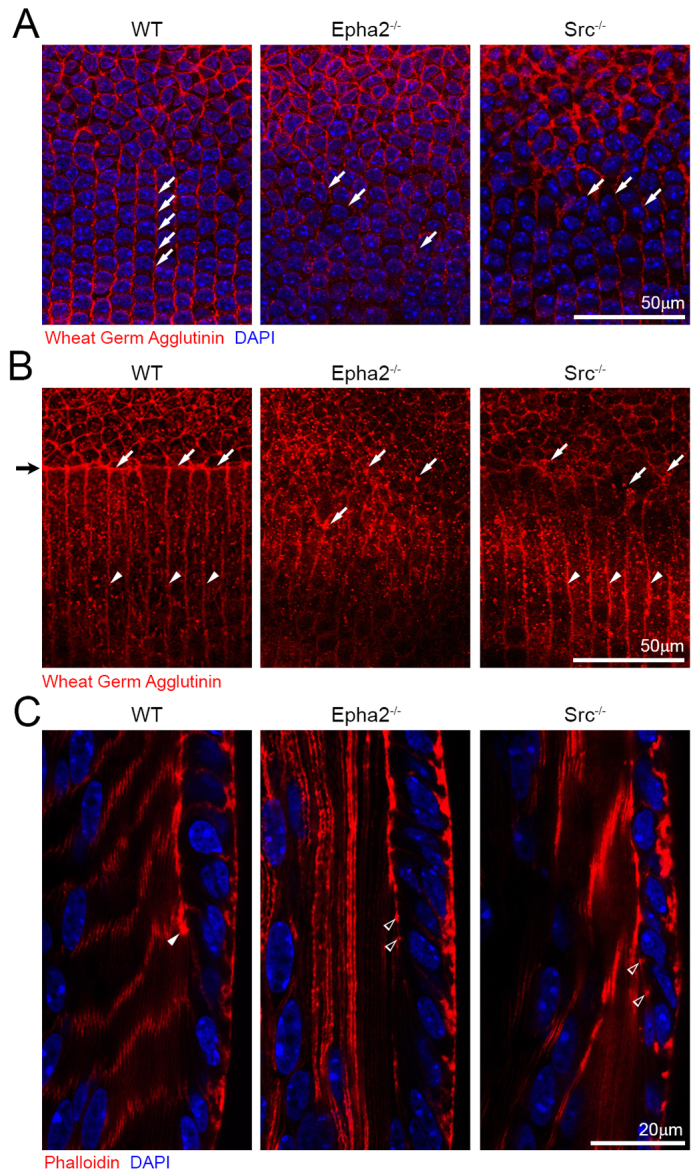
Wheat germ agglutinin and phalloidin staining of P21 WT, Src-/- and Epha2-/- lenses and frozen sections. (A) Confocal images of the equatorial region of P21 whole fixed lenses labeled with wheat germ agglutinin (WGA, red) and stained with DAPI show that the WT lens has aligned meridional rows (left, arrows), whereas both Epha2-/- and Src-/- lenses have disorganized meridional epithelial cells (middle and right, arrows). (B) Confocal images of the lens equator where epithelial cells transition to fiber cells show that the lens fulcrum (black arrow on far left) is strongly stained by WGA (left, arrows, red) in the WT lens. By contrast, Epha2-/- and Src-/- lenses lack a distinct lens fulcrum and have WGA-stained aggregates (middle and right, arrows) at the boundary between epithelial and fiber cells. In the WT lens, peripheral lens fibers are neatly aligned (left, arrowheads), and, similarly, Src-/- lens fibers appear aligned as well (right, arrowheads). No obvious fiber cell boundaries are visible in the Epha2-/- lens (middle). (C) Phalloidin staining of the WT lens section shows that F-actin is enriched at the lens fulcrum (arrowhead). However, in the Epha2-/- and Src-/- lens sections, there are multiple F-actin aggregates rather than a distinct lens fulcrum (open arrowheads).
EphA2 proteins accumulate at the vertices of hexagonal epithelial cells
To investigate the molecular basis for how EphA2 signaling controls epithelial cell shape and alignment at the lens equator, we examined EphA2 protein localization in these cells. Double immunostaining of the flat-mounted lens epithelium (attached to the lens capsule) revealed that both EphA2 and β-actin were enriched and colocalized at the vertices of the equatorial hexagonal epithelial cells that formed organized meridional rows in the WT sample (Fig. 2, arrowheads). By contrast, β-actin clusters were no longer restricted to the cell vertices and appeared at other points along the cell membrane in Epha2-/- equatorial epithelial cells (Fig. 2, arrows). E-cadherin was detected at cell-cell boundaries of WT hexagonal epithelial cells, but were not enriched at the cell vertices (Fig. 3A). However, we were unable to detect any obvious change in E-cadherin protein distribution in these equatorial epithelial cells between WT and Epha2-/- flat-mounted samples (Fig. 3A).
Fig. 2.
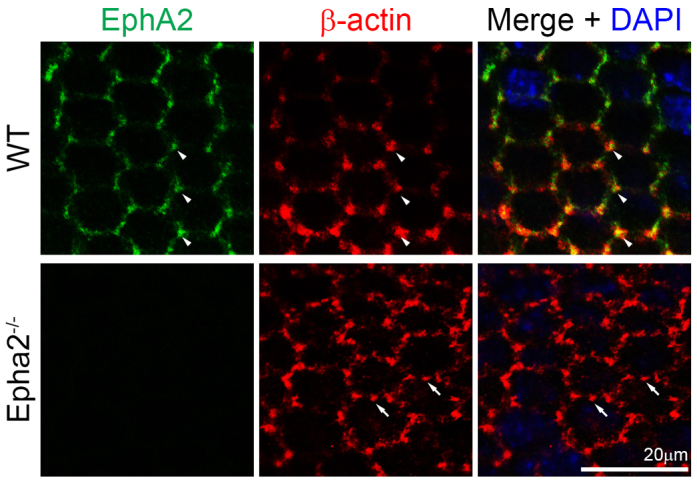
EphA2 and β-actin distribution in WT and Epha2-/- equatorial epithelial cells. Double immunolabeling of β-actin (red) and EphA2 (green) with DAPI staining (blue, nuclei) of equatorial lens epithelial cells from lens capsule flat-mounts of P21 WT and Epha2-/- mice. When equatorial cells organize into meridional rows, β-actin and EphA2 are enriched and colocalize at the vertices of hexagonal WT epithelial cells (arrowheads). By contrast, β-actin forms abnormal aggregates on the membranes of Epha2-/- epithelial cells that fail to pack into organized meridional rows (arrows). Antibody specificity is demonstrated by the lack of EphA2 staining in Epha2-/- cells.
Fig. 3.
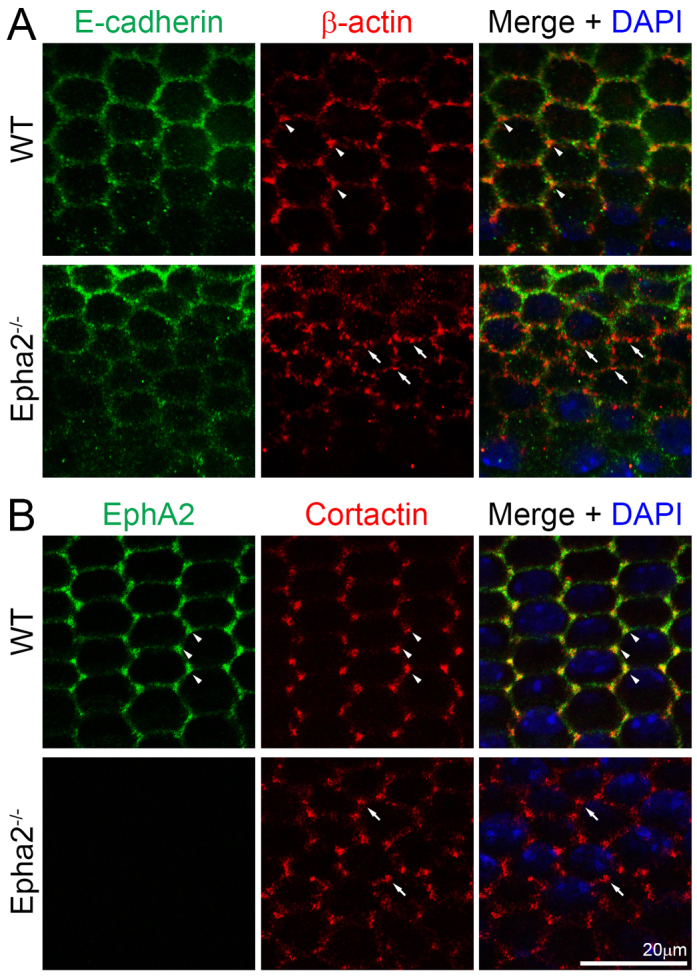
E-cadherin and total cortactin distribution in WT and Epha2-/- equatorial epithelial cells. (A) Double immunolabeling of E-cadherin (green) and β-actin (red) with DAPI staining (blue, nuclei) of equatorial lens epithelial cells from lens capsule flat-mounts of P21 WT and Epha2-/- mice. Confocal images reveal colocalization of β-actin and E-cadherin at the cell membranes of hexagonal epithelial cells in the WT sample. β-actin is enriched at the cell vertices of WT equatorial epithelial cells (arrowheads). However, there is only weak E-cadherin staining at the membranes of the Epha2-/- disorganized equatorial epithelial cells. β-actin (red) forms numerous abnormal aggregates on the membranes of Epha2-/- cells (arrows). (B) Double immunolabeling of EphA2 (green) and total cortactin (red) with DAPI staining (blue) of equatorial lens epithelial cells from lens capsule flat-mounts of P21 WT and Epha2-/- mice. EphA2 and total cortactin are enriched and colocalize at the vertices of hexagonal WT epithelial cells packed into organized meridional rows (arrowheads). By contrast, staining for total cortactin shows abnormal clusters at the cell membranes of disorganized equatorial Epha2-/- epithelial cells (arrows).
In order to understand the mechanism for the alteration of β-actin clusters in the Epha2-/- lens, we characterized the localization of cortactin, a filamentous actin (F-actin)-binding protein. Cortactin specifically accumulated at the vertices of the hexagonal WT epithelial cells (Fig. 3B, arrowheads). Moreover, cortactin partially colocalized with EphA2 at cell vertices. Interestingly, similar to the change in β-actin staining, cortactin proteins accumulated in abnormal clusters on the membranes of Epha2-/- epithelial cells (Fig. 3B, arrows). Thus, cortactin is likely to play an important role in the regulation of F-actin needed to maintain equatorial epithelial cell shape and alignment.
Activation of cortactin and Src is EphA2 dependent
The function of cortactin is regulated by phosphorylation mediated by Src kinases (Knöll and Drescher, 2004). The activation of Src kinase is regulated by its upstream kinases, including EphA2 (Baldwin et al., 2006; Faoro et al., 2010; Parri et al., 2007). Thus, we examined the phosphorylated forms of cortactin and Src. Immunostaining revealed that cortactin phosphorylated at Y466 (cortactin-pY466) was enriched at the vertices of hexagonal WT equatorial epithelial cells. However, cortactin-pY466 was not only absent from cell-cell boundaries, but also formed intracellular aggregates (or particles) in Epha2-/- equatorial epithelial cells (Fig. 4A). In addition, Src phosphorylated at Y424 (Src-pY424), which was also enriched at the vertices of WT hexagonal equatorial epithelial cells, was missing from cell-cell boundaries and formed intracellular aggregates (or particles) in Epha2-/- equatorial epithelial cells (Fig. 4B). Immunostaining with secondary antibodies alone did not produce any intracellular aggregates (or particles) in flat-mounted WT or Epha2-/- equatorial epithelial cells (data not shown).
Fig. 4.
EphA2, phosphorylated cortactin, β-actin and phosphorylated Src distribution in WT and Epha2-/- equatorial epithelial cells. (A) Triple immunolabeling of EphA2 (green), phosphorylated cortactin-Y466 (cortactin-pY466, red) and β-actin (purple) with DAPI staining (blue, nuclei) of equatorial lens epithelial cells from lens capsule flat-mounts of P21 WT and Epha2-/- mice. In hexagonal WT equatorial epithelial cells, EphA2, cortactin-pY466 and β-actin are localized at the cell membrane and are enriched and colocalize at cell vertices (arrowheads). By contrast, only punctate staining signals for cortactin-pY466 can be found in the disorganized Epha2-/- epithelial cells. (B) Similarly, double immunolabeling of phosphorylated Src-Y424 (Src-pY424, red) and EphA2 (green) reveals that both proteins are enriched and colocalize at the vertices of hexagonal WT epithelial cells (arrowheads). However, in the disorganized Epha2-/- epithelial cells, only random punctate signals from Src-pY424 are observed.
Quantitative analysis of fluorescence intensities of EphA2, β-actin, E-cadherin, cortactin, cortactin-pY466 and Src-pY424 immunostaining signal distribution in WT hexagonal equatorial epithelial cells confirmed the enrichment of these proteins, except E-cadherin, at cell vertices (supplementary material Fig. S1). Owing to the irregular shape of Epha2-/- equatorial epithelial cells, it was difficult to accurately determine the location of cell vertices and to perform similar quantitative analysis on mutant samples. Thus, EphA2 probably regulates cell-to-cell alignment by spatially controlling the activation of Src and cortactin as well as F-actin recruitment to the vertices of lens equatorial epithelial cells. Other kinases probably mediate the activation of mislocalized intracellular Src and cortactin in Epha2-/- cells.
Immunostaining of frozen lens sections confirmed that EphA2, cortactin-pY466 and Src-pY424 proteins were restricted to the apical and basal sides of equatorial epithelial cells in WT lens sections (Fig. 5). However, cortactin-pY466 and Src-pY424 were almost absent from Epha2-/- lens sections (Fig. 5). Src-pY424 was also absent at the lens fulcrum in the Epha2-/- lens. EphA2 and Src-pY424, but not cortactin-pY466, were also localized and enriched at the lens fulcrum (Fig. 5, arrowheads). Sporadically distributed intracellular aggregates of cortactin-pY466 and Src-pY424, as observed in flat-mounted samples (Fig. 4), were not seen in lens sections (Fig. 5). If we look at transverse sections of the data collected in Fig. 4 (a similar view to frozen lens sections), it would be difficult to discern the sporadically distributed intracellular aggregates in Epha2-/- cells. In addition, flat-mount staining of the lens epithelial cells requires different fixation and sample processing than frozen lens sections.
Src has been shown to play a role in the downregulation of E-cadherin-mediated adhesion junctions for promoting the epithelial-to-mesenchymal transition (EMT) (Walker et al., 2007). Lens epithelial-to-fiber cell differentiation is not a typical EMT process, but shares similar cellular processes, such as cell rearrangement, migration and elongation. Thus, we further examined the E-cadherin distribution near the lens equator. As expected, in the WT lens section E-cadherin proteins were located at both the apical and basal junctions of equatorial epithelial cells and accumulated at the lens fulcrum (Fig. 6, arrowheads), similar to Src-pY424 and EphA2 (Fig. 5, arrowheads), and disappeared in newly differentiating fiber cells. However, in the Epha2-/- lens section, E-cadherin accumulated at multiple areas in the apical, basal and lateral sides of equatorial epithelial cells without a normal lens fulcrum (Fig. 6, open arrowheads). In addition, the E-cadherin signal was sustained in newly differentiated Epha2-/- fiber cells (Fig. 6, arrows). Phalloidin-stained F-actin accumulated strongly at the apical interface and lens fulcrum in the WT lens section (Fig. 6, arrowhead in top middle panel), whereas the Epha2-/- lens section showed F-actin signals in multiple areas without a typical lens fulcrum (Fig. 6, open arrowheads in the bottom middle panel).
In order to determine the roles of Src in lens fulcrum and meridional row formation during lens equatorial cell morphogenesis, we examined Src-/- lenses labeled with Rhodamine-conjugated wheat germ agglutinin (WGA) and Src-/- lens sections co-stained with phalloidin. The WGA-stained WT lens showed aligned meridional rows (Fig. 7A, left, arrows), whereas both Epha2-/- and Src-/- lenses revealed misaligned meridional cells (Fig. 7A, middle and right, arrows). The lens fulcrum was detected as a WGA-stained sharp boundary separating surface epithelial cells and underlying fibers in the WT lens (Fig. 7B, left, arrows). No obvious WGA-stained boundary or lens fulcrum was detected in Epha2-/- and Src-/- lenses (Fig. 7B, middle and right, arrows). Compared with a severe disruption of fiber-to-fiber organization in Epha2-/- lenses, a mild perturbation of the fiber-to-fiber organization occurred in Src-/- lenses (Fig. 7B, right, arrowheads). Phalloidin staining results confirmed a typical lens fulcrum in the bow region of the WT lens section (Fig. 7C, arrowhead), but no obvious lens fulcrum in Epha2-/- or Src-/- lens sections (Fig. 7C, open arrowheads). Thus, both EphA2 and Src play an essential role in the formation of the lens fulcrum and organized meridional rows.
DISCUSSION
This work reveals that EphA2 signaling and Src activation are essential parts of the signaling cascade that controls the formation of the lens fulcrum and the organization of meridional rows of hexagonal epithelial cells during lens equatorial cell morphogenesis (Fig. 8). EphA2/ephrin bidirectional signaling phosphorylates downstream molecules, including Src and cortactin, to regulate the dynamics of the actin cytoskeleton. The actin cytoskeleton controls cell shape, cell-to-cell interaction and/or migration, which are needed for the formation of organized meridional rows. Both Epha2-/- and Src-/- lenses show disrupted lens fulcrums and misaligned equatorial epithelial cells. Without EphA2, neither Src nor cortactin is phosphorylated (activated) at cell vertices, and altered actin filaments disrupt the formation of organized meridional rows and the hexagonal shape of Epha2-/- equatorial epithelial cells. Thus, EphA2 and Src are the key signaling molecules that control the morphogenesis and differentiation of lens equatorial cells during development.
Fig. 8.
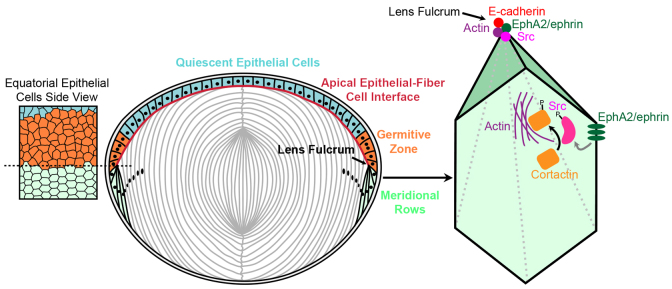
Model for EphA2 functions in the organization of hexagonal equatorial epithelial cells into meridional rows. Hexagonal equatorial epithelial cells are packed into organized meridional rows at the lens fulcrum. On the basal-lateral sides of equatorial epithelial cells, EphA2 is likely to interact with an ephrin ligand to cluster at the vertices of the hexagonal cells. EphA2 phosphorylates Src, which consequently activates cortactin to trigger actin to cluster at cell vertices. This signaling cascade and cell adhesion events lead to the organization of equatorial epithelial cells into meridional rows, which is probably a prerequisite for fiber cell organization. On the apical side of equatorial epithelial cells, EphA2 clustering phosphorylates and activates Src to recruit E-cadherin and actin to form the lens fulcrum, which serves as an anchor point for the transition from epithelial cells to fiber cells.
The initiation mechanism for EphA2 accumulation at the vertices of equatorial epithelial cells remains unclear. The clustering of EphA is important for its kinase activity to regulate downstream targets (Groves and Kuriyan, 2010; Salaita et al., 2010; Xu et al., 2011). Presumably, clustering of EphA2 (or ephrin ligands) is one of the early or initiating events that trigger downstream signaling through Src and cortactin to recruit actin to the vertices of hexagonal cells in the meridional rows. EphA2 receptors are known to undergo clustering at the cell membrane by self-oligomerization or through interaction with ephrin ligands (Himanen et al., 2010; Salaita et al., 2010). The center of the EphA2 cluster has the greatest tyrosine phosphorylation and tightest cell adhesion to the substrate, which results in reorganization of the actin cytoskeleton. Further studies of ephrin ligands will be needed to elucidate the mechanism for EphA2 clustering in equatorial lens epithelial cells.
It is remarkable that both phosphorylated Src and phosphorylated cortactin are absent from Epha2-/- epithelial cells. Src activation mainly relies on EphA2 signaling at the lens equator. The data from Src-/- lenses confirmed that Src activation is essential for the formation of the lens fulcrum and for organization of the meridional rows, and Src also seems to be partly responsible for fiber cell organization. Src activation plays a key role in lens equatorial cell morphogenesis probably by regulating changes in the actin cytoskeleton. Src is known to participate in cell homeostasis and in a vast range of physiological functions including cell proliferation and survival, cell shape control, regulation of the cytoskeleton, maintenance of normal intercellular contacts, cell-matrix adhesion dynamics, motility and migration (Thomas and Brugge, 1997; Yeatman, 2004). Src mediates major signaling events from receptor tyrosine kinases (RTKs) and from adhesion receptors, including integrins and E-cadherin (Bjorge et al., 2000). RTKs and adhesion receptors can act synergistically to affect cell survival, proliferation, cytoskeleton reorganization and invasion, and these receptors can directly associate with signaling complexes in which Src is used as a common signaling molecule (Abram and Courtneidge, 2000; Huveneers and Danen, 2009; McLean et al., 2005).
Interestingly, this work suggests that Src activation is spatially and temporally controlled by EphA2 to regulate cell-to-cell alignment at the lens equator. It is known that EphA2 can directly phosphorylate Src in other cell types (Parri et al., 2007). It might be important to study other molecules that are involved in the restricted activation of Src and cortactin that establish organized meridional rows prior to fiber cell elongation and differentiation. It has recently been reported that Fyn, another member of the Src family, is utilized to regulate N-cadherin junctions in the chick lens fiber cell (Leonard et al., 2013). Since the disruption of Src-/- lens fiber cells is less severe than that of Epha2-/- lens fiber cells (Fig. 7B), EphA2 might also control other members of Src family, such as Fyn, to regulate cell junctions and the F-actin recruitment needed for the organization and maintenance of fiber cells in mouse lenses. Studies in chick and quail lens explant models suggest that Src might be involved in posterior capsular cataracts and cortical cataracts (Leonard et al., 2013; Walker et al., 2007; Zhou et al., 2007; Zhou and Menko, 2004). However, Src has not been reported to be directly associated with human cataracts. Future studies will be necessary to understand the involvement of the Src family of proteins in human lens disorders.
Cortactin phosphorylation depends on the presence of EphA2 in equatorial epithelial cells packed into organized meridional rows. Previous work has suggested that cortactin phosphorylation promotes cell migration. Cortactin associates with the F-actin cytoskeleton through the F-actin-binding tandem cortactin repeats and the N-terminal acidic domain that interacts with the actin-related protein (Arp) 2/3 complex for dendritic actin nucleation (Kelley et al., 2010; May, 2001). The C-terminus of cortactin interacts with FAK at focal adhesions and may be phosphorylated by the FAK-Src complex (Tomar et al., 2012; Wang et al., 2011). The tyrosine phosphorylation of cortactin by the FAK-Src complex reduces its interaction with FAK, most likely to increase its turnover at focal adhesions to promote cell motility in other cell types. A similar mechanism might govern cell movement and restructuring as equatorial epithelial cells undergo cell shape changes to align into meridional rows. However, the changes in cortactin could be either the cause or effect of altered organization in Epha2-/- equatorial epithelial cells.
It has been suggested that the Eph-ephrin bidirectional signaling pathway and epithelial cell junction molecules work closely together to regulate epithelial cell integrity or to accomplish cell sorting processes during epithelial cell proliferation or differentiation (Miao and Wang, 2009). The activation of EphA2 changes the expression of E-cadherin and reduces cell-cell or cell-matrix adhesion (Zantek et al., 1999), and the upregulation or downregulation of E-cadherin can also alter the expression of Eph receptors or ephrin ligands (Orsulic and Kemler, 2000). Ephs and ephrins have dual roles in facilitating cell sorting into opposing compartments by shutting down intercompartmental communication through gap junctions (Mellitzer et al., 1999). Our data suggest that EphA2-dependent activation of Src and cortactin is likely to provide key signaling that recruits actin filaments to form basal-lateral cell vertices for establishing the hexagonal cell shape and for controlling the precise organization of epithelial cells into meridional rows. However, at the lens fulcrum, EphA2-dependent Src activation initiates the accumulation of E-cadherin and F-actin without the phosphorylation of cortactin. Presumably, a different F-actin-binding protein is involved at the lens fulcrum. A loss of EphA2 prevents Src-Y424 activation and cortactin-Y466 phosphorylation, which results in alterations in F-actin and E-cadherin protein distribution, leading to changes in basal-lateral cell shape and cell-cell alignment in the transition from epithelial to fiber cells at the lens equator.
Our results suggest that EphA2 and Src control lens fulcrum formation at the apical ends of equatorial epithelial cells as well as cortactin/F-actin complex localization at the basal-lateral vertices of these hexagonal cells at lens equator. The activation of Src at the apical tips (lens fulcrum) and at the vertices of hexagonal equatorial epithelial cells is solely controlled by EphA2. Src activation is important for the formation of the lens fulcrum and the organization of meridional rows (cell-to-cell alignment) at the lens equator. Src-/- lenses do not have obvious opacities, and no defects are apparent in Src-/- retina and cornea under the dissection microscope. Therefore, the defects in Src-/- lenses are probably not secondary effects due to abnormalities in other ocular tissues. Further work is needed to investigate the development of other ocular tissues in Src-/- mice. Interestingly, in contrast to Epha2-/- lenses that have normal lens weight but severely disrupted fiber cell organization, Src-/- lenses show a ∼15% reduction in lens weight with mild fiber cell alterations (data not shown). The phenotypic variation between Epha2-/- and Src-/- lens fiber cells suggests that other RTKs are likely to control Src activation to regulate the proliferation of lens epithelial cells and lens size; other members of the Src family, such as Fyn, may act as downstream targets of EphA2/ephrin signaling in the regulation of lens fiber cell organization by affecting N-cadherin localization (Jun et al., 2009; Leonard et al., 2013). This work is consistent with a recent study revealing disorganized lens cells and altered lens refractive index in Epha2-/- lenses (Shi et al., 2012). Dysfunction of EphA2 due to mutations and other insults is likely to perturb lens equatorial cell morphogenesis, alters the organization and interaction among lens fiber cells, causes abnormal refractive index and leads to both congenital and age-related cataracts in humans and mice (Jun et al., 2009; Kaul et al., 2010; Park et al., 2012; Shiels et al., 2008; Sundaresan et al., 2012; Tan et al., 2011; Zhang et al., 2009).
Supplementary Material
Acknowledgments
We thank Dr Chun-hong Xia for her critical reading of this manuscript.
Footnotes
Funding
This work was supported by grants from the National Institutes of Health [R01-EY013849 and R01-EY021519 to X.G.; R01-CA41072 and R01-NS080194 to J.A.C.]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Author contributions
C.C. and X.G. designed experiments, interpreted data and wrote the manuscript. C.C. performed experiments. M.M.A. and J.A.C provided and processed Src-/- samples. J.A.C. interpreted data and edited the manuscript.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.100727/-/DC1
References
- Abram C. L., Courtneidge S. A. (2000). Src family tyrosine kinases and growth factor signaling. Exp. Cell Res. 254, 1–13 [DOI] [PubMed] [Google Scholar]
- Arvanitis D., Davy A. (2008). Eph/ephrin signaling: networks. Genes Dev. 22, 416–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbell P. A., Dualan I., Mindel J., Brocks D., Ahmad M., Epstein S. (2005). Age-related cataract. Lancet 365, 599–609 [DOI] [PubMed] [Google Scholar]
- Baldwin C., Chen Z. W., Bedirian A., Yokota N., Nasr S. H., Rabb H., Lemay S. (2006). Upregulation of EphA2 during in vivo and in vitro renal ischemia-reperfusion injury: role of Src kinases. Am. J. Physiol. 291, F960–F971 [DOI] [PubMed] [Google Scholar]
- Bassnett S., Missey H., Vucemilo I. (1999). Molecular architecture of the lens fiber cell basal membrane complex. J. Cell Sci. 112, 2155–2165 [DOI] [PubMed] [Google Scholar]
- Bjorge J. D., Jakymiw A., Fujita D. J. (2000). Selected glimpses into the activation and function of Src kinase. Oncogene 19, 5620–5635 [DOI] [PubMed] [Google Scholar]
- Bond J., Green C., Donaldson P., Kistler J. (1996). Liquefaction of cortical tissue in diabetic and galactosemic rat lenses defined by confocal laser scanning microscopy. Invest. Ophthalmol. Vis. Sci. 37, 1557–1565 [PubMed] [Google Scholar]
- Cheng C., Gong X. (2011). Diverse roles of Eph/ephrin signaling in the mouse lens. PLoS ONE 6, e28147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. A., Son A. I., Komlos D., Sun Y., Kleiman N. J., Zhou R. (2008). Loss of ephrin-A5 function disrupts lens fiber cell packing and leads to cataract. Proc. Natl. Acad. Sci. USA 105, 16620–16625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A., Gale N. W., Murray E. W., Klinghoffer R. A., Soriano P., Feuerstein C., Robbins S. M. (1999). Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes Dev. 13, 3125–3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faoro L., Singleton P. A., Cervantes G. M., Lennon F. E., Choong N. W., Kanteti R., Ferguson B. D., Husain A. N., Tretiakova M. S., Ramnath N., et al. (2010). EphA2 mutation in lung squamous cell carcinoma promotes increased cell survival, cell invasion, focal adhesions, and mammalian target of rapamycin activation. J. Biol. Chem. 285, 18575–18585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X., Li E., Klier G., Huang Q., Wu Y., Lei H., Kumar N. M., Horwitz J., Gilula N. B. (1997). Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell 91, 833–843 [DOI] [PubMed] [Google Scholar]
- Groves J. T., Kuriyan J. (2010). Molecular mechanisms in signal transduction at the membrane. Nat. Struct. Mol. Biol. 17, 659–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen J. P., Chumley M. J., Lackmann M., Li C., Barton W. A., Jeffrey P. D., Vearing C., Geleick D., Feldheim D. A., Boyd A. W., et al. (2004). Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat. Neurosci. 7, 501–509 [DOI] [PubMed] [Google Scholar]
- Himanen J. P., Saha N., Nikolov D. B. (2007). Cell-cell signaling via Eph receptors and ephrins. Curr. Opin. Cell Biol. 19, 534–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen J. P., Yermekbayeva L., Janes P. W., Walker J. R., Xu K., Atapattu L., Rajashankar K. R., Mensinga A., Lackmann M., Nikolov D. B., et al. (2010). Architecture of Eph receptor clusters. Proc. Natl. Acad. Sci. USA 107, 10860–10865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S. J., Gale N. W., Mbamalu G., Yancopoulos G. D., Henkemeyer M., Pawson T. (1996). Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature 383, 722–725 [DOI] [PubMed] [Google Scholar]
- Huveneers S., Danen E. H. (2009). Adhesion signaling - crosstalk between integrins, Src and Rho. J. Cell Sci. 122, 1059–1069 [DOI] [PubMed] [Google Scholar]
- Jun G., Guo H., Klein B. E., Klein R., Wang J. J., Mitchell P., Miao H., Lee K. E., Joshi T., Buck M., et al. (2009). EPHA2 is associated with age-related cortical cataract in mice and humans. PLoS Genet. 5, e1000584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul H., Riazuddin S. A., Shahid M., Kousar S., Butt N. H., Zafar A. U., Khan S. N., Husnain T., Akram J., Hejtmancik J. F., et al. (2010). Autosomal recessive congenital cataract linked to EPHA2 in a consanguineous Pakistani family. Mol. Vis. 16, 511–517 [PMC free article] [PubMed] [Google Scholar]
- Kelley L. C., Hayes K. E., Ammer A. G., Martin K. H., Weed S. A. (2010). Cortactin phosphorylated by ERK1/2 localizes to sites of dynamic actin regulation and is required for carcinoma lamellipodia persistence. PLoS ONE 5, e13847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöll B., Drescher U. (2004). Src family kinases are involved in EphA receptor-mediated retinal axon guidance. J. Neurosci. 24, 6248–6257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander K., Klein R. (2002). Mechanisms and functions of Eph and ephrin signalling. Nat. Rev. Mol. Cell Biol. 3, 475–486 [DOI] [PubMed] [Google Scholar]
- Kuszak J. R. (1995). The ultrastructure of epithelial and fiber cells in the crystalline lens. Int. Rev. Cytol. 163, 305–350 [DOI] [PubMed] [Google Scholar]
- Leonard M., Zhang L., Bleaken B. M., Menko A. S. (2013). Distinct roles for N-Cadherin linked c-Src and fyn kinases in lens development. Dev. Dyn. 242, 469–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoodi T. A., Shammari S. A., Al-Muammar M. N., Almubrad T. M., Alhamdan A. A. (2012). Screening and structural evaluation of deleterious Non-Synonymous SNPs of ePHA2 gene involved in susceptibility to cataract formation. Bioinformation 8, 562–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May R. C. (2001). The Arp2/3 complex: a central regulator of the actin cytoskeleton. Cell. Mol. Life Sci. 58, 1607–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean G. W., Carragher N. O., Avizienyte E., Evans J., Brunton V. G., Frame M. C. (2005). The role of focal-adhesion kinase in cancer - a new therapeutic opportunity. Nat. Rev. Cancer 5, 505–515 [DOI] [PubMed] [Google Scholar]
- Mellitzer G., Xu Q., Wilkinson D. G. (1999). Eph receptors and ephrins restrict cell intermingling and communication. Nature 400, 77–81 [DOI] [PubMed] [Google Scholar]
- Miao H., Wang B. (2009). Eph/ephrin signaling in epithelial development and homeostasis. Int. J. Biochem. Cell Biol. 41, 762–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak R. B., Fischer R. S., Zoltoski R. K., Kuszak J. R., Fowler V. M. (2009). Tropomodulin1 is required for membrane skeleton organization and hexagonal geometry of fiber cells in the mouse lens. J. Cell Biol. 186, 915–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe M., Ikawa M., Kominami K., Nakanishi T., Nishimune Y. (1997). ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 407, 313–319 [DOI] [PubMed] [Google Scholar]
- Orsulic S., Kemler R. (2000). Expression of Eph receptors and ephrins is differentially regulated by E-cadherin. J. Cell Sci. 113, 1793–1802 [DOI] [PubMed] [Google Scholar]
- Park J. E., Son A. I., Hua R., Wang L., Zhang X., Zhou R. (2012). Human cataract mutations in EPHA2 SAM domain alter receptor stability and function. PLoS ONE 7, e36564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parri M., Buricchi F., Giannoni E., Grimaldi G., Mello T., Raugei G., Ramponi G., Chiarugi P. (2007). EphrinA1 activates a Src/focal adhesion kinase-mediated motility response leading to rho-dependent actino/myosin contractility. J. Biol. Chem. 282, 19619–19628 [DOI] [PubMed] [Google Scholar]
- Pasquale E. B. (2008). Eph-ephrin bidirectional signaling in physiology and disease. Cell 133, 38–52 [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. (1981). Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation 19, 134–153 [DOI] [PubMed] [Google Scholar]
- Poliakov A., Cotrina M., Wilkinson D. G. (2004). Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev. Cell 7, 465–480 [DOI] [PubMed] [Google Scholar]
- Salaita K., Nair P. M., Petit R. S., Neve R. M., Das D., Gray J. W., Groves J. T. (2010). Restriction of receptor movement alters cellular response: physical force sensing by EphA2. Science 327, 1380–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., De Maria A., Bennett T., Shiels A., Bassnett S. (2012). A role for epha2 in cell migration and refractive organization of the ocular lens. Invest. Ophthalmol. Vis. Sci. 53, 551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A., Bennett T. M., Knopf H. L., Maraini G., Li A., Jiao X., Hejtmancik J. F. (2008). The EPHA2 gene is associated with cataracts linked to chromosome 1p. Mol. Vis. 14, 2042–2055 [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. (1991). Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 64, 693–702 [DOI] [PubMed] [Google Scholar]
- Sugiyama Y., Akimoto K., Robinson M. L., Ohno S., Quinlan R. A. (2009). A cell polarity protein aPKClambda is required for eye lens formation and growth. Dev. Biol. 336, 246–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y., Stump R. J., Nguyen A., Wen L., Chen Y., Wang Y., Murdoch J. N., Lovicu F. J., McAvoy J. W. (2010). Secreted frizzled-related protein disrupts PCP in eye lens fiber cells that have polarised primary cilia. Dev. Biol. 338, 193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan P., Ravindran R. D., Vashist P., Shanker A., Nitsch D., Talwar B., Maraini G., Camparini M., Nonyane B. A., Smeeth L., et al. (2012). EPHA2 polymorphisms and age-related cataract in India. PLoS ONE 7, e33001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto M., Fukuda T., Sonoda R., Murakami F., Tanaka H., Yamamoto N. (2002). Ephrin-B3-EphA4 interactions regulate the growth of specific thalamocortical axon populations in vitro. Eur. J. Neurosci. 16, 1168–1172 [DOI] [PubMed] [Google Scholar]
- Tan W., Hou S., Jiang Z., Hu Z., Yang P., Ye J. (2011). Association of EPHA2 polymorphisms and age-related cortical cataract in a Han Chinese population. Mol. Vis. 17, 1553–1558 [PMC free article] [PubMed] [Google Scholar]
- Thomas S. M., Brugge J. S. (1997). Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13, 513–609 [DOI] [PubMed] [Google Scholar]
- Tomar A., Lawson C., Ghassemian M., Schlaepfer D. D. (2012). Cortactin as a target for FAK in the regulation of focal adhesion dynamics. PLoS ONE 7, e44041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. L., Wolff I. M., Zhang L., Menko A. S. (2007). Activation of SRC kinases signals induction of posterior capsule opacification. Invest. Ophthalmol. Vis. Sci. 48, 2214–2223 [DOI] [PubMed] [Google Scholar]
- Wang W., Liu Y., Liao K. (2011). Tyrosine phosphorylation of cortactin by the FAK-Src complex at focal adhesions regulates cell motility. BMC Cell Biol. 12, 49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Lin W. C., Petit R. S., Groves J. T. (2011). EphA2 receptor activation by monomeric Ephrin-A1 on supported membranes. Biophys. J. 101, 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman T. J. (2004). A renaissance for SRC. Nat. Rev. Cancer 4, 470–480 [DOI] [PubMed] [Google Scholar]
- Zampighi G. A., Eskandari S., Kreman M. (2000). Epithelial organization of the mammalian lens. Exp. Eye Res. 71, 415–435 [DOI] [PubMed] [Google Scholar]
- Zantek N. D., Azimi M., Fedor-Chaiken M., Wang B., Brackenbury R., Kinch M. S. (1999). E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth Differ. 10, 629–638 [PubMed] [Google Scholar]
- Zhang T., Hua R., Xiao W., Burdon K. P., Bhattacharya S. S., Craig J. E., Shang D., Zhao X., Mackey D. A., Moore A. T., et al. (2009). Mutations of the EPHA2 receptor tyrosine kinase gene cause autosomal dominant congenital cataract. Hum. Mutat. 30, E603–E611 [DOI] [PubMed] [Google Scholar]
- Zhou J., Menko A. S. (2004). Coordinate signaling by Src and p38 kinases in the induction of cortical cataracts. Invest. Ophthalmol. Vis. Sci. 45, 2314–2323 [DOI] [PubMed] [Google Scholar]
- Zhou J., Leonard M., Van Bockstaele E., Menko A. S. (2007). Mechanism of Src kinase induction of cortical cataract following exposure to stress: destabilization of cell-cell junctions. Mol. Vis. 13, 1298–1310 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.